Abstract
Background
Here, we report a laboratory-based study of Streptococcus pneumoniae recovered from patients with meningitis in Rio de Janeiro State, Brazil.
Methods
The aim of this study was to determine the evolution of β-lactam resistance, antimicrobial susceptibility pattern, serotypes, and genetic diversity of S. pneumoniae, isolated from meningitis patients between 2000 and 2008.
Results
A total of 264 S. pneumoniae recovered from patients between 2000 and 2008 were included. Susceptibility testing (E-test) of S. pneumoniae showed resistance to penicillin, ceftriaxone, oxacillin, cotrimoxazole, tetracycline, ofloxacin, erythromycin, chloramphenicol, and rifampicin. Penicillin resistance (PEN-R, minimal inhibitory concentration [MIC] ≥0.12 μg/mL) increased from 8% of isolates in 2000–2002, to 12% in 2003–2005, and to 20% in 2006–2008. Ceftriaxone resistance (MIC ≥1.0 μg/mL) was detected among some PEN-R isolates (13%) from 2004 onward. Within the PEN-R isolates, serotypes that are included in 10-valent pneumococcal conjugate vaccine predominated (90%), and resistance was detected mostly in isolates of serotypes 14 (61%), 23F (16%), 6B (10%), and 19F (3%). Multilocus sequence typing showed that 52% of the PEN-R isolates, and 89% of those with MICs ≥0.5 μg/mL, were sequence type (ST)-156 or single-locus variants of this ST (ST-557 or ST-4388); all of these isolates were serotype 14 and were assigned to the Spain9V-3 clone.
Conclusions
β-lactam resistance increased recently among cerebrospinal fluid isolates and was mainly due to the surge of the ST-4388, a previously undescribed gki single-locus variants of ST-156. Regional surveillance is shown to be essential to provide optimal antimicrobial therapy, monitor highly successful clones, and formulate adequate vaccination strategy.
Keywords: Streptococcus pneumoniae, meningitis, β-lactam resistance, MLST, serotypes
Although conjugate vaccines are having a major impact in those countries in which they have been introduced, Streptococcus pneumoniae remains a major cause of human morbidity and mortality worldwide, particularly in the very young and the very old population,1 and a major cause of community-acquired bacteremia normally associated with pneumonia, meningitis, or septicemia. Meningitis is a relatively rare clinical manifestation of pneumococcal disease, but it is associated with a high probability of a fatal outcome or neurologic damage.2–4 The clinical outcome of meningitis varies according to socioeconomic aspects, age, timing of antibiotic therapy, and host genetics,5–7 and the impact of meningitis (and other invasive pneumococcal diseases) is far greater in low-income countries than in high-income ones.1,3–5
The emergence of resistance of pneumococci to β-lactams and multiple classes of antibiotics during the 1960s and 1970s has raised concerns about the treatment of patients with central nervous system infection or clinical manifestations that require intravenous antibiotic therapy.1,8–11 These concerns were reinforced by the rapid increase in the prevalence of resistance in many countries and the emergence of several major penicillin-resistant (PEN-R) and multiply antibiotic-resistant pneumococcal clones that now have spread globally.
The Meningitis Advisory Committee of the Rio de Janeiro State Department of Health has records of pneumococcal meningitis since 1986 (Fig. 1). In the early 1990s, a survey of serotype prevalence and resistance to antibiotics in S. pneumoniae was carried out in the Rio de Janeiro State, and at that time 3.5% of isolates showed resistance to penicillin (minimal inhibitory concentrations [MICs], ≥0.12–<1 μg/mL).12 Here, we report a further laboratory-based study to determine the evolution of β-lactam resistance, antimicrobial susceptibility patterns, serotypes, and the genetic diversity of S. pneumoniae recovered from patients with meningitis between 2000 and 2008.
FIGURE 1.
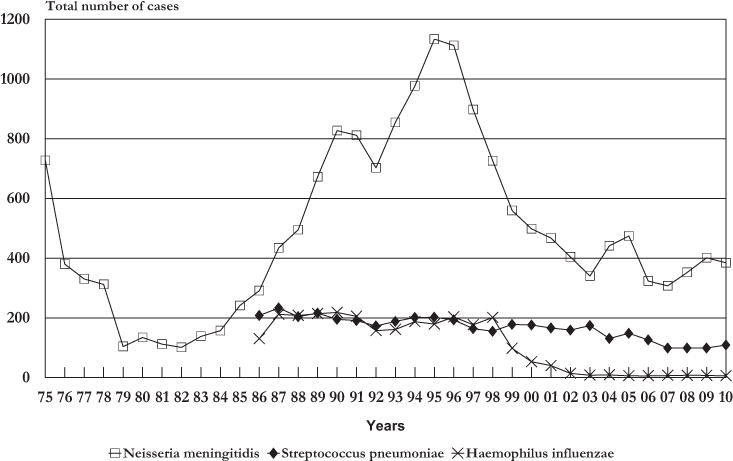
Most common etiologic sources of acute bacterial meningitis, Rio de Janeiro, 1975–2010.
MATERIALS AND METHODS
Setting, Study Population, and Study Design
In 2000, Rio de Janeiro State had a total population of approximately 14 million inhabitants, with 6 million living in the state capital city of Rio de Janeiro. Between 2000 and 2008, we conducted a laboratory-based survey of S. pneumoniae meningitis in association with the Central Laboratory Noel Nutels and the microbiology laboratory of the Infectious Diseases State Institute São Sebastião of the Rio de Janeiro State Department of Health. These laboratories receive cerebrospinal fluid (CSF) samples and/or bacteria isolated from meningitis patients from all over the Rio de Janeiro State, but mainly from state or city public hospitals in the Rio de Janeiro Metropolitan area. We also had the collaboration of 2 public federal hospital laboratories and 5 private microbiology laboratories that isolate and identify bacterial meningitis pathogens in the city of Rio de Janeiro. The associated laboratories have been providing pneumococcal isolates to the Laboratory of Biochemical Systematics of the Oswaldo Cruz Institute with demographic data for antibiotic susceptibility testing, where isolates of stored specimens were selected from reference and clinical laboratories. During the study period, most of the pneumococcal isolates were provided by the Central Laboratory Noel Nutels (in 2000–2008, n = 61) or the Infectious Diseases State Institute São Sebastião (in 2000–2008, n = 179). The other laboratories have provided fewer isolates (n = 24) since 2003. No changes occurred in the collection and storage protocols or patient population during the study period. Also, there were no significant differences in the number of isolates annually sent by the reference laboratories, or in the criteria for sending isolates to the Laboratory of Biochemical Systematics during the period of study; 30 isolates were not viable on receipt.
Demographic and clinical characteristics reported of the patients with pneumococcal meningitis were provided by the Meningitis Advisory Committee, Rio de Janeiro State Department of Health. Clinical data of patients enrolled were obtained retrospectively by patient record review. Clinical and epidemiological data were analyzed using Epi Info (Version 3.5.3, CDC). The heterogeneity of proportions between groups was compared using the χ2 test with Yates correction for statistical significance. All results for continuous variables are expressed by means.
Isolation and Identification of S. pneumoniae
Heated blood agar slants were used to culture bacteria from the CSF or blood culture of patients with clinically diagnosed meningitis. The growth of α-hemolytic isolates on blood agar plates with inhibition zones >15 mm using a 5-μg optochin disc (Oxoid, Cambridge, England) after incubation in a 5% CO2/95% air atmosphere (candle jar) was used to identify S. pneumoniae. Additional confirmatory tests included bile solubility and capsular serotyping. Serotyping was determined by the Neufeld-Quellung reaction in the Department of Microbiology, John Radcliffe Hospital, Oxford, United Kingdom, between 2000 and 2002, and in the Meningitis, Pneumonia and Pneumococcal Infections Core (National Reference Laboratory), Bacteriology Centre of the Adolfo Lutz Institute, São Paulo, Brazil, between 2003 and 2008. The National Reference Laboratory provides serotyping results to the Department of Health of each State as part of the meningitis surveillance program of the Brazilian Ministry of Health.
Antibiotic Susceptibility Testing
MICs of penicillin, ceftriaxone, oxacillin, rifampin, vancomycin, chloramphenicol, erythromycin, ofloxacin, tetracycline, and cotrimoxazole were determined by means of the E-test (AB Biodisk, Solna, Sweden) according to the manufacturer’s instruction; MICs of meropenem and gemifloxacin—2 alternative drugs to the treatment of pneumococcal meningitis4,9—were determined for isolates showing resistance to β-lactams. Briefly, a single E-test strip was placed onto blood agar (Oxoid Müeller-Hinton base with 5% sheep blood) plates that were incubated at 35°C in a 5% CO2/95% air atmosphere for 20 to 24 hours. The disc diffusion method to predict resistance to β-lactams and macrolides was performed with 1-μg oxacillin and 2-μg clindamycin discs (Oxoid) following the procedures described for the E-test. The reference strain Streptococcus pneumoniae ATCC 49619 was included as quality control. Zone diameter and MIC interpretative standards for S. pneumoniae were based on the Clinical and Laboratory Standards Institute document M100-S20.13 Isolates were stored in freezer in vials containing tryptone soy broth with 20% glycerol at −70°C.
Sequencing of Housekeeping Gene Fragments
Molecular characterization of 76 penicillin-susceptible (PEN-S; 2000–2002) and 31 PEN-R (2000–2008) S. pneumoniae was performed by multilocus sequence typing (MLST) according to the procedures previously described.14 One isolated colony from each isolate was subcultured on catalase tryptic soy agar plate (Oxoid) with 1000 units/mL of Sigma catalase, and DNA was isolated from the resuspended bacteria after overnight culture, using a QIAamp DNA Mini Kit (QIAGEN, Hilden, Germany), according to the manufacturer’s protocol for DNA purification from Gram-positive bacteria. Internal fragments of aroE, gdh, gki, recP, spi, xpt, and ddl were amplified and sequenced using an Applied Biosystems 3700 DNA analyzer (PE Applied Biosystems, Foster City, CA). Allele and sequence type (ST) numbers were assigned by submission to the pneumococcal MLST database (http://spneumoniae.mlst.net). Data generated by MLST were analyzed against all STs found in the online database to organize the population into clonal complexes, which is defined here as a group of STs in a population that shares 6 to 7 alleles with at least one other ST in the group and named following the nomenclature proposed.15 Bionumerics software (version 5.10; Applied Maths, Sint-Martens-Latem, Belgium) was used to create minimum spanning trees (Figs., Supplemental Digital Content 1–3, http://links.lww.com/INF/A934).16
Ethical Considerations
The study was reviewed and approved by the Ethical Committee of the Evandro Chagas Research Institute of the Oswaldo Cruz Foundation (FIOCRUZ), Brazilian Ministry of Health (protocol 0007.0.009.011–07).
RESULTS
Since 1999, S. pneumoniae has been reported as the second most common cause of bacterial meningitis in the State of Rio de Janeiro (Fig. 1). Between 2000 and 2008, 1272 cases of pneumococcal meningitis (G00.1, ISCD-10) were reported to the Rio de Janeiro State Department of Health; 51% (653) were culture-proven cases and 45% (573) were confirmed by the latex agglutination test (46 missing data). The average annual incidence rate was 1 case/100,000 inhabitants (range, 0.6–1.2). Of the total, 464 cases died with an overall case fatality rate of 36% (range, 31%–47%). The highest case fatality rates were recorded for young children (40%) and elderly patients (52%).
All isolates stored in the bacteria collection were selected from each year for this study. Between 2000 and 2008, culture-proven pneumococcal meningitis patients were retrospectively enrolled by selection of 264 stored S. pneumoniae isolates from reference and clinical laboratories. Of the 264 patients selected, 56% were male and 44% were female. The medium interval between the first symptoms and hospital admission was 1.9 days (range, 0–6). History of recurrent pneumococcal meningitis was recorded in 16 (6%) patients during the study period (2–6 episodes), mainly due to head trauma resulting in basilar skull fracture with leakage of CSF. A concurrent site of infection (otitis, sinusitis, or pneumonia) was detected in 18%, and HIV infection in 2% of the 202 patients with clinical characteristics recorded (62 missing data). At presentation, 24% had convulsions, 18% were comatose, and 4% had a hemorrhagic rash. For treatment, ceftriaxone (70%) and, to lesser extent (15%), ceftriaxone in combination with a second drug (ampicillin, oxacillin, or vancomycin) were the most common therapeutic options used, followed by penicillin (13%) or ampicillin (2%).
S. pneumoniae isolates analyzed were isolated from CSF (n = 248) or blood (n = 16). CSF results were available for 231 (88%) patients, which could be classified by visual inspection as follows: clear (19%), cloudy (73%), purulent (5%), or hemorrhagic (3%). The mean CSF white blood cell count was 3095/mm3 (range, 1–48,000/mm3); 14% had fewer than 100/mm3.
The age of patients enrolled in this study varied from 1 month to 78 years. The average age of patients infected with PEN-R S. pneumoniae was 8 years (median = 1 y) and for patients from whom PEN-S isolates were recovered, it was 23 years (median = 18 y). Most (71%; 22/31) of the PEN-R isolates were recovered from children <5 years of age (Fig. 2), whereas only 27% (61/227; 6 missing data) of the PEN-S isolates were recovered from this age group (χ2 = 22; P < 0.01). Also, a significant proportion of infants (39%) were infected with PEN-R isolates compared with the proportion of infants (19%) infected with PEN-S isolates (χ2 = 5; P = 0.02). For the 250 of the 264 culture-proven pneumococcal meningitis cases where full antibiotic susceptibility data were available, there was no difference in mortality between patients infected with PEN-R (35%, 11/31) or PEN-S (37%, 81/219) isolates (P = 0.97). Nevertheless, all patients treated solely with penicillin, ampicillin, or oxacillin (1 adult and 3 young children) for infection with PEN-R (MICs, 0.19–2 μg/mL) S. pneumoniae died within 72 hours; they had history of illness for less than 48 hours.
FIGURE 2.
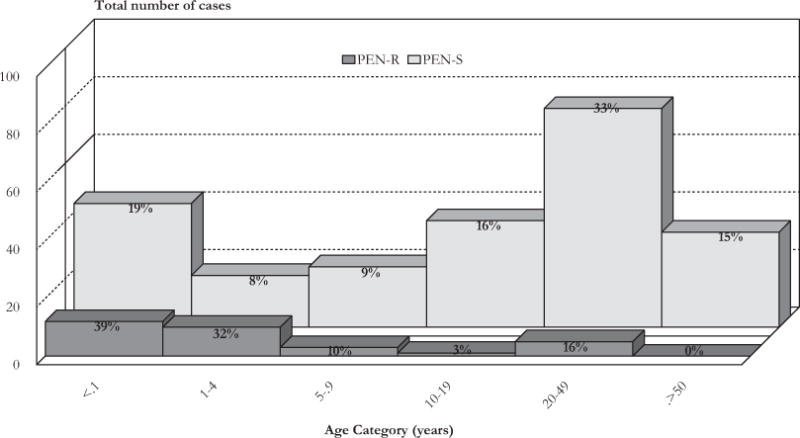
Distribution of patients by age category and penicillin-susceptibility profile (penicillin resistant: PEN-R; penicillin susceptible: PEN-S) of infecting S. pneumoniae enrolled in the study, 2000–2008.
The 1-μg oxacillin disc test identified 24% (64/264) of isolates having zone diameters of <20 mm. Although this test correctly identified all PEN-R (MIC, ≥0.12 μg/mL) isolates (12%, 31/264), which had inhibition zones of 6 to 14 mm, 14% of isolates that were susceptible to penicillin had zones <20 mm (9–19 mm). The latter isolates had oxacillin MICs of 0.38 to 2 μg/mL but penicillin MICs <0.12 μg/mL.
Penicillin resistance (MICs ranging from 0.12–2 μg/mL, in the present study) increased from 8% of isolates in 2000–2002, to 12% in 2003–2005, and to 20% in 2006–2008. Ceftriaxone MICs of PEN-R isolates were 0.023 to 0.094 μg/mL (29%), 0.125 to 0.38 μg/mL (29%), 0.50 to 0.75 μg/mL (29%), and 1 μg/mL (13%). The 4 ceftriaxone-resistant isolates (MIC of 1.0 μg/mL) were also PEN-R and were first detected in 2004; all were from children up to 2 years of age. Using the E-test, PEN-R S. pneumoniae also showed resistance to cotrimoxazole (74% MICs, 1–12 μg/mL; I 29%, R 45%), tetracycline (10% MICs, 8–16 μg/mL), ofloxacin (single isolate MIC, 4 μg/mL), and erythromycin (single isolate MIC, >256 μg/mL). All these PEN-R isolates were susceptible to vancomycin (MICs, 0.25–0.75 μg/mL), meropenem (MICs, 0.004–0.19 μg/mL), and gemifloxacin (MICs, 0.008–0.047 μg/mL).
Within the PEN-S group of isolates, 35% were resistant to cotrimoxazole (MICs, 1–32 μg/mL; I = 16%, R = 19%), 17% to tetracycline (MICs, 4–32 μg/mL; I = 5%, R = 12%), 8% to ofloxacin (MICs, 4–>32 μg/mL; I = 7%, R = 1%), 5% to erythromycin (MICs, 24–>256 μg/mL), 2% to chloramphenicol (MICs, 8–12 μg/mL), and 0.5% to rifampicin (MIC, 4 μg/mL); in 3% ceftriaxone MICs were 0.12 to 0.19 μg/mL, with oxacillin MICs of 0.5 to 2 μg/mL. The PEN-S isolates were fully susceptible to vancomycin (MICs, 0.25–0.75 μg/mL). All erythromycin-resistant isolates were also resistant to clindamycin; 80% had erythromycin MIC >256 μg/mL.
Among all meningitis isolates, the most frequent serotypes were 14 (17%), 6A (10%), 6B (6%), 19F (6%), 23F (6%), 3 (5%), 10A (4%), 8 (3%), 18C (3%), 9N (3%), and 11A (3%). Within the 31 PEN-R isolates (Fig., Supplemental Digital Content 1, http://links.lww.com/INF/A934), serotypes that are included in the 10-valent pneumococcal conjugate vaccine predominated (90%; 28/31): 14 (61%), 23F (16%), 6B (10%), 19F (3%). Single PEN-R isolates were serotype 23B and 11F. PEN-S isolates expressed a wider range of serotypes (Fig., Supplemental Digital Content 2, http://links.lww.com/INF/A934), with only 36% (78/216; 17 missing data) of these covered by the 10-valent conjugate vaccine: 14 (10%), 6B (6%), 19F (6%), 23F (4%), 18C (3%), 9V (2%), 5 (2%), 4 (1%), 1 (1%), and 7F (1%).
MLST showed that 52% of the PEN-R isolates and 89% of those with MICs ≥0.5 μg/mL (Table 1) were ST-156 (20%), or ST-557 (2%; a single-locus variant [SLV] of ST-156 that differs at xpt) or ST-4388 (30%; an SLV of ST-156 that differs at gki). Isolates of these STs were serotype 14, showed resistance only to β-lactams and cotrimoxazole (Table 1), and were assigned to the Spain9V-3 clone (Fig., Supplemental Digital Content 1, http://links.lww.com/INF/A934). The second most common clone encountered was Columbia23F-26, exhibiting either serotype 23F or 14 (Fig., Supplemental Digital Content 1, http://links.lww.com/INF/A934), which was resistant to penicillin and cotrimoxazole (Table 1). Isolates of the Spain9V-3 (2004–2008) clone and a single isolate of the Taiwan19F-14 (2007) clone were the only ones that showed reduced susceptibility (MIC, 1.0 μg/mL) to ceftriaxone (Table 1). This single isolate of the Taiwan19F-14 (ST-271) clone was also resistant to erythromycin, tetracycline, and cotrimoxazole. In addition to ST-4388, we found 6 new STs in isolates that were resistant to penicillin, mostly of serotype 6B (Fig., Supplemental Digital Content 1, http://links.lww.com/INF/A934; Table 1).
TABLE 1.
Major Clonal Complexes of PEN-R Streptococcus pneumoniae Isolates Identified in the Rio de Janeiro Region With Corresponding Serotype, Age of Patient, and MICs for Penicillin (PG), Ceftriaxone (TX), and Cotrimoxazole (TS)
| Year | Clonal Complex | ST | Serotype | Age | PG | TX | TS |
|---|---|---|---|---|---|---|---|
| 2001 | England14-9 | 15 | 14 | 35 y | 0.19 | 0.064 | 0.75 |
| 2001 | England14-9 | 15 | 14 | 4 mo | 0.125 | 0.125 | 2 |
| 2001* | Columbia23F-26 | 732 | 23F | 7 y | 0.125 | 0.064 | 2 |
| 2003 | Columbia23F-26 | 2919 | 23F | 1 y | 0.125 | 0.032 | 3 |
| 2005 | Columbia23F-26 | 2919 | 14 | 1 mo | 0.125 | 0.125 | 0.125 |
| 2006 | Columbia23F-26 | 338 | 23F | 7 y | 0.125 | 0.125 | 4 |
| 2007 | Columbia23F-26 | 338 | 23F | 2 y | 0.125 | 0.125 | 2 |
| 2001 | Spain9V-3 | 156 | 14 | 1 y | 0.75 | 0.38 | 1 |
| 2002 | Spain9V-3 | 156 | 14 | 2 y | 1.5 | 0.50 | 4 |
| 2002* | Spain9V-3 | 4388 | 14 | 30 y | 1 | 0.25 | 0.50 |
| 2003* | Spain9V-3 | 4388 | 14 | 8 mo | 0.75 | 0.50 | 4 |
| 2003 | Spain9V-3 | 156 | 14 | 7 mo | 0.50 | 0.25 | 3 |
| 2003* | Spain9V-3 | 4388 | 14 | 9 mo | 2 | 0.38 | 0.75 |
| 2004* | Spain9V-3 | 4388 | 14 | 5 mo | 1 | 1 | 1.5 |
| 2004* | Spain9V-3 | 4388 | 14 | 43 y | 1 | 0.50 | 3 |
| 2005* | Spain9V-3 | 4388 | 14 | 4 mo | 1 | 0.75 | 0.75 |
| 2005 | Spain9V-3 | 156 | 14 | 4 y | 0.50 | 0.38 | 2 |
| 2006* | Spain9V-3 | 4388 | 14 | 7 mo | 0.75 | 0.75 | 0.75 |
| 2006* | Spain9V-3 | 4388 | 14 | 4 mo | 1.5 | 0.75 | 6 |
| 2006 | Spain9V-3 | 557 | 14 | 1 y | 1 | 1 | 12 |
| 2007 | Spain9V-3 | 156 | 14 | 1 y | 1 | 0.50 | 4 |
| 2008* | Spain9V-3 | 4388 | 14 | 4 mo | 1 | 0.75 | 2 |
| 2008 | Spain9V-3 | 156 | 14 | 8 mo | 2 | 1 | 2 |
| 2007 | Taiwan19F-14 | 271 | 19F | 2 y | 1 | 1 | 8 |
| 2002 | SLV ST-753 | 812† | 11F | 16 y | 0.125 | 0.094 | 0.38 |
| 2003* | SLV ST-353 | 5961 | 23F | 47 y | 0.19 | 0.094 | 2 |
| 2004* | SLV ST-751 | 4426‡ | 6B | 8 y | 0.125 | 0.094 | 0.38 |
| 2001* | Singleton | 748 | 6B | 6 mo | 0.125 | 0.047 | 1.5 |
| 2002* | Singleton | 5962 | 23B | 4 y | 0.19 | 0.023 | 8 |
| 2004* | Singleton | 4431 | 23B | 26 y | 0.19 | 0.064 | 3 |
| 2006* | Singleton | 4425 | 6B | 2 y | 0.75 | 0.50 | 3 |
New sequence types.
ddl SLV of ST-753 present here as serotype 6A PEN-S isolate (Fig., Supplemetal Digital Content 3, http://links.lww.com/INF/A934).
gki SLV of ST-751 present here as serotype 6B PEN-S isolate (Fig., Supplemetal Digital Content 3, http://links.lww.com/INF/A934).
PEN-S isolates were a more genetically diverse population (Figs., Supplemental Digital Content 2 and 3, http://links.lww.com/INF/A934), including new STs (37%) all singletons unrelated to other isolates in the database, new STs (34%) related to other isolates in the database, and STs (29%) belonging to previously described major PEN-R and multiply antibiotic-resistant pneumococcal clones. Only 3 of these global clones, that is, England14-9, Columbia23F-26, and Spain9V-3, were also found within the PEN-R group of isolates (Figs., Supplemental Digital Content 1 and 3, http://links.lww.com/INF/A934). Erythromycin resistance was mainly found among PEN-S isolates; most were assigned to the England14-9 clone (ST-15), a widespread highly successful clone with isolates in this study exhibiting serotype 14 (Figs., Supplemental Digital Content 2 and 3, http://links.lww.com/INF/A934); the others were the Netherlands8-33 (ST-53; serotype 8) clone or ST-721 (serotype 11A), an SLV of ST-15 that differs at ddl. Two isolates of the ST-722 (serotype 14) were SLV of ST-15 that differs at recP, but susceptible to erythromycin. The ST-764 (serotype 5) found here exhibiting resistance to oxacillin (MIC, 1.5 μg/mL), tetracycline, and cotrimoxazole was an xpt SLV of ST-289 assigned to the global clone Columbia5-19 (Fig., Supplemental Digital Content 3, http://links.lww.com/INF/A934), originally associated with resistance to chloramphenicol and tetracycline. The Tennesse14-18 clone was represented here by the ST-66, the ST-737 (a recP SLV of ST-66), or the ST-738 (a ddl SLV of ST-66), which were associated with cotrimoxazole or oxacillin resistance and multiple capsules (Figs., Supplemental Digital Content 2 and 3, http://links.lww.com/INF/A934). The ST-754 (serotype NT) also found here exhibiting resistance to oxacillin (MIC, 2 μg/mL) and chloramphenicol (Fig., Supplemental Digital Content 3, http://links.lww.com/INF/A934) is related in the database to 4 nasopharynx isolates recovered in Brazil of the 3 STs 2315, 2982, and 4746, all ddl SLV of ST-754. The ST-752 is a gdh SLV of ST-162 assigned to the global clone Spain9V-3, both present here as serotype 9V and showing resistance only to cotrimoxazole (Fig., Supplemental Digital Content 3, http://links.lww.com/INF/A934).
DISCUSSION
S. pneumoniae is the second most common bacterium recovered from patients with suppurative meningitis in the Rio de Janeiro region and is responsible for the majority of fatal outcomes; the number of survivors who develop permanent neurologic sequelae is unknown.17 Until recently, penicillin resistance among meningitis-causing S. pneumoniae remained at low frequency in Rio de Janeiro, but it increased, together with the emergence of ceftriaxone resistance, after 2003. Although a similar trend was also reported elsewhere in Brazil (2000–2004),18 it has not been associated with a clonal complex as we have shown earlier.
Pediatric patients were the most affected by pneumococcal meningitis due to β-lactam-resistant organisms, which is in accordance with other published studies.8,9,18–21 Delayed administration of a thought-through empiric antibiotic regimen by injection and the limited availability of supportive intensive care might explain the high case fatality rates found among patients in this study, in particular in infants, patients older than 50 years of age, and disease caused by PEN-R isolates.
Antibiotic resistance is a particular threat in regions where there are a large number of meningitis cases of unknown cause, or where the bacteria cannot be cultured from clinical specimens and the etiologic agent is identified solely by antigen detection,17 because β-lactams resistance can go undetected if clinical specimens from a sterile site cannot be cultured. Another challenge involving the treatment of meningitis patients is the reduced number of local hospitals routinely performing pneumococcal susceptibility testing or capable of determining antibiotics MICs to bacterial meningitis pathogens, which might influence therapeutic decision and patient outcome.
Erythromycin resistance was mostly found among PEN-S isolates in every instance detected by the 2-μg clindamycin disc test. This antimicrobial susceptibility pattern suggests that the main mechanism of resistance is mediated by target modification by the rRNA methylase encoded by ermB, carried by the conjugative transposon Tn1545, which also confers resistance to tetracycline and streptomycin.2 However, clindamycin disc for screening S. pneumoniae for resistance to macrolides due to antibiotic efflux mediated by the mefA gene is of no value, because susceptibility to clindamycin is retained in isolates with this mechanism of resistance to macrolides, including erythromycin and clarithromycin.22
A 10-valent (1, 5, 4, 6B, 7F, 9V, 14, 18C, 19F, 23F) pneumococcal conjugate vaccine was introduced in the National Immunization Programme for young children (<2 y) during 2010.23 The introduction of such a vaccine in the Rio de Janeiro region should have a significant impact on the reduction of pneumococcal invasive disease caused by resistant clones,21 because the pediatric population is the most affected by these. In contrast, this strategy is not expected to cause a significant impact on the overall number of cases of invasive disease, because both the serotype profile of clinical isolates and the chosen target population.
In a previous study, analysis by pulsed-field gel electrophoresis of PEN-R S. pneumoniae recovered from young children with invasive disease (91 isolates from Brazilian patients) from 6 Latin-American countries (1993–1996) showed that 80% were represented by the “Spanish/USA” (serotype 23F) and the “French/Spanish” (serotypes 9 or 14) clones,24 later referred to as the Spain23F-1 and Spain9V-3 clones.25 MLST analysis of only PEN-S invasive isolates (21 isolates from Brazilian meningitis patients) from the same Latin-American countries (late 1990s–2002) revealed extensive genetic diversity,25 as we have shown here. Another study with isolates recovered from meningitis patients (2000–2001) living in the city of Salvador, northeast Brazil, found that most (56%; 33/59) of the PEN-R isolates belonged to the Tennesse14-18 clone (ST-66; serotype 14),26 which was found here exhibiting multiple capsules, fully susceptible to penicillin, and were either ST-66 or SLVs of this ST. Subsequently, studies with more recent data of molecular surveillance of PEN-R S. pneumoniae using MLST in Brazil have not been published. The data presented here, together with other studies,23,25 suggest regional differences in the prevalence of international PEN-R clones associated with pneumococcal meningitis. However, these studies were carried out at different times, which makes it difficult to address regional differences.
Some of the major multiresistant pneumococci clones were recovered within Rio de Janeiro during the 2000s. The majority of PEN-R and multiresistant S. pneumoniae isolates appear to be similar to those previously encountered in Western Europe and in the United States.2 A rapid increase in the proportion of PEN-R isolates has been related to the establishment within a community of multiply antibiotic-resistant pneumococci originally imported from Spain.2 This can be the first step to the emergence of novel PEN-R and multiresistant isolates within a community. Among the diversity of PEN-R isolates described here, there were successful isolates of the international resistant Spain9V-3 clone present here as serotype 14 variant that have entrenched and spread β-lactam resistance in Rio de Janeiro during the 2000s. It was associated here mainly due to the surge of the ST-4388, a previously undescribed gki SLV of ST-156. Continuing molecular surveillance will be necessary to monitor the ability of ST-4388 to spread within this country and change its capsule serotype.
A critical independent factor that influences the outcome for a patient with suppurative bacterial meningitis is the time lapse after disease onset.6 High degree of alertness of the attendant physician and the immediate administration of a well-considered empiric antibiotic regimen are of paramount importance for patient survival. We have shown the rates of β-lactams resistance steadily increasing in Rio de Janeiro; therefore, initial combination therapy with vancomycin is recommended.1,4,27 Subsequently, antibiotic therapy should be ideally guided by laboratory results of local hospital microbiology. The advice is to perform the MICs of penicillin and ceftriaxone in parallel, because cephalosporins should be used alone to the treatment of pneumococcal meningitis just for isolates with MICs to cephalosporins of <0.5 μg/mL.2,28 Also, there are S. pneumoniae isolates with reduced susceptibility to ceftriaxone, but fully susceptible to penicillin.28 In this context, our results confirm the value of the 1-μg oxacillin disc test for screening β-lactam resistance and guide initial therapy, even in laboratories capable to perform MICs. Nevertheless, laboratory investigations to detect meningitis pathogen are time-consuming, with an additional limitation of culture-based methods to detect vancomycin-tolerant isolates.10,11 Finally, it is necessary to improve access to multiple classes of antibiotics for initial combination therapy according to age category at the emergency room and rapid admission to an intensive care unit whenever it is indicated, as well as the implementation of pneumococcal susceptibility testing in the hospital laboratories. Unfortunately, these will not overcome the problems of poor access to hospitals and the tragic consequences of delayed treatment in areas with social deprivation.
Supplementary Material
Acknowledgments
The authors thank Prof. Brian G. Spratt at the Department of Infectious Disease Epidemiology, Imperial College School of Medicine, London, United Kingdom, for his critical review on this manuscript and support for this study. They thank David Griffiths, Department of Microbiology, John Radcliffe Hospital, Oxford, United Kingdom, and the staff of the Meningitis, Pneumonia and Pneumococcal Infections Core (National Reference Laboratory), Bacteriology Centre of the Adolfo Lutz Institute, São Paulo, Brazil, for serotyping results. Many thanks to Andréia Rodrigues Gonçalves Ayres, Cristiane Barcelos Ribeiro, and Elaine de Oliveira Cerqueira at the Meningitis Advisory Committee, Rio de Janeiro State Department of Health, for providing the epidemiological surveillance data. They acknowledge the staff of the microbiology laboratories of the Laboratório Central de Saúde Pública Noel Nutels, Instituto Estadual de Infectologia São Sebastião, Hospital Federal Cardoso Fontes, Hospital Federal de Bonsucesso, Hospitais da Rede INFECTO Consultoria, Hospital Barra D’Or, Neurolife, and Richet Laboratório for providing pneumococcal isolates. They also thank Jane W. Marsh, Infectious Diseases Epidemiology Research Unit, University of Pittsburgh School of Medicine and Graduate School of Public Health, Pittsburgh, PA, for her technical support. They acknowledge the curator Cynthia Bishop of the MLST database for Streptococcus pneumoniae.
Supported in part by a Fogarty International Center Global Infectious Diseases Research Training Program grant, National Institutes of Health, to the University of Pittsburgh (D43TW006592). L.H.H. receives research support and lecture fees from Sanofi Pasteur; lecture fees from Novartis Vaccines; and has served as a consultant to GlaxoSmithKline, Novartis Vaccines, Sanofi Pasteur, and Pfizer.
Footnotes
Presented in part at the 7th International Symposium on Pneumococci and Pneumococcal Diseases 2010, Tel Aviv, Israel.
The authors have no other funding or conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.pidj.com).
References
- 1.Scarborough M, Thwaites GE. The diagnosis and management of acute bacterial meningitis in resource-poor settings. Lancet Neurol. 2008;7:637–648. doi: 10.1016/S1474-4422(08)70139-X. [DOI] [PubMed] [Google Scholar]
- 2.Crook DW, Spratt BG. Multiple antibiotic resistance in Streptococcus pneumoniae. Br Med Bull. 1998;54:595–610. doi: 10.1093/oxfordjournals.bmb.a011713. [DOI] [PubMed] [Google Scholar]
- 3.Koedel U, Scheld WM, Pfister HW. Pathogenesis and pathophysiology of pneumococcal meningitis. Lancet Infect Dis. 2002;2:721–736. doi: 10.1016/s1473-3099(02)00450-4. [DOI] [PubMed] [Google Scholar]
- 4.Klein M, Pfister HW, Leib SL, et al. Therapy of community-acquired acute bacterial meningitis: the clock is running. Expert Opin Pharmacother. 2009;10:2609–2623. doi: 10.1517/14656560903277210. [DOI] [PubMed] [Google Scholar]
- 5.Hoen B, Viel JF, Gerard A, et al. Mortality in pneumococcal meningitis: a multivariate analysis of prognostic factors. Eur J Med. 1993;2:28–32. [PubMed] [Google Scholar]
- 6.Auburtin M, Wolff M, Charpentier J, et al. Detrimental role of delayed antibiotic administration and penicillin-nonsusceptible isolates in adult intensive care unit patients with pneumococcal meningitis: the PNEU-MOREA prospective multicenter study. Crit Care Med. 2006;34:2758–2765. doi: 10.1097/01.CCM.0000239434.26669.65. [DOI] [PubMed] [Google Scholar]
- 7.Brouwer MC, Gans J, Heckenberg SG, et al. Host genetic susceptibility to pneumococcal and meningococcal disease: a systematic review and meta-analysis. Lancet Infect Dis. 2009;9:31–44. doi: 10.1016/S1473-3099(08)70261-5. [DOI] [PubMed] [Google Scholar]
- 8.Appelbaum PC. World-wide development of antibiotic resistance in pneumococci. Eur J Clin Microbiol. 1987;6:367–377. doi: 10.1007/BF02013089. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan SL, Mason EO. Management of infections due to antibiotic-resistant Streptococcus pneumoniae. Clin Microbiol Rev. 1998;11:628–644. doi: 10.1128/cmr.11.4.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Novak R, Henriques B, Charpentier E, et al. Vancomycin tolerance in Streptococcus pneumoniae. Nature. 1999;399:590–593. doi: 10.1038/21202. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell L, Tuomanen EI. Vancomycin-tolerant Streptococcus pneumoniae and its clinical significance. Pediatr Infect Dis J. 2001;20:531–533. doi: 10.1097/00006454-200105000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Teixeira LM, Carvalho MG, Castineiras TM, et al. Serotyping distribution and antimicrobial resistance of Streptococcus pneumoniae isolated in Brazil (1992–1996) Adv Exp Med Biol. 1997;418:269–271. doi: 10.1007/978-1-4899-1825-3_66. [DOI] [PubMed] [Google Scholar]
- 13.Clinical Laboratory Standard Institute (CLSI) CLSI document M100-S20. Wayne, PA: CLSI; 2010. Performance Standards for Antimicrobial Susceptibility Testing: 20th Informational Supplement. [Google Scholar]
- 14.Enright MC, Spratt BG. A multilocus sequence typing scheme for Streptococcus pneumoniae: identification of clones associated with serious invasive disease. Microbiology. 1998;144:3049–3060. doi: 10.1099/00221287-144-11-3049. [DOI] [PubMed] [Google Scholar]
- 15.McGee L, McDougal L, Zhou J, et al. Nomenclature of major antimicrobial-resistant clones of Streptococcus pneumoniae defined by the pneumococcal molecular epidemiology network. J Clin Micrbiol. 2001;39:2565–2571. doi: 10.1128/JCM.39.7.2565-2571.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feil EJ, Li BC, Aanensen DM, et al. eBURST: inferring patterns of evolutionary descent among clusters of related bacterial genotypes from multilocus sequence typing data. J Bacteriol. 2004;186:1518–1530. doi: 10.1128/JB.186.5.1518-1530.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tuyama M, Boente RF, Rebelo MC, et al. The utility of the polymerase chain reaction assay for aetiologic definition of unspecified bacterial meningitis cases. Mem Inst Oswaldo Cruz. 2008;103:138–142. doi: 10.1590/s0074-02762008000200003. [DOI] [PubMed] [Google Scholar]
- 18.Brandileone MC, Casagrande ST, Guerra ML, et al. Increase in numbers of beta-lactam-resistant invasive Streptococcus pneumoniae in Brazil and the impact of conjugate vaccine coverage. J Med Microbiol. 2006;55:567–574. doi: 10.1099/jmm.0.46387-0. [DOI] [PubMed] [Google Scholar]
- 19.Pelton SI, Huot H, Finkelstein JA, et al. Emergence of 19A as virulent and multidrug resistant pneumococcus in Massachusetts following universal immunization of infants with pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2007;26:468–472. doi: 10.1097/INF.0b013e31803df9ca. [DOI] [PubMed] [Google Scholar]
- 20.Farrell DJ, Klugman KP, Pichichero M. Increased antimicrobial resistance among nonvaccine serotypes of Streptococcus pneumoniae in the pediatric population after the introduction of 7-valent pneumococcal vaccine in the United States. Pediatr Infect Dis J. 2007;26:123–128. doi: 10.1097/01.inf.0000253059.84602.c3. [DOI] [PubMed] [Google Scholar]
- 21.Kyaw MH, Lynfield R, Schaffner W, et al. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N Engl J Med. 2006;354:1455–1463. doi: 10.1056/NEJMoa051642. [DOI] [PubMed] [Google Scholar]
- 22.Waites K, Johnson C, Gray B, et al. Use of clindamycin disks to detect macrolide resistance mediated by ermB and mefE in Streptococcus pneumoniae isolates from adults and children. J Clin Microbiol. 2000;38:1731–1734. doi: 10.1128/jcm.38.5.1731-1734.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sartori AM, Soárez PC, Novaes HM. Cost-effectiveness of introducing the 10-valent pneumococcal conjugate vaccine into the universal immunisation of infants in Brazil. J Epidemiol Community Health. doi: 10.1136/jech.2010.111880. [DOI] [PubMed] [Google Scholar]
- 24.Tomasz A, Corso A, Severina EP, et al. Molecular epidemiologic characterization of penicillin-resistant Streptococcus pneumoniae invasive pediatric isolates recovered in six Latin-American countries: an overview. PAHO/Rockefeller University Workshop. Pan American Health Organization. Microb Drug Resist. 1998;4:195–207. doi: 10.1089/mdr.1998.4.195. [DOI] [PubMed] [Google Scholar]
- 25.Zemlicková H, Crisóstomo MI, Brandileone MC, et al. Serotypes and clonal types of penicillin-susceptible Streptococcus pneumoniae causing invasive disease in children in five Latin American countries. Microb Drug Resist. 2005;11:195–204. doi: 10.1089/mdr.2005.11.195. [DOI] [PubMed] [Google Scholar]
- 26.Reis JN, Palma T, Ribeiro GS, et al. Transmission of Streptococcus pneumoniae in an urban slum community. J Infect. 2008;57:204–213. doi: 10.1016/j.jinf.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bradley JS, Kaplan SL, Klugman KP, et al. Consensus, management of infections in children caused by Streptococcus pneumoniae with decreased susceptibility to penicillin. Pedtatr Infect Dis J. 1995;14:1037–1041. [PubMed] [Google Scholar]
- 28.De Champs C, Constantin JM, Bonnet R, et al. Decreased susceptibility to extended-spectrum cephalosporins of a penicillin-susceptible Streptococcus pneumoniae in meningitis. Infection. 2000;28:58–59. doi: 10.1007/s150100050016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


