Abstract
Emerging evidence supports the importance of nitroso-redox balance in the cardiovascular system. Xanthine oxidoreductase (XOR) is a major oxidative enzyme and increased XOR activity, leading to both increased production of reactive oxygen species and uric acid, is implicated in heart failure. Within the heart, XOR activity stimulates cardiomyocyte hypertrophy, apoptosis, and impairs matrix structure. The underpinnings of these derangements can be linked not solely to oxidative stress, but may also involve the process of nitroso-redox imbalance. In this regard, XOR interacts with nitric oxide signaling at numerous levels, including a direct protein-protein interaction with neuronal nitric oxide synthase (NOS1) in the sarcoplasmic reticulum. Deficiency or translocation of NOS1 away from this microdomain leads to increased activity of XOR, which in turn impairs excitation-contraction coupling and myofilament calcium sensitivity. There is a mounting abundance of preclinical data supporting beneficial effects of inhibiting XOR, but translation to the clinic continues to be incomplete. A growing understanding of XOR and its role in nitroso-redox imbalance has great potential to lead to improved pathophysiologic insights and possibly therapeutic advances.
Keywords: Xanthine Oxidoreductase, Nitric Oxide, Nitroso-Redox Imbalance, Heart Failure, S-nitrosylation, Review
2. INTRODUCTION
Heart failure (HF) is an important cause of morbidity and mortality worldwide (1, 2), and currently represents the leading cause of hospitalization in individuals older than 65 years (2, 3). Despite the significant progress in its management, 5-year mortality rates are still as high as 50% and are even greater in the more advanced stages of the disease (1, 2). The prevalence of HF increases with age and therefore the number of patients with HF is expected to rise with the progressive aging of the population (3). The improved survival of patients with myocardial infarction (MI) is an added driver of the increasing prevalence of HF (1, 4).
The regulated production of reactive oxygen species (ROS) is crucial for cellular homeostasis (5). In this regard, there is accumulating evidence that ROS play signaling roles and participate in the regulation of many cellular functions (5). However, oxidative stress denotes a state of imbalance between the production of ROS and antioxidant mechanisms in which the former prevail over the latter (6). Oxidative stress is intimately involved in the pathogenesis of HF, regardless of its underlying cause (coronary heart disease or non-ischemic cardiomyopathy) (7). Elevated levels of a variety of markers of oxidative stress have been reported in both plasma and pericardial fluid in patients with HF and positively correlate with the degree of left ventricular dilation and the severity of HF (8–11).
The imbalance between ROS production and antioxidant mechanisms leads to myocyte dysfunction, injury and necrosis resulting in the development and progression of HF (7). ROS reduce Ca+2 entry in the cardiomyocytes, attenuate myocardial contractility, and augment cardiomyocyte apoptosis (5, 12). Oxidative stress also contributes to the development of HF by inducing heart remodeling (13). ROS are potent stimulators of matrix metalloproteinases (MMP), which in turn mediate left ventricular (LV) remodeling (13–19).
A variety of sources contribute to the increased ROS production in HF, including xanthine oxidoreductase (XOR) (20, 21), the mitochondria (22, 23), nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (24–26) and uncoupled nitric oxide synthase (NOS, both neuronal (NOS1) and endothelial (NOS3)) (27, 28) (Figure 1). In contrast, reduced levels of antioxidant mechanisms have been reported in these patients and are inversely associated with the stage of HF (11, 20, 29). Among oxidative enzymes, XOR has been the focus of significant research as a principal culprit participating in the propagation of oxidative stress in HF (20). Here we review existing data on the role of XOR in the pathogenesis of HF and cardiac remodeling, and address the potential therapeutic implications of XOR inhibition.
Figure 1.
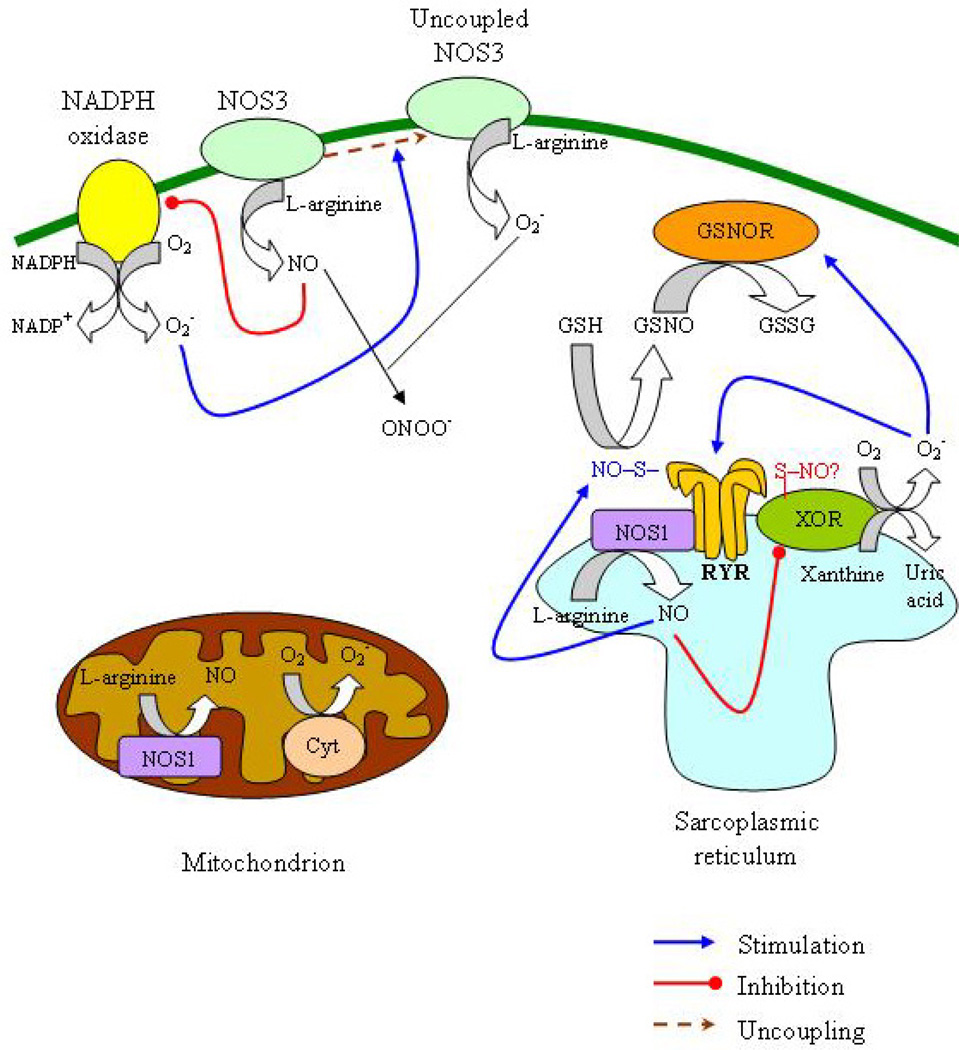
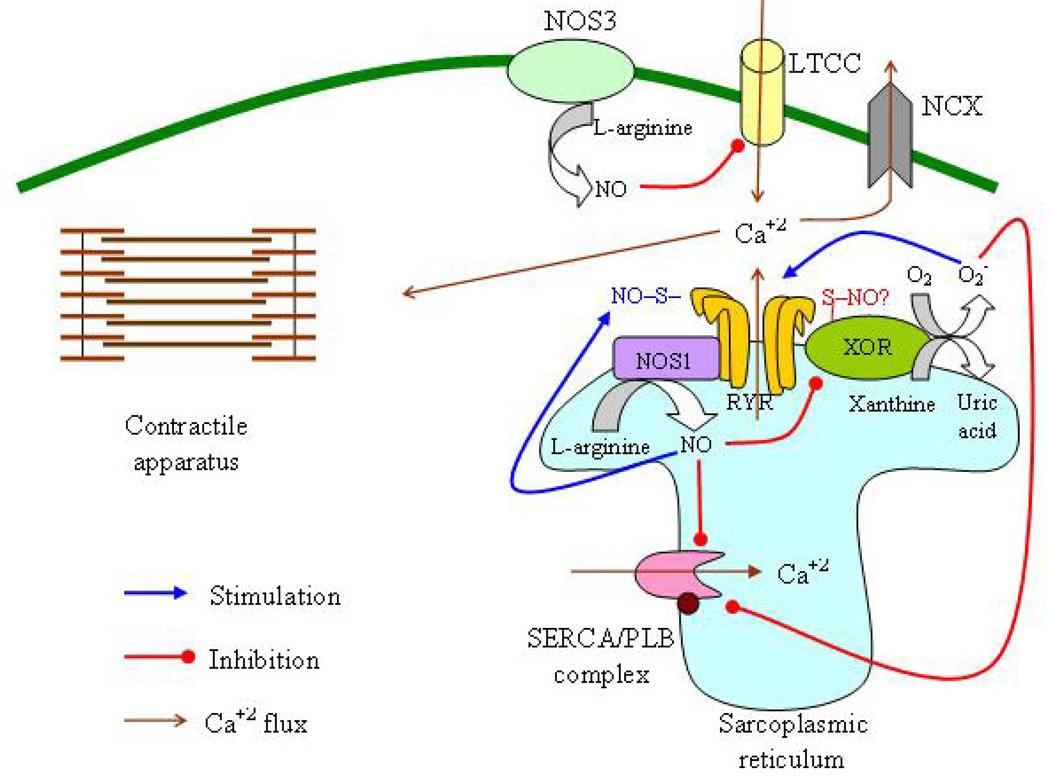
Interaction of oxidative and nitrosative pathways in heart failure. The principal sources of reactive oxygen species in the cardiomyocyte are xanthine oxidoreductase (XOR), nicotinamide adenine dinucleotide phosphate oxidase (NADPH) and the mitochondria. Nitric oxide (NO) is produced by neuronal nitric oxide synthase (NOS1, situated in the sarcoplasmic reticulum (SR) and in the mitochondria) and endothelial nitric oxide synthase (eNOS, situated in the caveolae in the cell membrane). XOR and NOS1 colocalize in the SR and this allows the inhibition of XOR by NOS1, possibly through S-nitrosylation In turn, XOR reduces S-nitrosoglutathione (GSNO), leading to the regeneration of glutathione (GSH), the enzyme that reduces SNO moieties in proteins and preserves the S-nitrosylation equilibrium. In SR, NOS1 regulates the activity of the ryanodine receptor (RyR) through S-nitrosylation; in contrast, XOR-generated superoxide (O2−) irreversibly activates RyR, precluding this regulatory action of NO. In the cell membrane, NOS3 suppresses NADPH activity whereas NADPH-produced O2− can induce NOS3 uncoupling resulting in O2− production and reduced NO synthesis. O2− interacts with NO and generates peroxynitrite (ONOO−). (GSSG, oxidized GSH; GSNOR, GSNO reductase; Cyt, cytochrome).
3. XOR – BIOCHEMISTRY AND FUNCTION
Among oxidative enzymes, XOR is a key regulator of oxidative stress (30). XOR belongs to the molybdoenzyme family and exists as a homodimer in 2 potentially interconvertible forms, xanthine dehydrogenase (XDH) and xanthine oxidase (XO) (30, 31). Each monomer consists of a molybdenum center, 2 distinct iron-sulfur centers and a flavin adenine dinucleotide (FAD) center (30, 31) (Figure 2). XOR catalyzes the conversion of hypoxanthine to xanthine and xanthine to uric acid (30). During XOR reoxidation, both hydrogen peroxide (H2O2) and superoxide (O2−) are produced (30) (Figure 3). XO appears to be more potent ROS generator than XDH since both can react with either NAD+ or molecular oxygen but the reactivity of XO toward NAD+ is negligible (32). However, XDH can be converted to XOR either by a reversible process involving thiol oxidation or by proteolytic cleavage (30).
Figure 2.
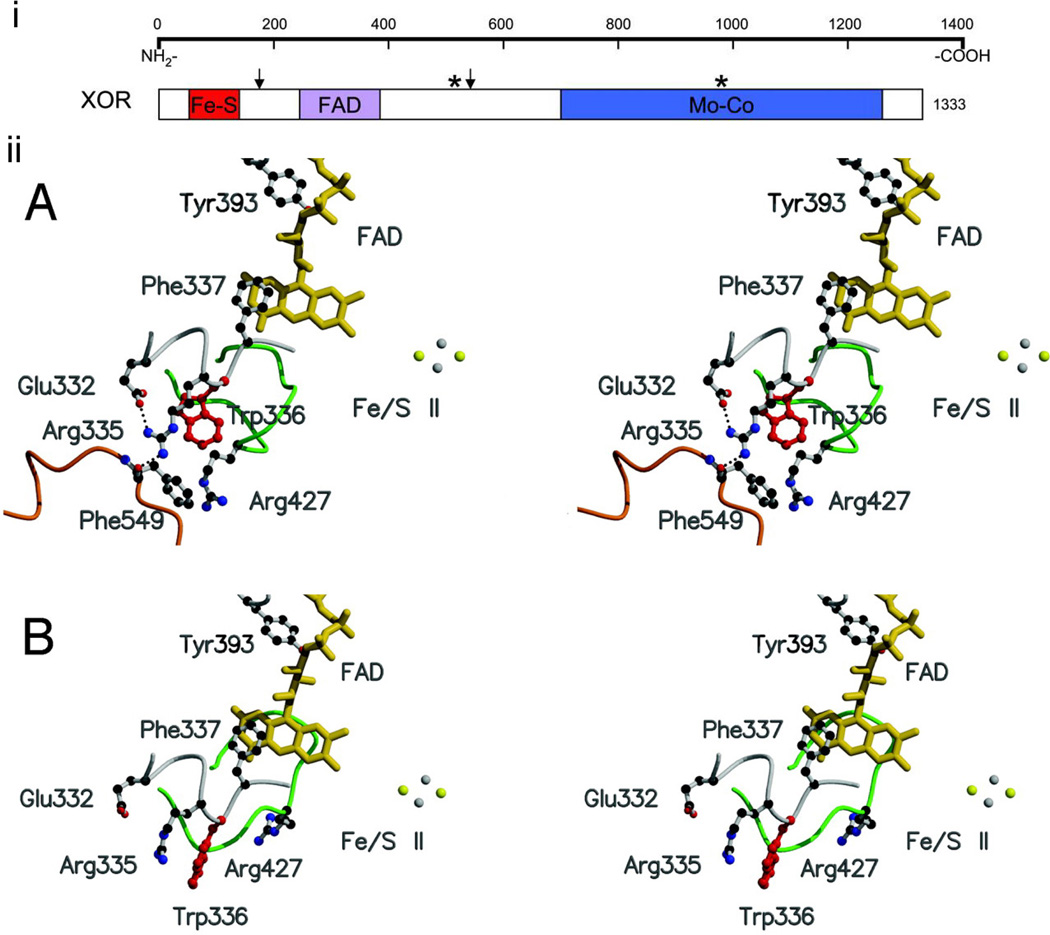
i) Secondary structure of xanthine oxidoreductase (XOR). Arrows designate trypsin sites; stars designate cysteine residues modified in reversible XOR conversion. Reproduced with permission from (30). Copyright 2004, The Physiological Society. ii) Structure of the active-site loop (Gln-423–Lys-433, shown in green) of the xanthine dehydrogenase (A) and xanthine oxidase (B) forms of bovine xanthine oxidoreductase. Reproduced with permission from (31). Copyright 2003, National Academy of Sciences, U.S.A.
Figure 3.
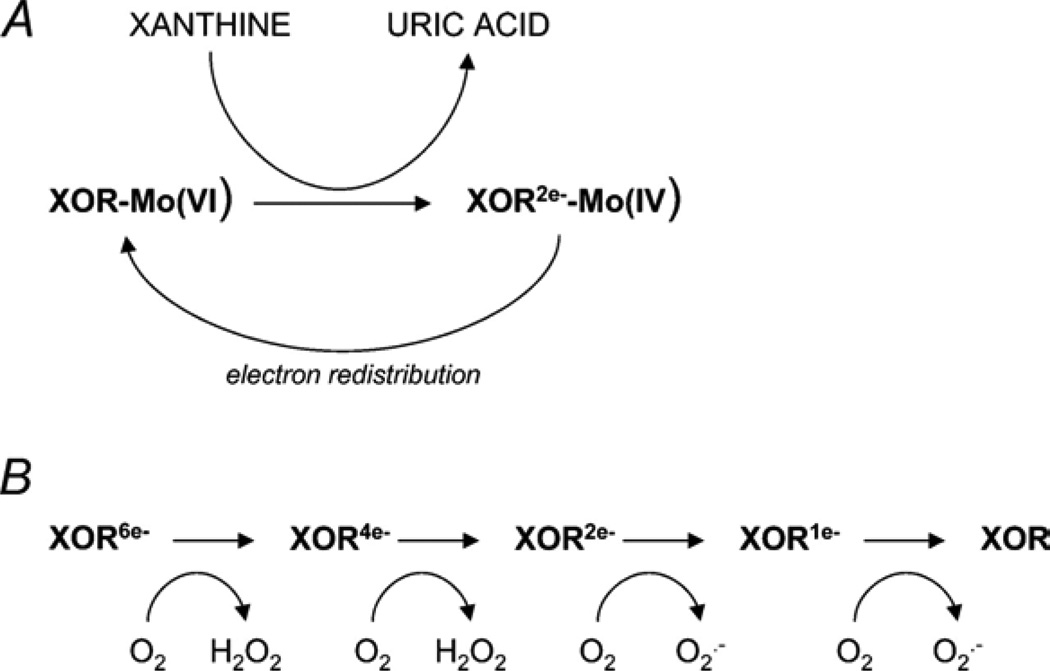
Mechanism of superoxide production by xanthine oxidoreductase (XOR). Reproduced with permission from (30). Copyright 2004, The Physiological Society.
O2− was identified as a product of XOR activity in 1968 and is a potent free radical produced by the 1-electron reduction of molecular oxygen (33). Except from its direct effects, O2− can also transform to other potent ROS (5, 34). O2− can react with H2O2 leading to the formation of hydroxyl radicals (OH−) (Haber-Weiss reaction) (34). Production of OH− through this reaction has also been demonstrated in the failing myocardium (35). O2− can also generate peroxynitrite by interacting with NO (5). O2− is converted by superoxide dismutase (SOD) to H2O2, which is less potent but can also exert cardiotoxic effects (5, 36). H2O2 can also convert to OH− through the Fenton reaction (H2O2 + Fe2+ → OH− + OH− + Fe3+) and this conversion was shown to occur in myocardial tissue obtained from animal models of heart failure (22, 36).
4. XOR IS UPREGULATED IN HF
An upregulation of cardiac XOR expression and activity has been consistently reported in various animal models of HF (28, 37–40) and in humans with HF (21). This upregulation could be a result of the activation of the renin-angiotensin system – a hallmark of HF (41) – since angiotensin II appears to stimulate XOR (42). Moreover, HF is characterized by increased circulating levels of proinflammatory cytokines (43, 44), which could also augment XOR activity (45, 46). Hypoxia also stimulates XOR activity (47–49) and might therefore play a role in XOR upregulation in HF, particularly in patients with ischemic cardiomyopathy.
5. EFFECTS OF XOR IN MYOCARDIAL CONTRACTILITY
5.1. Ex vivo studies
Whether XOR is implicated in the pathogenesis of HF and the specific mechanisms involved have been evaluated in several studies. XOR-produced O2− diminishes maximal Ca2+-activated force in isolated cardiac myofilaments (50). Moreover, XOR inhibition with allopurinol augments contractile force (51). A XOR-induced decrease in the Ca2+ sensitivity of cardiac myofilaments could help explain this negative inotropic effect of XOR (51–53). Furthermore, XOR reduces myofibrillar Ca2+-ATPase activity (54). An increase in the maximal force-generating capacity of cardiomyocytes may also contribute to the positive inotropic action of XOR inhibition (55). Maximal force-generating capacity is greatly reduced in ventricular muscle isolated from rats with HF (56). It should also be noted that allopurinol had no significant effect in the contractility of ventricular trabeculae isolated from normal hearts (55). This finding supports the importance of XOR upregulation in HF in the pathogenesis of myocardial dysfunction (55). In addition to O2−, OH−, produced by the interaction of O2− with H2O2, can also reduce myocardial contractility (13, 57).
5.2. In vivo studies
The negative inotropic effects of XOR in the in vitro studies were also assessed in animal models of HF. In an early experiment from our lab, acute inhibition of XOR with allopurinol (200 mg infused as 3.3. mg/min in the right atrium) improved myocardial contractility in dogs with pacing-induced HF (38). These observations were subsequently reproduced by other investigators (58). In accordance with the results of the ex vivo studies, allopurinol did not substantially modulate the contractility of normal hearts (38, 58).
In order to decipher the mechanisms accounting for these beneficial actions of XOR inhibition, we compared the effects of allopurinol (200 mg given as 6.6. mg/min intravenously for 30 min) with intravenously administered vitamin C – an antioxidant agent – in dogs with pacing-induced HF (28). Allopurinol exerted a positive inotropic effect to a degree equivalent to vitamin C (28). In addition, when allopurinol was infused immediately after vitamin C, it had no additional effect on myocardial contractility (28). These findings supported the idea that XOR-induced oxidative stress is primarily responsible for the detrimental effects of XOR on myocardial systolic function and that the latter effects can be abrogated with allopurinol treatment (28).
In a genetic model of dilated cardiomyopathy, the spontaneously hypertensive/HF (SHHF) rat, oxypurinol – the active metabolite of allopurinol – when administered po for 4 weeks (1 mmol/l in drinking water) improved fractional shortening and increased LV ejection fraction (LVEF) (59). Again, these effects of oxypurinol appeared to result from the amelioration of XOR-induced oxidative stress (59). Indeed, SHHF rats showed an increase in oxidative stress and more specifically, in the production of O2− in their cardiomyocytes; this altered redox state was restored with oxypurinol treatment (59). We also observed that oxidative stress was primarily due to increased XOR activity whereas NADPH oxidase activity was similar in HF and control rats (59).
5.3. Mechanisms involved in the negative inotropic action of XOR
Overall, there is now considerable evidence supporting the importance of XOR in the impairment of myocardial contractility in HF. XOR-induced oxidative stress emerges as the principal mechanism of this effect of XOR. We will next discuss in more detail the putative mechanisms that could contribute to this negative inotropic action of XOR-induced oxidative stress. We will schematically divide them in effects on myocardial energetics, Ca+2 cycling and structural changes in the myofilaments.
5.3.1. Myocardial energetics
XOR could contribute to the development of HF through its adverse effects on myocardial energetics (60). Phosphocreatine (PCr) is the principal energy reserve molecule in the heart (61). Creatine kinase (CK) uses PCr as a substrate in order to produce ATP, the cellular energy “currency” (61). HF is characterized by a depletion of both ATP and PCr and the reduction in the levels of these energy molecules correlates directly with the severity of HF (62). The PCr/ATP ratio is also reduced in HF, suggesting an imbalance between energy demand and supply (63). In patients with dilated cardiomyopathy, the decreased PCr/ATP ratio represents an independent predictor of mortality (64). Energy depletion in the failing myocardium appears to result principally from a reduction in CK activity (65, 66). In turn, inhibition of CK activity in isolated rat hearts diminishes contractile reserve (67, 68) and it has been proposed that the same may apply in the failing human heart (61). Therefore, a tight link appears to exit between energy starvation in the failing myocardium and the development and progression of heart failure (61).
A number of ex vivo studies supports a role for XOR in the impairment of myocardial energetics in HF (36, 57). XOR reduces CK activity in rat hearts in vitro through the production of O2− (36). H2O2 also suppressed CK activity in the same report (36). Moreover, OH− reduced the ATP content of cardiomyocytes in vitro (57).
There are preliminary findings that suggest that XOR inhibition can prevent the deterioration of myocardial energy status and thus prevent the development of HF. Treatment with either allopurinol or oxypurinol (0.5. mmol/l and 1 mmol/l in the drinking water for 4 weeks, respectively) after the induction of MI in mice prevented the decrease in the PCr/ATP ratio (60). This resulted in attenuation of ventricular dilation and of the fall in LVEF (60).
It has been proposed that energy depletion in the failing human heart has also deleterious consequences on mechanical efficiency (61). In isolated rat hearts, inhibition of CK activity induces mechanoenergetic uncoupling (67, 68). Mechanoenergetic uncoupling is a core characteristic of HF and refers to a mismatch between the depressed myocardial contractility and the energy consumption, which is disproportionately high (38, 69). The pivotal role of XOR in the regulation of myocardial energetics is supported by the findings of several studies that evaluated the effects of XOR inhibition on mechanical efficiency. We showed that allopurinol consistently improves mechanical efficiency in animal models of HF (28, 38). In dogs with pacing-induced HF, acute inhibition of XOR with allopurinol increased myocardial contractility; at the same time, a reduction in myocardial oxygen consumption was observed and therefore an improvement in mechanical efficiency was achieved with allopurinol treatment (38). Again, attenuation of XOR-induced oxidative stress appears to be implicated in this improvement in myocardial energetics with allopurinol treatment (28). Indeed, in dogs with pacing-induced HF, allopurinol and vitamin C ameliorated mechanoenergetic uncoupling to a similar extent (28). In addition, when allopurinol was infused immediately after vitamin C, it did not yield any further improvement in cardiac mechanical efficiency (28).
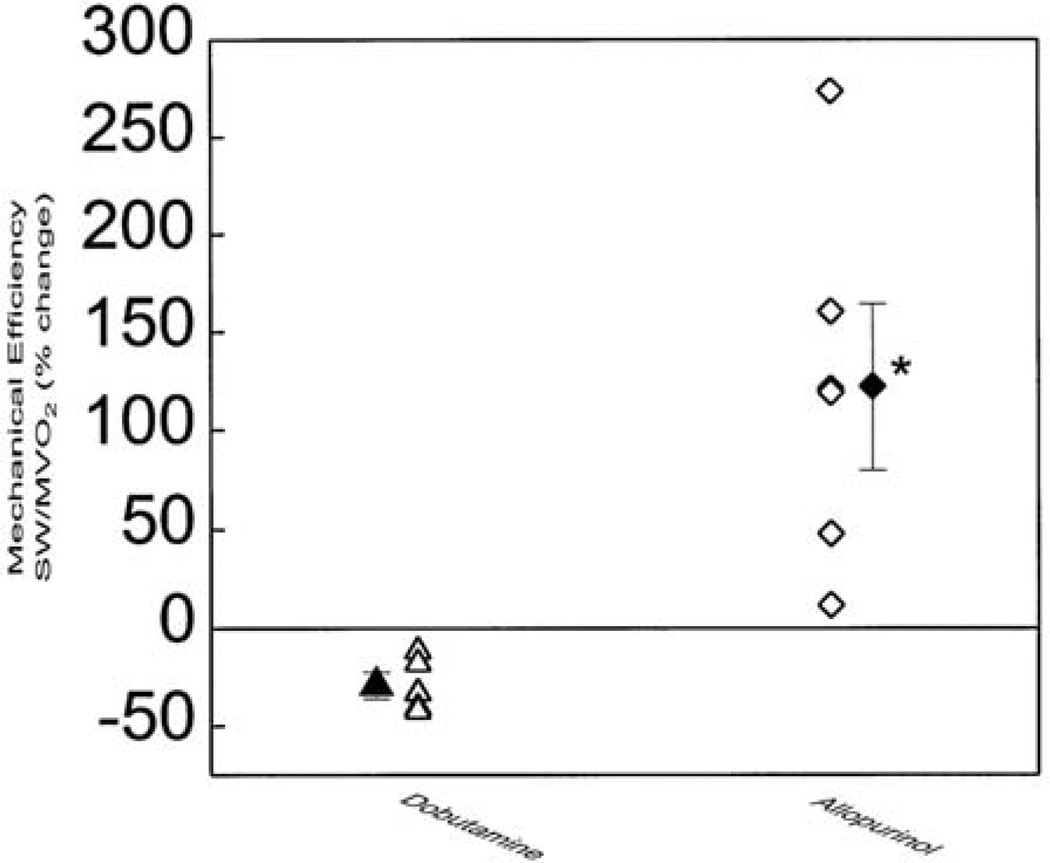
The amelioration of myocardial energetics with allopurinol appears paradoxical in view of its positive inotropic action. Conventional positive inotropic agents disproportionately increase myocardial energy consumption and further aggravate the mechanoenergetic uncoupling in the failing heart (69). In dogs with HF, we reported that dobutamine improves contractility but at the same time induces a significant increase in myocardial oxygen consumption culminating in a decrease in mechanical efficiency (38) (Figure 4). A similar effect in humans was theorized as an explanation for the increased morbidity and mortality of patients with HF treated with positive inotropic agents (70, 71). The Ca2+-sensititizing effect of XOR-inhibition was proposed to account for the improvement in mechanoenergetic uncoupling with allopurinol despite the increase in contractility (39).
Figure 4.
Comparison of the effects of allopurinol and dobutamine on myocardial efficiency in dogs with pacing-induced heart failure. Compared with baseline, allopurinol significantly improves efficiency whereas dobutamines significantly decreases it. Solid symbols represent mean±SEM. *p < 0.05 vs dobutamine. (SW, stroke work; MVO2, myocardial oxygen consumption per unit time). Reproduced with permission from (38). Copyright 1999, American Heart Association.
5.3.2. Ca2+ cycling
Perturbations in physiological cardiomyocyte Ca+2 cycling might be involved in the pathogenesis of HF (39, 72). The major players in Ca+2 cycling are the cardiac calcium release channel (ryanodine receptor, RyR), the sarcoplasmic reticulum (SR) Ca2+-ATPase 2alpha (SERCA2alpha)/phospholamban (PLB) complex and the Na+-Ca+2 transporter (NCX) (72) (Figure 5). RyR is responsible for the release of Ca+2 from the SR in response to the influx of Ca+2 through the L-type (or voltage dependent) Ca+2 channels (LTCC) (72). SERCA2alpha controls Ca2+ uptake in to the SR during diastole after its release from the myofilaments (72). SERCA2alpha activity is directly associated with cardiac contractility (72). PLB is another integral protein of the SR and plays an important role in the regulation of cardiac contractility (72). PLB inhibits SERCA in the unphosphorylated state; in contrast, phosphorylation of PLB is associated with enhanced SERCA activity (72). NCX participates in diastolic Ca2+ extrusion in the extracellular space (72).
Figure 5.
Regulation of excitation-contraction coupling. The ryanodine receptor (RyR) is responsible for the release of Ca+2 from the SR in response to the influx of Ca+2 through the L-type Ca+2 channels (LTCC). Sarcoplasmic reticulum (SR) Ca2+-ATPase (SERCA) controls Ca2+ uptake in to the SR during diastole after its release from the myofilaments. Phospholamban (PLB) is another integral protein of the SR and inhibits SERCA in the unphosphorylated state. Na+-Ca+2 transporter (NCX) participates in diastolic Ca2+ extrusion in the extracellular space. In HF, there is a reduction in SERCA levels, an increase in NCX levels and an increase in PLB levels and activity. There is also impairment in the RyR function resulting in its uncoupling with the LTCC. Xanthine oxidoreductase (XOR) upregulation appears to contribute to these changes in Ca+2 cycling. On the other hand, nitric oxide (NO) synthase 1 (NOS1) inhibits SERCA and activates RyR whereas NOS3 inhibits LTCC. The actions of NOS1 and NOS3 on Ca+2 flux are based on their close proximity with Ca+2 cycling proteins and are dependent on the redox milieu.
HF is characterized by abnormalities in the abundance and/or the activity of all major Ca+2 cycling proteins. More specifically, impairment in the RyR function has been reported in human HF resulting in uncoupling with the LTCC (73). SERCA2alpha levels and activity are reduced in human HF and result in both systolic and diastolic dysfunction (74, 75). In addition, PLB phosphorylation is impaired in HF and this leads to a further suppression of SERCA2alpha function (75). Regarding NCX, an upregulation in its expression has been reported in the failing human myocardium (74). It has been hypothesized that the subsequent increased Ca2+ extrusion in the extracellular space combined with a reduced SR Ca2+ uptake contributes to the pathogenesis of HF (76).
XOR appears to affect Ca2+ cycling. Early studies showed that XOR inhibition blunts Ca2+ amplitude (51). It was suggested that this effect might stem from increased binding of Ca2+ to the myofilaments and that this decrease in intracellular Ca2+ concentration may be beneficial (51). More recent studies offer new insights in the interaction of XOR with Ca2+ cycling proteins. We found that SERCA levels decrease whereas NCX levels increase in SHHF rats during the development of HF; more importantly, oxypurinol normalized NCX levels and blunted the reduction in SERCA levels (59). In order to identify the level where XOR controls the synthesis of Ca2+-cycling proteins, we studied the effects of XOR inhibition in dogs with pacing-induced HF (77). Allopurinol treatment (100 mg po daily) during the HF-induction period attenuated the decrease in myocardial contractility (77). In accordance with our earlier findings in mice, allopurinol attenuated the increase in NCX mRNA and protein levels in dogs as well (77). Furthermore, allopurinol prevented the upregulation of PLB mRNA and protein levels and the fall in phosphorylated PLB levels (77). In rats with established diastolic HF, XOR inhibition with oxypurinol also increased phosphorylated PLB levels (40). Therefore, it is intriguing to speculate that XOR-derived ROS could influence transcription of genes encoding the synthesis of Ca2+-cycling proteins. In addition, it controls Ca2+-cycling by post-translational mechanisms as well, such as the regulation of PLB activity by affecting its phosphorylation.
5.3.3. Structural changes in the myofilaments
XOR upregulation in HF also has detrimental effects on myofilament structure that apparently contribute to its negative inotropic action. In mice with ischemic cardiomyopathy, allopurinol reduced the levels of 4-hydroxy-2-nonenal (HNE)-modified myocardial proteins (39). HNE is a lipid peroxidation product that can disrupt the structure of myocardial proteins (78, 79). In another study, transgenic mice with truncated troponin I, a model of dilated cardiomyopathy, exhibited a 3-fold increase in XOR activity compared with wild type littermates (80). These transgenic mice showed excessive oxidative damage in myofibrillar proteins, particularly actin, that was attenuated with allopurinol treatment (26 mg/dl in the drinking water for 2 months) (80). In the same report, XOR inhibition with allopurinol restored cardiac muscle force in isolated cardiac muscle and prevented LV dilation (80). These findings support the notion that the myofibrillar damage induced by XOR is implicated in the pathogenesis of contractile dysfunction in HF.
6. CARDIAC REMODELING – THE ROLE OF XOR
Cardiac remodeling is the intermediate step in the development of overt HF regardless of its underlying cause (81). The principal mechanisms leading to remodeling are cardiomyocyte hypertrophy and apoptosis as well as alterations in matrix structure (81). Oxidative stress is intimately implicated in the development of cardiac remodeling by stimulating all 3 pathways (13). Among the various sources of ROS, XOR is a key factor in the development of remodeling. Several studies showed that allopurinol prevents LV remodeling both after experimental MI in mice (39, 60, 82) as well as in established HF (59, 83).
We will next briefly discuss the pathophysiologic mechanisms culminating in cardiac remodeling, with a specific focus on the existing data implicating XOR in these pathways.
6.1. Hypertrophy
Cardiac hypertrophy represents, to some extent, a physiological adaptive response to hemodynamic stress (84). However, it might also progress to overt HF and is associated with increased cardiovascular morbidity and mortality in the general population (84, 85). Significant progress has been made during the last decade in elucidating the signaling pathways involved in the pathogenesis of myocardial hypertrophy (86). In this labyrinth of interacting control mechanisms, the mitogen-activated protein kinase (MAPK) pathway appears to have a prominent role (86). The final effectors in this system are p38, c-Jun N-terminal kinases (JNK) and extracellular signal-regulated kinases (ERK) (86). Among these, ERK was proposed to be particularly significant in terms of regulating cardiac hypertrophy (86, 87).
Recent studies propose a role for XOR in the stimulation of ERK-induced myocardial hypertrophy (Figure 6). In isolated rat cardiomyocytes exposed to hypoxia-reoxygenation, there was an upregulation of XOR activity and the resulting oxidative stress induced ERK phosphorylation (88). In addition, allopurinol dose-dependently reduced this ERK activation (88). OH− also induce myocardial hypertrophy after experimental MI in mice (13). In Dahl salt-sensitive hypertensive rats with established diastolic HF, an increase in ERK phosphorylation was noticed that was attenuated with oxypurinol treatment (40). In SHHF rats, we also reported an increased phosphorylated/unphosphorylated ERK ratio and a restoring of this imbalance with oxypurinol (59). More importantly, in the same study, XOR inhibition induced a regression in cardiomyocyte hypertrophy and reduced LV mass (59) (Figure 7). These preliminary observations implicate XOR in the pathophysiology of cardiac hypertrophy through the activation of ERK.
Figure 6.
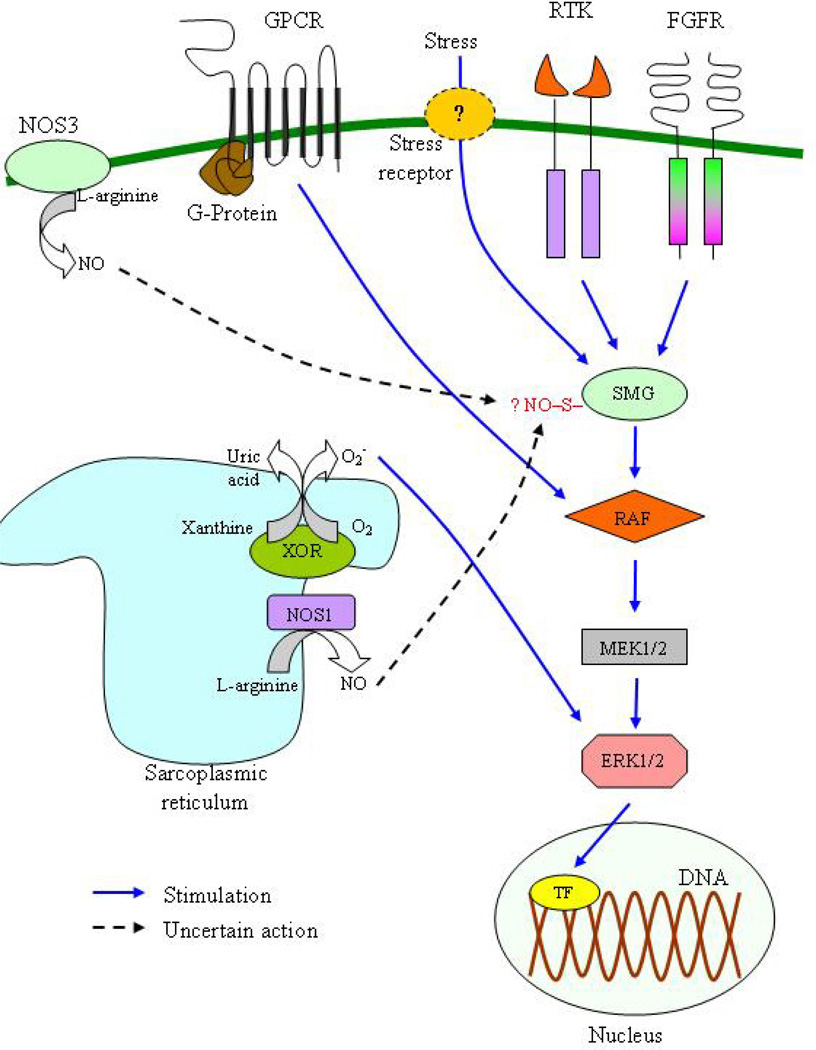
Simplified representation of the extracellular signal-regulated kinases (ERK)1/2 branch of the mitogen-activated protein kinase (MAPK) signaling pathway and its interaction with xanthine oxidoreductase (XOR) and nitric oxide (NO). The activation of cell membrane receptors (including G-protein coupled receptors (GPCR), receptors tyrosine kinase (RTK) and fibroblast growth factor receptors (FGFR)) by receptor-specific external stimuli (e.g. endothelin-1 for GPCR, epidermal growth factor for RTK) leads to the sequential activation of a series of intracellular messengers. Stress acts either directly or through a putative receptor. The first intracellular messengers that become activated are the small GTPases (SMG), including members of the Ras (mainly) and Rho/Rac/cdc42 subfamilies, which then activate the RAF family (A-RAF, B-RAF and C-RAF), which in turn activate MEK1/2. The final effectors are ERK1/2, which in turn stimulate hypertrophy by either phosphorylating transcription factors (TF) or by regulating ribosomal RNA transcription and protein translation. ERK1/2 possibly translocates to the nucleus in order to activate TF. XOR activates ERK1/2 and this could partly mediate its hypertrophic actions in the myocardium. The actions of NO on ERK1/2 pathway are a matter of debate. NO appears to act on Ras and both inhibitory and stimulating effects have been reported. These actions are possibly mediated through S-nitrosylation. The relative contribution of nitric oxide synthase 1 (NOS1) and NOS3 on these NO-effects on ERK1/2 pathway is also uncertain.
Figure 7.
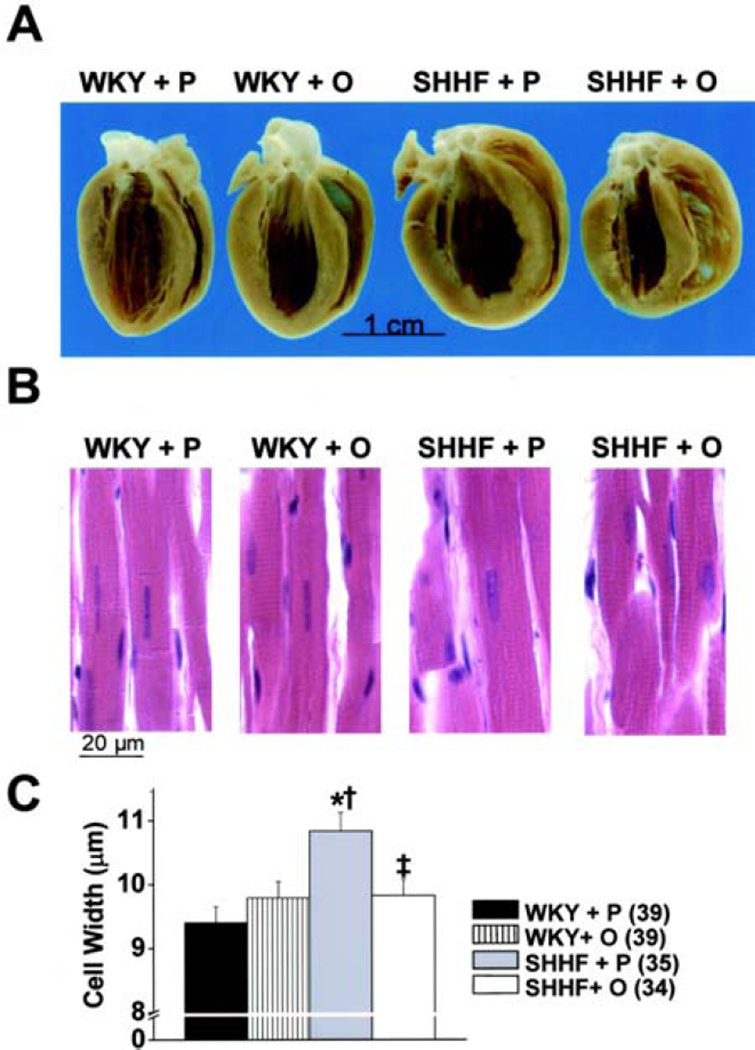
Oxypurinol induces reverse remodeling in rats with dilated cardiomyopathy. A. Gross pathology showing increased left ventricular (LV) diameter in spontaneously hypertensive/HF (SHHF) rats treated with placebo (SHHF+P); after treatment with oxypurinol (SHHF+O), LV volume is similar to control rats (Wistar Kyoto rats, WKY). B. Light microscopy with hematoxylin/eosin staining shows increased LV myocyte width in SHHF+P rats, which is decreased with oxypurinol treatment (SHHF+O rats) to levels comparable to control rats (WKY). C. Graphic representation of LV myocyte width; there is a significant increase in SHHF+P rats witch is normalized with oxypurinol treatment. *p < 0.001 compared with WKY+P, †p < 0.05 compared with WKY+O, ‡p < 0.01 compared with SHHF+P. Reproduced with permission from (59). Copyright 2006, American Heart Association.
Cardiac hypertrophy is also characterized by an upregulation of a series of genes that is collectively characterized as the fetal gene program and includes atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), beta-isoform of myosin heavy chain (beta-MHC) and the alpha skeletal muscle isoform of actin (alphaSA) (89). This overexpression might represent a causative agent for myocardial hypertrophy (89). We found that mRNA levels of all these genes are increased in SHHF rats with established HF; we also showed that oxypurinol blunted the upregulation of ANP and alphaSA and completely normalized BNP and beta-MHC expression (59).
6.2. Apoptosis
In animal models of heart failure, apoptotic cardiomyocyte death is a precursor of overt heart failure (90). Increased apoptosis of cardiomyocytes is also frequently observed in patients with HF and may play a role in its progression (91, 92). Our knowledge of the signaling routes that regulate cardiomyocyte apoptosis has increased exponentially during the last decade (84). These routes are often identical or tightly linked to the ones controlling myocardial hypertrophy (84, 86). Thus, apoptosis signal-regulating kinase 1 (ASK1), which stimulates cardiomyocyte apoptosis and contributes to the pathogenesis of LV remodeling (93, 94), belongs to the above-mentioned MAPK family and is a selective upstream activator of p38 and JNK (86, 93, 95). Both p38 and JNK have also been implicated as apoptosis-promoting kinases, although the existing data are conflicting (86, 96). The final effectors of apoptotic cell death are caspases, a group of proteolytic enzymes, which are under the control of the Bcl-2 family of proteins (97, 98). The Bcl-2 family includes members with both pro- and anti-apoptotic actions (97, 98). Among the former, the Bax subfamily was recently shown to interact with p38 resulting in the induction of cardiomyocyte apoptosis (99).
Oxidative stress is intimately involved in the stimulation of apoptotic death of cardiomyocytes (12, 90). XOR was also shown to stimulate apoptosis of rat cardiomyocytes in vitro by increasing Bax mRNA levels (100). Oxidative stress was also shown to activate ASK (95, 101) and ASK appears to be essential for the oxidative stress-induced apoptotic cell death (94, 95). In Dahl salt-sensitive hypertensive rats with established diastolic HF, an increase in ASK1 phosphorylation has been reported and was reversed with oxypurinol treatment (40). Moreover, XOR inhibition prevented LV dilation and improved the survival rate in the same study (40). It is apparent that more studies are necessary in order to clarify the role of ASK in XOR-mediated prevention of cardiac remodeling.
6.3. Alterations in matrix structure
In the heart, extracellular matrix is not a static skeleton but a dynamic system (102–104). In contrast, it has become clear that altered matrix turnover is an active component of the process of cardiac remodeling (102–104). MMP is a family of enzymes that includes more than 20 members (collagenases, gelatinases and stromelysins) and is the major regulator of extracellular tissue breakdown (102–104). In this context, it is of interest that XOR activates MMP-2, MMP-9 and MMP-13 in cardiac fibroblasts and vascular smooth muscle cells in vitro (16, 17). OH− is also a major player in the pathogenesis of post-MI remodeling in mice, possibly by activating MMP-2 (13). Interestingly, myocardial MMP-2, MMP-9 and MMP-13 protein levels and activity are elevated in patients with HF compared with controls (19, 105, 106). In addition, in animal models, non-selective inhibition of MMP activity prevented the development of cardiac remodeling (15, 18). MMP-9 knockout mice are protected from post-MI remodeling (107) whereas transgenic mice overexpressing MMP-2 develop advanced remodeling (108). Preliminary studies in humans also suggest that suppression of MMP-2 and MMP-9 expression in patients with acute MI prevents remodeling (109). Therefore, the in vitro stimulatory effects of XOR on MMP-2, MMP-9 and MMP-13 could be implicated in the beneficial effects of XOR-inhibitions on cardiac remodeling.
7. ISCHEMIC CARDIOMYOPATHY – THE ROLE OF XOR
The impact of XOR inhibition in ischemic cardiomyopathy deserves special mention, since this is the commonest cause of HF (4, 110). There is considerable evidence suggesting that allopurinol preserves myocardial contractility and improves survival after experimental MI in mice (39, 60, 82) (Figure 8). Interestingly, an increased XOR activity was also demonstrated in the remote LV myocardium in these reports (39, 82). Even more importantly, in rats with established ischemic HF, chronic administration of allopurinol (50 mg/kg/day po for 10 weeks) induced reverse remodeling and decreased LV weight and fibrosis (83). Therefore, XOR inhibition seems not only to prevent the development of HF after MI but is also effective in stimulating reverse remodeling in established ischemic HF.
Figure 8.
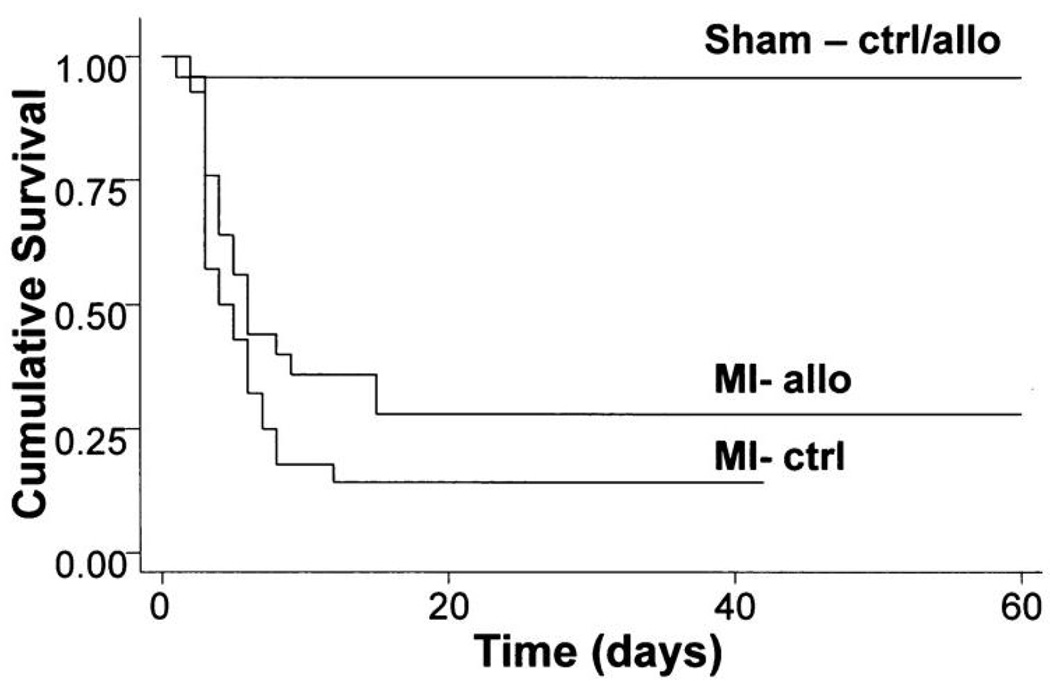
Allopurinol improves survival after experimental myocardial infarction in mice. Mice subjected to myocardial infarction and treated with allopurinol after recovery (1 mmol/l in drinking water) were twice as likely to survive for 60 days as compared with mice treated with placebo (39% versus 19%). (ctrl, placebo-treated mice; allo; allopurinol-treated mice; MI, myocardial infarction; Sham-ctrl/allo, pooled survival in sham-operated mice receiving either allopurinol or placebo). Reproduced with permission from (39). Copyright 2004, American Heart Association.
8. DIASTOLIC DYSFUNCTION AND XOR
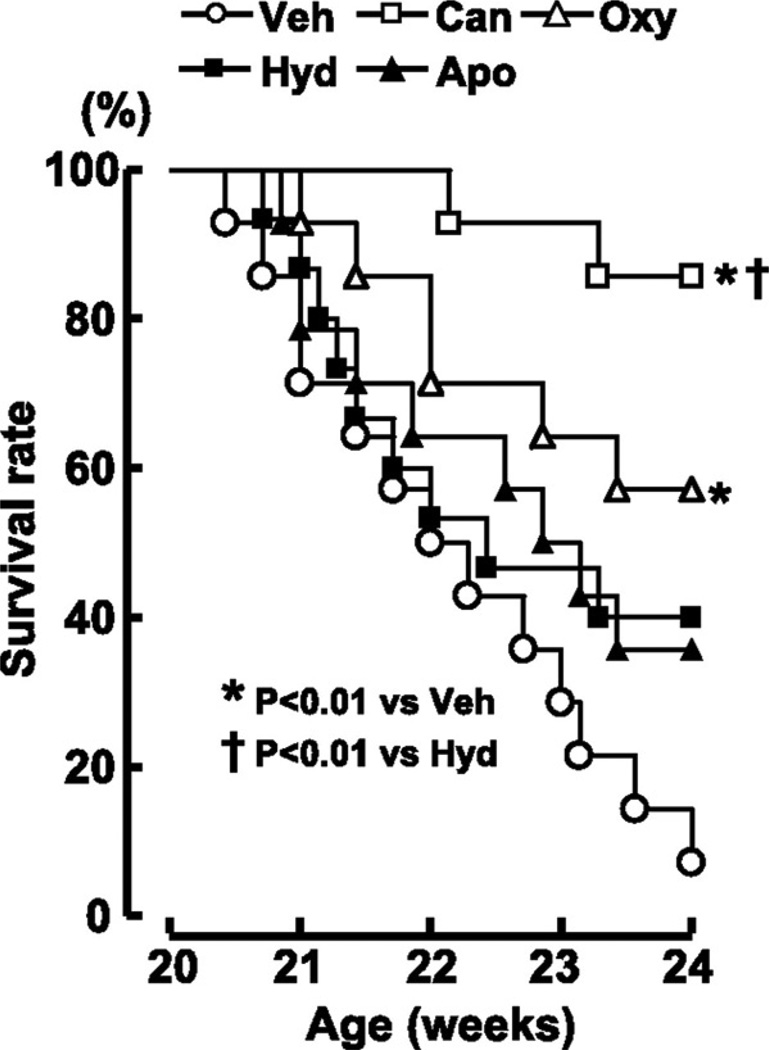
It has already been mentioned that XOR inhibition exerts a Ca2+ sensitizing action in cardiac myofilaments (39, 51–53). One could anticipate impairment in diastolic relaxation as a result of this effect; indeed, other Ca2+ sensitizing agents induce diastolic dysfunction by lowering the threshold of (Ca2+)I where myocardial contraction is activated (111). In contrast, XOR inhibition does not shift the range of Ca2+ activation and can actually improve diastolic dysfunction (39, 40). In an early study in mice with post-MI HF, allopurinol improved diastolic relaxation (39). In a more recent report, Dahl salt-sensitive hypertensive rats with established diastolic HF were treated with oxypurinol (40 mg/kg per day for 4 weeks) (40). XOR inhibition reduced interstitial fibrosis, prevented LV dilation and the survival rate of rats treated with this agent was significantly (p < 0.01) greater than rats given vehicle (0.5% carboxymethyl cellulose) (40) (Figure 9). As already mentioned, a reduction in ERK and ASK1 phosphorylation, an augmentation in PLB phosphorylation and an attenuation in cardiomyocyte apoptosis were observed with oxypurinol treatment and could explain its beneficial effects (40). The role of renin-angiotensin system inhibition in patients with systolic HF is well established and they might also be useful in diastolic HF (4). It is thus of interest that the beneficial effects of candesartan in this study were mediated through its effects on cardiac XOR (40). Therefore, XOR inhibition might partly contribute to the salutary effects of angiotensin receptor blockers in patients with HF.
Figure 9.
Treatment with oxypurinol (40 mg/kg per day from 20 to 24 weeks of age) improves survival rate in Dahl salt-sensitive hypertensive rats with established diastolic HF. (veh, vehicle (0.5% carboxymethyl cellulose); can, candesartan; oxy, oxypurinol; hyd, hydralazine; apo, apocynin). Reproduced with permission from (40). Copyright 2007, American Heart Association.
9. INTERACTION OF XOR WITH OTHER SOURCES OF ROS IN HF
NADPH oxidases are family of 5 enzymes that transfer electrons from NADPH to molecular oxygen leading to the formation of O2−. Similar to XOR, NADPH oxidases are also stimulated – among other agonists – by cytokines and angiotensin II (112). We recently showed that NADPH oxidase activity is similar in HF and control rats (59). In the same study we observed an upregulation of the NADPH oxidase subunits Gp91phox and p67phox in HF rats whereas p22phox and p47phox did not change significantly (59). However, NADPH oxidase activity is increased in the failing human myocardium (26, 113, 114) and may promote the development of cardiac hypertrophy, remodeling and ultimately HF (112, 115). It should be noted that there appears to be a cross-talk between NADPH and XOR in that the former increases the production of O2− from the latter by inducing the conversion of XDH to XO (116).
The mitochondria might also generate ROS in the failing heart (22). This was attributed to a decreased activity of complex I leading to an impairment in electron transport (22). However, the mitochondria also represent the main energy source through oxidative phosphorylation and this essential function is disrupted by XOR-produced O2− (117).
10. INTERACTION OF XOR WITH ANTIOXIDANT SYSTEMS IN HF
SOD converts O2− to H2O2 and exists in 3 isoforms, cytosolic or copper-zing SOD (SOD1), manganese SOD (SOD2, localized in mitochondria) and extracellular SOD (SOD3) (118). A relative deficit of SOD appears to be implicated in the development and progression of HF (23, 100, 119). Heart/muscle-specific SOD2-deficient mutant mice demonstrate increased production of O2− leading to HF (23). In humans, a polymorphism in the SOD2 gene that reduces the antioxidant activity of SOD also increases the risk of developing HF (119). Regarding the implicated mechanisms, inhibition of SOD1 and SOD3 induced apoptosis of rat cardiomyocytes in vitro and increased Bax mRNA levels (100). SOD2-deficient mutant mice showed suppressed oxidative phosphorylation and decreased levels of ATP in the heart (23). These detrimental consequences of the deficiency of SOD, the principal superoxide-inactivating antioxidant enzyme, further support the pivotal role of O2− in the pathogenesis of HF.
11. CROSS-TALK OF OXIDATIVE AND NITROSATIVE PATHWAYS IN HF – THE ROLE OF XOR
Since its original description as endothelium-derived relaxing factor in the pivotal experiments of Furchgott and Zawadski in 1980 (120), intense interest has been focused on the nitric oxide (NO) signaling pathway (5). Prototypically, NO exerts signaling by activation of guanylyl cyclase, which in turn converts guanosine triphosphate (GTP) to cyclic guanosine monophosphate (cGMP), an important second messenger (121). However, another mechanism of action of NO has been the topic of intensive research during the last decade, namely S-nitrosylation, which is the covalent attachment of NO to cysteine thiol (5). This ubiquitous signaling mode represents a post-translational modification system akin to phosphorylation, and is regulated and capable of tight molecular regulation by virtue of its reversibility. An important corollary to the molecular regulation exerted by SNO, is that NOSs are frequently found in signaling modules participating in protein-protein interactions with effector proteins. This is particularly true in the cardiomyocyte in which NOSs are compartmentalized in various key organelles (122). NOS1 is situated in the SR and mitochondria and NOS3 in specialized sarcolemmal signal-transducing domains termed caveolae (123–125). In this context, it is also important to stress that, in HF, NOS1 redistributes from the SR to the cell membrane (126) and this could have important implications in NO signaling in the failing myocardium (122).
NO is an important modulator of excitation/contraction coupling and myocardial energetics (127, 128) (Figure 5). NO regulates all major Ca+2 cycling proteins. NOS1 progressively activates RyR, possibly by S-nitrosylation (129, 130). NOS1 inhibits SERCA (123). NOS3 inhibits the LTCC in a cGMP-dependent way; however, it can also regulate it through S-nitrosylation and whether it exerts stimulatory or inhibitory actions depends on the redox milieu (131, 132).
Overall, NO appears to protect against the development of myocardial hypertrophy (133, 134). However, it has been reported that NO activates the hypertrophy-inducing MAPK, ERK1/2, in animal and human cell lines (135, 136). More specifically, NO activates Ras through S-nitrosylation and this leads to ERK1/2 activation (137). It was also suggested that NO mediates the stimulating effect of vascular endothelial growth factor (VEGF) on ERK1/2 in human endothelial cells; this effect was cGMP-dependent (138, 139). This is controversial as others did not observe effects of NO on ERK1/2 in murine macrophages (140). More recent studies reported that NOS1 inhibits Ras activity by S-nitrosylation and impairs downstream activation of RAF and ERK1/2 (141).
Besides these effects of NO on hypertrophy-regulating kinases, NO modulates the activity of specific phosphatases that are implicated in myocardial hypertrophy. Calcineurin is a Ca+2-activated phosphatase that dephosphorylates nuclear factor of activated T-cells (NFAT), which then translocates to the nucleus and stimulates hypertrophic gene expression (142). In cardiomyocytes and in vascular smooth muscle cells, NO prevents the activation of calcineurin and exerts anti-hypertrophic actions by regulating Ca+2 concentration (143, 144). This was mediated through a cGMP-dependent inhibition of Ca+2 entry via the LTCC (144).
NO can both induce and inhibit apoptosis depending on its concentration, cell type and redox status (145). It has already been mentioned that XOR can activate ASK-1, which then stimulates p38 and JNK resulting in increased apoptosis. In contrast, NO inhibits ASK-1 through S-nitrosylation (146). It was also recently reported that NOS inhibition induces cardiomyocyte apoptosis in wild type mice but not in ASK-1 deficient mice (147). These findings highlight the significance of ASK-1 inhibition in the anti-apoptotic actions of NO. Despite the inhibitory action of NO on ASK-1, the effects of NO on the downstream effectors of ASK-1 in the MAPK pathway, p38 and JNK, might promote apoptosis. Thus, NO activates p38 in various cell lines resulting in pro-apoptotic effects (135, 136, 140, 148). NO-induced p38 activation is both cGMP-dependent and independent (149, 150). Regarding the effect of NO on JNK, both inhibitory and stimulating actions have been reported, depending on the cell line assessed (136, 140, 151–153). Again, these actions of NO were either mediated through the guanylyl cyclase pathway or through S-nitrosylation (151–153). Overall, the effects of NO on both the apoptosis-stimulating (p38/JNK) and the hypertrophy-inducing branches (ERK1/2) of the MAPK pathway are complex and require further study.
ROS and NO interact in multiple levels (Figure 1). On one hand, XOR has multiple effects on NO-signaling pathways. As previously mentioned, O2− interacts with NO to form peroxynitrite; thus, elevated O2− levels decrease NO bioavailability (5). XOR also appears to regulate the S-nitrosylation pathway. In recent years it has become clear that nitrosative and oxidative pathways share similar targets and therefore compete for the same binding sites (5). Moreover, the modification of a protein by one signaling route could alter this protein’s susceptibility to the effects of the other route (5). It was also shown that XOR reduces S-nitrosothiols and particularly S-nitrosoglutathione (GSNO), leading to the regeneration of glutathione, the enzyme that reduces SNO moieties in proteins and preserves the S-nitrosylation equilibrium (154). Interestingly, the reduction of GSNO by XOR depends on the production of O2− (154). However, XOR can also augment NO production by catalyzing nitrite reduction, particularly in ischemic conditions (155).
On the other hand, NO regulates XOR activity. Earlier biochemical studies showed that NO can inhibit both XO and XDH (156, 157). More specifically, NO reacts with an essential sulphur in the molybdenum center of XOR and removes it (156). Furthermore, NOS1 deficiency augments the production of O2− by XOR in the cardiomyocytes; in contrast, NOS3 deficiency has no impact on XOR expression (52, 158, 159). This effect of NOS1 deficiency is due to an increase in XOR activity whereas XOR mRNA and protein levels are not affected; this suggests that NOS1 via a post-translational effect (very possibly S-nitrosylation) constrains XOR activity (52, 159). NOS1 deficiency also results in increased activation of p38 kinase, and it has been proposed that the latter could activate XOR by phosphorylation (158). It is also of interest that XOR and NOS1 coimmunoprecipitate in the SR of cardiomyocytes, suggesting a direct protein-protein interaction (52). The co-localization of XOR and NOS1 could also stem from an attachment to a common adapter protein (52). Therefore, inside the cardiomyocytes, this NO-XOR interaction appears to be NOS1-specific and spatially confined to the SR (52). It is also of interest that NOS3 suppresses NADPH activity conferring cardioprotection (160) whereas NADPH-generated O2− can induce NOS3 uncoupling thereby augmenting O2− production and reducing NO bioavailability (161). It is apparent that the cross-talk between oxidative and nitrosative pathways is not limited to XOR and NOS1 but extends to other major players of these systems.
The NO-XOR interplay is an important regulator of myocardial function (5, 162). It has been already mentioned that S-nitrosylation progressively activates RyR; however, oxidation hampers this regulatory action by inducing irreversible activation of RyR (129, 130). In addition, the effect of NOS3 on LTCC depends on the redox status (131, 132). Peroxynitrite, the product on NO and O2− reaction, suppresses myocardial contractility by impairing Ca+2 responsiveness of myofilaments (57). This was attributed to a reduction in the activity of NCX resulting in an increase in intracellular Ca+2 concentration (57). Peroxynitrite also inhibits mitochondrial function and this could result in a reduction in ATP levels that could also be implicated in its negative inotropic actions (163). Besides systolic function, peroxynitrite increases resting tension in isolated heart muscle and this could potentially result in impairment in diastolic function (164). The suppressing effects of peroxynitrite on myocardial contractility could also be related to its proapoptotic actions on cardiomyocytes (165). In addition, peroxynitrite also triggers the cell death of endothelial cells (165) and this might adversely affect coronary vasculature, myocardial perfusion, and inotropy.
Overall, these findings suggest that XOR has an important role in the interplay between oxidative and nitrosative mechanisms and the preservation of nitroso-redox balance (6). In vivo studies lend further support to this hypothesis. In dogs with HF, we showed that pretreatment with the non-specific NOS-inhibitor NG-monomethyl-l-arginine (L-NMMA) prevents the increase in myocardial contractility and the improvement in mechanoenergeting uncoupling induced by allopurinol (28). This finding implies that suppression of O2− production is not sufficient to restore myocardial function but additionally requires an intact NO-signaling pathway (28). We extended these findings by studying NOS1 knockout mice; these mice exhibited impaired contractility that was reversed by allopurinol (52). This suggests that part of the positive inotropic effects of allopurinol stem from a reduction in O2− levels–induced rise in the bioavailability of NO (52). In addition, in dogs with normal heart function, L-NMMA increased myocardial oxygen consumption and depressed myocardial efficiency and this effect was alleviated by either vitamin C or allopurinol coinfusion (28). These findings suggest that a physiological NO signaling is essential for the control of XOR-induced oxidative stress in normal hearts and prevents XOR from depressing cardiac efficiency (28).
The pivotal role of nitroso-redox balance in cardiovascular homeostasis and more specifically in HF is also supported by clinical studies showing that combined administration of nitrates and hydralazine reduced mortality in HF regardless of its severity (166, 167). Since nitrates act as NO donors (168) and hydralazine scavenges ROS and is implicated as possibly inhibiting NADPH oxidases (169, 170), it is attractive to contemplate that their beneficial effects in HF are mediated through the restoration of nitroso-redox balance (162).
12. PERIPHERAL EFFECTS OF XOR INHIBITION IN HF
Even though XOR is located in cardiomyocytes and exerts a multitude of autocrine effects as delineated above, the liver and small intestine show the most abundant expression of this enzyme in humans (30). In addition, XOR is expressed in endothelial cells and can also be released in the circulation (30). Therefore, apart from the direct effects of XOR on myocardial structure and function, peripheral mechanisms may also be implicated in the XOR-induced impairment of myocardial function. In dogs with pacing-induced HF, allopurinol treatment (100 mg po daily during the pacing period) attenuates the increase in arterial elastance, an index of afterload, as well as the decrease in ventricular elastance, a marker of contractility (37). Together these effects lead to the preservation of ventricular-vascular coupling ratio (ventricular/arterial elastance) and subsequently to an improved systolic function compared with the placebo group (37). Similar results were observed with both acute (5-day) and chronic (10-week) administration of allopurinol in rats with left coronary artery ligation-induced HF (83).
Studies in both animals and humans have shown that oxidative stress plays a role in the development of endothelial dysfunction (ED) in HF (171). ED is frequently present in HF and correlates with its severity ( (172–177). Endothelium-bound XOR has a critical role in the pathogenesis of ED in these patients since it is more than 2-fold more active in HF and its activity strongly correlates with the degree of ED (20). In addition, several studies showed an improvement in ED in patients with HF with XOR inhibition (178–180). It must be emphasized that systemic ED is associated with arterial stiffening and increased vascular tone which in turn increase afterload and lead to HF progression (181–183). In addition, ED in the coronary circulation might compromise myocardial oxygenation and could also contribute to the worsening of myocardial function (184, 185). More importantly, prospective studies showed that ED in HF represents an independent risk factor for readmission with worsening HF, heart transplantation and mortality (186–188). Therefore, it is reasonable to assume that the salutary effects of XOR inhibition on ED could translate into long-term clinical benefits in patients with HF; however, this remains to be established in prospective studies.
Another important aspect of XOR inhibition is the reduction in serum uric acid (SUA) levels. Uric acid per se is a powerful ROS-scavenger (189) and also activates other endogenous antioxidant systems, particularly SOD (190). Interestingly, uric acid is metabolized by uricase in most mammals whereas in humans the uricase gene is nonfunctional (191). It has thus been proposed that this might confer a survival advantage to humans due to increased antioxidant defense (192). However, uric acid can also promote the development of cardiovascular disease since it has proinflammatory actions (193–195), activates platelets (196), stimulates the growth of vascular smooth muscle cells (197, 198) and induces ED (199). Prospective studies suggested that elevated SUA levels represent an independent risk factor both in the general population (200–202) and in patients with established cardiovascular disease, including coronary heart disease (203, 204) and acute stroke (205). Elevated SUA levels are frequently observed in patients with HF and directly correlate with its severity (206–208). The upregulation of XOR in HF might contribute to this phenomenon; decreased renal excretion and increased XOR substrate resulting from enhanced ATP breakdown are other putative explanations (209). Elevated SUA levels in HF reflect – to a certain degree – the activation of the XOR oxidative pathway; however, uric acid per se may also contribute to HF pathophysiology (209). In patients with HF, elevated SUA levels are associated with systemic inflammation (207), increased peripheral vascular resistance (210, 211), decreased functional capacity (206) and with the presence of diastolic dysfunction (212). More importantly, prospective studies showed that elevated SUA levels in HF independently predict mortality and the need for transplantation (213–215). Interestingly, elevated SUA levels also predict allograft vasculopathy in cardiac transplant recipients (216). In addition, in the Losartan Intervention For Endpoint reduction in hypertension (LIFE) study, a losartan-induced reduction in SUA levels in patients with left ventricular hypertrophy reduced cardiovascular morbidity and mortality (217). Therefore, by reducing SUA levels, XOR inhibition might offset an important risk factor in patients with HF.
13. XOR AND ADIPOGENESIS
Our knowledge of the mechanisms through which XOR contributes to the pathogenesis of cardiovascular disease continues to expand. A recent study showed that XOR can stimulate adipogenesis and that this effect is mediated through a XOR-induced increase in the activity of peroxisome proliferator-activated receptor gamma (PPARgamma) (218). PPARgamma is an important regulator of adipose differentiation and when activated, enhances adipogenesis through the induction of transcription of specific adipogenic genes (219). In the same study, a reduction in adipose mass was observed in XOR null mice, further supporting the importance of XOR in adipogenesis (218). Interestingly, XOR null mice do not live more than 40 days (220) and this is additional evidence that XOR serves important physiological roles.
If the association of XOR with adipogenesis is confirmed in humans, XOR inhibition could emerge as a promising strategy in the management of obesity and obesity-related disorders, including type 2 diabetes mellitus and the metabolic syndrome. Paradoxically, obesity is associated with improved survival in patients with HF, a phenomenon termed reverse epidemiology (221). However, it appears that obesity is not truly protective but merely reflects a lower inflammatory response in obese patients with HF (221). HF is increasingly being recognized as an inflammatory state and increased inflammation contributes to the development and progression of HF (222). Clearly, more studies are required to unravel the complex links between obesity and HF and the potential role of XOR in this interplay.
14. CLINICAL STUDIES OF XOR INHIBITION IN HF
The beneficial effects of XOR inhibition in the above-mentioned studies in a variety of animal models of HF support the pivotal role of XOR in the pathogenesis of HF. They also suggest that XOR inhibition might represent a valuable adjunct in the treatment of HF in humans as well. Based on these promising results, a number of studies have evaluated the effect of this treatment strategy in the clinical setting. In a preliminary study from our group in patients with dilated cardiomyopathy, intracoronary infusion of allopurinol (0.5., 1.0. and 1.5. mg/min, each administered for 15 min) decreased myocardial oxygen consumption without affecting myocardial contractility (21). Therefore, XOR is involved in the pathogenesis of mechanoenergetic uncoupling in HF i.e. the discordance between impaired LV work and myocardial energy consumption, which impairs the mechanical efficiency of contraction (21).
In another study assessing acute XOR inhibition, a single intravenous dose of oxypurinol (400 mg) significantly reduced LV end-systolic volume and increased LVEF (p = 0.03 and p = 0.003, respectively) in patients with ischemic cardiomyopathy (223).
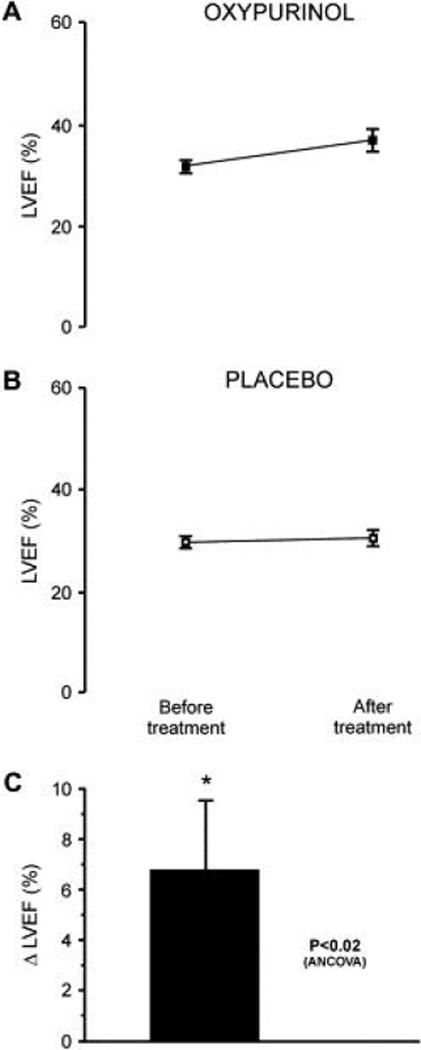
Short-term XOR inhibition has also been evaluated in HF. In a randomized placebo-controlled trial (n = 50), allopurinol (300 mg/day po for 3 months) significantly (p = 0.035) reduced plasma BNP concentrations (224). It is well established that elevated BNP concentrations represent a strong and independent risk factor in HF (225, 226). In another randomized placebo-controlled trial (n = 60), oxypurinol (600 mg/day po for 1 month) induced a non-significant increase in LVEF (p = 0.08) (227). However, when patients with baseline LVEF ≤ 40% were analyzed separately, there was a significant (p < 0.02) improvement in LVEF with oxypurinol treatment (227) (Figure 10).
Figure 10.
Treatment with oxypurinol (600 mg/day po for 1 month) significantly (p < 0.02) increases left ventricular ejection fraction (LVEF) in patients with heart failure and baseline LVEF ≤ 40% compared with placebo. (A, B) Averaged LVEF data for oxypurinol and placebo, respectively, before and after treatment. (C) Adjusted change in LVEF in the oxypurinol group relative to placebo. * p < 0.02 vs. pretreatment value. Reproduced with permission from (227). Copyright 2006, Elsevier Inc.
The largest study of the effects of an XO inhibitor in HF patients is the Oxypurinol Therapy for Chronic HF study (OPT-CHF) (228, 229). In this double-blind study, 405 patients with NYHA class III-IV heart failure were randomized to receive either oxypurinol 600 mg/day or placebo for 24 weeks (229). Overall, there was no difference beween groups in the composite endpoint comprising CHF morbidity, mortality, and quality of life (229). However, in a post-hoc exploratory analysis oxypurinol improved the outcome of patients with elevated SUA levels at baseline (> 9.5. mg/dl) (229). This cutoff value was selected on the basis of a previous study in patients with CHF which showed that these SUA levels are the best for predicting mortality (213). In addition, within the entire oxypurinol group, a larger decrease in SUA levels was associated with a better outcome (229). These data strongly support the concept that patients with CHF and elevated SUA levels, potentially a surrogate for increased XOR activity, might benefit the most from XOR inhibition. Finally, in the placebo group of the OPT-CHF study, baseline SUA levels were associated with adverse outcome, further supporting earlier reports on the predictive role of SUA levels in CHF and other cardiovascular disorders (213–216).
It should be noticed that the effects of XOR inhibition in the aforementioned studies were observed in the context of standard pharmacological treatment for HF, including beta-blockers and rennin-angiotensin system inhibitors (223, 227, 229). Therefore, it appears that XOR inhibition can provide additive benefits to those of current drug therapy for HF.
An important limitation of all studies employing allopurinol treatment is that this agent has other effects besides XOR inhibition, including antioxidant action, copper chelation, inhibition of lipid peroxidation and down-regulation of heat shock protein expression (230). Allopurinol can also directly scavenge OH− (231). Therefore, not all effects of allopurinol can be attributed to XOR inhibition. On the other hand, it has been suggested that cell-associated XOR may be less susceptible to allopurinol inhibition (232). This finding could imply that the effects of allopurinol administration cannot totally capture the significance of XOR.
15. PERSPECTIVE
XOR is intimately involved in the pathophysiology of HF and exerts regulatory actions in multiple levels of both ROS and NO signaling pathways. Preclinical studies and preliminary clinical studies assessing XOR inhibition showed promising findings. Given the rising burden of HF and its high morbidity and mortality rates, there is a clear rationale for pursuing further prospective, large-scale clinical studies designed to evaluate the potential therapeutic role of XOR inhibition in these patients. Finally, in this emerging era of individualized medicine, identifying and targeting specific subgroups of patients – such as patients with elevated SUA levels as the OPT-CHF study suggests – might also maximize the benefits of XOR inhibition in the management of HF.
Acknowledgments
Joshua M. Hare is supported by grants NIH 2RO1 HL-65455-05, NIA RO1 AG025017, NIH U54HL081028-01 and NIH/NHLBI R01HL084275. Konstantinos Tziomalos is supported by a grant from the Hellenic Antihypertensive Society. Joshua M. Hare serves as a consultant to Cardiome Pharma Corporation.
Abbreviations
- ANP
atrial natriuretic peptide
- aSA
a skeletal muscle isoform of actin
- ASK1
apoptosis signal-regulating kinase 1
- β-MHC
β-isoform of myosin heavy chain
- BNP
brain natriuretic peptide
- cGMP
cyclic guanosine monophosphate
- CK
creatine kinase
- ED
endothelial dysfunction
- ERK
extracellular signal-regulated kinases
- GTP
guanosine triphosphate
- HF
heart failure
- HNE
4-hydroxy-2-nonenal
- JNK
c-Jun N-terminal kinases
- L-NMMA
NG-monomethyl-L-arginine
- LTCC
L-type Ca+2 channels
- LV
left ventricular
- LVEF
left ventricular ejection fraction
- MAPK
mitogen-activated protein kinase
- MI
myocardial infarction
- MMP
matrix metalloproteinases
- NADPH
nicotinamide adenine dinucleotide phosphate
- NCX
Na+-Ca+2 transporter
- NFAT
nuclear factor of activated T-cells
- NO
nitric oxide
- NOS
nitric oxide synthase
- NOS1
neuronal nitric oxide synthase
- NOS3
endothelial nitric oxide synthase
- PCr
Phosphocreatine
- PLB
phospholamban
- PPARgamma
peroxisome proliferator-activated receptor gamma
- ROS
reactive oxygen species
- RYR
ryanodine receptor
- SERCA
sarcoplasmic reticulum Ca2+-ATPase
- SOD
superoxide dismutase
- SR
sarcoplasmic reticulum
- SUA
serum uric acid
- VEGF
vascular endothelial growth factor
- XDH
xanthine dehydrogenase
- XO
xanthine oxidase
- XOR
xanthine oxidoreductase
REFERENCES
- 1.Tendera M. The epidemiology of heart failure. J Renin Angiotensin Aldosterone Syst. 2004;5(Suppl. 1):S2–S6. doi: 10.3317/jraas.2004.020. [DOI] [PubMed] [Google Scholar]
- 2.American Heart Association. Heart disease and stroke statistics; 2005 update. 2007 [Google Scholar]
- 3.Masoudi FA, Havranek EP, Krumholz HM. The burden of chronic congestive heart failure in older persons: magnitude and implications for policy and research. Heart Fail Rev. 2002;7:9–16. doi: 10.1023/a:1013793621248. [DOI] [PubMed] [Google Scholar]
- 4.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 5.Hare JM, Stamler JS. NO/redox disequilibrium in the failing heart and cardiovascular system. J Clin Invest. 2005;115:509–517. doi: 10.1172/JCI200524459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zimmet JM, Hare JM. Nitroso-redox interactions in the cardiovascular system. Circulation. 2006;114:1531–1544. doi: 10.1161/CIRCULATIONAHA.105.605519. [DOI] [PubMed] [Google Scholar]
- 7.Seddon M, Looi YH, Shah AM. Oxidative stress and redox signalling in cardiac hypertrophy and heart failure. Heart. 2007;93:903–907. doi: 10.1136/hrt.2005.068270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mallat Z, Philip I, Lebret M, Chatel D, Maclouf J, Tedgui A. Elevated levels of 8-iso-prostaglandin F2alpha in pericardial fluid of patients with heart failure: a potential role for in vivo oxidant stress in ventricular dilatation and progression to heart failure. Circulation. 1998;97:1536–1539. doi: 10.1161/01.cir.97.16.1536. [DOI] [PubMed] [Google Scholar]
- 9.Belch JJ, Bridges AB, Scott N, Chopra M. Oxygen free radicals and congestive heart failure. Br Heart J. 1991;65:245–248. doi: 10.1136/hrt.65.5.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nonaka-Sarukawa M, Yamamoto K, Aoki H, Takano H, Katsuki T, Ikeda U, Shimada K. Increased urinary 15-F2t-isoprostane concentrations in patients with non-ischaemic congestive heart failure: a marker of oxidative stress. Heart. 2003;89:871–874. doi: 10.1136/heart.89.8.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keith M, Geranmayegan A, Sole MJ, Kurian R, Robinson A, Omran AS, Jeejeebhoy KN. Increased oxidative stress in patients with congestive heart failure. J Am Coll Cardiol. 1998;31:1352–1356. doi: 10.1016/s0735-1097(98)00101-6. [DOI] [PubMed] [Google Scholar]
- 12.Hare JM. Oxidative stress and apoptosis in heart failure progression. Circ Res. 2001;89:198–200. [PubMed] [Google Scholar]
- 13.Kinugawa S, Tsutsui H, Hayashidani S, Ide T, Suematsu N, Satoh S, Utsumi H, Takeshita A. Treatment with dimethylthiourea prevents left ventricular remodeling and failure after experimental myocardial infarction in mice: role of oxidative stress. Circ Res. 2000;87:392–398. doi: 10.1161/01.res.87.5.392. [DOI] [PubMed] [Google Scholar]
- 14.Kameda K, Matsunaga T, Abe N, Hanada H, Ishizaka H, Ono H, Saitoh M, Fukui K, Fukuda I, Osanai T, Okumura K. Correlation of oxidative stress with activity of matrix metalloproteinase in patients with coronary artery disease. Possible role for left ventricular remodelling. Eur Heart J. 2003;24:2180–2185. doi: 10.1016/j.ehj.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 15.Peterson JT, Hallak H, Johnson L, Li H, O’Brien PM, Sliskovic DR, Bocan TM, Coker ML, Etoh T, Spinale FG. Matrix metalloproteinase inhibition attenuates left ventricular remodeling and dysfunction in a rat model of progressive heart failure. Circulation. 2001;103:2303–2309. doi: 10.1161/01.cir.103.18.2303. [DOI] [PubMed] [Google Scholar]
- 16.Rajagopalan S, Meng XP, Ramasamy S, Harrison DG, Galis ZS. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stability. J Clin Invest. 1996;98:2572–2579. doi: 10.1172/JCI119076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siwik DA, Pagano PJ, Colucci WS. Oxidative stress regulates collagen synthesis and matrix metalloproteinase activity in cardiac fibroblasts. Am J Physiol Cell Physiol. 2001;280:C53–C60. doi: 10.1152/ajpcell.2001.280.1.C53. [DOI] [PubMed] [Google Scholar]
- 18.Spinale FG, Coker ML, Krombach SR, Mukherjee R, Hallak H, Houck WV, Clair MJ, Kribbs SB, Johnson LL, Peterson JT, Zile MR. Matrix metalloproteinase inhibition during the development of congestive heart failure : effects on left ventricular dimensions and function. Circ Res. 1999;85:364–376. doi: 10.1161/01.res.85.4.364. [DOI] [PubMed] [Google Scholar]
- 19.Thomas CV, Coker ML, Zellner JL, Handy JR, Crumbley AJ, III, Spinale FG. Increased matrix metalloproteinase activity and selective upregulation in LV myocardium from patients with end-stage dilated cardiomyopathy. Circulation. 1998;97:1708–1715. doi: 10.1161/01.cir.97.17.1708. [DOI] [PubMed] [Google Scholar]
- 20.Landmesser U, Spiekermann S, Dikalov S, Tatge H, Wilke R, Kohler C, Harrison DG, Hornig B, Drexler H. Vascular oxidative stress and endothelial dysfunction in patients with chronic heart failure: role of xanthine-oxidase and extracellular superoxide dismutase. Circulation. 2002;106:3073–3078. doi: 10.1161/01.cir.0000041431.57222.af. [DOI] [PubMed] [Google Scholar]
- 21.Cappola TP, Kass DA, Nelson GS, Berger RD, Rosas GO, Kobeissi ZA, Marban E, Hare JM. Allopurinol improves myocardial efficiency in patients with idiopathic dilated cardiomyopathy. Circulation. 2001;104:2407–2411. doi: 10.1161/hc4501.098928. [DOI] [PubMed] [Google Scholar]
- 22.Ide T, Tsutsui H, Kinugawa S, Utsumi H, Kang D, Hattori N, Uchida K, Arimura K, Egashira K, Takeshita A. Mitochondrial electron transport complex I is a potential source of oxygen free radicals in the failing myocardium. Circ Res. 1999;85:357–363. doi: 10.1161/01.res.85.4.357. [DOI] [PubMed] [Google Scholar]
- 23.Nojiri H, Shimizu T, Funakoshi M, Yamaguchi O, Zhou H, Kawakami S, Ohta Y, Sami M, Tachibana T, Ishikawa H, Kurosawa H, Kahn RC, Otsu K, Shirasawa T. Oxidative stress causes heart failure with impaired mitochondrial respiration. J Biol Chem. 2006;281:33789–33801. doi: 10.1074/jbc.M602118200. [DOI] [PubMed] [Google Scholar]
- 24.Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest. 2005;115:500–508. doi: 10.1172/JCI200524408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mollnau H, Oelze M, August M, Wendt M, Daiber A, Schulz E, Baldus S, Kleschyov AL, Materne A, Wenzel P, Hink U, Nickenig G, Fleming I, Munzel T. Mechanisms of increased vascular superoxide production in an experimental model of idiopathic dilated cardiomyopathy. Arterioscler Thromb Vasc Biol. 2005;25:2554–2559. doi: 10.1161/01.ATV.0000190673.41925.9B. [DOI] [PubMed] [Google Scholar]
- 26.Heymes C, Bendall JK, Ratajczak P, Cave AC, Samuel JL, Hasenfuss G, Shah AM. Increased myocardial NADPH oxidase activity in human heart failure. J Am Coll Cardiol. 2003;41:2164–2171. doi: 10.1016/s0735-1097(03)00471-6. [DOI] [PubMed] [Google Scholar]
- 27.Dixon LJ, Morgan DR, Hughes SM, McGrath LT, El Sherbeeny NA, Plumb RD, Devine A, Leahey W, Johnston GD, McVeigh GE. Functional consequences of endothelial nitric oxide synthase uncoupling in congestive cardiac failure. Circulation. 2003;107:1725–1728. doi: 10.1161/01.CIR.0000066283.13253.78. [DOI] [PubMed] [Google Scholar]
- 28.Saavedra WF, Paolocci N, St John ME, Skaf MW, Stewart GC, Xie JS, Harrison RW, Zeichner J, Mudrick D, Marban E, Kass DA, Hare JM. Imbalance between xanthine oxidase and nitric oxide synthase signaling pathways underlies mechanoenergetic uncoupling in the failing heart. Circ Res. 2002;90:297–304. doi: 10.1161/hh0302.104531. [DOI] [PubMed] [Google Scholar]
- 29.Chen Y, Hou M, Li Y, Traverse JH, Zhang P, Salvemini D, Fukai T, Bache RJ. Increased superoxide production causes coronary endothelial dysfunction and depressed oxygen consumption in the failing heart. Am J Physiol Heart Circ Physiol. 2005;288:H133–H141. doi: 10.1152/ajpheart.00851.2003. [DOI] [PubMed] [Google Scholar]
- 30.Berry CE, Hare JM. Xanthine oxidoreductase and cardiovascular disease: molecular mechanisms and pathophysiological implications. J Physiol. 2004;555:589–606. doi: 10.1113/jphysiol.2003.055913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuwabara Y, Nishino T, Okamoto K, Matsumura T, Eger BT, Pai EF, Nishino T. Unique amino acids cluster for switching from the dehydrogenase to oxidase form of xanthine oxidoreductase. Proc Natl Acad Sci U S A. 2003;100:8170–8175. doi: 10.1073/pnas.1431485100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olson JS, Ballou DP, Palmer G, Massey V. The mechanism of action of xanthine oxidase. J Biol Chem. 1974;249:4363–4382. [PubMed] [Google Scholar]
- 33.McCord JM, Fridovich I. The reduction of cytochrome c by milk xanthine oxidase. J Biol Chem. 1968;243:5753–5760. [PubMed] [Google Scholar]
- 34.Halliwell B, Gutteridge JM. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984;219:1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ide T, Tsutsui H, Kinugawa S, Suematsu N, Hayashidani S, Ichikawa K, Utsumi H, Machida Y, Egashira K, Takeshita A. Direct evidence for increased hydroxyl radicals originating from superoxide in the failing myocardium. Circ Res. 2000;86:152–157. doi: 10.1161/01.res.86.2.152. [DOI] [PubMed] [Google Scholar]
- 36.Genet S, Kale RK, Baquer NZ. Effects of free radicals on cytosolic creatine kinase activities and protection by antioxidant enzymes and sulfhydryl compounds. Mol Cell Biochem. 2000;210:23–28. doi: 10.1023/a:1007071617480. [DOI] [PubMed] [Google Scholar]
- 37.Amado LC, Saliaris AP, Raju SV, Lehrke S, St John M, Xie J, Stewart G, Fitton T, Minhas KM, Brawn J, Hare JM. Xanthine oxidase inhibition ameliorates cardiovascular dysfunction in dogs with pacing-induced heart failure. J Mol Cell Cardiol. 2005;39:531–536. doi: 10.1016/j.yjmcc.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 38.Ekelund UE, Harrison RW, Shokek O, Thakkar RN, Tunin RS, Senzaki H, Kass DA, Marban E, Hare JM. Intravenous allopurinol decreases myocardial oxygen consumption and increases mechanical efficiency in dogs with pacing-induced heart failure. Circ Res. 1999;85:437–445. doi: 10.1161/01.res.85.5.437. [DOI] [PubMed] [Google Scholar]
- 39.Stull LB, Leppo MK, Szweda L, Gao WD, Marban E. Chronic treatment with allopurinol boosts survival and cardiac contractility in murine postischemic cardiomyopathy. Circ Res. 2004;95:1005–1011. doi: 10.1161/01.RES.0000148635.73331.c5. [DOI] [PubMed] [Google Scholar]
- 40.Yamamoto E, Kataoka K, Yamashita T, Tokutomi Y, Dong YF, Matsuba S, Ogawa H, Kim-Mitsuyama S. Role of xanthine oxidoreductase in the reversal of diastolic heart failure by candesartan in the salt-sensitive hypertensive rat. Hypertension. 2007;50:657–662. doi: 10.1161/HYPERTENSIONAHA.107.095315. [DOI] [PubMed] [Google Scholar]
- 41.Schrier RW, Abraham WT. Hormones and hemodynamics in heart failure. N Engl J Med. 1999;341:577–585. doi: 10.1056/NEJM199908193410806. [DOI] [PubMed] [Google Scholar]
- 42.Mervaala EM, Cheng ZJ, Tikkanen I, Lapatto R, Nurminen K, Vapaatalo H, Muller DN, Fiebeler A, Ganten U, Ganten D, Luft FC. Endothelial dysfunction and xanthine oxidoreductase activity in rats with human renin and angiotensinogen genes. Hypertension. 2001;37:414–418. doi: 10.1161/01.hyp.37.2.414. [DOI] [PubMed] [Google Scholar]
- 43.Aukrust P, Ueland T, Muller F, Andreassen AK, Nordoy I, Aas H, Kjekshus J, Simonsen S, Froland SS, Gullestad L. Elevated circulating levels of C-C chemokines in patients with congestive heart failure. Circulation. 1998;97:1136–1143. doi: 10.1161/01.cir.97.12.1136. [DOI] [PubMed] [Google Scholar]
- 44.Torre-Amione G, Kapadia S, Benedict C, Oral H, Young JB, Mann DL. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: a report from the Studies of Left Ventricular Dysfunction (SOLVD) J Am Coll Cardiol. 1996;27:1201–1206. doi: 10.1016/0735-1097(95)00589-7. [DOI] [PubMed] [Google Scholar]
- 45.Ferdinandy P, Danial H, Ambrus I, Rothery RA, Schulz R. Peroxynitrite is a major contributor to cytokine-induced myocardial contractile failure. Circ Res. 2000;87:241–247. doi: 10.1161/01.res.87.3.241. [DOI] [PubMed] [Google Scholar]
- 46.Flanders KC, Bhandiwad AR, Winokur TS. Transforming growth factor-betas block cytokine induction of catalase and xanthine oxidase mRNA levels in cultured rat cardiac cells. J Mol Cell Cardiol. 1997;29:273–280. doi: 10.1006/jmcc.1996.0272. [DOI] [PubMed] [Google Scholar]
- 47.Hassoun PM, Yu FS, Shedd AL, Zulueta JJ, Thannickal VJ, Lanzillo JJ, Fanburg BL. Regulation of endothelial cell xanthine dehydrogenase xanthine oxidase gene expression by oxygen tension. Am J Physiol. 1994;266:L163–L171. doi: 10.1152/ajplung.1994.266.2.L163. [DOI] [PubMed] [Google Scholar]
- 48.Poss WB, Huecksteadt TP, Panus PC, Freeman BA, Hoidal JR. Regulation of xanthine dehydrogenase and xanthine oxidase activity by hypoxia. Am J Physiol. 1996;270:L941–L946. doi: 10.1152/ajplung.1996.270.6.L941. [DOI] [PubMed] [Google Scholar]
- 49.Hoshikawa Y, Ono S, Suzuki S, Tanita T, Chida M, Song C, Noda M, Tabata T, Voelkel NF, Fujimura S. Generation of oxidative stress contributes to the development of pulmonary hypertension induced by hypoxia. J Appl Physiol. 2001;90:1299–1306. doi: 10.1152/jappl.2001.90.4.1299. [DOI] [PubMed] [Google Scholar]
- 50.MacFarlane NG, Miller DJ. Depression of peak force without altering calcium sensitivity by the superoxide anion in chemically skinned cardiac muscle of rat. Circ Res. 1992;70:1217–1224. doi: 10.1161/01.res.70.6.1217. [DOI] [PubMed] [Google Scholar]
- 51.Perez NG, Gao WD, Marban E. Novel myofilament Ca2+-sensitizing property of xanthine oxidase inhibitors. Circ Res. 1998;83:423–430. doi: 10.1161/01.res.83.4.423. [DOI] [PubMed] [Google Scholar]
- 52.Khan SA, Lee K, Minhas KM, Gonzalez DR, Raju SV, Tejani AD, Li D, Berkowitz DE, Hare JM. Neuronal nitric oxide synthase negatively regulates xanthine oxidoreductase inhibition of cardiac excitation-contraction coupling. Proc Natl Acad Sci U S A. 2004;101:15944–15948. doi: 10.1073/pnas.0404136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gao WD, Liu Y, Marban E. Selective effects of oxygen free radicals on excitation-contraction coupling in ventricular muscle. Implications for the mechanism of stunned myocardium. Circulation. 1996;94:2597–2604. doi: 10.1161/01.cir.94.10.2597. [DOI] [PubMed] [Google Scholar]
- 54.Ventura C, Guarnieri C, Caldarera CM. Inhibitory effect of superoxide radicals on cardiac myofibrillar ATPase activity. Ital J Biochem. 1985;34:267–274. [PubMed] [Google Scholar]
- 55.Kogler H, Fraser H, McCune S, Altschuld R, Marban E. Disproportionate enhancement of myocardial contractility by the xanthine oxidase inhibitor oxypurinol in failing rat myocardium. Cardiovasc Res. 2003;59:582–592. doi: 10.1016/s0008-6363(03)00512-1. [DOI] [PubMed] [Google Scholar]
- 56.Perez NG, Hashimoto K, McCune S, Altschuld RA, Marban E. Origin of contractile dysfunction in heart failure: calcium cycling versus myofilaments. Circulation. 1999;99:1077–1083. doi: 10.1161/01.cir.99.8.1077. [DOI] [PubMed] [Google Scholar]
- 57.Ishida H, Genka C, Hirota Y, Hamasaki Y, Nakazawa H. Distinct roles of peroxynitrite and hydroxyl radical in triggering stunned myocardium-like impairment of cardiac myocytes in vitro. Mol Cell Biochem. 1999;198:31–38. doi: 10.1023/a:1006989826711. [DOI] [PubMed] [Google Scholar]
- 58.Ukai T, Cheng CP, Tachibana H, Igawa A, Zhang ZS, Cheng HJ, Little WC. Allopurinol enhances the contractile response to dobutamine and exercise in dogs with pacing-induced heart failure. Circulation. 2001;103:750–755. doi: 10.1161/01.cir.103.5.750. [DOI] [PubMed] [Google Scholar]
- 59.Minhas KM, Saraiva RM, Schuleri KH, Lehrke S, Zheng M, Saliaris AP, Berry CE, Barouch LA, Vandegaer KM, Li D, Hare JM. Xanthine oxidoreductase inhibition causes reverse remodeling in rats with dilated cardiomyopathy. Circ Res. 2006;98:271–279. doi: 10.1161/01.RES.0000200181.59551.71. [DOI] [PubMed] [Google Scholar]
- 60.Naumova AV, Chacko VP, Ouwerkerk R, Stull L, Marban E, Weiss RG. Xanthine oxidase inhibitors improve energetics and function after infarction in failing mouse hearts. Am J Physiol Heart Circ Physiol. 2006;290:H837–H843. doi: 10.1152/ajpheart.00831.2005. [DOI] [PubMed] [Google Scholar]
- 61.Ingwall JS, Weiss RG. Is the failing heart energy starved? On using chemical energy to support cardiac function. Circ Res. 2004;95:135–145. doi: 10.1161/01.RES.0000137170.41939.d9. [DOI] [PubMed] [Google Scholar]
- 62.Beer M, Seyfarth T, Sandstede J, Landschutz W, Lipke C, Kostler H, von Kienlin M, Harre K, Hahn D, Neubauer S. Absolute concentrations of high-energy phosphate metabolites in normal, hypertrophied, and failing human myocardium measured noninvasively with (31)P-SLOOP magnetic resonance spectroscopy. J Am Coll Cardiol. 2002;40:1267–1274. doi: 10.1016/s0735-1097(02)02160-5. [DOI] [PubMed] [Google Scholar]
- 63.Conway MA, Allis J, Ouwerkerk R, Niioka T, Rajagopalan B, Radda GK. Detection of low phosphocreatine to ATP ratio in failing hypertrophied human myocardium by 31P magnetic resonance spectroscopy. Lancet. 1991;338:973–976. doi: 10.1016/0140-6736(91)91838-l. [DOI] [PubMed] [Google Scholar]
- 64.Neubauer S, Horn M, Cramer M, Harre K, Newell JB, Peters W, Pabst T, Ertl G, Hahn D, Ingwall JS, Kochsiek K. Myocardial phosphocreatine-to-ATP ratio is a predictor of mortality in patients with dilated cardiomyopathy. Circulation. 1997;96:2190–2196. doi: 10.1161/01.cir.96.7.2190. [DOI] [PubMed] [Google Scholar]
- 65.Shen W, Spindler M, Higgins MA, Jin N, Gill RM, Bloem LJ, Ryan TP, Ingwall JS. The fall in creatine levels and creatine kinase isozyme changes in the failing heart are reversible: complex post-transcriptional regulation of the components of the CK system. J Mol Cell Cardiol. 2005;39:537–544. doi: 10.1016/j.yjmcc.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 66.Nascimben L, Ingwall JS, Pauletto P, Friedrich J, Gwathmey JK, Saks V, Pessina AC, Allen PD. Creatine kinase system in failing and nonfailing human myocardium. Circulation. 1996;94:1894–1901. doi: 10.1161/01.cir.94.8.1894. [DOI] [PubMed] [Google Scholar]
- 67.Tian R, Ingwall JS. Energetic basis for reduced contractile reserve in isolated rat hearts. Am J Physiol. 1996;270:H1207–H1216. doi: 10.1152/ajpheart.1996.270.4.H1207. [DOI] [PubMed] [Google Scholar]
- 68.Hamman BL, Bittl JA, Jacobus WE, Allen PD, Spencer RS, Tian R, Ingwall JS. Inhibition of the creatine kinase reaction decreases the contractile reserve of isolated rat hearts. Am J Physiol. 1995;269:H1030–H1036. doi: 10.1152/ajpheart.1995.269.3.H1030. [DOI] [PubMed] [Google Scholar]
- 69.Ishihara H, Yokota M, Sobue T, Saito H. Relation between ventriculoarterial coupling and myocardial energetics in patients with idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 1994;23:406–416. doi: 10.1016/0735-1097(94)90428-6. [DOI] [PubMed] [Google Scholar]
- 70.Packer M, Carver JR, Rodeheffer RJ, Ivanhoe RJ, DiBianco R, Zeldis SM, Hendrix GH, Bommer WJ, Elkayam U, Kukin ML, Mallis GI, Sollano JA, Shannon J, Tandon PK, DeMets DL. Effect of oral milrinone on mortality in severe chronic heart failure. The PROMISE Study Research Group. N Engl J Med. 1991;325:1468–1475. doi: 10.1056/NEJM199111213252103. [DOI] [PubMed] [Google Scholar]
- 71.O’Connor CM, Gattis WA, Uretsky BF, Adams KF, Jr, McNulty SE, Grossman SH, McKenna WJ, Zannad F, Swedberg K, Gheorghiade M, Califf RM. Continuous intravenous dobutamine is associated with an increased risk of death in patients with advanced heart failure: insights from the Flolan International Randomized Survival Trial (FIRST) Am Heart J. 1999;138:78–86. doi: 10.1016/s0002-8703(99)70250-4. [DOI] [PubMed] [Google Scholar]
- 72.del Monte F, Hajjar RJ. Targeting calcium cycling proteins in heart failure through gene transfer. J Physiol. 2003;546:49–61. doi: 10.1113/jphysiol.2002.026732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6. from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 74.Studer R, Reinecke H, Bilger J, Eschenhagen T, Bohm M, Hasenfuss G, Just H, Holtz J, Drexler H. Gene expression of the cardiac Na (+)-Ca2+ exchanger in end-stage human heart failure. Circ Res. 1994;75:443–453. doi: 10.1161/01.res.75.3.443. [DOI] [PubMed] [Google Scholar]
- 75.Schmidt U, Hajjar RJ, Helm PA, Kim CS, Doye AA, Gwathmey JK. Contribution of abnormal sarcoplasmic reticulum ATPase activity to systolic and diastolic dysfunction in human heart failure. J Mol Cell Cardiol. 1998;30:1929–1937. doi: 10.1006/jmcc.1998.0748. [DOI] [PubMed] [Google Scholar]
- 76.O’Rourke B, Kass DA, Tomaselli GF, Kaab S, Tunin R, Marban E. Mechanisms of altered excitation-contraction coupling in canine tachycardia-induced heart failure, I: experimental studies. Circ Res. 1999;84:562–570. doi: 10.1161/01.res.84.5.562. [DOI] [PubMed] [Google Scholar]
- 77.Saliaris AP, Amado LC, Minhas KM, Schuleri KH, Lehrke S, St John M, Fitton T, Barreiro C, Berry C, Zheng M, Kozielski K, Eneboe V, Brawn J, Hare JM. Chronic allopurinol administration ameliorates maladaptive alterations in Ca2+ cycling proteins and beta-adrenergic hyporesponsiveness in heart failure. Am J Physiol Heart Circ Physiol. 2007;292:H1328–H1335. doi: 10.1152/ajpheart.00461.2006. [DOI] [PubMed] [Google Scholar]
- 78.Blasig IE, Grune T, Schonheit K, Rohde E, Jakstadt M, Haseloff RF, Siems WG. 4-Hydroxynonenal, a novel indicator of lipid peroxidation for reperfusion injury of the myocardium. Am J Physiol. 1995;269:H14–H22. doi: 10.1152/ajpheart.1995.269.1.H14. [DOI] [PubMed] [Google Scholar]
- 79.Eaton P, Hearse DJ, Shattock MJ. Lipid hydroperoxide modification of proteins during myocardial ischaemia. Cardiovasc Res. 2001;51:294–303. doi: 10.1016/s0008-6363(01)00303-0. [DOI] [PubMed] [Google Scholar]
- 80.Duncan JG, Ravi R, Stull LB, Murphy AM. Chronic xanthine oxidase inhibition prevents myofibrillar protein oxidation and preserves cardiac function in a transgenic mouse model of cardiomyopathy. Am J Physiol Heart Circ Physiol. 2005;289:H1512–H1518. doi: 10.1152/ajpheart.00168.2005. [DOI] [PubMed] [Google Scholar]
- 81.Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348:2007–2018. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- 82.Engberding N, Spiekermann S, Schaefer A, Heineke A, Wiencke A, Muller M, Fuchs M, Hilfiker-Kleiner D, Hornig B, Drexler H, Landmesser U. Allopurinol attenuates left ventricular remodeling and dysfunction after experimental myocardial infarction: a new action for an old drug? Circulation. 2004;110:2175–2179. doi: 10.1161/01.CIR.0000144303.24894.1C. [DOI] [PubMed] [Google Scholar]
- 83.Mellin V, Isabelle M, Oudot A, Vergely-Vandriesse C, Monteil C, Di Meglio B, Henry JP, Dautreaux B, Rochette L, Thuillez C, Mulder P. Transient reduction in myocardial free oxygen radical levels is involved in the improved cardiac function and structure after long-term allopurinol treatment initiated in established chronic heart failure. Eur Heart J. 2005;26:1544–1550. doi: 10.1093/eurheartj/ehi305. [DOI] [PubMed] [Google Scholar]
- 84.van Empel VP, De Windt LJ. Myocyte hypertrophy and apoptosis: a balancing act. Cardiovasc Res. 2004;63:487–499. doi: 10.1016/j.cardiores.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 85.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 86.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 87.Bueno OF, De Windt LJ, Tymitz KM, Witt SA, Kimball TR, Klevitsky R, Hewett TE, Jones SP, Lefer DJ, Peng CF, Kitsis RN, Molkentin JD. The MEK1-ERK1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic mice. EMBO J. 2000;19:6341–6350. doi: 10.1093/emboj/19.23.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kang SM, Lim S, Song H, Chang W, Lee S, Bae SM, Chung JH, Lee H, Kim HG, Yoon DH, Kim TW, Jang Y, Sung JM, Chung NS, Hwang KC. Allopurinol modulates reactive oxygen species generation and Ca2+ overload in ischemia-reperfused heart and hypoxia-reoxygenated cardiomyocytes. Eur J Pharmacol. 2006;535:212–219. doi: 10.1016/j.ejphar.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 89.Dorn GW, Robbins J, Sugden PH. Phenotyping hypertrophy: eschew obfuscation. Circ Res. 2003;92:1171–1175. doi: 10.1161/01.RES.0000077012.11088.BC. [DOI] [PubMed] [Google Scholar]
- 90.Cesselli D, Jakoniuk I, Barlucchi L, Beltrami AP, Hintze TH, Nadal-Ginard B, Kajstura J, Leri A, Anversa P. Oxidative stress-mediated cardiac cell death is a major determinant of ventricular dysfunction and failure in dog dilated cardiomyopathy. Circ Res. 2001;89:279–286. doi: 10.1161/hh1501.094115. [DOI] [PubMed] [Google Scholar]
- 91.Narula J, Haider N, Virmani R, DiSalvo TG, Kolodgie FD, Hajjar RJ, Schmidt U, Semigran MJ, Dec GW, Khaw BA. Apoptosis in myocytes in end-stage heart failure. N Engl J Med. 1996;335:1182–1189. doi: 10.1056/NEJM199610173351603. [DOI] [PubMed] [Google Scholar]
- 92.Olivetti G, Abbi R, Quaini F, Kajstura J, Cheng W, Nitahara JA, Quaini E, Di Loreto C, Beltrami CA, Krajewski S, Reed JC, Anversa P. Apoptosis in the failing human heart. N Engl J Med. 1997;336:1131–1141. doi: 10.1056/NEJM199704173361603. [DOI] [PubMed] [Google Scholar]
- 93.Nagai H, Noguchi T, Takeda K, Ichijo H. Pathophysiological roles of ASK1-MAP kinase signaling pathways. J Biochem Mol Biol. 2007;40:1–6. doi: 10.5483/bmbrep.2007.40.1.001. [DOI] [PubMed] [Google Scholar]
- 94.Yamaguchi O, Higuchi Y, Hirotani S, Kashiwase K, Nakayama H, Hikoso S, Takeda T, Watanabe T, Asahi M, Taniike M, Matsumura Y, Tsujimoto I, Hongo K, Kusakari Y, Kurihara S, Nishida K, Ichijo H, Hori M, Otsu K. Targeted deletion of apoptosis signal-regulating kinase 1 attenuates left ventricular remodeling. Proc Natl Acad Sci U S A. 2003;100:15883–15888. doi: 10.1073/pnas.2136717100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tobiume K, Matsuzawa A, Takahashi T, Nishitoh H, Morita K, Takeda K, Minowa O, Miyazono K, Noda T, Ichijo H. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2001;2:222–228. doi: 10.1093/embo-reports/kve046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kaiser RA, Liang Q, Bueno O, Huang Y, Lackey T, Klevitsky R, Hewett TE, Molkentin JD. Genetic inhibition or activation of JNK1/2 protects the myocardium from ischemia-reperfusion-induced cell death in vivo . J Biol Chem. 2005;280:32602–32608. doi: 10.1074/jbc.M500684200. [DOI] [PubMed] [Google Scholar]
- 97.Adams JM, Cory S. Life-or-death decisions by the Bcl-2 protein family. Trends Biochem Sci. 2001;26:61–66. doi: 10.1016/s0968-0004(00)01740-0. [DOI] [PubMed] [Google Scholar]
- 98.Regula KM, Kirshenbaum LA. Apoptosis of ventricular myocytes: a means to an end. J Mol Cell Cardiol. 2005;38:3–13. doi: 10.1016/j.yjmcc.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 99.Dhingra S, Sharma AK, Singla DK, Singal PK. p38 and ERK 1/2 MAPkinases mediate interplay of TNF- and IL-10 in regulating oxidative stress and cardiac myocyte apoptosis. Am J Physiol Heart Circ Physiol. 2007;293:H3524–H3531. doi: 10.1152/ajpheart.00919.2007. [DOI] [PubMed] [Google Scholar]
- 100.Siwik DA, Tzortzis JD, Pimental DR, Chang DL, Pagano PJ, Singh K, Sawyer DB, Colucci WS. Inhibition of copper-zinc superoxide dismutase induces cell growth, hypertrophic phenotype, and apoptosis in neonatal rat cardiac myocytes in vitro. Circ Res. 1999;85:147–153. doi: 10.1161/01.res.85.2.147. [DOI] [PubMed] [Google Scholar]
- 101.Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gallagher GL, Jackson CJ, Hunyor SN. Myocardial extracellular matrix remodeling in ischemic heart failure. Front Biosci. 2007;12:1410–1419. doi: 10.2741/2157. [DOI] [PubMed] [Google Scholar]
- 103.Deschamps AM, Spinale FG. Pathways of matrix metalloproteinase induction in heart failure: bioactive molecules and transcriptional regulation. Cardiovasc Res. 2006;69:666–676. doi: 10.1016/j.cardiores.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 104.Spinale FG. Matrix metalloproteinases: regulation and dysregulation in the failing heart. Circ Res. 2002;90:520–530. doi: 10.1161/01.res.0000013290.12884.a3. [DOI] [PubMed] [Google Scholar]
- 105.Sivakumar P, Gupta S, Sarkar S, Sen S. Upregulation of lysyl oxidase and MMPs during cardiac remodeling in human dilated cardiomyopathy. Mol Cell Biochem. 2008;307:159–167. doi: 10.1007/s11010-007-9595-2. [DOI] [PubMed] [Google Scholar]
- 106.Li YY, Feldman AM, Sun Y, McTiernan CF. Differential expression of tissue inhibitors of metalloproteinases in the failing human heart. Circulation. 1998;98:1728–1734. doi: 10.1161/01.cir.98.17.1728. [DOI] [PubMed] [Google Scholar]
- 107.Ducharme A, Frantz S, Aikawa M, Rabkin E, Lindsey M, Rohde LE, Schoen FJ, Kelly RA, Werb Z, Libby P, Lee RT. Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J Clin Invest. 2000;106:55–62. doi: 10.1172/JCI8768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bergman MR, Teerlink JR, Mahimkar R, Li L, Zhu BQ, Nguyen A, Dahi S, Karliner JS, Lovett DH. Cardiac matrix metalloproteinase-2 expression independently induces marked ventricular remodeling and systolic dysfunction. Am J Physiol Heart Circ Physiol. 2007;292:H1847–H1860. doi: 10.1152/ajpheart.00434.2006. [DOI] [PubMed] [Google Scholar]
- 109.Fujiwara T, Matsunaga T, Kameda K, Abe N, Ono H, Higuma T, Yokoyama J, Hanada H, Osanai T, Okumura K. Nicorandil suppresses the increases in plasma level of matrix metalloproteinase activity and attenuates left ventricular remodeling in patients with acute myocardial infarction. Heart Vessels. 2007;22:303–309. doi: 10.1007/s00380-007-0975-z. [DOI] [PubMed] [Google Scholar]
- 110.Gheorghiade M, Bonow RO. Chronic heart failure in the United States: a manifestation of coronary artery disease. Circulation. 1998;97:282–289. doi: 10.1161/01.cir.97.3.282. [DOI] [PubMed] [Google Scholar]
- 111.Hajjar RJ, Schmidt U, Helm P, Gwathmey JK. Ca++ sensitizers impair cardiac relaxation in failing human myocardium. J Pharmacol Exp Ther. 1997;280:247–254. [PubMed] [Google Scholar]
- 112.Murdoch CE, Zhang M, Cave AC, Shah AM. NADPH oxidase-dependent redox signalling in cardiac hypertrophy, remodelling and failure. Cardiovasc Res. 2006;71:208–215. doi: 10.1016/j.cardiores.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 113.Maack C, Kartes T, Kilter H, Schafers HJ, Nickenig G, Bohm M, Laufs U. Oxygen free radical release in human failing myocardium is associated with increased activity of rac1-GTPase and represents a target for statin treatment. Circulation. 2003;108:1567–1574. doi: 10.1161/01.CIR.0000091084.46500.BB. [DOI] [PubMed] [Google Scholar]
- 114.Nediani C, Borchi E, Giordano C, Baruzzo S, Ponziani V, Sebastiani M, Nassi P, Mugelli A, d’Amati G, Cerbai E. NADPH oxidase-dependent redox signaling in human heart failure: relationship between the left and right ventricle. J Mol Cell Cardiol. 2007;42:826–834. doi: 10.1016/j.yjmcc.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 115.Qin F, Simeone M, Patel R. Inhibition of NADPH oxidase reduces myocardial oxidative stress and apoptosis and improves cardiac function in heart failure after myocardial infarction. Free Radic Biol Med. 2007;43:271–281. doi: 10.1016/j.freeradbiomed.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 116.McNally JS, Davis ME, Giddens DP, Saha A, Hwang J, Dikalov S, Jo H, Harrison DG. Role of xanthine oxidoreductase and NAD (P)H oxidase in endothelial superoxide production in response to oscillatory shear stress. Am J Physiol Heart Circ Physiol. 2003;285:H2290–H2297. doi: 10.1152/ajpheart.00515.2003. [DOI] [PubMed] [Google Scholar]
- 117.Makazan Z, Saini HK, Dhalla NS. Role of oxidative stress in alterations of mitochondrial function in ischemic-reperfused hearts. Am J Physiol Heart Circ Physiol. 2007;292:H1986–H1994. doi: 10.1152/ajpheart.01214.2006. [DOI] [PubMed] [Google Scholar]
- 118.Faraci FM, Didion SP. Vascular protection: superoxide dismutase isoforms in the vessel wall. Arterioscler Thromb Vasc Biol. 2004;24:1367–1373. doi: 10.1161/01.ATV.0000133604.20182.cf. [DOI] [PubMed] [Google Scholar]
- 119.Valenti L, Conte D, Piperno A, Dongiovanni P, Fracanzani AL, Fraquelli M, Vergani A, Gianni C, Carmagnola L, Fargion S. The mitochondrial superoxide dismutase A16V polymorphism in the cardiomyopathy associated with hereditary haemochromatosis. J Med Genet. 2004;41:946–950. doi: 10.1136/jmg.2004.019588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 121.Murad F. Shattuck Lecture. Nitric oxide and cyclic GMP in cell signaling and drug development. N Engl J Med. 2006;355:2003–2011. doi: 10.1056/NEJMsa063904. [DOI] [PubMed] [Google Scholar]
- 122.Hare JM. Spatial confinement of isoforms of cardiac nitric-oxide synthase: unravelling the complexities of nitric oxide’s cardiobiology. Lancet. 2004;363:1338–1339. doi: 10.1016/S0140-6736(04)16083-2. [DOI] [PubMed] [Google Scholar]
- 123.Xu KY, Huso DL, Dawson TM, Bredt DS, Becker LC. Nitric oxide synthase in cardiac sarcoplasmic reticulum. Proc Natl Acad Sci U S A. 1999;96:657–662. doi: 10.1073/pnas.96.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Feron O, Belhassen L, Kobzik L, Smith TW, Kelly RA, Michel T. Endothelial nitric oxide synthase targeting to caveolae. Specific interactions with caveolin isoforms in cardiac myocytes and endothelial cells. J Biol Chem. 1996;271:22810–22814. doi: 10.1074/jbc.271.37.22810. [DOI] [PubMed] [Google Scholar]
- 125.Kanai AJ, Pearce LL, Clemens PR, Birder LA, VanBibber MM, Choi SY, de Groat WC, Peterson J. Identification of a neuronal nitric oxide synthase in isolated cardiac mitochondria using electrochemical detection. Proc Natl Acad Sci U S A. 2001;98:14126–14131. doi: 10.1073/pnas.241380298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Damy T, Ratajczak P, Shah AM, Camors E, Marty I, Hasenfuss G, Marotte F, Samuel JL, Heymes C. Increased neuronal nitric oxide synthase-derived NO production in the failing human heart. Lancet. 2004;363:1365–1367. doi: 10.1016/S0140-6736(04)16048-0. [DOI] [PubMed] [Google Scholar]
- 127.Saraiva RM, Hare JM. Nitric oxide signaling in the cardiovascular system: implications for heart failure. Curr Opin Cardiol. 2006;21:221–228. doi: 10.1097/01.hco.0000221584.56372.dc. [DOI] [PubMed] [Google Scholar]
- 128.Hare JM. Nitric oxide and excitation-contraction coupling. J Mol Cell Cardiol. 2003;35:719–729. doi: 10.1016/s0022-2828(03)00143-3. [DOI] [PubMed] [Google Scholar]
- 129.Xu L, Eu JP, Meissner G, Stamler JS. Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science. 1998;279:234–237. doi: 10.1126/science.279.5348.234. [DOI] [PubMed] [Google Scholar]
- 130.Eu JP, Sun J, Xu L, Stamler JS, Meissner G. The skeletal muscle calcium release channel: coupled O2 sensor and NO signaling functions. Cell. 2000;102:499–509. doi: 10.1016/s0092-8674(00)00054-4. [DOI] [PubMed] [Google Scholar]
- 131.Campbell DL, Stamler JS, Strauss HC. Redox modulation of L-type calcium channels in ferret ventricular myocytes. Dual mechanism regulation by nitric oxide and S-nitrosothiols. J Gen Physiol. 1996;108:277–293. doi: 10.1085/jgp.108.4.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sun J, Picht E, Ginsburg KS, Bers DM, Steenbergen C, Murphy E. Hypercontractile female hearts exhibit increased S-nitrosylation of the L-type Ca2+ channel alpha1 subunit and reduced ischemia/reperfusion injury. Circ Res. 2006;98:403–411. doi: 10.1161/01.RES.0000202707.79018.0a. [DOI] [PubMed] [Google Scholar]
- 133.Barouch LA, Cappola TP, Harrison RW, Crone JK, Rodriguez ER, Burnett AL, Hare JM. Combined loss of neuronal and endothelial nitric oxide synthase causes premature mortality and age-related hypertrophic cardiac remodeling in mice. J Mol Cell Cardiol. 2003;35:637–644. doi: 10.1016/s0022-2828(03)00079-8. [DOI] [PubMed] [Google Scholar]
- 134.Cappola TP, Cope L, Cernetich A, Barouch LA, Minhas K, Irizarry RA, Parmigiani G, Durrani S, Lavoie T, Hoffman EP, Ye SQ, Garcia JG, Hare JM. Deficiency of different nitric oxide synthase isoforms activates divergent transcriptional programs in cardiac hypertrophy. Physiol Genomics. 2003;14:25–34. doi: 10.1152/physiolgenomics.00156.2002. [DOI] [PubMed] [Google Scholar]
- 135.Kim SJ, Ju JW, Oh CD, Yoon YM, Song WK, Kim JH, Yoo YJ, Bang OS, Kang SS, Chun JS. ERK-1/2 and p38 kinase oppositely regulate nitric oxide-induced apoptosis of chondrocytes in association with p53, caspase-3, and differentiation status. J Biol Chem. 2002;277:1332–1339. doi: 10.1074/jbc.M107231200. [DOI] [PubMed] [Google Scholar]
- 136.Lander HM, Jacovina AT, Davis RJ, Tauras JM. Differential activation of mitogen-activated protein kinases by nitric oxide-related species. J Biol Chem. 1996;271:19705–19709. doi: 10.1074/jbc.271.33.19705. [DOI] [PubMed] [Google Scholar]
- 137.Lander HM, Hajjar DP, Hempstead BL, Mirza UA, Chait BT, Campbell S, Quilliam LA. A molecular redox switch on p21 (ras) Structural basis for the nitric oxide-p21 (ras) interaction. J Biol Chem. 1997;272:4323–4326. doi: 10.1074/jbc.272.7.4323. [DOI] [PubMed] [Google Scholar]
- 138.Hood J, Granger HJ. Protein kinase G mediates vascular endothelial growth factor-induced Raf-1 activation and proliferation in human endothelial cells. J Biol Chem. 1998;273:23504–23508. doi: 10.1074/jbc.273.36.23504. [DOI] [PubMed] [Google Scholar]
- 139.Parenti A, Morbidelli L, Cui XL, Douglas JG, Hood JD, Granger HJ, Ledda F, Ziche M. Nitric oxide is an upstream signal of vascular endothelial growth factor-induced extracellular signal-regulated kinase1/2 activation in postcapillary endothelium. J Biol Chem. 1998;273:4220–4226. doi: 10.1074/jbc.273.7.4220. [DOI] [PubMed] [Google Scholar]
- 140.Jun CD, Oh CD, Kwak HJ, Pae HO, Yoo JC, Choi BM, Chun JS, Park RK, Chung HT. Overexpression of protein kinase C isoforms protects RAW 264.7. macrophages from nitric oxide-induced apoptosis: involvement of c-Jun N-terminal kinase/stress-activated protein kinase, p38 kinase, and CPP-32 protease pathways. J Immunol. 1999;162:3395–3401. [PubMed] [Google Scholar]
- 141.Raines KW, Cao GL, Lee EK, Rosen GM, Shapiro P. Neuronal nitric oxide synthase-induced S-nitrosylation of H-Ras inhibits calcium ionophore-mediated extracellular-signal-regulated kinase activity. Biochem J. 2006;397:329–336. doi: 10.1042/BJ20052002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Li SJ, Sun NL. Regulation of intracellular Ca2+ and calcineurin by NO/PKG in proliferation of vascular smooth muscle cells. Acta Pharmacol Sin. 2005;26:323–328. doi: 10.1111/j.1745-7254.2005.00049.x. [DOI] [PubMed] [Google Scholar]
- 144.Fiedler B, Lohmann SM, Smolenski A, Linnemuller S, Pieske B, Schroder F, Molkentin JD, Drexler H, Wollert KC. Inhibition of calcineurin-NFAT hypertrophy signaling by cGMP-dependent protein kinase type I in cardiac myocytes. Proc Natl Acad Sci U S A. 2002;99:11363–11368. doi: 10.1073/pnas.162100799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Raju SV, Barouch LA, Hare JM. Nitric oxide and oxidative stress in cardiovascular aging. Sci Aging Knowledge Environ. 2005;2005 doi: 10.1126/sageke.2005.21.re4. re4. [DOI] [PubMed] [Google Scholar]
- 146.Park HS, Yu JW, Cho JH, Kim MS, Huh SH, Ryoo K, Choi EJ. Inhibition of apoptosis signal-regulating kinase 1 by nitric oxide through a thiol redox mechanism. J Biol Chem. 2004;279:7584–7590. doi: 10.1074/jbc.M304183200. [DOI] [PubMed] [Google Scholar]
- 147.Yamashita T, Yamamoto E, Kataoka K, Nakamura T, Matsuba S, Tokutomi Y, Dong YF, Ichijo H, Ogawa H, Kim-Mitsuyama S. Apoptosis signal-regulating kinase-1 is involved in vascular endothelial and cardiac remodeling caused by nitric oxide deficiency. Hypertension. 2007;50:519–524. doi: 10.1161/HYPERTENSIONAHA.107.092049. [DOI] [PubMed] [Google Scholar]
- 148.Ghatan S, Larner S, Kinoshita Y, Hetman M, Patel L, Xia Z, Youle RJ, Morrison RS. p38 MAP kinase mediates bax translocation in nitric oxide-induced apoptosis in neurons. J Cell Biol. 2000;150:335–347. doi: 10.1083/jcb.150.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Browning DD, McShane MP, Marty C, Ye RD. Nitric oxide activation of p38 mitogen-activated protein kinase in 293T fibroblasts requires cGMP-dependent protein kinase. J Biol Chem. 2000;275:2811–2816. doi: 10.1074/jbc.275.4.2811. [DOI] [PubMed] [Google Scholar]
- 150.Han OJ, Joe KH, Kim SW, Lee HS, Kwon NS, Baek KJ, Yun HY. Involvement of p38 mitogen-activated protein kinase and apoptosis signal-regulating kinase-1 in nitric oxide-induced cell death in PC12 cells. Neurochem Res. 2001;26:525–532. doi: 10.1023/a:1010917129951. [DOI] [PubMed] [Google Scholar]
- 151.Park HS, Huh SH, Kim MS, Lee SH, Choi EJ. Nitric oxide negatively regulates c-Jun N-terminal kinase/stress-activated protein kinase by means of S-nitrosylation. Proc Natl Acad Sci U S A. 2000;97:14382–14387. doi: 10.1073/pnas.97.26.14382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Park DS, Stefanis L, Yan CY, Farinelli SE, Greene LA. Ordering the cell death pathway. Differential effects of BCL2, an interleukin-1-converting enzyme family protease inhibitor, and other survival agents on JNK activation in serum/nerve growth factor-deprived PC12 cells. J Biol Chem. 1996;271:21898–21905. doi: 10.1074/jbc.271.36.21898. [DOI] [PubMed] [Google Scholar]
- 153.So HS, Park RK, Kim MS, Lee SR, Jung BH, Chung SY, Jun CD, Chung HT. Nitric oxide inhibits c-Jun N-terminal kinase 2 (JNK2) via S-nitrosylation. Biochem Biophys Res Commun. 1998;247:809–813. doi: 10.1006/bbrc.1998.8788. [DOI] [PubMed] [Google Scholar]
- 154.Trujillo M, Alvarez MN, Peluffo G, Freeman BA, Radi R. Xanthine oxidase-mediated decomposition of S-nitrosothiols. J Biol Chem. 1998;273:7828–7834. doi: 10.1074/jbc.273.14.7828. [DOI] [PubMed] [Google Scholar]
- 155.Li H, Samouilov A, Liu X, Zweier JL. Characterization of the magnitude and kinetics of xanthine oxidase-catalyzed nitrite reduction. Evaluation of its role in nitric oxide generation in anoxic tissues. J Biol Chem. 2001;276:24482–24489. doi: 10.1074/jbc.M011648200. [DOI] [PubMed] [Google Scholar]
- 156.Ichimori K, Fukahori M, Nakazawa H, Okamoto K, Nishino T. Inhibition of xanthine oxidase and xanthine dehydrogenase by nitric oxide. Nitric oxide converts reduced xanthine-oxidizing enzymes into the desulfo-type inactive form. J Biol Chem. 1999;274:7763–7768. doi: 10.1074/jbc.274.12.7763. [DOI] [PubMed] [Google Scholar]
- 157.Hassoun PM, Yu FS, Zulueta JJ, White AC, Lanzillo JJ. Effect of nitric oxide and cell redox status on the regulation of endothelial cell xanthine dehydrogenase. Am J Physiol. 1995;268:L809–L817. doi: 10.1152/ajplung.1995.268.5.L809. [DOI] [PubMed] [Google Scholar]
- 158.Kinugawa S, Huang H, Wang Z, Kaminski PM, Wolin MS, Hintze TH. A defect of neuronal nitric oxide synthase increases xanthine oxidase-derived superoxide anion and attenuates the control of myocardial oxygen consumption by nitric oxide derived from endothelial nitric oxide synthase. Circ Res. 2005;96:355–362. doi: 10.1161/01.RES.0000155331.09458.A7. [DOI] [PubMed] [Google Scholar]
- 159.Saraiva RM, Minhas KM, Raju SV, Barouch LA, Pitz E, Schuleri KH, Vandegaer K, Li D, Hare JM. Deficiency of neuronal nitric oxide synthase increases mortality and cardiac remodeling after myocardial infarction: role of nitroso-redox equilibrium. Circulation. 2005;112:3415–3422. doi: 10.1161/CIRCULATIONAHA.105.557892. [DOI] [PubMed] [Google Scholar]
- 160.Smith RS, Jr, Agata J, Xia CF, Chao L, Chao J. Human endothelial nitric oxide synthase gene delivery protects against cardiac remodeling and reduces oxidative stress after myocardial infarction. Life Sci. 2005;76:2457–2471. doi: 10.1016/j.lfs.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 161.Kalinowski L, Malinski T. Endothelial NADH/NADPH-dependent enzymatic sources of superoxide production: relationship to endothelial dysfunction. Acta Biochim Pol. 2004;51:459–469. [PubMed] [Google Scholar]
- 162.Hare JM. Nitroso-redox balance in the cardiovascular system. N Engl J Med. 2004;351:2112–2114. doi: 10.1056/NEJMe048269. [DOI] [PubMed] [Google Scholar]
- 163.Radi R, Rodriguez M, Castro L, Telleri R. Inhibition of mitochondrial electron transport by peroxynitrite. Arch Biochem Biophys. 1994;308:89–95. doi: 10.1006/abbi.1994.1013. [DOI] [PubMed] [Google Scholar]
- 164.Digerness SB, Harris KD, Kirklin JW, Urthaler F, Viera L, Beckman JS, Darley-Usmar V. Peroxynitrite irreversibly decreases diastolic and systolic function in cardiac muscle. Free Radic Biol Med. 1999;27:1386–1392. doi: 10.1016/s0891-5849(99)00184-7. [DOI] [PubMed] [Google Scholar]
- 165.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cohn JN, Archibald DG, Ziesche S, Franciosa JA, Harston WE, Tristani FE, Dunkman WB, Jacobs W, Francis GS, Flohr KH, Goldman S, Cobb FR, Shah PM, Saunders R, Fletcher RD, Loeb HS, Hughes VC, Baker B. Effect of vasodilator therapy on mortality in chronic congestive heart failure. Results of a Veterans Administration Cooperative Study. N Engl J Med. 1986;314:1547–1552. doi: 10.1056/NEJM198606123142404. [DOI] [PubMed] [Google Scholar]
- 167.Taylor AL, Ziesche S, Yancy C, Carson P, D’Agostino R, Jr, Ferdinand K, Taylor M, Adams K, Sabolinski M, Worcel M, Cohn JN. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med. 2004;351:2049–2057. doi: 10.1056/NEJMoa042934. [DOI] [PubMed] [Google Scholar]
- 168.Ignarro LJ, Napoli C, Loscalzo J. Nitric oxide donors and cardiovascular agents modulating the bioactivity of nitric oxide: an overview. Circ Res. 2002;90:21–28. doi: 10.1161/hh0102.102330. [DOI] [PubMed] [Google Scholar]
- 169.Munzel T, Kurz S, Rajagopalan S, Thoenes M, Berrington WR, Thompson JA, Freeman BA, Harrison DG. Hydralazine prevents nitroglycerin tolerance by inhibiting activation of a membrane-bound NADH oxidase. A new action for an old drug. J Clin Invest. 1996;98:1465–1470. doi: 10.1172/JCI118935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Daiber A, Oelze M, Coldewey M, Kaiser K, Huth C, Schildknecht S, Bachschmid M, Nazirisadeh Y, Ullrich V, Mulsch A, Munzel T, Tsilimingas N. Hydralazine is a powerful inhibitor of peroxynitrite formation as a possible explanation for its beneficial effects on prognosis in patients with congestive heart failure. Biochem Biophys Res Commun. 2005;338:1865–1874. doi: 10.1016/j.bbrc.2005.10.106. [DOI] [PubMed] [Google Scholar]
- 171.Hornig B, Arakawa N, Kohler C, Drexler H. Vitamin C improves endothelial function of conduit arteries in patients with chronic heart failure. Circulation. 1998;97:363–368. doi: 10.1161/01.cir.97.4.363. [DOI] [PubMed] [Google Scholar]
- 172.Kubo SH, Rector TS, Bank AJ, Williams RE, Heifetz SM. Endothelium-dependent vasodilation is attenuated in patients with heart failure. Circulation. 1991;84:1589–1596. doi: 10.1161/01.cir.84.4.1589. [DOI] [PubMed] [Google Scholar]
- 173.Drexler H, Hayoz D, Munzel T, Hornig B, Just H, Brunner HR, Zelis R. Endothelial function in chronic congestive heart failure. Am J Cardiol. 1992;69:1596–1601. doi: 10.1016/0002-9149(92)90710-g. [DOI] [PubMed] [Google Scholar]
- 174.Katz SD, Biasucci L, Sabba C, Strom JA, Jondeau G, Galvao M, Solomon S, Nikolic SD, Forman R, LeJemtel TH. Impaired endothelium-mediated vasodilation in the peripheral vasculature of patients with congestive heart failure. J Am Coll Cardiol. 1992;19:918–925. doi: 10.1016/0735-1097(92)90271-n. [DOI] [PubMed] [Google Scholar]
- 175.Katz SD, Schwarz M, Yuen J, LeJemtel TH. Impaired acetylcholine-mediated vasodilation in patients with congestive heart failure. Role of endothelium-derived vasodilating and vasoconstricting factors. Circulation. 1993;88:55–61. doi: 10.1161/01.cir.88.1.55. [DOI] [PubMed] [Google Scholar]
- 176.Chong AY, Blann AD, Patel J, Freestone B, Hughes E, Lip GY. Endothelial dysfunction and damage in congestive heart failure: relation of flow-mediated dilation to circulating endothelial cells, plasma indexes of endothelial damage, and brain natriuretic peptide. Circulation. 2004;110:1794–1798. doi: 10.1161/01.CIR.0000143073.60937.50. [DOI] [PubMed] [Google Scholar]
- 177.Ishibashi Y, Shimada T, Sakane T, Takahashi N, Sugamori T, Ohhata S, Inoue S, Katoh H, Sano K, Murakami Y, Hashimoto M. Contribution of endogenous nitric oxide to basal vasomotor tone of peripheral vessels and plasma B-type natriuretic peptide levels in patients with congestive heart failure. J Am Coll Cardiol. 2000;36:1605–1611. doi: 10.1016/s0735-1097(00)00920-7. [DOI] [PubMed] [Google Scholar]
- 178.Doehner W, Schoene N, Rauchhaus M, Leyva-Leon F, Pavitt DV, Reaveley DA, Schuler G, Coats AJ, Anker SD, Hambrecht R. Effects of xanthine oxidase inhibition with allopurinol on endothelial function and peripheral blood flow in hyperuricemic patients with chronic heart failure: results from 2 placebo-controlled studies. Circulation. 2002;105:2619–2624. doi: 10.1161/01.cir.0000017502.58595.ed. [DOI] [PubMed] [Google Scholar]
- 179.Farquharson CA, Butler R, Hill A, Belch JJ, Struthers AD. Allopurinol improves endothelial dysfunction in chronic heart failure. Circulation. 2002;106:221–226. doi: 10.1161/01.cir.0000022140.61460.1d. [DOI] [PubMed] [Google Scholar]
- 180.George J, Carr E, Davies J, Belch JJ, Struthers A. High-dose allopurinol improves endothelial function by profoundly reducing vascular oxidative stress and not by lowering uric acid. Circulation. 2006;114:2508–2516. doi: 10.1161/CIRCULATIONAHA.106.651117. [DOI] [PubMed] [Google Scholar]
- 181.Lopez FA, Casado S. Heart failure, redox alterations, and endothelial dysfunction. Hypertension. 2001;38:1400–1405. doi: 10.1161/hy1201.099612. [DOI] [PubMed] [Google Scholar]
- 182.Drexler H. Endothelium as a therapeutic target in heart failure. Circulation. 1998;98:2652–2655. doi: 10.1161/01.cir.98.24.2652. [DOI] [PubMed] [Google Scholar]
- 183.Tziomalos K, Athyros VG, Karagiannis A, Mikhailidis DP. Endothelial function, arterial stiffness and lipid lowering drugs. Expert Opin Ther Targets. 2007;11:1143–1160. doi: 10.1517/14728222.11.9.1143. [DOI] [PubMed] [Google Scholar]
- 184.Arimura K, Egashira K, Nakamura R, Ide T, Tsutsui H, Shimokawa H, Takeshita A. Increased inactivation of nitric oxide is involved in coronary endothelial dysfunction in heart failure. Am J Physiol Heart Circ Physiol. 2001;280:H68–H75. doi: 10.1152/ajpheart.2001.280.1.H68. [DOI] [PubMed] [Google Scholar]
- 185.van den Heuvel AF, van Veldhuisen DJ, van der Wall EE, Blanksma PK, Siebelink HM, Vaalburg WM, van Gilst WH, Crijns HJ. Regional myocardial blood flow reserve impairment and metabolic changes suggesting myocardial ischemia in patients with idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 2000;35:19–28. doi: 10.1016/s0735-1097(99)00499-4. [DOI] [PubMed] [Google Scholar]
- 186.Katz SD, Hryniewicz K, Hriljac I, Balidemaj K, Dimayuga C, Hudaihed A, Yasskiy A. Vascular endothelial dysfunction and mortality risk in patients with chronic heart failure. Circulation. 2005;111:310–314. doi: 10.1161/01.CIR.0000153349.77489.CF. [DOI] [PubMed] [Google Scholar]
- 187.Heitzer T, Baldus S, von Kodolitsch Y, Rudolph V, Meinertz T. Systemic endothelial dysfunction as an early predictor of adverse outcome in heart failure. Arterioscler Thromb Vasc Biol. 2005;25:1174–1179. doi: 10.1161/01.ATV.0000166516.52477.81. [DOI] [PubMed] [Google Scholar]
- 188.Fischer D, Rossa S, Landmesser U, Spiekermann S, Engberding N, Hornig B, Drexler H. Endothelial dysfunction in patients with chronic heart failure is independently associated with increased incidence of hospitalization, cardiac transplantation, or death. Eur Heart J. 2005;26:65–69. doi: 10.1093/eurheartj/ehi001. [DOI] [PubMed] [Google Scholar]
- 189.Waring WS, Webb DJ, Maxwell SR. Systemic uric acid administration increases serum antioxidant capacity in healthy volunteers. J Cardiovasc Pharmacol. 2001;38:365–371. doi: 10.1097/00005344-200109000-00005. [DOI] [PubMed] [Google Scholar]
- 190.Hink HU, Santanam N, Dikalov S, McCann L, Nguyen AD, Parthasarathy S, Harrison DG, Fukai T. Peroxidase properties of extracellular superoxide dismutase: role of uric acid in modulating in vivo activity. Arterioscler Thromb Vasc Biol. 2002;22:1402–1408. doi: 10.1161/01.atv.0000027524.86752.02. [DOI] [PubMed] [Google Scholar]
- 191.Johnson RJ, Kang DH, Feig D, Kivlighn S, Kanellis J, Watanabe S, Tuttle KR, Rodriguez-Iturbe B, Herrera-Acosta J, Mazzali M. Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension. 2003;41:1183–1190. doi: 10.1161/01.HYP.0000069700.62727.C5. [DOI] [PubMed] [Google Scholar]
- 192.Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci U S A. 1981;78:6858–6862. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Netea MG, Kullberg BJ, Blok WL, Netea RT, van der Meer JW. The role of hyperuricemia in the increased cytokine production after lipopolysaccharide challenge in neutropenic mice. Blood. 1997;89:577–582. [PubMed] [Google Scholar]
- 194.Kanellis J, Watanabe S, Li JH, Kang DH, Li P, Nakagawa T, Wamsley A, Sheikh-Hamad D, Lan HY, Feng L, Johnson RJ. Uric acid stimulates monocyte chemoattractant protein-1 production in vascular smooth muscle cells via mitogen-activated protein kinase and cyclooxygenase-2. Hypertension. 2003;41:1287–1293. doi: 10.1161/01.HYP.0000072820.07472.3B. [DOI] [PubMed] [Google Scholar]
- 195.Kang DH, Park SK, Lee IK, Johnson RJ. Uric acid-induced C-reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. J Am Soc Nephrol. 2005;16:3553–3562. doi: 10.1681/ASN.2005050572. [DOI] [PubMed] [Google Scholar]
- 196.Ginsberg MH, Kozin F, O’Malley M, McCarty DJ. Release of platelet constituents by monosodium urate crystals. J Clin Invest. 1977;60:999–1007. doi: 10.1172/JCI108880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kang DH, Han L, Ouyang X, Kahn AM, Kanellis J, Li P, Feng L, Nakagawa T, Watanabe S, Hosoyamada M, Endou H, Lipkowitz M, Abramson R, Mu W, Johnson RJ. Uric acid causes vascular smooth muscle cell proliferation by entering cells via a functional urate transporter. Am J Nephrol. 2005;25:425–433. doi: 10.1159/000087713. [DOI] [PubMed] [Google Scholar]
- 198.Rao GN, Corson MA, Berk BC. Uric acid stimulates vascular smooth muscle cell proliferation by increasing platelet-derived growth factor A-chain expression. J Biol Chem. 1991;266:8604–8608. [PubMed] [Google Scholar]
- 199.Waring WS, Webb DJ, Maxwell SR. Uric acid as a risk factor for cardiovascular disease. QJM. 2000;93:707–713. doi: 10.1093/qjmed/93.11.707. [DOI] [PubMed] [Google Scholar]
- 200.Bos MJ, Koudstaal PJ, Hofman A, Witteman JC, Breteler MM. Uric acid is a risk factor for myocardial infarction and stroke: the Rotterdam study. Stroke. 2006;37:1503–1507. doi: 10.1161/01.STR.0000221716.55088.d4. [DOI] [PubMed] [Google Scholar]
- 201.Fang J, Alderman MH. Serum uric acid and cardiovascular mortality the NHANES I epidemiologic follow-up study, 1971–1992. National Health and Nutrition Examination Survey. JAMA. 2000;283:2404–2410. doi: 10.1001/jama.283.18.2404. [DOI] [PubMed] [Google Scholar]
- 202.Niskanen LK, Laaksonen DE, Nyyssonen K, Alfthan G, Lakka HM, Lakka TA, Salonen JT. Uric acid level as a risk factor for cardiovascular and all-cause mortality in middle-aged men: a prospective cohort study. Arch Intern Med. 2004;164:1546–1551. doi: 10.1001/archinte.164.14.1546. [DOI] [PubMed] [Google Scholar]
- 203.Athyros VG, Elisaf M, Papageorgiou AA, Symeonidis AN, Pehlivanidis AN, Bouloukos VI, Milionis HJ, Mikhailidis DP. Effect of statins versus untreated dyslipidemia on serum uric acid levels in patients with coronary heart disease: a subgroup analysis of the GREek Atorvastatin and Coronary-heart-disease Evaluation (GREACE) study. Am J Kidney Dis. 2004;43:589–599. doi: 10.1053/j.ajkd.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 204.Athyros VG, Mikhailidis DP, Liberopoulos EN, Kakafika AI, Karagiannis A, Papageorgiou AA, Tziomalos K, Ganotakis ES, Elisaf M. Effect of statin treatment on renal function and serum uric acid levels and their relation to vascular events in patients with coronary heart disease and metabolic syndrome: a subgroup analysis of the GREek Atorvastatin and Coronary heart disease Evaluation (GREACE) Study. Nephrol Dial Transplant. 2007;22:118–127. doi: 10.1093/ndt/gfl538. [DOI] [PubMed] [Google Scholar]
- 205.Karagiannis A, Mikhailidis DP, Tziomalos K, Sileli M, Savvatianos S, Kakafika A, Gossios T, Krikis N, Moschou I, Xochellis M, Athyros VG. Serum uric acid as an independent predictor of early death after acute stroke. Circ J. 2007;71:1120–1127. doi: 10.1253/circj.71.1120. [DOI] [PubMed] [Google Scholar]
- 206.Leyva F, Anker S, Swan JW, Godsland IF, Wingrove CS, Chua TP, Stevenson JC, Coats AJ. Serum uric acid as an index of impaired oxidative metabolism in chronic heart failure. Eur Heart J. 1997;18:858–865. doi: 10.1093/oxfordjournals.eurheartj.a015352. [DOI] [PubMed] [Google Scholar]
- 207.Leyva F, Anker SD, Godsland IF, Teixeira M, Hellewell PG, Kox WJ, Poole-Wilson PA, Coats AJ. Uric acid in chronic heart failure: a marker of chronic inflammation. Eur Heart J. 1998;19:1814–1822. doi: 10.1053/euhj.1998.1188. [DOI] [PubMed] [Google Scholar]
- 208.Kittleson MM, St John ME, Bead V, Champion HC, Kasper EK, Russell SD, Wittstein IS, Hare JM. Increased levels of uric acid predict haemodynamic compromise in patients with heart failure independently of B-type natriuretic peptide levels. Heart. 2007;93:365–367. doi: 10.1136/hrt.2006.090845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Hare JM, Johnson RJ. Uric acid predicts clinical outcomes in heart failure: insights regarding the role of xanthine oxidase and uric acid in disease pathophysiology. Circulation. 2003;107:1951–1953. doi: 10.1161/01.CIR.0000066420.36123.35. [DOI] [PubMed] [Google Scholar]
- 210.Doehner W, Rauchhaus M, Florea VG, Sharma R, Bolger AP, Davos CH, Coats AJ, Anker SD. Uric acid in cachectic and noncachectic patients with chronic heart failure: relationship to leg vascular resistance. Am Heart J. 2001;141:792–799. doi: 10.1067/mhj.2001.114367. [DOI] [PubMed] [Google Scholar]
- 211.Anker SD, Leyva F, Poole-Wilson PA, Kox WJ, Stevenson JC, Coats AJ. Relation between serum uric acid and lower limb blood flow in patients with chronic heart failure. Heart. 1997;78:39–43. doi: 10.1136/hrt.78.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Cicoira M, Zanolla L, Rossi A, Golia G, Franceschini L, Brighetti G, Zeni P, Zardini P. Elevated serum uric acid levels are associated with diastolic dysfunction in patients with dilated cardiomyopathy. Am Heart J. 2002;143:1107–1111. doi: 10.1067/mhj.2002.122122. [DOI] [PubMed] [Google Scholar]
- 213.Anker SD, Doehner W, Rauchhaus M, Sharma R, Francis D, Knosalla C, Davos CH, Cicoira M, Shamim W, Kemp M, Segal R, Osterziel KJ, Leyva F, Hetzer R, Ponikowski P, Coats AJ. Uric acid and survival in chronic heart failure: validation and application in metabolic, functional, and hemodynamic staging. Circulation. 2003;107:1991–1997. doi: 10.1161/01.CIR.0000065637.10517.A0. [DOI] [PubMed] [Google Scholar]
- 214.Sakai H, Tsutamoto T, Tsutsui T, Tanaka T, Ishikawa C, Horie M. Serum level of uric acid, partly secreted from the failing heart, is a prognostic marker in patients with congestive heart failure. Circ J. 2006;70:1006–1011. doi: 10.1253/circj.70.1006. [DOI] [PubMed] [Google Scholar]
- 215.Jankowska EA, Ponikowska B, Majda J, Zymlinski R, Trzaska M, Reczuch K, Borodulin-Nadzieja L, Banasiak W, Ponikowski P. Hyperuricaemia predicts poor outcome in patients with mild to moderate chronic heart failure. Int J Cardiol. 2007;115:151–155. doi: 10.1016/j.ijcard.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 216.Kittleson MM, Bead V, Fradley M, St John ME, Champion HC, Kasper EK, Russell SD, Wittstein IS, Hare JM. Elevated uric acid levels predict allograft vasculopathy in cardiac transplant recipients. J Heart Lung Transplant. 2007;26:498–503. doi: 10.1016/j.healun.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 217.Hoieggen A, Alderman MH, Kjeldsen SE, Julius S, Devereux RB, De Faire U, Fyhrquist F, Ibsen H, Kristianson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H, Chen C, Dahlof B. The impact of serum uric acid on cardiovascular outcomes in the LIFE study. Kidney Int. 2004;65:1041–1049. doi: 10.1111/j.1523-1755.2004.00484.x. [DOI] [PubMed] [Google Scholar]
- 218.Cheung KJ, Tzameli I, Pissios P, Rovira I, Gavrilova O, Ohtsubo T, Chen Z, Finkel T, Flier JS, Friedman JM. Xanthine oxidoreductase is a regulator of adipogenesis and PPARgamma activity. Cell Metab. 2007;5:115–128. doi: 10.1016/j.cmet.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 219.Evans RM, Barish GD, Wang YX. PPARs and the complex journey to obesity. Nat Med. 2004;10:355–361. doi: 10.1038/nm1025. [DOI] [PubMed] [Google Scholar]
- 220.Ohtsubo T, Rovira II, Starost MF, Liu C, Finkel T. Xanthine oxidoreductase is an endogenous regulator of cyclooxygenase-2. Circ Res. 2004;95:1118–1124. doi: 10.1161/01.RES.0000149571.96304.36. [DOI] [PubMed] [Google Scholar]
- 221.Kalantar-Zadeh K, Block G, Horwich T, Fonarow GC. Reverse epidemiology of conventional cardiovascular risk factors in patients with chronic heart failure. J Am Coll Cardiol. 2004;43:1439–1444. doi: 10.1016/j.jacc.2003.11.039. [DOI] [PubMed] [Google Scholar]
- 222.Yndestad A, Damas JK, Oie E, Ueland T, Gullestad L, Aukrust P. Systemic inflammation in heart failure--the whys and wherefores. Heart Fail Rev. 2006;11:83–92. doi: 10.1007/s10741-006-9196-2. [DOI] [PubMed] [Google Scholar]
- 223.Baldus S, Mullerleile K, Chumley P, Steven D, Rudolph V, Lund GK, Staude HJ, Stork A, Koster R, Kahler J, Weiss C, Munzel T, Meinertz T, Freeman BA, Heitzer T. Inhibition of xanthine oxidase improves myocardial contractility in patients with ischemic cardiomyopathy. Free Radic Biol Med. 2006;41:1282–1288. doi: 10.1016/j.freeradbiomed.2006.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Gavin AD, Struthers AD. Allopurinol reduces B-type natriuretic peptide concentrations and haemoglobin but does not alter exercise capacity in chronic heart failure. Heart. 2005;91:749–753. doi: 10.1136/hrt.2004.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Latini R, Masson S, Anand I, Salio M, Hester A, Judd D, Barlera S, Maggioni AP, Tognoni G, Cohn JN. The comparative prognostic value of plasma neurohormones at baseline in patients with heart failure enrolled in Val-HeFT. Eur Heart J. 2004;25:292–299. doi: 10.1016/j.ehj.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 226.Stanek B, Frey B, Hulsmann M, Berger R, Sturm B, Strametz-Juranek J, Bergler-Klein J, Moser P, Bojic A, Hartter E, Pacher R. Prognostic evaluation of neurohumoral plasma levels before and during beta-blocker therapy in advanced left ventricular dysfunction. J Am Coll Cardiol. 2001;38:436–442. doi: 10.1016/s0735-1097(01)01383-3. [DOI] [PubMed] [Google Scholar]
- 227.Cingolani HE, Plastino JA, Escudero EM, Mangal B, Brown J, Perez NG. The effect of xanthine oxidase inhibition upon ejection fraction in heart failure patients: La Plata Study. J Card Fail. 2006;12:491–498. doi: 10.1016/j.cardfail.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 228.Freudenberger RS, Schwarz RP, Jr, Brown J, Moore A, Mann D, Givertz MM, Colucci WS, Hare JM. Rationale, design and organisation of an efficacy and safety study of oxypurinol added to standard therapy in patients with NYHA class. Expert Opin Investig Drugs. 2004;13:1509–1516. doi: 10.1517/13543784.13.11.1509. [DOI] [PubMed] [Google Scholar]
- 229.Hare JM, Mangal B, Brown J, Fisher C, Freudenberger RS, Colucci WS, Mann DL, Liu P, Givertz MM, Schwarz RP. Impact of Oxypurinol in Patients with Symptomatic Heart failure: Results of the OPT-CHF Study. J Am Coll Cardiol. 2008 doi: 10.1016/j.jacc.2008.01.068. In press. [DOI] [PubMed] [Google Scholar]
- 230.Pacher P, Nivorozhkin A, Szabo C. Therapeutic effects of xanthine oxidase inhibitors: renaissance half a century after the discovery of allopurinol. Pharmacol Rev. 2006;58:87–114. doi: 10.1124/pr.58.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Moorhouse PC, Grootveld M, Halliwell B, Quinlan JG, Gutteridge JM. Allopurinol and oxypurinol are hydroxyl radical scavengers. FEBS Lett. 1987;213:23–28. doi: 10.1016/0014-5793(87)81458-8. [DOI] [PubMed] [Google Scholar]
- 232.Kelley EE, Trostchansky A, Rubbo H, Freeman BA, Radi R, Tarpey MM. Binding of xanthine oxidase to glycosaminoglycans limits inhibition by oxypurinol. J Biol Chem. 2004;279:37231–37234. doi: 10.1074/jbc.M402077200. [DOI] [PubMed] [Google Scholar]