Abstract
Primary sites of tumor are the focal triggers of cancers, yet it is the subsequent metastasis events that cause the majority of the morbidity and mortality. Metastatic tumor cells exhibit a phenotype that differs from that of the parent cells, as they represent a resistant, invasive subpopulation of the original tumor, may have acquired additional genetic or epigenetic alterations under exposure to prior chemotherapeutic or radiotherapeutic treatments, and reside in a microenvironment differing from that of its origin. This combination of resistant phenotype and distal location make tracking and treating metastases particularly challenging. In this review, we highlight some of the unique biological traits of metastasis, which in turn, inspire emerging strategies for targeted imaging of metastasized tumors and metastasis-directed delivery of therapeutics.
Keywords: cancer stem cell, intravasation, extravasation, metastasis, targeted delivery
1. Introduction: the challenge of targeting metastases
The concept of targeted delivery is well established for tumors but has been applied less extensively to metastases. Clearly, the development of effective delivery methods is critical for cancer, for which it is essential to direct as much drug to the cancer cells while sparing healthy cells to the greatest extent possible. Considerable research progress has been made in understanding the challenges and in exploiting the biology of tumors for drug delivery. Passive targeting via the enhanced permeation and retention (EPR) effect and active targeting via integrins, growth factor receptors and other cell surface ligands are well studied approaches for enhancing drug delivery to established tumors [1-3]. While the traditional focus has been on delivering cancer therapeutics to primary tumors, a yet more significant challenge that is only now becoming recognized is targeting secondary tumors. Here, the challenge to eradicating cancers shifts to the identification and elimination of metastases that have spread from the primary tumor site and have the capability to grow new tumors in distant tissues.
Indeed, 90% of cancer deaths come from metastasis [4]. Metastases are frequently multifocal, are notoriously difficult to eradicate, and consequently have low response and cure rates [5]. They result from the summation of a series of low probability events: the acquisition of stem cell-like properties by the primary tumor cells, shedding and dissemination to a distant tissue, colonization mediated by bidirectional signaling between tumor cell and stroma, a period of dormancy, and eventually reawakening of micrometastases and outgrowth of full-blown metastatic disease [6]. The inherent selection process required for this process results in metastases that are separated in time and space from the primary tumor, are often present at multiple sites, and that are endowed with genetic and epigenetic alterations that render them resistant to treatment and often even tracking [7, 8].
Metastases present unique challenges from a drug delivery standpoint. In order to head off the morbidity and mortality associated with metastatic disease, targeting micrometastases before they manifest themselves in overt disease and/or spread further is necessary. One consequence is that reliance upon the EPR effect for drug accumulation is unlikely to be effective for metastases whose vasculature is not yet developed. Once established, metastases have characteristics of host tissue and primary tumor that may complicate targeting strategies that would be effective for the primary tumor. As such, novel approaches to the sensitive and accurate detection of microlesions are needed. These will require sophisticated targeting strategies that allow an imaging agent to find and discriminate the microlesion from the host environment. At the same time, it may be possible to exploit the dual nature of metastases, with characteristics of both tumor (seed) and stroma (soil), for effective targeting [9].
In this review, we outline emerging strategies and approaches for targeted delivery of agents to metastases for the integrated purposes of detection, tracking and therapy. First, we review the main features of metastasis with a focus on features that may be utilized for targeted detection and delivery. Second, we explore mechanisms by which the metastatic process itself may be targeted. Third, we review approaches that have been developed for targeting metastases that have taken hold in particular organs and are beginning to emerge. Fourth, we motivate the need for and application of targeting approaches to high resolution imaging of metastases and metastatic processes.
2. The biology of metastasis: implications for delivery and tracking
Metastasis, a multistage process, involves spread of malignant cells from primary tumor to distant organs in new microenvironments [10]. Jean Claude Recamier in 1829 was the first to reference and document metastasis - “métastase”, hematogenous spread of disease [11]. Stephen Paget's seed and soil theory of metastases [12], stating that a permissive microenvironment (the soil) promotes growth of the disseminated tumor cell (the seed), remains the basis of research to date and is widely accepted to explain the mechanism of metastasis. The “metastatic cascade”, a phenomenon first reported in 1975, involves distinct steps involving local invasion, intravasation into adjacent blood and lymphatic vessels, transit through circulation and evasion of host immune systems, extravasation into the parenchyma of distant organs, and colonization and formation of micro-metastases, followed by proliferation and progression to macro-metastases (Figure 1) [13-15]. Understanding the molecular basis of interaction between primary tumor and distant metastases and their niche will be central to designing distinctive molecularly targeted strategies for the primary versus metastatic tumors.
Figure 1. The metastatic cascade -- from primary tumor to macrometastases.
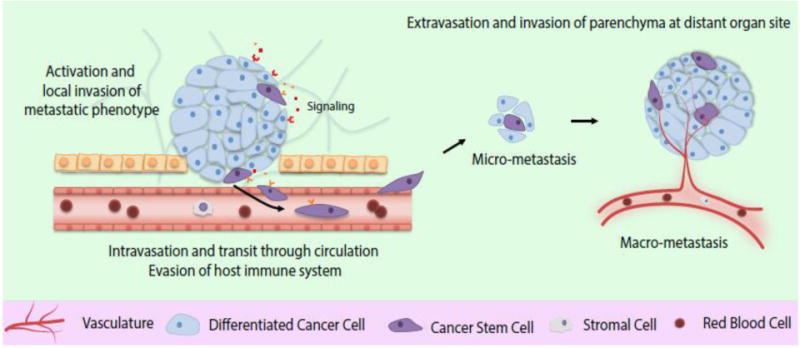
Beginning with tumor initiating (stem) cells, a stepwise progression occurs from the tumors exiting the primary site of growth (local invasion and activation of metastatic phenotype) followed by systemic translocation (intravasation and transit through circulation) and finally adaptation and reawakening in distant metastatic sites (extravasation, micrometastases formation and colonization leading to clinically detectable active macrometastases). The metastatic process requires activation of cancer stem cells and interaction with surrounding stromal cells throughout the program.
Current thinking says that heterogeneity within a tumor, both physical and functional in nature, can be explained by the cancer stem cell model [16, 17]. One of the posits of the model is that cancer stem cells, a minor fraction of all tumor cells, drive tumor progression though either innate or acquired therapy resistance and by formation of metastases [18, 19]. Cancer cell heterogeneity is frequently explained by the clonal evolutionary theory [20, 21], though this does not encompass the heterogeneity that arises from genetic evolution and epigenetic changes. Theoretically, cancer stem cells (CSCs) can arise from the cell-of-origin of tumor or from transformed cells within the parent tumor as a result of genetic and epigenetic alterations, as has been studied in a diverse range of cancers, including glioblastoma multiforme [22-24], prostate cancer [25-29], colorectal cancers [30-36] and breast cancers [37-39] models, among others. However, due to patient-to-patient variability and lack of consistent results from xenograft models, the cancer stem cell model is not universally accepted.
During each step in the metastatic cascade, tumor cells interact with their immediate non-tumor microenvironment. For instance, the interaction of disseminating tumor cells in circulation with macrophages, leukocytes and other immune components has been studied in depth and form the basis for molecularly targeted therapies [40-44]. The molecular signaling between tumor and stromal cells is only beginning to be unraveled. The physical microenvironment, e.g. flow in the case of circulating tumor cells, also plays a major role in the adaptation that disseminating cells must undergo and the phenotypes that they acquire [45].
In metastases, the interaction of specialized cancer cell(s) (“seed”) and the host (“soil”) promotes the emergence of a metastatic phenotype that is evolved from that of the parent tumor. Numerous studies have shown the molecular differences between primary and distant metastases affecting treatment decisions. For instance, variable Her2 expression levels in primary tumors lead to subsequent clonal expansion of Her2- populations and a discordance in Her2 levels between the primary tumor and distant metastases in breast cancer [46]. Similar discordance in ER and PR receptor expression has been observed between the primary breast tumor and the corresponding metastatic lesions [47]. In colorectal cancer patients with wild-type KRAS, failure of EGFR antibody therapy was observed due to activating BRAF or PIK3CA mutations underlying intratumoral heterogeneity. Increased heterogeneity between primary tumors and lymph node metastases presented a major challenge for targeted EGFR therapy as a choice for these patients [48]. The basic question of how seed and soil heterogeneity synergize to promote metastases remains unanswered.
Finally, inter-tumor heterogeneity (between tumors of the same tissue type arising in different patients) and intra-tumor heterogeneity (within a single patient tumor) additionally pose challenges in identification of effective cancer biomarkers, prediction of treatment response, and the design of targeted therapies. Concepts of cancer stem cell biology and Paget's theory will need to be adapted to emerging new technologies and experimentally testable hypotheses with clinical relevance developed for targeting tumors.
Given their origin from interactions between “seed” and “soil,” metastases may exhibit phenotypic properties partially of the parent tumor, partially of the host tissue and potentially novel properties resulting from the unique interaction of tumor cells with their new host. As a result, a therapy that might have had excellent efficacy on the parent tumor might be ineffective on metastases. On the other hand, understanding the interactions of metastases with their host might provide opportunities for targeting a therapy to the correct cells with fairly high specificity. That is, a metastasis might be targeted based on the properties of tumor cells themselves, based on the mechanics of the metastatic process, or based on characteristics of the host tissues. These strategies are discussed further in the following sections.
3. Targeting metastatic processes
The successes and limitations of molecularly targeted therapies can inform the development of targeted delivery strategies for metastasis. One of the drivers toward precision medicine has been an explosive growth of promising targets identified in the past decade resulting from extensive understanding of metastatic tumor biology. The predominant targets include growth factor receptors [49-51], tyrosine and serine-threonine kinase receptors [52-54] and non-receptor signaling molecules [41, 55, 56], which drive cancer cell survival, proliferation, and progression. Herceptin, a monoclonal antibody against receptor tyrosine kinase HER2 (ErbB2) was the first targeted therapy, approved in 1998 by the FDA for treating patients with HER2-positive metastatic breast cancer [50, 57]. This was closely followed by the Bcr-Abl targeted drug, Gleevec, for chronic myeloid leukemia [58-61]. Although the FDA has since approved a large number of drugs developed against a range of both therapeutic and non-therapeutic targets, it has become apparent that a single targeted therapy is not sufficient to cure most cancers, due in significant part to inter-tumor heterogeneity and to drug resistance associated with heterogeneity of metastases. Thus, detection of and delivery to nascent and established metastases remain as critical barriers to reaping the benefits of precision medicine and to cancer therapy in general.
A precursor to targeted delivery to disseminated metastases is the use of immunoconjugates for treating leukemias. For instance, Mylotarg was developed as a conjugate of the potent antitumor antibiotic, calicheamicin, with the anti-CD33 antibody. Similarly, Zevalin [62] and Bexxar [63] employed anti-CD20 antibodies to target radioisotopes to cancer cells. These agents, though clinically effective, exhibited lethal side effects most likely due to non-specific binding between the targeting agent and non-target moieties on the cell surface and possible interaction with the target expressed on non-cancerous cells.
The targeting paradigm can be adapted to metastases by addressing unique site tags on the populations of cells that form metastases or by exploiting the metastatic phenotype. The recent explosion of research on cancer stem cells (CSCs), in addition to modifying how we think about resistance and metastasis, has brought to fore several cell surface markers that can be exploited to deliver therapeutic agents to this otherwise hard-to-reach subpopulation of cells. The most commonly applied approach has centered on hyaluronic acid (HA), which is a natural glycosaminoglycan and intrinsic ligand for CD44 expressed on many cancer stem cells. HA has been functionalized to a variety of nanoparticles and used to target and increase the effectiveness of drug delivery in mammary fat pad and lung metastasis models [64, 65]. Another common cancer stem cell marker is CD133, which does not have an easily utilized natural ligand. Nonetheless, it has been targeted for nanoparticle drug delivery in mouse models using an evolved aptamer and a monoclonal antibody as ligands [66, 67]. While not exclusive to cancer stem cells, EGFR is a common oncogene, and the anti-EGFR monoclonal antibody cetuximab has been approved for treatment of metastatic colon cancer and head and neck squamous cell carcinoma. This antibody is being investigated as a means to target nanoparticles administered via convection-enhanced delivery to glioblastoma with the intent of reaching glioma CSCs [68]. Other cell surface markers such as EpCAM may emerge as additional receptors for targeted delivery to CSCs [69].
From a physical standpoint [4, 45], the metastatic process can be conceptualized in terms of a series of steps including detachment, intravasation, circulation, extravasation, colonization and eventually reactivation. The molecular basis for each of these processes is at least partially understood and provides a potential avenue for delivering therapeutic agents. For example, growth factors, chemokines, integrins and matrix metalloproteinases are all viable targets for cell motility, and indeed motility inhibitors are in clinical development [70]. Integrins, which are frequently associated with angiogenic tumors, are also involved in cellular invasion, as the invasive cell switches from cell-cell to cell-ECM interactions. This interaction has been targeted using RGD peptides conjugated to either liposomes or albumin nanoparticles to modulate metastasis in vivo [71, 72]. Cell surface receptors associated with motility, such as CXCR4, represent another targeting option for delivery of drugs and siRNA [73]. Likewise, nanoparticles carrying a substrate for MMPs have been used to target invading cell populations [74].
Once a tumor cell has reached the circulation, it is a hard-to-find entity in the 3 L of plasma circulating in the average human. Its ultimate landing in a distant tissue is mediated by adhesion ligands that can capture cells in flow, most notably the selectins. Nanoparticles functionalized with selectins could thus be used to capture circulating tumor cells [75] or metastases that have sprouted in distant tissues [76]. In order for such a strategy to be effective, nanoparticles would need to be long circulating. This has been achieved in various applications by extensive functionalization with PEG or by creating flexible, disc-like nanoparticles that mimic red blood cells and share their flow and adhesion characteristics in the circulation [75, 77].
4. Targeting organ-specific metastases
The concept that metastases exhibit organ specificity was first described in 1980 [78]. Owing to differences in barriers of infiltration and the microenvironment of individual distant metastatic sites, metastatic propensities and dynamics to different organs can vary. Bone marrow, lungs, liver and brain are the most common clinically relevant sites of metastases. From an infiltration perspective, fenestrated sinusoid capillaries of bone marrow are more permissive to cancer cell infiltration than the contiguous structure of lung capillary walls and brain capillaries, which are more difficult to penetrate. Infiltration through these barriers selects for those tumor cells that express the necessary extravasation functions. Thus, in colorectal carcinoma, the mesenteric circulation from the bowels and the permissiveness of the liver capillary sinusoids are thought to favor liver metastasis [79, 80]. However, to cross the blood-brain barrier (BBB) and metastasize to the brain, cancer cells require specialized mechanisms [81-85]. Thus, therapeutic agents could conceivably be designed to target these organ systems via mimicry of the mechanisms utilized by cancer cells during the metastatic process (Figure 2).
Figure 2.
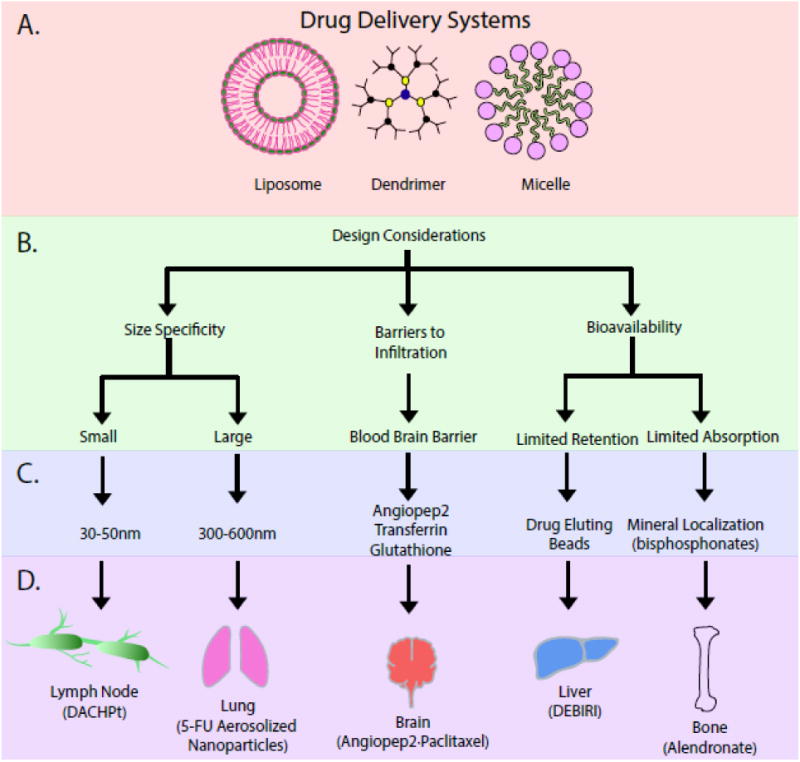
Drug delivery systems for targeting organ-specific metastases. A. Nanoarchitectures provide sizes favorable for delivery as well as the presentation of multiple targeting ligands for increased avidity and the concentration of payload. B. In targeting a particular tissue, design considerations include the filtering characteristics of the organ and specialized physiological barriers that hinder uptake. C. These considerations lead to a restricted set of variables to be optimized experimentally, and D. implemented for successful targeting and imaging/eradication of metastases.
Metastasis to Lymph Nodes
A variety of cancers initiate their metastatic spread via the lymphatic system. Nanoparticles can be used to extend the circulation time of therapeutics such that they distribute to and reach the lymphatics [86]. A number of studies have suggested that the lower size end of therapeutic nanoparticles may be beneficial for this application. For example, sub-50 nm polymeric micelles have been used to deliver platinum-based chemotherapies to lymph and brain metastases [87, 88], while ∼15 nm particles were used to deliver doxorubicin successfully to breast cancer metastases in the lymph [89]. In addition to size, targeting of lymph can be accomplished using the LyP-1 peptide, which localizes with lymphatic endothelium and tumor associated macrophages. For example, LyP-1 has been conjugated to PEGylated liposomes for delivery of doxorubicin leading to inhibition of lymph metastases [90]. More generic tumor targeting motifs such as the integrin-targeting RGD peptide have also demonstrated effectiveness is delivery of therapeutics to minimize metastatic formation and growth in lymph [91]. A promising new avenue is the targeting of lymphatics with nanoparticles presenting antigens and adjuvants for immunotherapy [92].
Metastasis to the Lungs
Drug delivery for treatment of lung metastases can be achieved by taking advantage of the large surface area, thin alveolar epithelium, rapid absorption, lack of first-pass metabolism, high bioavailability, and the capacity to absorb large quantities of drug of the lung. The first study with 5-fluorouracil delivered in a nebulized form showed higher accumulation of the drug in the tumor than in the surrounding normal tissue [93]. Several studies, clinical and preclinical, are underway to explore aerosol chemotherapy [94] using nanoparticles as a means of targeting lung metastases [95-100]. Aerosol administration can be coupled with molecularly targeted therapy, as demonstrated using gelatin based nanoparticle formulation targeting epidermal growth factor receptors in mice [101-103]. Systemic delivery of drugs to lungs can be improved using vehicles that extend circulation time, such as small cationic DOTAP liposomes with nitric oxide synthase gene and low-dose cisplatin [104]. Furthermore, molecularly targeted liposomal and polymeric nanoparticle formulations have been applied to lung metastases [105, 106].
Metastasis to the Brain
Brain metastases are most commonly seen from cancers of the lung, breast and skin (melanoma) [107]. The development of brain-targeted therapeutics and chemotherapeutics has been extremely challenging due to limited permeability of the blood-brain barrier (BBB). Nonetheless, some agents that mediate passage across the BBB have been identified and exploited. For example, angiopep-2, a 19-amino acid peptide that binds the low-density lipoprotein receptor related protein (LRP) receptors at the BBB, was conjugated to paclitaxel and greater than 50-fold enhanced delivery across the BBB was observed [108]. This agent has now entered clinical trials. Conjugates of angiopep-2 with molecularly targeted therapies such as anti-HER2 monoclonal antibodies are emerging [109]. Alternative ligands that mediate passage across the BBB include transferrin and glutathione. Of note, glutathione-conjugated PEGylated liposomes have been utilized in preclinical and clinical studies for delivery of numerous agents to treat a variety of central nervous system disorders, including brain metastases. Recent Phase 1/2a clinical trial results from the use of this platform for doxorubicin delivery against brain metastases are promising [110].
A number of other approaches are under investigation to overcome the significant challenge of delivery across the BBB [111]. A semi-targeted approach is the use of surfactants that can mediate BBB passage as coatings for nanoparticles used in detection of and delivery to brain metastases [112]. Physical disruption, as with focused ultrasound, is an alternative to biochemical mediators [113]. As immune cells such as macrophages and stem cells have been found to exhibit homing to tumor cells, their use as targeted carriers loaded with therapeutic molecules or nanotherapeutics is an area of active interest [114, 115].
Metastasis to the Liver
Liver (hepatic) metastases are observed with a variety of with cancers but are particularly common with colorectal cancer. Infusion via the hepatic artery is frequently used to achieve regional delivery of chemotherapeutics to the liver, thereby increasing the therapeutic index [116]. To improve further the hepatic distribution, chemotherapeutic drugs have been loaded into beads for controlled release, most notably in the DEBIRI (drug eluting beads loaded with irinotecan) protocol, which has been used in a number of European and U.S. clinical trials [117, 118] and is CE approved. Targeted delivery strategies are also under investigation for reaching hepatic metastases, including folic acid targeting of RNA nanoparticles and mannosylated liposomes that target the non-parenchymal cells of the liver [119, 120].
Metastasis to Bone
Drug delivery to bone has been accomplished frequently in the context of biomaterials serving as bone grafts while delivering therapeutics; this approach is suitable for established bone neoplasms [121]. The fenestrated architecture of bone may allow penetration of nanoparticles with an extended circulation time, as has been shown, for example, in antisense delivery with PLGA particles [122]. The most common strategy for targeting to bone with systemic carriers relies on bisphosphonates, such as alendronate, that localize to the mineralized regions associated with bone neoplasms. Alendronate is readily functionalized to a variety of polymers and liposomes, and its activity assayed via hydroxyapatite binding assay [123-125]. An interesting approach to increasing the specificity of targeting is to attach the therapeutic to the carrier via a linkage susceptible to proteases such as cathepsin K that are highly expressed at bone resorption sites and in neoplasm [126].
5. “Targeted Imaging” of Metastases
A prerequisite for treatment of metastatic disease is its early identification. The detection of metastases is particularly challenging given their distance from the original disease site, initial small size, and potentially limited contrast relative to its surrounding environment. The targeting approaches being developed to improve the therapeutic index for delivery to metastases are also being applied to develop imaging agents that can be addressed to metastatic lesions. Nanotheranostic strategies where agents are used for detection and treatment are also emerging [127].
A standard-of-care imaging method for the detection of cancers is magnetic resonance imaging (MRI). Nanoformulations incorporating gadolinium for MRI contrast and displaying antibodies or other ligands have been designed to detect metastases in a variety of organs [128]. Surfactant coatings have extended this approach to imaging of brain metastases [112, 129]. Multimodal imaging where MRI is integrated with another imaging method has been demonstrated by utilizing the absorption properties of iron oxide nanoparticles for fluorescence molecular tomography [130]. Positron emission tomography (PET) tracers have been directed to metastatic lesions by conjugation to antibodies for surface markers associated with metastases, such as anti-carcinoembryonic antigen for tracking hepatic metastases of colorectal cancer [131]. Multimodal PET/MRI agents have also been developed using “porphysomes” and directed to bone metastases [132]. Development of a molecularly targeted approach to imaging a malignant lesion leads naturally to theranostic approaches, such as imaging followed by photothermal ablation of metastases traveling through the lymph [133].
Optical imaging of cellular processes associated with metastasis is widely implemented in the laboratory, yet optical imaging of cancer and in particular of metastasis is less developed. Non-invasive molecular imaging using fluorescent probes [134] and multi-photon microscopy [135] have provided us with greater insights beyond the cellular dynamics of tumor progression such as tumor growth [136], macrometastasis [137], and tumor angiogenesis [138], and have enabled functional readouts of subcellular biological processes such as protein–protein interactions [139, 140]. While optical imaging using fluorescent proteins has helped to advance the study of cancer dynamics in situ, drawbacks relating to interference with tissue absorption and auto-fluorescence leading to low sensitivity of detection of exogenously labeled cells continue to limit adequate cancer resolution in vivo.
The use of near-infrared (NIR) dyes such as quantum dots (QDs) reduces the extent of tissue absorption; however, interference from tissue components and passivation and targeting of QDs remain formidable challenges. An alternative may be the development of “upconversion” fluorophores that absorb light in the NIR and emit in the visible range [141], though in this case attenuation of the emitted signal remains an issue. Recently, fluorophores that are bright, stable, tunable and biocompatible, and emit in the short wave infrared (SWIR) region, which overlaps with the “second and third optical windows” from 1000-1600 nm, have been advanced to overcome the issues of tissue absorption and interference from autofluorescence. Specifically, NaYF4 doped with Yb and Er can be produced as nanocrystals whose emission properties are tunable via size control. These highly luminescent rare-earth nanomaterials offer superior detection sensitivity over other SWIR emitters while offering the capability of multispectral in vivo SWIR imaging [142]. Human serum albumin encapsulated rare-earth (RE) nanoparticles were used to detect emerging and disseminated tumors in melanoma mouse models [143].
A major impediment to capitalizing on the power of new optical imaging fluorophores is the lack of sufficient contrast between the diseased lesion and healthy tissue. Albumin nanoshells provide a facile platform as targeting ligands can be either adsorbed directly on via the drug binding pockets inherent to albumin or by chemical bioconjugation. Both methods have been utilized for improved targeting of functionalized rare earth-albumin nanocomposites evaluated first using in vitro models of cancers [144, 145] and more recently to identify microscale (∼25 mm3) metastases in the lungs of mice [146]. These emerging molecular imaging approaches may soon provide avenues to directly visualize biological processes that promote tumor metastasis in a clinical setting. These and similar molecularly tailored imaging technologies [147] could elucidate the dynamics of metastases and profile the molecular expression of metastatic cells, thus illuminating more precise therapeutic targets, which will be an integral tool to personalize therapeutic interventions early in the clinic especially for the hard-to-treat cancers.
6. Perspectives
Metastases are responsible for a major portion of the morbidity and mortality of cancer. Modern research tools have helped us to characterize cancer stem cells as a distinct phenotype within tumors, to understand the physical processes that permit extravasation, circulation and invasion of colonizing cells, and to compare comprehensively at the genomic and proteomic level the alterations occurring in metastatic cells and heterogeneity across tumors and metastases. In parallel, the potency and specificity of probes for imaging and for therapy continues to improve. By exploiting previously unexplored wavelength ranges and capturing nanoscale electronic transitions, optical imaging probes of tremendous brightness and with the capability of spectral tuning are emerging. Additionally, antibodies, peptides and other ligands can be “evolved” to have very high specificity for a target identified as playing a role in metastatic lesions or in the metastatic process. These have been incorporated in many cases into multifunctional nanoformulations of increasing complexity. These biomimetic approaches may be supplemented with more organic, biologically derived strategies involving tumor exosomes [148] or cells possessing innate homing capacity for tumors and metastases.
Even with high affinity imaging probes and therapeutic antagonists, there remains the enduring challenge of how to target these agents to metastases in such a way that they arrive in sufficient density to provide imaging contrast or pharmacological effect, while maintaining the specificity to distinguish diseased from host tissue. Strategies for targeting agents to metastases can fall into three categories: target the CSCs that give rise to metastases before they occur, disrupt one or more of the mechanistic steps required for metastasis to occur, or if necessary target the nascent tumor in its new microenvironment. The key challenge with any of these approaches remains specificity and potency. By its nature, a metastatic lesion consists of a small number of cells that have evolved resistance mechanisms to conventional chemotherapy and that is in a location distant from the original site. As such, targeting probes that need to seek out the metastases from the 37 trillion cells in the human body poses a major challenge, analogous to the classic “needle in a haystack” problem.
The challenges discussed above notwithstanding, substantial progress has been made to identify metastases, via the application of a combination of integrative physical and molecular recognition approaches. In addition to the leaky vasculature associated with more established tumors, size-dependent filtering occurs in a variety of organs, including the fenestrated endothelium of liver and spleen, capillaries of bone, and the alveolar space of lungs. These physical effects can be potentially combined with one or more ligand-directed events to create a vehicle that directs imaging probes and modern therapeutics to the nascent metastasis with high levels of precision. Translating the many promising preclinical examples into clinical practice will require continued convergence of precision technologies, advances in molecular biology, and a greater systems-level understanding of the tumor heterogeneity.
Acknowledgments
The authors thank Varun Arvind for assistance with the figures. Financial support was provided by the Nationals Institutes of Health under grant 1R01 EB018378-02.
References
- 1.Ediriwickrema A, Saltzman WM. Nanotherapy for Cancer: Targeting and Multifunctionality in the Future of Cancer Therapies. ACS Biomater Sci Eng. 2015;1:64–78. doi: 10.1021/ab500084g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kobayashi H, Turkbey B, Watanabe R, Choyke PL. Cancer drug delivery: considerations in the rational design of nanosized bioconjugates. Bioconjug Chem. 2014;25:2093–2100. doi: 10.1021/bc500481x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Srinivasarao M, Galliford CV, Low PS. Principles in the design of ligand-targeted cancer therapeutics and imaging agents. Nat Rev Drug Discov. 2015;14:203–219. doi: 10.1038/nrd4519. [DOI] [PubMed] [Google Scholar]
- 4.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 5.Eckhardt BL, Francis PA, Parker BS, Anderson RL. Strategies for the discovery and development of therapies for metastatic breast cancer. Nat Rev Drug Discov. 2012;11:479–497. doi: 10.1038/nrd2372. [DOI] [PubMed] [Google Scholar]
- 6.Wan L, Pantel K, Kang Y. Tumor metastasis: moving new biological insights into the clinic. Nature medicine. 2013;19:1450–1464. doi: 10.1038/nm.3391. [DOI] [PubMed] [Google Scholar]
- 7.Klemm F, Joyce JA. Microenvironmental regulation of therapeutic response in cancer. Trends Cell Biol. 2015;25:198–213. doi: 10.1016/j.tcb.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Byler S, Goldgar S, Heerboth S, Leary M, Housman G, Moulton K, Sarkar S. Genetic and epigenetic aspects of breast cancer progression and therapy. Anticancer research. 2014;34:1071–1077. [PubMed] [Google Scholar]
- 9.Buijs JT, Kuijpers CC, van der Pluijm G. Targeted therapy options for treatment of bone metastases; beyond bisphosphonates. Current pharmaceutical design. 2010;16:3015–3027. doi: 10.2174/138161210793563536. [DOI] [PubMed] [Google Scholar]
- 10.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited, Nature reviews. Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 11.Carr J, Carr I. The origin of cancer metastasis. Canadian bulletin of medical history = Bulletin canadien d'histoire de la medecine. 2005;22:353–358. doi: 10.3138/cbmh.22.2.353. [DOI] [PubMed] [Google Scholar]
- 12.Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer metastasis reviews. 1989;8:98–101. [PubMed] [Google Scholar]
- 13.Shibue T, Weinberg RA. Metastatic colonization: settlement, adaptation and propagation of tumor cells in a foreign tissue environment. Seminars in cancer biology. 2011;21:99–106. doi: 10.1016/j.semcancer.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Ombrato L, Malanchi I. The EMT universe: space between cancer cell dissemination and metastasis initiation. Critical reviews in oncogenesis. 2014;19:349–361. doi: 10.1615/critrevoncog.2014011802. [DOI] [PubMed] [Google Scholar]
- 15.Li L, Li W. Epithelial-mesenchymal transition in human cancer: Comprehensive reprogramming of metabolism, epigenetics, and differentiation. Pharmacology & therapeutics. 2015 doi: 10.1016/j.pharmthera.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Dick JE. Stem cell concepts renew cancer research. Blood. 2008;112:4793–4807. doi: 10.1182/blood-2008-08-077941. [DOI] [PubMed] [Google Scholar]
- 17.Shackleton M, Quintana E, Fearon ER, Morrison SJ. Heterogeneity in cancer: cancer stem cells versus clonal evolution. Cell. 2009;138:822–829. doi: 10.1016/j.cell.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 18.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magee JA, Piskounova E, Morrison SJ. Cancer stem cells: impact, heterogeneity, and uncertainty. Cancer cell. 2012;21:283–296. doi: 10.1016/j.ccr.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang T, Rycaj K, Liu ZM, Tang DG. Cancer stem cells: constantly evolving and functionally heterogeneous therapeutic targets. Cancer research. 2014;74:2922–2927. doi: 10.1158/0008-5472.CAN-14-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greaves M, Maley CC. Clonal evolution in cancer. Nature. 2012;481:306–313. doi: 10.1038/nature10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beier D, Wischhusen J, Dietmaier W, Hau P, Proescholdt M, Brawanski A, Bogdahn U, Beier CP. CD133 expression and cancer stem cells predict prognosis in high-grade oligodendroglial tumors. Brain pathology. 2008;18:370–377. doi: 10.1111/j.1750-3639.2008.00130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beier D, Hau P, Proescholdt M, Lohmeier A, Wischhusen J, Oefner PJ, Aigner L, Brawanski A, Bogdahn U, Beier CP. CD133(+) and CD133(-) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer research. 2007;67:4010–4015. doi: 10.1158/0008-5472.CAN-06-4180. [DOI] [PubMed] [Google Scholar]
- 24.Beier CP, Kumar P, Meyer K, Leukel P, Bruttel V, Aschenbrenner I, Riemenschneider MJ, Fragoulis A, Rummele P, Lamszus K, Schulz JB, Weis J, Bogdahn U, Wischhusen J, Hau P, Spang R, Beier D. The cancer stem cell subtype determines immune infiltration of glioblastoma. Stem cells and development. 2012;21:2753–2761. doi: 10.1089/scd.2011.0660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang ZA, Mitrofanova A, Bergren SK, Abate-Shen C, Cardiff RD, Califano A, Shen MM. Lineage analysis of basal epithelial cells reveals their unexpected plasticity and supports a cell-of-origin model for prostate cancer heterogeneity. Nature cell biology. 2013;15:274–283. doi: 10.1038/ncb2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X, Kruithof-de Julio M, Economides KD, Walker D, Yu H, Halili MV, Hu YP, Price SM, Abate-Shen C, Shen MM. A luminal epithelial stem cell that is a cell of origin for prostate cancer. Nature. 2009;461:495–500. doi: 10.1038/nature08361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leong KG, Wang BE, Johnson L, Gao WQ. Generation of a prostate from a single adult stem cell. Nature. 2008;456:804–808. doi: 10.1038/nature07427. [DOI] [PubMed] [Google Scholar]
- 28.Goldstein AS, Huang J, Guo C, Garraway IP, Witte ON. Identification of a cell of origin for human prostate cancer. Science. 2010;329:568–571. doi: 10.1126/science.1189992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldstein AS, Drake JM, Burnes DL, Finley DS, Zhang H, Reiter RE, Huang J, Witte ON. Purification and direct transformation of epithelial progenitor cells from primary human prostate. Nature protocols. 2011;6:656–667. doi: 10.1038/nprot.2011.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vermeulen L, Morrissey E, van der Heijden M, Nicholson AM, Sottoriva A, Buczacki S, Kemp R, Tavare S, Winton DJ. Defining stem cell dynamics in models of intestinal tumor initiation. Science. 2013;342:995–998. doi: 10.1126/science.1243148. [DOI] [PubMed] [Google Scholar]
- 31.Vermeulen L, De Sousa EMF, van der Heijden M, Cameron K, de Jong JH, Borovski T, Tuynman JB, Todaro M, Merz C, Rodermond H, Sprick MR, Kemper K, Richel DJ, Stassi G, Medema JP. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nature cell biology. 2010;12:468–476. doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- 32.Rao GH, Liu HM, Li BW, Hao JJ, Yang YL, Wang MR, Wang XH, Wang J, Jin HJ, Du L, Chen Q. Establishment of a human colorectal cancer cell line P6C with stem cell properties and resistance to chemotherapeutic drugs. Acta pharmacologica Sinica. 2013;34:793–804. doi: 10.1038/aps.2013.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rao G, Wang H, Li B, Huang L, Xue D, Wang X, Jin H, Wang J, Zhu Y, Lu Y, Du L, Chen Q. Reciprocal interactions between tumor-associated macrophages and CD44-positive cancer cells via osteopontin/CD44 promote tumorigenicity in colorectal cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:785–797. doi: 10.1158/1078-0432.CCR-12-2788. [DOI] [PubMed] [Google Scholar]
- 34.Du L, Rao G, Wang H, Li B, Tian W, Cui J, He L, Laffin B, Tian X, Hao C, Liu H, Sun X, Zhu Y, Tang DG, Mehrpour M, Lu Y, Chen Q. CD44-positive cancer stem cells expressing cellular prion protein contribute to metastatic capacity in colorectal cancer. Cancer research. 2013;73:2682–2694. doi: 10.1158/0008-5472.CAN-12-3759. [DOI] [PubMed] [Google Scholar]
- 35.Lee TK, Cheung VC, Ng IO. Liver tumor-initiating cells as a therapeutic target for hepatocellular carcinoma. Cancer letters. 2013;338:101–109. doi: 10.1016/j.canlet.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 36.Lee TK, Castilho A, Cheung VC, Tang KH, Ma S, Ng IO. CD24(+) liver tumor-initiating cells drive self-renewal and tumor initiation through STAT3-mediated NANOG regulation. Cell stem cell. 2011;9:50–63. doi: 10.1016/j.stem.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 37.Luo M, Zhao X, Chen S, Liu S, Wicha MS, Guan JL. Distinct FAK activities determine progenitor and mammary stem cell characteristics. Cancer research. 2013;73:5591–5602. doi: 10.1158/0008-5472.CAN-13-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Atkinson RL, Yang WT, Rosen DG, Landis MD, Wong H, Lewis MT, Creighton CJ, Sexton KR, Hilsenbeck SG, Sahin AA, Brewster AM, Woodward WA, Chang JC. Cancer stem cell markers are enriched in normal tissue adjacent to triple negative breast cancer and inversely correlated with DNA repair deficiency. Breast cancer research : BCR. 2013;15:R77. doi: 10.1186/bcr3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Creighton CJ, Li X, Landis M, Dixon JM, Neumeister VM, Sjolund A, Rimm DL, Wong H, Rodriguez A, Herschkowitz JI, Fan C, Zhang X, He X, Pavlick A, Gutierrez MC, Renshaw L, Larionov AA, Faratian D, Hilsenbeck SG, Perou CM, Lewis MT, Rosen JM, Chang JC. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features, Proceedings of the National Academy of Sciences of the United States of America. 2009;106:13820–13825. doi: 10.1073/pnas.0905718106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Erez N, Coussens LM. Leukocytes as paracrine regulators of metastasis and determinants of organ-specific colonization, International journal of cancer. Journal international du cancer. 2011;128:2536–2544. doi: 10.1002/ijc.26032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DeNardo DG, Johansson M, Coussens LM. Immune cells as mediators of solid tumor metastasis. Cancer metastasis reviews. 2008;27:11–18. doi: 10.1007/s10555-007-9100-0. [DOI] [PubMed] [Google Scholar]
- 42.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 43.Wyckoff J, Wang W, Lin EY, Wang Y, Pixley F, Stanley ER, Graf T, Pollard JW, Segall J, Condeelis J. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer research. 2004;64:7022–7029. doi: 10.1158/0008-5472.CAN-04-1449. [DOI] [PubMed] [Google Scholar]
- 44.Rolny C, Mazzone M, Tugues S, Laoui D, Johansson I, Coulon C, Squadrito ML, Segura I, Li X, Knevels E, Costa S, Vinckier S, Dresselaer T, Akerud P, De Mol M, Salomaki H, Phillipson M, Wyns S, Larsson E, Buysschaert I, Botling J, Himmelreich U, Van Ginderachter JA, De Palma M, Dewerchin M, Claesson-Welsh L, Carmeliet P. HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF. Cancer cell. 2011;19:31–44. doi: 10.1016/j.ccr.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 45.Wirtz D, Konstantopoulos K, Searson PC. The physics of cancer: the role of physical interactions and mechanical forces in metastasis. Nature reviews Cancer. 2011;11:512–522. doi: 10.1038/nrc3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tapia C, Savic S, Wagner U, Schonegg R, Novotny H, Grilli B, Herzog M, Barascud AD, Zlobec I, Cathomas G, Terracciano L, Feichter G, Bubendorf L. HER2 gene status in primary breast cancers and matched distant metastases. Breast cancer research : BCR. 2007;9:R31. doi: 10.1186/bcr1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Curtit E, Nerich V, Mansi L, Chaigneau L, Cals L, Villanueva C, Bazan F, Montcuquet P, Meneveau N, Perrin S, Algros MP, Pivot X. Discordances in estrogen receptor status, progesterone receptor status, and HER2 status between primary breast cancer and metastasis. The oncologist. 2013;18:667–674. doi: 10.1634/theoncologist.2012-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baldus SE, Schaefer KL, Engers R, Hartleb D, Stoecklein NH, Gabbert HE. Prevalence and heterogeneity of KRAS, BRAF, and PIK3CA mutations in primary colorectal adenocarcinomas and their corresponding metastases. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16:790–799. doi: 10.1158/1078-0432.CCR-09-2446. [DOI] [PubMed] [Google Scholar]
- 49.Shin DM, Zhang H, Saba NF, Chen AY, Nannapaneni S, Amin AR, Muller S, Lewis M, Sica G, Kono S, Brandes JC, Grist WJ, Moreno-Williams R, Beitler JJ, Thomas SM, Chen Z, Shin HJ, Grandis JR, Khuri FR, Chen ZG. Chemoprevention of head and neck cancer by simultaneous blocking of epidermal growth factor receptor and cyclooxygenase-2 signaling pathways: preclinical and clinical studies. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:1244–1256. doi: 10.1158/1078-0432.CCR-12-3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hudziak RM, Schlessinger J, Ullrich A. Increased expression of the putative growth factor receptor p185HER2 causes transformation and tumorigenesis of NIH 3T3 cells. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:7159–7163. doi: 10.1073/pnas.84.20.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dannenberg AJ, Lippman SM, Mann JR, Subbaramaiah K, DuBois RN. Cyclooxygenase-2 and epidermal growth factor receptor: pharmacologic targets for chemoprevention. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:254–266. doi: 10.1200/JCO.2005.09.112. [DOI] [PubMed] [Google Scholar]
- 52.Ganapathy V, Ge R, Grazioli A, Xie W, Banach-Petrosky W, Kang Y, Lonning S, McPherson J, Yingling JM, Biswas S, Mundy GR, Reiss M. Targeting the Transforming Growth Factor-beta pathway inhibits human basal-like breast cancer metastasis. Molecular cancer. 2010;9:122. doi: 10.1186/1476-4598-9-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dong L, Ge XY, Wang YX, Yang LQ, Li SL, Yu GY, Gao Y, Fu J. Transforming growth factor-beta and epithelial-mesenchymal transition are associated with pulmonary metastasis in adenoid cystic carcinoma. Oral oncology. 2013;49:1051–1058. doi: 10.1016/j.oraloncology.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 54.Dieci MV, Arnedos M, Andre F, Soria JC. Fibroblast growth factor receptor inhibitors as a cancer treatment: from a biologic rationale to medical perspectives. Cancer discovery. 2013;3:264–279. doi: 10.1158/2159-8290.CD-12-0362. [DOI] [PubMed] [Google Scholar]
- 55.Hui AB, Bruce JP, Alajez NM, Shi W, Yue S, Perez-Ordonez B, Xu W, O'Sullivan B, Waldron J, Cummings B, Gullane P, Siu L, Liu FF. Significance of dysregulated metadherin and microRNA-375 in head and neck cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:7539–7550. doi: 10.1158/1078-0432.CCR-11-2102. [DOI] [PubMed] [Google Scholar]
- 56.Dupont Jensen J, Laenkholm AV, Knoop A, Ewertz M, Bandaru R, Liu W, Hackl W, Barrett JC, Gardner H. PIK3CA mutations may be discordant between primary and corresponding metastatic disease in breast cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:667–677. doi: 10.1158/1078-0432.CCR-10-1133. [DOI] [PubMed] [Google Scholar]
- 57.Hudziak RM, Lewis GD, Winget M, Fendly BM, Shepard HM, Ullrich A. p185HER2 monoclonal antibody has antiproliferative effects in vitro and sensitizes human breast tumor cells to tumor necrosis factor. Molecular and cellular biology. 1989;9:1165–1172. doi: 10.1128/mcb.9.3.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mauro MJ, O'Dwyer ME, Druker BJ. ST1571, a tyrosine kinase inhibitor for the treatment of chronic myelogenous leukemia: validating the promise of molecularly targeted therapy. Cancer chemotherapy and pharmacology. 2001;48(1):S77–78. doi: 10.1007/s002800100310. [DOI] [PubMed] [Google Scholar]
- 59.Mauro MJ, Druker BJ. STI571: a gene product-targeted therapy for leukemia. Current oncology reports. 2001;3:223–227. doi: 10.1007/s11912-001-0054-z. [DOI] [PubMed] [Google Scholar]
- 60.Mauro MJ, Druker BJ. STI571: targeting BCR-ABL as therapy for CML. The oncologist. 2001;6:233–238. doi: 10.1634/theoncologist.6-3-233. [DOI] [PubMed] [Google Scholar]
- 61.Druker BJ. ST1571: a paradigm for clinical trials of molecularly targeted agents. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2001;55:529–530. doi: 10.1016/s0753-3322(01)00137-8. [DOI] [PubMed] [Google Scholar]
- 62.Grillo-Lopez AJ. Zevalin: the first radioimmunotherapy approved for the treatment of lymphoma. Expert review of anticancer therapy. 2002;2:485–493. doi: 10.1586/14737140.2.5.485. [DOI] [PubMed] [Google Scholar]
- 63.Garber K. ODAC panel gives nod to Bexxar. Journal of the National Cancer Institute. 2003;95:189. doi: 10.1093/jnci/95.3.189. [DOI] [PubMed] [Google Scholar]
- 64.Shen H, Shi S, Zhang Z, Gong T, Sun X. Coating Solid Lipid Nanoparticles with Hyaluronic Acid Enhances Antitumor Activity against Melanoma Stem-like Cells. Theranostics. 2015;5:755–771. doi: 10.7150/thno.10804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hu K, Zhou H, Liu Y, Liu Z, Liu J, Tang J, Li J, Zhang J, Sheng W, Zhao Y, Wu Y, Chen C. Hyaluronic acid functional amphipathic and redox-responsive polymer particles for the co-delivery of doxorubicin and cyclopamine to eradicate breast cancer cells and cancer stem cells. Nanoscale. 2015;7:8607–8618. doi: 10.1039/c5nr01084e. [DOI] [PubMed] [Google Scholar]
- 66.Ni M, Xiong M, Zhang X, Cai G, Chen H, Zeng Q, Yu Z. Poly(lactic-co-glycolic acid) nanoparticles conjugated with CD133 aptamers for targeted salinomycin delivery to CD133+ osteosarcoma cancer stem cells. International journal of nanomedicine. 2015;10:2537–2554. doi: 10.2147/IJN.S78498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Swaminathan SK, Roger E, Toti U, Niu L, Ohlfest JR, Panyam J. CD133-targeted paclitaxel delivery inhibits local tumor recurrence in a mouse model of breast cancer. Journal of controlled release : official journal of the Controlled Release Society. 2013;171:280–287. doi: 10.1016/j.jconrel.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 68.Kaluzova M, Bouras A, Machaidze R, Hadjipanayis CG. Targeted therapy of glioblastoma stem-like cells and tumor non-stem cells using cetuximab-conjugated iron-oxide nanoparticles. Oncotarget. 2015;6:8788–8806. doi: 10.18632/oncotarget.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen J, Liu Q, Xiao J, Du J. EpCAM-Antibody-Labeled Noncytotoxic Polymer Vesicles for Cancer Stem Cells-Targeted Delivery of Anticancer Drug and siRNA. Biomacromolecules. 2015 doi: 10.1021/acs.biomac.5b00551. [DOI] [PubMed] [Google Scholar]
- 70.Palmer TD, Ashby WJ, Lewis JD, Zijlstra A. Targeting tumor cell motility to prevent metastasis. Advanced drug delivery reviews. 2011;63:568–581. doi: 10.1016/j.addr.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang F, Chen L, Zhang R, Chen Z, Zhu L. RGD peptide conjugated liposomal drug delivery system for enhance therapeutic efficacy in treating bone metastasis from prostate cancer. Journal of controlled release : official journal of the Controlled Release Society. 2014;196:222–233. doi: 10.1016/j.jconrel.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 72.Ji S, Xu J, Zhang B, Yao W, Xu W, Wu W, Xu Y, Wang H, Ni Q, Hou H, Yu X. RGD-conjugated albumin nanoparticles as a novel delivery vehicle in pancreatic cancer therapy. Cancer biology & therapy. 2012;13:206–215. doi: 10.4161/cbt.13.4.18692. [DOI] [PubMed] [Google Scholar]
- 73.Guo P, You JO, Yang J, Jia D, Moses MA, Auguste DT. Inhibiting metastatic breast cancer cell migration via the synergy of targeted, pH-triggered siRNA delivery and chemokine axis blockade. Mol Pharm. 2014;11:755–765. doi: 10.1021/mp4004699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Veiseh O, Kievit FM, Ellenbogen RG, Zhang M. Cancer cell invasion: treatment and monitoring opportunities in nanomedicine. Advanced drug delivery reviews. 2011;63:582–596. doi: 10.1016/j.addr.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li J, Sharkey CC, Huang D, King MR. Nanobiotechnology for the Therapeutic Targeting of Cancer Cells in Blood. Cell Mol Bioeng. 2015;8:137–150. doi: 10.1007/s12195-015-0381-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mai J, Huang Y, Mu C, Zhang G, Xu R, Guo X, Xia X, Volk DE, Lokesh GL, Thiviyanathan V, Gorenstein DG, Liu X, Ferrari M, Shen H. Bone marrow endothelium-targeted therapeutics for metastatic breast cancer. Journal of controlled release : official journal of the Controlled Release Society. 2014;187:22–29. doi: 10.1016/j.jconrel.2014.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yoo JW, Irvine DJ, Discher DE, Mitragotri S. Bio-inspired, bioengineered and biomimetic drug delivery carriers. Nat Rev Drug Discov. 2011;10:521–535. doi: 10.1038/nrd3499. [DOI] [PubMed] [Google Scholar]
- 78.Hart IR, Fidler IJ. Role of organ selectivity in the determination of metastatic patterns of B16 melanoma. Cancer research. 1980;40:2281–2287. [PubMed] [Google Scholar]
- 79.Schluter K, Gassmann P, Enns A, Korb T, Hemping-Bovenkerk A, Holzen J, Haier J. Organ-specific metastatic tumor cell adhesion and extravasation of colon carcinoma cells with different metastatic potential. The American journal of pathology. 2006;169:1064–1073. doi: 10.2353/ajpath.2006.050566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Paku S, Kopper L, Nagy P. Development of the vasculature in “pushing-type” liver metastases of an experimental colorectal cancer. International journal of cancer Journal international du cancer. 2005;115:893–902. doi: 10.1002/ijc.20886. [DOI] [PubMed] [Google Scholar]
- 81.Carbonell WS, Ansorge O, Sibson N, Muschel R. The vascular basement membrane as “soil” in brain metastasis. PloS one. 2009;4:e5857. doi: 10.1371/journal.pone.0005857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kienast Y, von Baumgarten L, Fuhrmann M, Klinkert WE, Goldbrunner R, Herms J, Winkler F. Real-time imaging reveals the single steps of brain metastasis formation. Nature medicine. 2010;16:116–122. doi: 10.1038/nm.2072. [DOI] [PubMed] [Google Scholar]
- 83.Lorger M, Felding-Habermann B. Capturing changes in the brain microenvironment during initial steps of breast cancer brain metastasis. The American journal of pathology. 2010;176:2958–2971. doi: 10.2353/ajpath.2010.090838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Serres S, Soto MS, Hamilton A, McAteer MA, Carbonell WS, Robson MD, Ansorge O, Khrapitchev A, Bristow C, Balathasan L, Weissensteiner T, Anthony DC, Choudhury RP, Muschel RJ, Sibson NR. Molecular MRI enables early and sensitive detection of brain metastases. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:6674–6679. doi: 10.1073/pnas.1117412109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wu L, Li X, Janagam DR, Lowe TL. Overcoming the blood-brain barrier in chemotherapy treatment of pediatric brain tumors. Pharmaceutical research. 2014;31:531–540. doi: 10.1007/s11095-013-1196-z. [DOI] [PubMed] [Google Scholar]
- 86.Ryan GM, Kaminskas LM, Porter CJ. Nano-chemotherapeutics: maximising lymphatic drug exposure to improve the treatment of lymph-metastatic cancers. Journal of controlled release : official journal of the Controlled Release Society. 2014;193:241–256. doi: 10.1016/j.jconrel.2014.04.051. [DOI] [PubMed] [Google Scholar]
- 87.Rafi M, Cabral H, Kano MR, Mi P, Iwata C, Yashiro M, Hirakawa K, Miyazono K, Nishiyama N, Kataoka K. Polymeric micelles incorporating (1,2-diaminocyclohexane)platinum (II) suppress the growth of orthotopic scirrhous gastric tumors and their lymph node metastasis. Journal of controlled release : official journal of the Controlled Release Society. 2012;159:189–196. doi: 10.1016/j.jconrel.2012.01.038. [DOI] [PubMed] [Google Scholar]
- 88.Cabral H, Makino J, Matsumoto Y, Mi P, Wu H, Nomoto T, Toh K, Yamada N, Higuchi Y, Konishi S, Kano MR, Nishihara H, Miura Y, Nishiyama N, Kataoka K. Systemic Targeting of Lymph Node Metastasis through the Blood Vascular System by Using Size-Controlled Nanocarriers. ACS nano. 2015;9:4957–4967. doi: 10.1021/nn5070259. [DOI] [PubMed] [Google Scholar]
- 89.Qin L, Zhang F, Lu X, Wei X, Wang J, Fang X, Si D, Wang Y, Zhang C, Yang R, Liu C, Liang W. Polymeric micelles for enhanced lymphatic drug delivery to treat metastatic tumors. Journal of controlled release : official journal of the Controlled Release Society. 2013;171:133–142. doi: 10.1016/j.jconrel.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 90.Yan Z, Wang F, Wen Z, Zhan C, Feng L, Liu Y, Wei X, Xie C, Lu W. LyP-1-conjugated PEGylated liposomes: a carrier system for targeted therapy of lymphatic metastatic tumor. Journal of controlled release : official journal of the Controlled Release Society. 2012;157:118–125. doi: 10.1016/j.jconrel.2011.07.034. [DOI] [PubMed] [Google Scholar]
- 91.Murphy EA, Majeti BK, Barnes LA, Makale M, Weis SM, Lutu-Fuga K, Wrasidlo W, Cheresh DA. Nanoparticle-mediated drug delivery to tumor vasculature suppresses metastasis. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:9343–9348. doi: 10.1073/pnas.0803728105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jeanbart L, Ballester M, de Titta A, Corthesy P, Romero P, Hubbell JA, Swartz MA. Enhancing efficacy of anticancer vaccines by targeted delivery to tumor-draining lymph nodes. Cancer Immunol Res. 2014;2:436–447. doi: 10.1158/2326-6066.CIR-14-0019-T. [DOI] [PubMed] [Google Scholar]
- 93.Tatsumura T, Koyama S, Tsujimoto M, Kitagawa M, Kagamimori S. Further study of nebulisation chemotherapy, a new chemotherapeutic method in the treatment of lung carcinomas: fundamental and clinical. British journal of cancer. 1993;68:1146–1149. doi: 10.1038/bjc.1993.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rao RD, Markovic SN, Anderson PM. Aerosol therapy for malignancy involving the lungs. Current cancer drug targets. 2003;3:239–250. doi: 10.2174/1568009033481895. [DOI] [PubMed] [Google Scholar]
- 95.Guma SR, Lee DA, Ling Y, Gordon N, Kleinerman ES. Aerosol interleukin-2 induces natural killer cell proliferation in the lung and combination therapy improves the survival of mice with osteosarcoma lung metastasis. Pediatric blood & cancer. 2014;61:1362–1368. doi: 10.1002/pbc.25019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hirota K, Oishi Y, Taniguchi H, Sawachi K, Inagawa H, Kohchi C, Soma G, Terada H. Antitumor effect of inhalatory lipopolysaccharide and synergetic effect in combination with cyclophosphamide. Anticancer research. 2010;30:3129–3134. [PubMed] [Google Scholar]
- 97.Latimer P, Menchaca M, Snyder RM, Yu W, Gilbert BE, Sanders BG, Kline K. Aerosol delivery of liposomal formulated paclitaxel and vitamin E analog reduces murine mammary tumor burden and metastases. Experimental biology and medicine. 2009;234:1244–1252. doi: 10.3181/0901-RM-8. [DOI] [PubMed] [Google Scholar]
- 98.Posch C, Weihsengruber F, Bartsch K, Feichtenschlager V, Sanlorenzo M, Vujic I, Monshi B, Ortiz-Urda S, Rappersberger K. Low-dose inhalation of interleukin-2 bio-chemotherapy for the treatment of pulmonary metastases in melanoma patients. British journal of cancer. 2014;110:1427–1432. doi: 10.1038/bjc.2014.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kaminskas LM, McLeod VM, Ryan GM, Kelly BD, Haynes JM, Williamson M, Thienthong N, Owen DJ, Porter CJ. Pulmonary administration of a doxorubicin-conjugated dendrimer enhances drug exposure to lung metastases and improves cancer therapy. Journal of controlled release : official journal of the Controlled Release Society. 2014;183:18–26. doi: 10.1016/j.jconrel.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 100.Otterson GA, Villalona-Calero MA, Sharma S, Kris MG, Imondi A, Gerber M, White DA, Ratain MJ, Schiller JH, Sandler A, Kraut M, Mani S, Murren JR. Phase I study of inhaled Doxorubicin for patients with metastatic tumors to the lungs. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:1246–1252. doi: 10.1158/1078-0432.CCR-06-1096. [DOI] [PubMed] [Google Scholar]
- 101.Tseng CL, Su WY, Yen KC, Yang KC, Lin FH. The use of biotinylated-EGF-modified gelatin nanoparticle carrier to enhance cisplatin accumulation in cancerous lungs via inhalation. Biomaterials. 2009;30:3476–3485. doi: 10.1016/j.biomaterials.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 102.Tseng CL, Wang TW, Dong GC, Yueh-Hsiu Wu S, Young TH, Shieh MJ, Lou PJ, Lin FH. Development of gelatin nanoparticles with biotinylated EGF conjugation for lung cancer targeting. Biomaterials. 2007;28:3996–4005. doi: 10.1016/j.biomaterials.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 103.Tseng CL, Wu SY, Wang WH, Peng CL, Lin FH, Lin CC, Young TH, Shieh MJ. Targeting efficiency and biodistribution of biotinylated-EGF-conjugated gelatin nanoparticles administered via aerosol delivery in nude mice with lung cancer. Biomaterials. 2008;29:3014–3022. doi: 10.1016/j.biomaterials.2008.03.033. [DOI] [PubMed] [Google Scholar]
- 104.Ye S, Yang W, Wang Y, Ou W, Ma Q, Yu C, Ren J, Zhong G, Shi H, Yuan Z, Su X, Zhu W. Cationic liposome-mediated nitric oxide synthase gene therapy enhances the antitumor effects of cisplatin in lung cancer. International journal of molecular medicine. 2013;31:33–42. doi: 10.3892/ijmm.2012.1171. [DOI] [PubMed] [Google Scholar]
- 105.Li SD, Chono S, Huang L. Efficient oncogene silencing and metastasis inhibition via systemic delivery of siRNA, Molecular therapy : the journal of the American Society of Gene Therapy. 2008;16:942–946. doi: 10.1038/mt.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Guan YY, Luan X, Xu JR, Liu YR, Lu Q, Wang C, Liu HJ, Gao YG, Chen HZ, Fang C. Selective eradication of tumor vascular pericytes by peptide-conjugated nanoparticles for antiangiogenic therapy of melanoma lung metastasis. Biomaterials. 2014;35:3060–3070. doi: 10.1016/j.biomaterials.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 107.Steeg PS, Camphausen KA, Smith QR. Brain metastases as preventive and therapeutic targets. Nature reviews Cancer. 2011;11:352–363. doi: 10.1038/nrc3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Thomas FC, Taskar K, Rudraraju V, Goda S, Thorsheim HR, Gaasch JA, Mittapalli RK, Palmieri D, Steeg PS, Lockman PR, Smith QR. Uptake of ANG1005, a novel paclitaxel derivative, through the blood-brain barrier into brain and experimental brain metastases of breast cancer. Pharmaceutical research. 2009;26:2486–2494. doi: 10.1007/s11095-009-9964-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Regina A, Demeule M, Tripathy S, Lord-Dufour S, Currie JC, Iddir M, Annabi B, Castaigne JP, Lachowicz JE. ANG4043, a novel brain-penetrant peptide-mAb conjugate, is efficacious against HER2-positive intracranial tumors in mice. Molecular cancer therapeutics. 2015;14:129–140. doi: 10.1158/1535-7163.MCT-14-0399. [DOI] [PubMed] [Google Scholar]
- 110.Brandsma D, Milojkovic Kerklaan B, Dieras V, Altintas S, Anders CK, Arnedos Ballester MG, H, Soetekouw PMMB, Gladdines W, Lonnqvist F, Jager A, van Linde ME, Schellens J, Aftimos P. Phase 1/2a study of glutathione PEGylated liposomal doxorubicin (2B3-101) in patients with brain metastases (BM) from solid tumors or recurrent high grade gliomas (HGG) abstractt. Ann Oncol. 2014;25:iv146–iv164. [Google Scholar]
- 111.Parrish KE, Sarkaria JN, Elmquist WF. Improving drug delivery to primary and metastatic brain tumors: strategies to overcome the blood-brain barrier. Clin Pharmacol Ther. 2015;97:336–346. doi: 10.1002/cpt.71. [DOI] [PubMed] [Google Scholar]
- 112.Li J, Cai P, Shalviri A, Henderson JT, He C, Foltz WD, Prasad P, Brodersen PM, Chen Y, DaCosta R, Rauth AM, Wu XY. A multifunctional polymeric nanotheranostic system delivers doxorubicin and imaging agents across the blood-brain barrier targeting brain metastases of breast cancer. ACS nano. 2014;8:9925–9940. doi: 10.1021/nn501069c. [DOI] [PubMed] [Google Scholar]
- 113.Park EJ, Zhang YZ, Vykhodtseva N, McDannold N. Ultrasound-mediated blood-brain/blood-tumor barrier disruption improves outcomes with trastuzumab in a breast cancer brain metastasis model. Journal of controlled release : official journal of the Controlled Release Society. 2012;163:277–284. doi: 10.1016/j.jconrel.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Choi MR, Bardhan R, Stanton-Maxey KJ, Badve S, Nakshatri H, Stantz KM, Cao N, Halas NJ, Clare SE. Delivery of nanoparticles to brain metastases of breast cancer using a cellular Trojan horse. Cancer nanotechnology. 2012;3:47–54. doi: 10.1007/s12645-012-0029-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bagci-Onder T, Du W, Figueiredo JL, Martinez-Quintanilla J, Shah K. Targeting breast to brain metastatic tumours with death receptor ligand expressing therapeutic stem cells. Brain. 2015;138:1710–1721. doi: 10.1093/brain/awv094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Karanicolas PJ, Metrakos P, Chan K, Asmis T, Chen E, Kingham TP, Kemeny N, Porter G, Fields RC, Pingpank J, Dixon E, Wei A, Cleary S, Zogopoulos G, Dey C, D'Angelica M, Fong Y, Dowden S, Ko YJ. Hepatic arterial infusion pump chemotherapy in the management of colorectal liver metastases: expert consensus statement. Curr Oncol. 2014;21:e129–136. doi: 10.3747/co.21.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jones RP, Sutton P, Greensmith RM, Santoyo-Castelazo A, Carr DF, Jenkins R, Rowe C, Hamlett J, Park BK, Terlizzo M, O'Grady E, Ghaneh P, Fenwick SW, Malik HZ, Poston GJ, Kitteringham NR. Hepatic activation of irinotecan predicts tumour response in patients with colorectal liver metastases treated with DEBIRI: exploratory findings from a phase II study. Cancer chemotherapy and pharmacology. 2013;72:359–368. doi: 10.1007/s00280-013-2199-5. [DOI] [PubMed] [Google Scholar]
- 118.Fiorentini G, Aliberti C, Tilli M, Mulazzani L, Graziano F, Giordani P, Mambrini A, Montagnani F, Alessandroni P, Catalano V, Coschiera P. Intra-arterial infusion of irinotecan-loaded drug-eluting beads (DEBIRI) versus intravenous therapy (FOLFIRI) for hepatic metastases from colorectal cancer: final results of a phase III study. Anticancer research. 2012;32:1387–1395. [PubMed] [Google Scholar]
- 119.Opanasopit P, Sakai M, Nishikawa M, Kawakami S, Yamashita F, Hashida M. Inhibition of liver metastasis by targeting of immunomodulators using mannosylated liposome carriers. Journal of controlled release : official journal of the Controlled Release Society. 2002;80:283–294. doi: 10.1016/s0168-3659(02)00006-8. [DOI] [PubMed] [Google Scholar]
- 120.Rychahou P, Haque F, Shu Y, Zaytseva Y, Weiss HL, Lee EY, Mustain W, Valentino J, Guo P, Evers BM. Delivery of RNA nanoparticles into colorectal cancer metastases following systemic administration. ACS nano. 2015;9:1108–1116. doi: 10.1021/acsnano.5b00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Marques C, Ferreira JM, Andronescu E, Ficai D, Sonmez M, Ficai A. Multifunctional materials for bone cancer treatment. International journal of nanomedicine. 2014;9:2713–2725. doi: 10.2147/IJN.S55943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Elazar V, Adwan H, Rohekar K, Zepp M, Lifshitz-Shovali R, Berger MR, Golomb G. Biodistribution of antisense nanoparticles in mammary carcinoma rat model. Drug Deliv. 2010;17:408–418. doi: 10.3109/10717541003777225. [DOI] [PubMed] [Google Scholar]
- 123.Wang D, Miller S, Sima M, Kopeckova P, Kopecek J. Synthesis and evaluation of water-soluble polymeric bone-targeted drug delivery systems. Bioconjug Chem. 2003;14:853–859. doi: 10.1021/bc034090j. [DOI] [PubMed] [Google Scholar]
- 124.Wang G, Mostafa NZ, Incani V, Kucharski C, Uludag H. Bisphosphonate-decorated lipid nanoparticles designed as drug carriers for bone diseases. J Biomed Mater Res A. 2012;100:684–693. doi: 10.1002/jbm.a.34002. [DOI] [PubMed] [Google Scholar]
- 125.Chen H, Li G, Chi H, Wang D, Tu C, Pan L, Zhu L, Qiu F, Guo F, Zhu X. Alendronate-conjugated amphiphilic hyperbranched polymer based on Boltorn H40 and poly(ethylene glycol) for bone-targeted drug delivery. Bioconjug Chem. 2012;23:1915–1924. doi: 10.1021/bc3003088. [DOI] [PubMed] [Google Scholar]
- 126.Segal E, Pan H, Ofek P, Udagawa T, Kopeckova P, Kopecek J, Satchi-Fainaro R. Targeting angiogenesis-dependent calcified neoplasms using combined polymer therapeutics. PloS one. 2009;4:e5233. doi: 10.1371/journal.pone.0005233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kim JW, Galanzha EI, Zaharoff DA, Griffin RJ, Zharov VP. Nanotheranostics of circulating tumor cells, infections and other pathological features in vivo. Mol Pharm. 2013;10:813–830. doi: 10.1021/mp300577s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kievit FM, Stephen ZR, Veiseh O, Arami H, Wang T, Lai VP, Park JO, Ellenbogen RG, Disis ML, Zhang M. Targeting of primary breast cancers and metastases in a transgenic mouse model using rationally designed multifunctional SPIONs. ACS nano. 2012;6:2591–2601. doi: 10.1021/nn205070h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Patil R, Ljubimov AV, Gangalum PR, Ding H, Portilla-Arias J, Wagner S, Inoue S, Konda B, Rekechenetskiy A, Chesnokova A, Markman JL, Ljubimov VA, Li D, Prasad RS, Black KL, Holler E, Ljubimova JY. MRI virtual biopsy and treatment of brain metastatic tumors with targeted nanobioconjugates: nanoclinic in the brain. ACS nano. 2015;9:5594–5608. doi: 10.1021/acsnano.5b01872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Peiris PM, Toy R, Doolittle E, Pansky J, Abramowski A, Tam M, Vicente P, Tran E, Hayden E, Camann A, Mayer A, Erokwu BO, Berman Z, Wilson D, Baskaran H, Flask CA, Keri RA, Karathanasis E. Imaging metastasis using an integrin-targeting chain-shaped nanoparticle. ACS nano. 2012;6:8783–8795. doi: 10.1021/nn303833p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rajkumar V, Goh V, Siddique M, Robson M, Boxer G, Pedley RB, Cook GJ. Texture analysis of (125)I-A5B7 anti-CEA antibody SPECT differentiates metastatic colorectal cancer model phenotypes and anti-vascular therapy response. British journal of cancer. 2015;112:1882–1887. doi: 10.1038/bjc.2015.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liu TW, Macdonald TD, Jin CS, Gold JM, Bristow RG, Wilson BC, Zheng G. Inherently multimodal nanoparticle-driven tracking and real-time delineation of orthotopic prostate tumors and micrometastases. ACS nano. 2013;7:4221–4232. doi: 10.1021/nn400669r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chen Q, Liang C, Wang X, He J, Li Y, Liu Z. An albumin-based theranostic nano-agent for dual-modal imaging guided photothermal therapy to inhibit lymphatic metastasis of cancer post surgery. Biomaterials. 2014;35:9355–9362. doi: 10.1016/j.biomaterials.2014.07.062. [DOI] [PubMed] [Google Scholar]
- 134.Hoffman RM. Application of GFP imaging in cancer. Laboratory investigation; a journal of technical methods and pathology. 2015;95:432–452. doi: 10.1038/labinvest.2014.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Timpson P, McGhee EJ, Anderson KI. Imaging molecular dynamics in vivo--from cell biology to animal models. Journal of cell science. 2011;124:2877–2890. doi: 10.1242/jcs.085191. [DOI] [PubMed] [Google Scholar]
- 136.Yang M, Luiken G, Baranov E, Hoffman RM. Facile whole-body imaging of internal fluorescent tumors in mice with an LED flashlight. BioTechniques. 2005;39:170, 172. doi: 10.2144/05392BM02. [DOI] [PubMed] [Google Scholar]
- 137.Tsuji K, Yamauchi K, Yang M, Jiang P, Bouvet M, Endo H, Kanai Y, Yamashita K, Moossa AR, Hoffman RM. Dual-color imaging of nuclear-cytoplasmic dynamics, viability, and proliferation of cancer cells in the portal vein area. Cancer research. 2006;66:303–306. doi: 10.1158/0008-5472.CAN-05-2958. [DOI] [PubMed] [Google Scholar]
- 138.Brown EB, Campbell RB, Tsuzuki Y, Xu L, Carmeliet P, Fukumura D, Jain RK. In vivo measurement of gene expression, angiogenesis and physiological function in tumors using multiphoton laser scanning microscopy. Nature medicine. 2001;7:864–868. doi: 10.1038/89997. [DOI] [PubMed] [Google Scholar]
- 139.Massoud TF, Paulmurugan R, Gambhir SS. A molecularly engineered split reporter for imaging protein-protein interactions with positron emission tomography. Nature medicine. 2010;16:921–926. doi: 10.1038/nm.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fan-Minogue H, Cao Z, Paulmurugan R, Chan CT, Massoud TF, Felsher DW, Gambhir SS. Noninvasive molecular imaging of c-Myc activation in living mice. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:15892–15897. doi: 10.1073/pnas.1007443107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Qiao R, Liu C, Liu M, Hu H, Hou Y, Wu K, Lin Y, Liang J, Gao M. Ultrasensitive in vivo detection of primary gastric tumor and lymphatic metastasis using upconversion nanoparticles. ACS nano. 2015;9:2120–2129. doi: 10.1021/nn507433p. [DOI] [PubMed] [Google Scholar]
- 142.van Saders B, Al-Baroudi L, Tan MC, Riman RE. Rare-earth doped particles with tunable infrared emissions for biomedical imaging. Opt Mater Express. 2013;3:566–573. [Google Scholar]
- 143.Naczynski DJ, Tan MC, Zevon M, Wall B, Kohl J, Kulesa A, Chen S, Roth CM, Riman RE, Moghe PV. Rare-earth-doped biological composites as in vivo shortwave infrared reporters. Nature communications. 2013;4:2199. doi: 10.1038/ncomms3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cui M, Naczynski DJ, Zevon M, Griffith CK, Sheihet L, Poventud-Fuentes I, Chen S, Roth CM, Moghe PV. Multifunctional Albumin Nanoparticles As Combination Drug Carriers for Intra-Tumoral Chemotherapy. Advanced healthcare materials. 2013 doi: 10.1002/adhm.201200467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Naczynski DJ, Andelman T, Pal D, Chen S, Riman RE, Roth CM, Moghe PV. Albumin Nanoshell Encapsulation of Near-Infrared-Excitable Rare-Earth Nanoparticles Enhances Biocompatibility and Enables Targeted Cell Imaging. Small. 2010;6:1631–1640. doi: 10.1002/smll.200902403. [DOI] [PubMed] [Google Scholar]
- 146.Zevon M, Ganapathy V, Kantamneni H, Mnigozzi M, Kim P, Adler D, Pierce MC, Riman RE, Roth CM, Moghe PV. Engineering of Rare-Earth Albumin Nanocomposites for Breast Cancer Metastasis-Targeted Short Wave Infrared Imaging. Small. 2015 doi: 10.1002/smll.201502202. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chowdhury R, Ganeshan B, Irshad S, Lawler K, Eisenblatter M, Milewicz H, Rodriguez-Justo M, Miles K, Ellis P, Groves A, Punwani S, Ng T. The use of molecular imaging combined with genomic techniques to understand the heterogeneity in cancer metastasis. Br J Radiol. 2014;87:20140065. doi: 10.1259/bjr.20140065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lee EY, Park KS, Yoon YJ, Lee J, Moon HG, Jang SC, Choi KH, Kim YK, Gho YS. Therapeutic effects of autologous tumor-derived nanovesicles on melanoma growth and metastasis. PloS one. 2012;7:e33330. doi: 10.1371/journal.pone.0033330. [DOI] [PMC free article] [PubMed] [Google Scholar]


