Abstract
Background
Renin angiotensin system (RAS) inhibitor use after acute myocardial infarction (AMI) is a quality indicator, but there may also be reasons not to use this therapy. We sought to determine how chronic kidney disease (CKD) and acute kidney injury (AKI) affected RAS inhibitor prescription after AMI in patients with and without decreased ejection fraction (EF).
Methods
Participants from the TRIUMPH registry were categorized by admission estimated glomerular filtration rate (eGFR in ml/min/1.73m2; severe [<30], moderate [30–59], mild [60–89] and no [≥90] CKD) and occurrence of AKI (an increase in creatinine ≥0.3mg/dl or ≥50%). RAS inhibitor prescriptions at discharge were compared across categories of CKD, AKI and decreased EF (<40% vs. ≥40%) using a hierarchical modified Poisson model.
Results
Among 4223 AMI patients (mean age 59.0 years, 67.0% male, 67.3% white), RAS inhibitor use decreased significantly with lower eGFR (P <0.001), but there was no effect of decreased EF on this relationship (interaction P = 0.40). Without AKI, severe and moderate CKD were associated with significantly less RAS inhibitor use; relative risks (RRs) = 0.67 (95% CIs, 0.58–0.78) and 0.94 (0.90–0.99), respectively. When AKI occurred, CKD was associated with less RAS inhibitor use: RR 0.84 (0.76–0.93) for mild CKD, 0.78 (0.68–0.88) for moderate CKD, and 0.50 (0.42–0.61) for severe CKD. EF <40% was associated with use (RR 1.11, 1.03–1.18), independent of renal function.
Conclusions
CKD and AKI are associated with fewer RAS inhibitor prescriptions at discharge, but in both AKI and non-AKI patients, eGFR was more strongly associated with use than EF.
Keywords: Acute myocardial infarction, heart failure, chronic kidney disease, acute kidney injury, renin-angiotensin system inhibitors
Introduction
Inhibition of the renin–angiotensin system (RAS) decreases cardiovascular (CV) morbidity and mortality in patients with atherosclerosis[1–3] and with systolic heart failure (HF).[4, 5] As such, the American College of Cardiology/American Heart Association (ACC/AHA) guidelines recommend use of angiotensin converting enzyme inhibitors (ACEIs) in patients with acute myocardial infarction (AMI) or stable ischemic heart disease (IHD), particularly if systolic heart failure is present, unless contraindications exist.[6–8] ACEIs and angiotensin receptor blockers (ARBs, collectively classified with ACEIs as RAS inhibitors) are used virtually interchangeably for IHD and HF, and their use after AMI in patients with ejection fraction (EF) <40% is endorsed as a quality performance measure.[9]
Despite the proven benefits of these agents, their use is not without risk. One relative contraindication to prescribing RAS inhibitors is a low estimated glomerular filtration rate (eGFR) or a recent rise in serum creatinine. As such, clinical judgment is relied upon to determine the risks and benefits of RAS inhibitor use in individual circumstances. While RAS inhibitors have important benefits in the setting of chronic kidney disease (CKD),[10–13] physicians may miss an opportunity to use RAS therapy in AMI patients with elevated serum creatinine,[14] possibly due to the fear that renal function may deteriorate or that hyperkalemia will ensue. This is of substantial clinical relevance since worsening renal function (either acutely or more chronically) correlates with adverse CV outcomes in patients admitted with AMI and could potentially indicate a population with a greater absolute risk reduction from RAS therapy.[15–20]
In light of the complexity of balancing the risks and benefits of RAS therapy in patients with impaired renal function at the time of an AMI, more insight into current practice patterns is needed. To our knowledge, no large studies have investigated patterns of RAS inhibitor use upon hospital discharge following AMI in patients with kidney dysfunction. We therefore examined demographic, clinical, and socioeconomic factors associated with RAS inhibitor use in patients recovering from an AMI with a specific focus on the association of CKD, AKI and left ventricular function on treatment patterns. We hypothesized that among AMI patients eligible to receive RAS inhibitors at discharge, RAS inhibitor prescription would be substantially lower in patients with more advanced CKD (whether or not HF was present).
Methods
Study population and protocol
Details regarding the study design of the TRIUMPH study have been previously published.[21] Briefly, 4340 patients from 24 U.S. hospitals were enrolled between April 2005 and December 2008. Inclusion criteria included patients ≥ 18 years of age with a diagnosis of AMI, biomarker evidence of myocardial necrosis and either prolonged (≥20 minutes) ischemic signs/symptoms or electrocardiographic ST-wave changes. Patients included must have had creatinine available on admission and subsequent in-hospital creatinine values and been discharged alive. Exclusion criteria included: (a) a documented contraindication to RAS inhibitor, which was prospectively collected; (b) lack of a serum creatinine value on admission; or (c) lack of a second creatinine value during hospitalization. RAS inhibitor prescription at discharge was recorded.
The study was undertaken in accordance with the principles of the Declarations of Helsinki. Each participating hospital obtained Institutional Research Board approval, and all patients provided written informed consent.
Definition of terms
Moderate to severe systolic HF was defined as having a left ventricular ejection fraction (LVEF) of less than 40%.[22] Renal function at baseline was determined by calculating the estimated glomerular filtration rate (eGFR) from admission creatinine using the CKD-EPI equation.[23] Patients with eGFR>90 ml/min/1.73m2 were designated as “normal kidney function”, while patients with eGFR 60 – 89 ml/min/1.73m2, 30 – 59 ml/min/1.73m2, and < 30 ml/min/1.73m2 were designated as having “mild”, “moderate”, and “severe” CKD, respectively. End-stage renal disease (ESRD) was a separate category in our analysis, which comprised patients on chronic dialysis. AKI was defined as an increase in the serum creatinine level of 0.3 mg/dL or a 50% increase in the creatinine level during the hospital stay, as compared with the admission value.[24]
Statistical Analysis
We divided the cohort into five groups of renal function according to eGFR (normal kidney function, mild CKD, moderate CKD, severe CKD, and dialysis-dependent ESRD) and compared the baseline characteristics across each category. Categorical variables were compared with Chi-squared or Fisher exact tests and continuous variables were compared with an independent one-way analysis of variance. We then compared the frequencies of RAS inhibitor use at discharge between all renal function categories using the Mantel-Haenszel trend test, stratified by presence or absence of AKI as well as by presence or absence of moderate to severe systolic HF. A Hierarchical modified Poisson model was used to assess the independent association between renal function and RAS inhibitor use at discharge. Site was entered as a random effect to account for patient clustering, and site-level variation was explored with a median rate ratio (MRR), which estimates the average likelihood of the same patient being treated with RAS at one random hospital as compared with another. To avoid overestimation of effect sizes, modified Poisson model was used because RAS inhibitor use occurred >10% of the time.[25]
To examine whether or not the occurrence of AKI had more of an impact in certain renal function categories, we assessed the interaction of renal function category and AKI (of note, cause of AKI was not available). A sensitivity analysis eliminated individuals who experienced hyperkalemia during the admission. Covariates included race, gender, age, insurance status, presence of LVEF dysfunction, occurrence of an STEMI, smoking status obesity, and histories of myocardial infarction (MI)/percutaneous coronary intervention (PCI)/coronary artery bypass grafting (CABG), congestive heart failure (CHF), atrial fibrillation (AF), peripheral vascular disease (PVD), cerebrovascular accident (CVA), hypertension (HTN), diabetes mellitus (DM, and dyslipidemia. Race, which was classified as White/Caucasian, Black/African-American, or Other, was self-reported by the participant and recorded by the study nurse coordinators; information on race was considered to be essential since utilization of recommended medications varies by race. Approximately, 9.5% of patients had missing covariate data: 9.2% were missing only 1 value and 0.3% were missing 2 or 3 values, with the highest missing rate for any single variables being 5.3% (for obesity). Missing covariate data was accounted for using single imputation with IVEWARE.[26] All analyses were conducted using SAS v9.3 (SAS Institute, Inc., Cary, NC), and statistical significance was determined by a 2-sided p-value of <0.05.
The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents. The sources of funding were an NIH (NIDDK) grant (K23 DK085378, to JBW) and, for TRIUMPH, a grant from the NIH (NHLBI) to Washington University School of Medicine (SCCOR Grant #P50HL077113-01).
Results
Study population
Of the 4316 TRIUMPH patients who survived to discharge, 39 had a documented contraindication to RAS inhibitors and 54 were missing an admission or subsequent creatinine, leaving 4223 participants in the analytic cohort (Figure 1). Their characteristics are shown in Table 1. The mean age was 59.0 years, 67.0% were male, 67.3% were white, 31.0% had diabetes, and 66.7% had hypertension. Among the participants, 1220 (28.9%) had normal kidney function, 1958 (46.5%) had mild CKD, 818 (19.4%) had moderate CKD, and 146 (3.5%) had severe CKD based upon patients’ admission eGFR. Eighty-one (1.9%) had dialysis-dependent ESRD. ST-elevation AMI was diagnosed in 1814 (43.3%) participants, while inhospital PCI and CABG were performed in 65.4% and 9.0%, respectively. During hospitalization, 498 (12.4%) participants developed AKI. Upon discharge, moderate to severe systolic dysfunction was documented in 783 (18.6%) of participants.
Figure 1.
Flowchart demonstrating the creation of the study cohort.
Abbreviations: RAS, renin-angiotensin system; Cr, creatinine; eGFR, estimated glomerular filtration rate
Table 1.
Characteristics of the participants.
| Initial estimated glomerular filtration rate (mL/min/1.73m2) | Total n = 4223 |
P Value | |||||
|---|---|---|---|---|---|---|---|
| Dialysis n = 81 |
< 30 n = 146 |
30 – 59 n = 818 |
60 – 89 n = 1958 |
≥90 n = 1220 |
|||
| Demographics | |||||||
| Age (yrs) | 62.2 ± 11.8 | 65.1 ± 12.9 | 65.7 ± 12.1 | 59.1 ± 11.8 | 53.4 ± 10.6 | 59.0 ± 12.4 | < 0.001 |
| Sex | < 0.001 | ||||||
| Male | 44 (54.3%) | 82 (56.2%) | 462 (56.5%) | 1363 (69.6%) | 879 (72.0%) | 2830 (67.0%) | |
| Female | 37 (45.7%) | 64 (43.8%) | 356 (43.5%) | 595 (30.4%) | 341 (28.0%) | 1393 (33.0%) | |
| Race | < 0.001 | ||||||
| White/Caucasian | 38 (46.9%) | 91 (62.3%) | 582 (71.1%) | 1384 (70.7%) | 746 (61.1%) | 2841 (67.3%) | |
| Black/African-American | 41 (50.6%) | 46 (31.5%) | 192 (23.5%) | 435 (22.2%) | 383 (31.4%) | 1097 (26.0%) | |
| Other | 2 (2.5%) | 9 (6.2%) | 44 (5.4%) | 139 (7.1%) | 91 (7.5%) | 285 (6.7%) | |
| Ethnicity | < 0.001 | ||||||
| Hispanic/Latino | 5 (6.4%) | 10 (7.1%) | 35 (4.4%) | 107 (5.6%) | 104 (8.8%) | 261 (6.4%) | |
| Non-Hispanic/Latino | 73 (93.6%) | 131 (92.9%) | 760 (95.6%) | 1800 (94.4%) | 1080 (91.2%) | 3844 (93.6%) | |
| Unknown | 3 | 5 | 23 | 51 | 36 | 118 | |
| Payor | |||||||
| None/self-pay | 11 (13.9%) | 18 (12.6%) | 121 (14.9%) | 443 (23.1%) | 341 (28.4%) | 934 (22.5%) | < 0.001 |
| unknown | 2 | 3 | 6 | 37 | 21 | 69 | |
| Medical History | |||||||
| Obesity1 | 24 (32.4%) | 59 (45.4%) | 328 (42.6%) | 775 (41.4%) | 456 (39.5%) | 1642 (41.0%) | 0.26 |
| missing | 7 | 16 | 48 | 86 | 65 | 222 | |
| Smoking2 | 13 (16.0%) | 25 (17.1%) | 197 (24.1%) | 772 (39.4%) | 608 (49.8%) | 1615 (38.2%) | < 0.001 |
| Hypertension | 71 (87.7%) | 133 (91.1%) | 655 (80.1%) | 1251 (63.9%) | 707 (58.0%) | 2817 (66.7%) | < 0.001 |
| Diabetes | 57 (70.4%) | 93 (63.7%) | 339 (41.4%) | 500 (25.5%) | 319 (26.1%) | 1308 (31.0%) | < 0.001 |
| Dyslipidemia | 32 (39.5%) | 82 (56.2%) | 464 (56.7%) | 940 (48.0%) | 546 (44.8%) | 2064 (48.9%) | < 0.001 |
| Prior MI/PCI/CABG | 42 (51.9%) | 82 (56.2%) | 325 (39.7%) | 584 (29.8%) | 309 (25.3%) | 1342 (31.8%) | < 0.001 |
| Chronic Heart Failure | 19 (23.5%) | 43 (29.5%) | 119 (14.5%) | 137 (7.0%) | 45 (3.7%) | 363 (8.6%) | < 0.001 |
| Atrial fibrillation | 8 (9.9%) | 14 (9.6%) | 65 (7.9%) | 88 (4.5%) | 31 (2.5%) | 206 (4.9%) | < 0.001 |
| Pacemaker implantation | 1 (1.2%) | 3 (2.1%) | 15 (1.8%) | 13 (0.7%) | 5 (0.4%) | 37 (0.9%) | 0.004 |
| Prior cerebrovascular accident | 15 (18.5%) | 19 (13.0%) | 50 (6.1%) | 83 (4.2%) | 39 (3.2%) | 206 (4.9%) | < 0.001 |
| Peripheral vascular disease | 9 (11.1%) | 15 (10.3%) | 54 (6.6%) | 77 (3.9%) | 37 (3.0%) | 192 (4.5%) | < 0.001 |
| Clinical variables during admission | |||||||
| Final MI diagnosis | < 0.001 | ||||||
| STEMI | 14 (17.3%) | 22 (15.6%) | 306 (37.9%) | 897 (46.0%) | 575 (47.4%) | 1814 (43.3%) | |
| NSTEMI | 67 (82.7%) | 119 (84.4%) | 501 (62.1%) | 1053 (54.0%) | 638 (52.6%) | 2378 (56.7%) | |
| missing | 5 | 11 | 8 | 7 | 31 | ||
| In-hospital PCI | 34 (42.0%) | 45 (30.8%) | 469 (57.3%) | 1352 (69.1%) | 863 (70.7%) | 2763 (65.4%) | < 0.001 |
| In-hospital CABG | 7 (8.6%) | 19 (13.0%) | 84 (10.3%) | 169 (8.6%) | 102 (8.4%) | 381 (9.0%) | 0.25 |
| Decreased LVSF3 | 19 (23.5%) | 42 (28.8%) | 163 (20.0%) | 353 (18.0%) | 206 (16.9%) | 783 (18.6%) | 0.005 |
| missing | 2 | 1 | 2 | 5 | |||
| Acute kidney injury | < 0.001 | ||||||
| No AKI | 0 (0.0%) | 93 (63.7%) | 645 (78.9%) | 1780 (90.9%) | 1084 (88.9%) | 3602 (85.3%) | |
| AKI | 0 (0.0%) | 53 (36.3%) | 173 (21.1%) | 178 (9.1%) | 136 (11.1%) | 540 (12.8%) | |
| Dialysis-dependence | 81 (100.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 81 (1.9%) | |
Continuous variables compared using one-way analysis of variance; categorical variables compared using chi-square or Fisher’s exact test.
Defined as body mass index > 30kg/m2
Defined as occurring within the past 1 month
Defined as moderate or severe
Abbreviations: MI, myocardial infarction; PCI, percutaneous coronary intervention; CABG, coronary artery bypass surgery; STEMI, ST-elevation MI; NSTEMI, non-ST-elevation MI; LVSF, left ventricular systolic function; AKI, acute kidney injury
RAS inhibitor prescription: effect of CKD
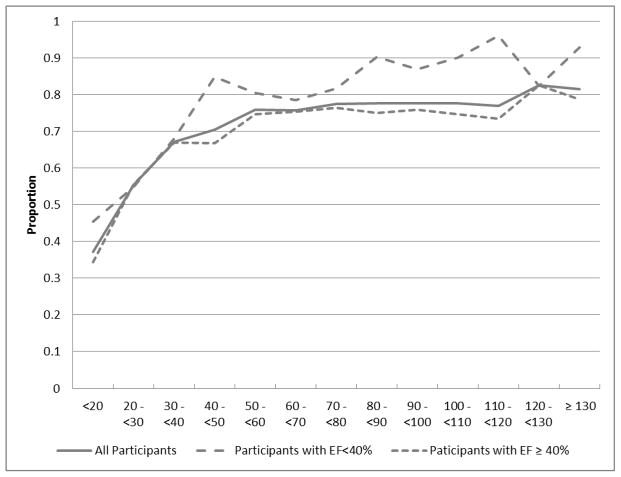
Overall, 3177 (75.2%) participants were prescribed RAS inhibitors at the time of discharge, which varied by the presence of systolic dysfunction. Among those with EF < 40%, 81.7% received RAS inhibitors as compared with 73.7% of those with EF ≥ 40% (P<0.001). The patterns of RAS inhibitor use by level of eGFR, stratified by presence of systolic HF are shown in Figure 2 and Table 2. Generally, RAS inhibitor use decreased significantly as level of eGFR decreased in both those with systolic HF and those without (Mantel-Haenszel trend test P<0.001). For example, in patients with systolic HF, the rate decreased from 89.3% in participants with eGFR ≥ 90 mL/min/1.73m2 to 52.4% in participants with eGFR < 30 mL/min/1.73m2 (P for trend <0.001). The same general pattern was present in participants with intact EF, where 76.1% received RAS inhibitors in the eGFR > 90 mL/min/1.73m2 group and 49.0% in the eGFR < 30 mL/min/1.73m2 group (P for trend <0.001). Absolute prescribing rates were higher for participants with EF < 40%, regardless of eGFR. In the eGFR ≥ 90 mL/min/1.73m2 group, RAS inhibitors were prescribed 13.2% less often, in absolute terms, in participants with EF ≥ 40% compared to EF < 40%, while in the eGFR < 30 mL/min/1.73m2 group, this difference was 3.4%. However, formal interaction testing revealed that the difference by EF across categories of renal dysfunction was not significant (P=0.40).
Figure 2.
Renin-angiotensin system inhibitor use proportion by estimated glomerular filtration rate.
X-axis values are estimated glomerular filtration rates, in mL/min/1.73m2
Abbreviations: EF, ejection fraction
Table 2.
Use of renin-angiotensin system inhibitors upon discharge by level of glomerular filtration rate.
| Estimated glomerular filtration rate1
|
P value | |||||
|---|---|---|---|---|---|---|
| dialysis n = 81 |
<30 n = 146 |
30 – 59 n = 818 |
60 – 89 n = 1958 |
≥90 n = 1220 |
||
| All | 49 (60.5%) | 73 (50.0%) | 594 (72.6%) | 1505 (76.9%) | 956 (78.4%) | < 0.001 |
| EF < 40% | 11 (57.9%) | 22 (52.4%) | 130 (79.8%) | 293 (83.0%) | 184 (89.3%) | < 0.001 |
| EF ≥ 40% | 38 (61.3%) | 51 (49.0%) | 462 (70.8%) | 1212 (75.6%) | 770 (76.1%) | < 0.001 |
| No AKI | – | 51 (54.8%) | 486 (75.3%) | 1386 (77.9%) | 850 (78.4%) | < 0.001 |
| AKI | – | 22 (41.5%) | 108 (62.4%) | 119 (66.9%) | 106 (77.9%) | < 0.001 |
in mL/min/1.73m2
Abbreviations: EF, ejection fraction; AKI, acute kidney injury
Because patients on dialysis are qualitatively different from others, they were not included in the trend analyses. Patients on dialysis had rates of RAS inhibitor prescription between those with eGFR < 30 mL/min/1.73m2 and those with eGFR 30 – 59 mL/min/1.73m2, for both categories of EF.
RAS inhibitor prescription: effect of AKI
Overall, AKI was associated with decreased use of RAS inhibitors at discharge (Table 2). As baseline eGFR decreased, differential use between those with and without AKI tended to increase. For example, there was only a 1.5% difference in use by AKI status in patients with eGFR ≥ 90 mL/min/1.73m2 (78.4% versus 77.9%), but an 11.0% difference in the eGFR 60 – 89 mL/min/1.73m2 group and a 13.3% difference in the eGFR < 30 mL/min/1.73m2 group. The formal test of interaction of eGFR and AKI was significant (p < 0.01). Notably, there was no interaction between AKI and level of EF (P=0.25) for RAS inhibitor prescribing.
Factors associated with RAS inhibitor use
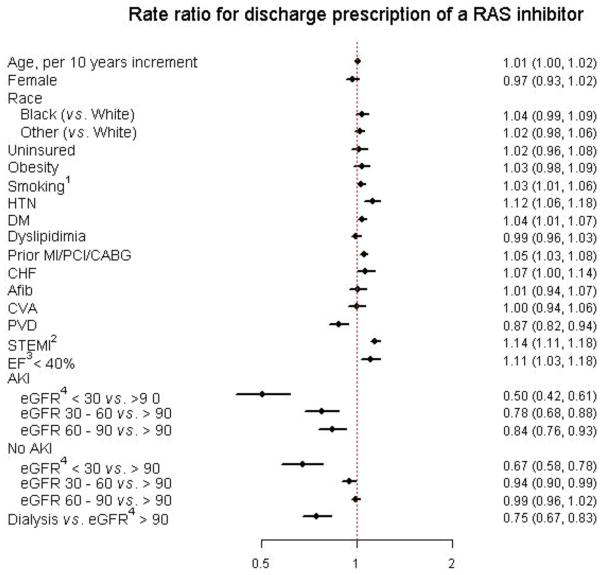
Figure 3 demonstrates the fully-adjusted strength of association between different patient characteristics and the use of RAS inhibitors at discharge. AKI was found to have a significant interaction with baseline kidney function. Among those without AKI, severe (eGFR < 30 ml/min/1.73m2) and moderate (eGFR of 30 – 59 ml/min/1.73m2) renal dysfunction were independently associated with significantly less use of RAS inhibitors compared to those with normal renal function, having observed relative risks of 0.67 (95% CIs 0.58 – 0.78) and 0.94 (0.90 – 0.99), respectively. There was no significant association between mild renal dysfunction (eGFR of 60 – 89 ml/min/1.73m2) and with normal renal function; RR = 0.99 (95% CI’s 0.96 – 1.02).
Figure 3.
Forest plot of rate ratios for discharge prescription of a renin-angiotensin system inhibitor.
Abbreviations: HTN, hypertension; DM, diabetes mellitus; MI, myocardial infarction; PCI, percutaneous coronary intervention; CABG; coronary artery bypass grafting; CHF, congestive heart failure; Afib, atrial fibrillation; CVA, cerebrovascular accident; PVD, peripheral vascular disease; STEMI, ST-elevation MI; EF, ejection fraction; AKI, acute kidney injury; eGFR, estimated glomerular filtration rate.
Among those with AKI, lower eGFR was associated with less use of RAS inhibitors: RR 0.50 (0.42 – 0.61) for eGFR < 30 ml/min/1.73m2, RR 0.78 (0.68 – 0.88) for eGFR 30 – 59 ml/min/1.73m2, and RR 0.84 (0.76 – 0.93) for 60 – 89 mL/min/1.73m2. Dialysis patients were also significantly less like to receive RAS inhibitors (RR 0.75, 0.67 – 0.83). Participants with LVEF < 40% as well participants who experienced a STEMI were also significantly more likely to receive a RAS inhibitor (RR 1.11 and 1.14, respectively), independent of renal function.
In a sensitivity analysis, individuals (n = 36) who experienced hyperkalemia during admission were excluded. Results of the model were nearly identical; among those without AKI, severe and moderate renal dysfunction were again independently associated with significantly less use of RAS inhibitors, with relative risks of 0.67 (0.58 – 0.78) and 0.95 (0.90 – 0.99), respectively; as before, there was no significant association with prescription and mild renal dysfunction and normal renal function. Among those with AKI, lower eGFR was associated with less use of RAS inhibitors: RR 0.52 (0.43 – 0.63) for eGFR < 30 ml/min/1.73m2, RR 0.78 (0.69 – 0.89) for eGFR 30 – 59 ml/min/1.73m2, and RR 0.85 (0.76 – 0.94) for 60 – 89 mL/min/1.73m2. Dialysis patients were again significantly less like to receive RAS inhibitors (RR 0.76, 0.69 – 0.85).
RAS inhibitor prescription: effect of site
The median rate ratio (MRR) for RAS inhibitor prescription across the 24 sites was 1.09, suggesting little variation across centers in the use of RAS inhibitors, after adjusting for patient characteristics.
Discussion
Prescription of RAS inhibitors is an evidence-based cornerstone of care for the secondary prevention of MI, particularly in patients with moderate to severe systolic HF (LVEF ≤ 40%).[4, 5] However, concern exists about the use of RAS inhibitors in the context of kidney disease, given that these agents can worsen renal function and promote hyperkalemia. Because AMI patients frequently harbor significant underlying CKD, or experience AKI during their hospital course, a fuller understanding of how RAS inhibitors are used in this setting can inform future quality-improvement efforts. As such, our study was designed to carefully examine the respective roles of decreased ejection fraction, CKD, and AKI in RAS inhibitor prescription after AMI. Using a large, multi-center AMI registry, we found that renal status, as determined by the presence of either CKD or AKI, appeared to be more strongly associated with RAS inhibitor use after AMI than decreased ejection fraction. While other studies have also demonstrated systematic underuse of several cardinal post-AMI therapies, such as aspirin, β-blockers, and ACE inhibitors, in patients with CKD and dialysis-dependent ESRD,[20, 27–29] our study suggests that prescribers may be preferentially weighing renal function, as opposed to EF, in their decision-making process. This is an important finding, given what is known about both the beneficial effects and the potential risks of these agents in the setting of AMI, heart failure and kidney disease.
There is substantial interest in the cardiology community concerning optimal allocation of cardiovascular treatments [30] so that the patients who are most likely to benefit from specific treatments are the ones preferentially receiving them. In patients with AMIs, RAS inhibitors should be prescribed unless contraindications are present, especially if systolic heart failure is present. RAS inhibitors slow the progression of diabetic kidney disease,[10] decrease proteinuria in CKD,[12] and inhibit fibrosis,[31] and therefore have a strong rationale for use in most types of kidney disease. As such, AMI patients with CKD, and especially those with low EF, would seem to be appropriate candidates for these drugs.
In contrast to this clinical logic, we found that RAS inhibitor use decreased significantly as CKD stage worsened, with even dialysis-dependent patients having a rate below that of individuals with eGFR 30 – 59 mL/min/1.73m2. Moreover, we found that while decreased ejection fraction appeared to be a factor driving prescribing decisions, it was at best only a modest one, given that our model revealed a RR of only 11% in a fully-adjusted model that accounted for renal function. Indeed, at each stage of CKD, prescribing percentage was only slightly higher in patients with low EF compared to those with intact EF, and there was no statistical interaction between EF and stage of CKD upon formal testing.
Our finding of apparent underuse of RAS inhibitors, as noted previously by others,[32, 33] suggests reluctance among prescribers to employ these agents in the setting of CKD. Such reluctance, however, may not be justified in light of the results from clinical trials. For example, the benefits of RAS inhibitors was studied in an analysis of 6867 participants with CKD from the two SYMPHONY trials, which demonstrated that the 90-day mortality benefits of ACE inhibitors were greatest in acute coronary syndrome patients with the most advanced CKD.[17] Another report from the ADVANCE study, which enrolled patients with new-onset coronary heart disease (CHD), suggested that the effect of CKD on subsequent cardiovascular events and death might be accounted for almost fully by the use of “cardioprotective” medications, such as HMG-CoA reductase inhibitors, β-blockers, calcium channel blockers, and RAS inhibitors.[34] Nihilism about the benefits of RAS inhibitors in preventing progression of CKD to ESRD should also not deter prescribing these agents. In a post hoc analysis[35] of the RENAAL trial,[13] randomization to an ARB (versus placebo) resulted in decreased risk of ESRD, an effect which increased, in relative terms, as eGFR decreased. As a result, hesitancy in prescribing these agents may mean that patients who might particularly benefit from RAS inhibitors, namely those with CKD and decreased EF,[36] are not receiving it.
Acute, as opposed to chronic, kidney disease was also associated with RAS inhibitor prescribing decisions. RAS inhibitor prescription rates decreased across strata of eGFR more strongly when accompanied by AKI, as formally demonstrated by the statistical interaction between CKD and AKI. This was not unexpected, given that worsening renal function during the post-AMI period is associated with adverse outcomes.[18] Information as to the cause of AKI was not available in our dataset; it is certainly possible that continuation of pre-admission RAS inhibitors or early introduction of these agents (that is, prior to discharge) was the cause of AKI in some patients. For some of these patients, withholding RAS inhibitors at discharge would have been the appropriate clinical response. However, while AKI is a undoubtedly a clinical scenario which demands particular scrutiny, careful consideration of the ramifications of RAS inhibitor-induced changes in creatinine and potassium is required when weighing the benefits and risks of therapy. An acute post-initiation rise in serum creatinine is most often a functional and reversible change, and is associated with improved renal outcomes in diabetic patients with CKD;[37] this is likely the case in patients with non-diabetic CKD as well. While a rise in serum potassium after introduction of an ACEI or ARB is not uncommon[38] and, over the long term, is associated with an increased rate of progression to ESRD, careful laboratory monitoring in the post-initiation period can be used to identify patients at risk for this outcome[39] and therapy can then be discontinued in those who manifest laboratory abnormalities. As such, patients recovering from AKI, especially those with CKD or reduced EF, might be prudently challenged with a RAS inhibitor at a future date following a return to baseline eGFR.
Perhaps most difficult to explain is our finding of low rates of use in patients undergoing chronic dialysis. While RAS inhibitors can promote hyperkalemia even in patients who are functionally anephric (hypothesized to be via inhibition of the intestinal Na/K ATPase),[40] there is some evidence that ACE inhibitors are beneficial in this patient population.[41–43] Further, both heart failure and its precursor, left ventricular hypertrophy, are common in dialysis patients, providing added rationale for use of these agents when safe and practical. Because dialysis patients have serum potassium levels measured routinely on a monthly basis, potentially untoward effects are relatively easy to detect and monitor.
Our findings should be interpreted in the context of several potentially important limitations. The use of the admission creatinine to estimate eGFR and therefore define the presence of CKD is imperfect, since an impaired eGFR at time of admission may be a result of the AMI itself rather than be reflective of baseline CKD. This could result in misclassification of chronic disease as acute. We may have underestimated the appropriateness of withholding these agents if, for example, patients had discomfiting rises in serum creatinine or potassium, untoward effects which a provider want to avoid in the post-AMI period. We also did not have information on hypotension, which would an appropriate reason to withhold RAS inhibitors. In addition, while the TRIUMPH study collected extensive patient-level data, unmeasured confounding may still be present. Finally,, little follow-up data was available to better define adverse renal consequences for using, or not using, RAS inhibitors at discharge, and so more work is required to examine post-discharge RAS inhibitor prescribing patterns.
Conclusions
We found, in a large, multi-center, US population of AMI patients, that use of RAS inhibitors decreased as eGFR declined. CKD appeared to play a larger role in RAS inhibitor prescriber decisions than did the presence of a reduced ejection fraction, and the combination of AKI and CKD was a particularly large barrier to the prescription of these drugs. However, given what is known about the benefits and risk of RAS inhibitors in both the post-AMI and CKD settings, it is possible that patients who would benefit from these drugs are not receiving them; more work is needed.
Acknowledgments
Funding/Support: Dr. Wetmore was supported by an NIH (NIDDK) grant (K23 DK085378). TRIUMPH was sponsored by a grant from the NIH (NHLBI): Washington University School of Medicine SCCOR Grant #P50HL077113-01.
Footnotes
Author contributions: Dr. Spertus had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Wetmore, Sharma, Spertus.
Acquisition, analysis, or interpretation of data: all authors.
Drafting of the manuscript: Wetmore, Sharma, Spertus.
Critical revision of the manuscript for important intellectual content: all authors.
Statistical analysis: Tang, Jones.
Obtained funding: Spertus.
Administrative, technical, or material support: Tang, Jones.
Study supervision: Wetmore, Spertus.
Disclosures: Dr. Wetmore has served on an advisory board for Alexion and receives research funding from Amgen. Dr. Spertus has research grants from Lilly, Gilead and Abbott Vascular. He is a consultant to United Healthcare, Novartis, Amgen and Regeneron. He holds the copyright to the Seattle Angina Questionnaire and has an equity stake in Health Outcomes Sciences.
Role of the Funder/Sponsor: The funding organizations did not play a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 2.Fox KM Investigators EUtOrocewPiscAd. Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double-blind, placebo-controlled, multicentre trial (the EUROPA study) Lancet. 2003;362:782–788. doi: 10.1016/s0140-6736(03)14286-9. [DOI] [PubMed] [Google Scholar]
- 3.Dagenais GR, Pogue J, Fox K, Simoons ML, Yusuf S. Angiotensin-converting-enzyme inhibitors in stable vascular disease without left ventricular systolic dysfunction or heart failure: a combined analysis of three trials. Lancet. 2006;368:581–588. doi: 10.1016/S0140-6736(06)69201-5. [DOI] [PubMed] [Google Scholar]
- 4.Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The SOLVD Investigators. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 5.Granger CB, McMurray JJ, Yusuf S, Held P, Michelson EL, Olofsson B, Ostergren J, Pfeffer MA, Swedberg K Investigators C Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet. 2003;362:772–776. doi: 10.1016/S0140-6736(03)14284-5. [DOI] [PubMed] [Google Scholar]
- 6.Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, Douglas PS, Foody JM, Gerber TC, Hinderliter AL, King SB, 3rd, Kligfield PD, Krumholz HM, Kwong RY, Lim MJ, Linderbaum JA, Mack MJ, Munger MA, Prager RL, Sabik JF, Shaw LJ, Sikkema JD, Smith CR, Jr, Smith SC, Jr, Spertus JA, Williams SV American College of Cardiology F, American Heart Association Task Force on Practice G, American College of P, American Association for Thoracic S, Preventive Cardiovascular Nurses A, Society for Cardiovascular A, Interventions, Society of Thoracic S. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60:e44–e164. doi: 10.1016/j.jacc.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 7.Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Jr, Chavey WE, 2nd, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Halperin JL, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura R, Ornato JP, Page RL, Riegel B American College of C, American Heart Association Task Force on Practice G, American College of Emergency P, Society for Cardiovascular A, Interventions, Society of Thoracic S, American Association of C, Pulmonary R, Society for Academic Emergency M. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-Elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol. 2007;50:e1–e157. doi: 10.1016/j.jacc.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 8.O’Gara PT, Kushner FG, Ascheim DD, Casey DE, Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis-Holland JE, Tommaso JE, Tracy CM, Woo YJ, Zhao DX, Force CAT. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:529–555. doi: 10.1161/CIR.0b013e3182742c84. [DOI] [PubMed] [Google Scholar]
- 9.Specification Manual for the Joint Commission International Library of Measures Version 1.0, effective for January 2011 discharges (1st Quarter 2011) 2011 http://www.jointcommissioninternational.org/assets/3/7/ILM-Index-Measure-Codes-Descriptions.pdf.
- 10.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329:1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 11.Maschio G, Alberti D, Janin G, Locatelli F, Mann JF, Motolese M, Ponticelli C, Ritz E, Zucchelli P. Effect of the angiotensin-converting-enzyme inhibitor benazepril on the progression of chronic renal insufficiency. The Angiotensin-Converting-Enzyme Inhibition in Progressive Renal Insufficiency Study Group. N Engl J Med. 1996;334:939–945. doi: 10.1056/NEJM199604113341502. [DOI] [PubMed] [Google Scholar]
- 12.Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia) Lancet. 1997;349:1857–1863. [PubMed] [Google Scholar]
- 13.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S, Investigators RS. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 14.Tessone A, Gottlieb S, Barbash IM, Garty M, Porath A, Tenenbaum A, Hod H, Boyko V, Mandelzweig L, Behar S, Leor J. Underuse of standard care and outcome of patients with acute myocardial infarction and chronic renal insufficiency. Cardiology. 2007;108:193–199. doi: 10.1159/000096777. [DOI] [PubMed] [Google Scholar]
- 15.Masoudi FA, Plomondon ME, Magid DJ, Sales A, Rumsfeld JS. Renal insufficiency and mortality from acute coronary syndromes. Am Heart J. 2004;147:623–629. doi: 10.1016/j.ahj.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 16.Mielniczuk LM, Pfeffer MA, Lewis EF, Blazing MA, de Lemos JA, Shui A, Mohanavelu S, Califf RM, Braunwald E. Estimated glomerular filtration rate, inflammation, and cardiovascular events after an acute coronary syndrome. Am Heart J. 2008;155:725–731. doi: 10.1016/j.ahj.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 17.Reddan DN, Szczech L, Bhapkar MV, Moliterno DJ, Califf RM, Ohman EM, Berger PB, Hochman JS, Van de Werf F, Harrington RA, Newby LK. Renal function, concomitant medication use and outcomes following acute coronary syndromes. Nephrol Dial Transplant. 2005;20:2105–2112. doi: 10.1093/ndt/gfh981. [DOI] [PubMed] [Google Scholar]
- 18.Amin AP, Spertus JA, Reid KJ, Lan X, Buchanan DM, Decker C, Masoudi FA. The prognostic importance of worsening renal function during an acute myocardial infarction on long-term mortality. Am Heart J. 2010;160:1065–1071. doi: 10.1016/j.ahj.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Fox CS, Muntner P, Chen AY, Alexander KP, Roe MT, Wiviott SD. Short-term outcomes of acute myocardial infarction in patients with acute kidney injury: a report from the national cardiovascular data registry. Circulation. 2012;125:497–504. doi: 10.1161/CIRCULATIONAHA.111.039909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fox CS, Muntner P, Chen AY, Alexander KP, Roe MT, Cannon CP, Saucedo JF, Kontos MC, Wiviott SD, Acute Coronary T Intervention Outcomes Network r. Use of evidence-based therapies in short-term outcomes of ST-segment elevation myocardial infarction and non-ST-segment elevation myocardial infarction in patients with chronic kidney disease: a report from the National Cardiovascular Data Acute Coronary Treatment and Intervention Outcomes Network registry. Circulation. 2010;121:357–365. doi: 10.1161/CIRCULATIONAHA.109.865352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arnold SV, Chan PS, Jones PG, Decker C, Buchanan DM, Krumholz HM, Ho PM, Spertus JA Cardiovascular Outcomes Research C. Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients’ Health Status (TRIUMPH): design and rationale of a prospective multicenter registry. Circ Cardiovasc Qual Outcomes. 2011;4:467–476. doi: 10.1161/CIRCOUTCOMES.110.960468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B, Lung T, Heart Rhythm S American College of C, American Heart Association Task Force on Practice G, American College of Chest P, International Society for H. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 23.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, Ckd EPI. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A Acute Kidney Injury N. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 26.Raghunathan TE, Solenberger PW, JVH . IVEware: Imputation and Variance Estimation Software -User Guide. Survey Research Center, Institute for Social Research, University of Michigan, Placed Published; 2002. [Google Scholar]
- 27.Peterson PN, Ambardekar AV, Jones PG, Krumholz HM, Schelbert E, Spertus JA, Rumsfeld JS, Masoudi FA. Increased mortality among survivors of myocardial infarction with kidney dysfunction: the contribution of gaps in the use of guideline-based therapies. BMC Cardiovasc Disord. 2009;9:29. doi: 10.1186/1471-2261-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ezekowitz J, McAlister FA, Humphries KH, Norris CM, Tonelli M, Ghali WA, Knudtson ML, Investigators A. The association among renal insufficiency, pharmacotherapy, and outcomes in 6,427 patients with heart failure and coronary artery disease. J Am Coll Cardiol. 2004;44:1587–1592. doi: 10.1016/j.jacc.2004.06.072. [DOI] [PubMed] [Google Scholar]
- 29.Berger AK, Duval S, Krumholz HM. Aspirin, beta-blocker, and angiotensin-converting enzyme inhibitor therapy in patients with end-stage renal disease and an acute myocardial infarction. J Am Coll Cardiol. 2003;42:201–208. doi: 10.1016/s0735-1097(03)00572-2. [DOI] [PubMed] [Google Scholar]
- 30.Amin AP, Spertus JA, Cohen DJ, Chhatriwalla A, Kennedy KF, Vilain K, Salisbury AC, Venkitachalam L, Lai SM, Mauri L, Normand SL, Rumsfeld JS, Messenger JC, Yeh RW. Use of drug-eluting stents as a function of predicted benefit: clinical and economic implications of current practice. Arch Intern Med. 2012;172:1145–1152. doi: 10.1001/archinternmed.2012.3093. [DOI] [PubMed] [Google Scholar]
- 31.Fogo AB. Mechanisms of progression of chronic kidney disease. Pediatr Nephrol. 2007;22:2011–2022. doi: 10.1007/s00467-007-0524-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winkelmayer WC, Charytan DM, Brookhart MA, Levin R, Solomon DH, Avorn J. Kidney function and use of recommended medications after myocardial infarction in elderly patients. Clin J Am Soc Nephrol. 2006;1:796–801. doi: 10.2215/CJN.00150106. [DOI] [PubMed] [Google Scholar]
- 33.Gibney EM, Casebeer AW, Schooley LM, Cunningham F, Grover FL, Bell MR, McDonald GO, Shroyer AL, Parikh CR. Cardiovascular medication use after coronary bypass surgery in patients with renal dysfunction: a national Veterans Administration study. Kidney Int. 2005;68:826–832. doi: 10.1111/j.1523-1755.2005.00463.x. [DOI] [PubMed] [Google Scholar]
- 34.Bansal N, Hsu CY, Chandra M, Iribarren C, Fortmann SP, Hlatky MA, Go AS. Potential role of differential medication use in explaining excess risk of cardiovascular events and death associated with chronic kidney disease: a cohort study. BMC Nephrol. 2011;12:44. doi: 10.1186/1471-2369-12-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Remuzzi G, Ruggenenti P, Perna A, Dimitrov BD, de Zeeuw D, Hille DA, Shahinfar S, Carides GW, Brenner BM, Group RS. Continuum of renoprotection with losartan at all stages of type 2 diabetic nephropathy: a post hoc analysis of the RENAAL trial results. J Am Soc Nephrol. 2004;15:3117–3125. doi: 10.1097/01.ASN.0000146423.71226.0C. [DOI] [PubMed] [Google Scholar]
- 36.Damman K, Tang WH, Felker GM, Lassus J, Zannad F, Krum H, McMurray JJ. Current evidence on treatment of patients with chronic systolic heart failure and renal insufficiency: practical considerations from published data. J Am Coll Cardiol. 2014;63:853–871. doi: 10.1016/j.jacc.2013.11.031. [DOI] [PubMed] [Google Scholar]
- 37.Holtkamp FA, de Zeeuw D, Thomas MC, Cooper ME, de Graeff PA, Hillege HJ, Parving HH, Brenner BM, Shahinfar S, Lambers Heerspink HJ. An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long-term renal function. Kidney Int. 2011;80:282–287. doi: 10.1038/ki.2011.79. [DOI] [PubMed] [Google Scholar]
- 38.Miao Y, Dobre D, Heerspink HJ, Brenner BM, Cooper ME, Parving HH, Shahinfar S, Grobbee D, de Zeeuw D. Increased serum potassium affects renal outcomes: a post hoc analysis of the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) trial. Diabetologia. 2011;54:44–50. doi: 10.1007/s00125-010-1922-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bakris GL, Weir MR. Angiotensin-converting enzyme inhibitor-associated elevations in serum creatinine: is this a cause for concern? Arch Intern Med. 2000;160:685–693. doi: 10.1001/archinte.160.5.685. [DOI] [PubMed] [Google Scholar]
- 40.Hatch M, Freel RW, Vaziri ND. Local upregulation of colonic angiotensin II receptors enhances potassium excretion in chronic renal failure. Am J Physiol. 1998;274:F275–282. doi: 10.1152/ajprenal.1998.274.2.F275. [DOI] [PubMed] [Google Scholar]
- 41.Efrati S, Zaidenstein R, Dishy V, Beberashvili I, Sharist M, Averbukh Z, Golik A, Weissgarten J. ACE inhibitors and survival of hemodialysis patients. Am J Kidney Dis. 2002;40:1023–1029. doi: 10.1053/ajkd.2002.36340. [DOI] [PubMed] [Google Scholar]
- 42.Anand IS, Bishu K, Rector TS, Ishani A, Kuskowski MA, Cohn JN. Proteinuria, chronic kidney disease, and the effect of an angiotensin receptor blocker in addition to an angiotensin-converting enzyme inhibitor in patients with moderate to severe heart failure. Circulation. 2009;120:1577–1584. doi: 10.1161/CIRCULATIONAHA.109.853648. [DOI] [PubMed] [Google Scholar]
- 43.Cice G, Di Benedetto A, D’Isa S, D’Andrea A, Marcelli D, Gatti E, Calabro R. Effects of telmisartan added to Angiotensin-converting enzyme inhibitors on mortality and morbidity in hemodialysis patients with chronic heart failure a double-blind, placebo-controlled trial. J Am Coll Cardiol. 2010;56:1701–1708. doi: 10.1016/j.jacc.2010.03.105. [DOI] [PubMed] [Google Scholar]