Abstract
Background
Vedolizumab (VDZ) demonstrated efficacy in Crohn's disease (CD) and ulcerative colitis (UC) in the GEMINI trials. Our aim was to evaluate the efficacy of VDZ at week 14 in inflammatory bowel disease (IBD) in a multicenter cohort of patients.
Methods
Patients at Massachusetts General Hospital and Brigham and Women's Hospital were considered for inclusion. VDZ (300mg) was administered at weeks 0, 2, 6 and 14. Efficacy was assessed using the Harvey Bradshaw index (HBI) for CD, the simple clinical colitis activity index (SCCAI) for UC and physician assessment, along with C-reactive protein (CRP) and decrease of corticosteroid therapy. Clinical response was defined as decrease in HBI ≥ 3 and SCCAI ≥ 3 and remission as HBI ≤ 4, SCCAI ≤ 2 and physician assessment of response and remission.
Results
Our study included 172 patients (107 CD, 59 UC, 6 IBD-U, male 48.3%, mean age 40 years and disease duration 14 years). Fourteen patients had an ostomy and 9 an ileoanal pouch and only 35.5% fulfilled eligibility for the GEMINI trials. Previous treatment failures with ≥ 2 anti-TNFs occurred in 70.9%, one-third were on an immunomodulator and 46% systemic steroids at baseline. In CD, 48.9% and 23.9% and in UC, 53.9% and 29.3% had clinical response and clinical remission at week 14. Adverse events occurred in 10.5%.
Conclusions
VDZ is safe and well tolerated in refractory IBD patients in a clinical practice with efficacy in UC and CD with responses similar to what was seen in clinical trials.
Keywords: Vedolizumab, Crohn's disease, ulcerative colitis
Introduction
The introduction of the biologic monoclonal antibodies to tumour necrosis factor α (anti-TNF) has transformed the management of patients with inflammatory bowel disease (IBD) over the past two decades. Despite their efficacy, many patients do not respond to anti-TNF therapy with approximately 30% of Crohn's disease (CD) patients and 35% of ulcerative colitis (UC) patients having a primary non-response.1 Of those who initially respond, a significant number lose response over time.1,2 Patients losing response to their first anti-TNF have lower rates of response to second or third anti-TNF therapies.3 In addition, anti-TNF therapy is associated with significant side effects including serious infections and malignancies.4,5
The integrin inhibitors, a newer class of therapy, which block leukocyte trafficking to the gut mucosa present an attractive option for IBD patients. Natalizumab, an α4β7 and α4β1 integrin antibody, was the first integrin inhibitor to demonstrate efficacy in CD, but concerns regarding reactivation of JC virus and development of progressive multifocal leukoencephalopathy (PML) have limited its use.6 Vedolizumab (VDZ), a gut selective α4β7 integrin antibody, without any cases of PML to date, was assessed in the phase 3 GEMINI studies and demonstrated efficacy in inducing and maintaining remission in both CD and UC.
Patients enrolled in clinical trials are not entirely representative of those encountered in the clinical practice setting. This was demonstrated in a retrospective study, in which only 31% of 206 patients with moderate to severe IBD would have been eligible to participate in the selected trials.7 Efficacy of VDZ in a clinical practice setting that includes patients who would have been excluded from clinical trials, such as those with an ostomy, Crohn's disease affecting an ileoanal pouch, or in the context of multiple prior therapy failures is unknown. Thus, establishing the efficacy and durability of VDZ in the context of real-world clinical practice is essential to appropriately position it in the treatment algorithms for CD and UC.
Our aim was to evaluate the efficacy of VDZ for induction of clinical response and remission at week 14 in patients with IBD and to assess for predictors of response to therapy. Specifically, we examined the effect of VDZ on clinical disease activity and resolution of inflammatory markers in a large multicenter cohort of patients with IBD.
Materials and Methods
This study included patients from two major academic hospitals in Boston: Massachusetts General Hospital (MGH) and Brigham and Women's Hospital (BWH). VDZ was administered intravenously at weeks 0, 2, 6 and 14 at a dose of 300mg.
Inclusion
All patients commencing VDZ for CD and UC at both institutions were considered for inclusion in this study. Patients who had at least completed the 3 infusion loading doses at the time of analysis (weeks 0, 2 and 6) were included.
Exclusion
Patients without clinically active disease at baseline as per clinical assessment or Harvey Bradshaw Index (HBI) ≤ 4 (CD) or simple clinical colitis activity index (SCCAI) ≤2 (UC) were excluded from the efficacy analysis.
MGH Protocol
Patients receiving VDZ were approached for inclusion and prospectively evaluated at weeks 0, 2, 6 and 14. Data collected at each visit included the Harvey Bradshaw Index (HBI) for CD8 the simple clinical colitis activity index (SCCAI) for UC9, along with serum C-reactive protein (CRP) and corticosteroid dose. Patients with clinically active CD at baseline (per clinical assessment and HBI score > 4) and patients with clinically active UC (per clinical assessment and SCCAI score > 2) were assessed for clinical efficacy. Patients with a pouch were assessed using the modified pouchitis disease activity index (mPDAI)10 with response defined as mPDAI reduction ≥ 2 and remission at mPDAI ≤ 4 and by physician assessment. Patients with a stoma were assessed by stoma emptying count, HBI and physician assessment.
BWH Protocol
All patients receiving VDZ were retrospectively assessed at weeks 0, 2, 6, and 14 by chart review. Additionally, the treating physician at BWH was asked to assess the patient's clinical response to VDZ at week 14 as either unchanged, clinical response or clinical remission. Laboratory tests including CRP were routinely collected and corticosteroid dose recorded.
Variables, outcomes and definitions
A review of the electronic medical record was performed for all patients in addition to patient interview to confirm the diagnosis of IBD, the use and dose of concomitant immunosuppression as well as demographic and disease related variables including age, gender, type of IBD, smoking history, surgical history, disease distribution and duration, and prior biologic therapies. Pertinent laboratory values were also collected.
The primary outcome was achievement of either clinical response or clinical remission at week 14. Clinical response was defined as either a decrease in HBI ≥3 and SCCAI ≥ 3 from baseline (MGH) or as physician assessment of clinical response (BWH). Clinical remission was defined as HBI ≤ 4 and SCCAI ≤ 2 (MGH) or as physician assessment of clinical remission (BWH).
The secondary outcomes included clinical response at week 6, normalisation in CRP at week 14, CRP values at weeks 2, 6 and 14 and prednisone dose reduction at week 14 compared to baseline. Patients with indeterminate colitis (IBD-U) were analysed with the UC population. The cut-off for normal CRP for our laboratory was ≤ 8.0mg/L. The week 14 data for both hospitals was combined to give an overall assessment of clinical response and remission.
Statistical analysis
All statistical analysis was carried out using Stata 13.1 (StataCorp, College Station, TX). Continuous variables were summarized using means and standard deviations and compared using the student t-test while categorical variables were expressed as proportions and compared using the chi-square test with Fisher's exact correction where appropriate. Univariate analysis was performed to identify predictors of response or remission at week 14. Multivariate analysis was then performed using a logistic regression model. Variables felt a-priori to be related to response or remission, were entered into the model. These included: disease type, smoking status, sex, perianal disease, immunomodulator or corticosteroid use at induction, number of prior anti-TNFs and CRP at induction. The study was approved by the Institutional Review Board of Partners Healthcare
Results
Between June 2014 and April 2015, 193 patients treated with VDZ had received at least 3 infusions. A total of 21 patients refused consent and were excluded from the analysis. 172 patients between MGH (86) and BWH (86) were analysed (107 CD, 59 UC, 6 IBD-U). Three were excluded from week 6 and 14 analysis due to inactive disease at baseline. The cohort was 48.3% male with a mean age of 40.0 years (SD ± 13.8) and the mean disease duration was 14.0 years (SD ± 9.9). There were 14 patients with an ostomy and 9 with an ileoanal pouch. Only 5 patients (2.9%) were anti-TNF naïve. Previous treatment failures included at least 2 anti-TNFs in 70.9%. Just over half (58.9%) of CD patients had previously undergone surgery. At baseline 32.1% of the patients on VDZ were on concomitant therapy with an immunomodulator (51.0% thiopurines, 49.0% methotrexate) and 46.0% were on systemic steroids. Amongst the 172 patients in our study, only 61 (35.5%,) would have been eligible to participate in the GEMINI trials. Other baseline patient characteristics for Crohn's disease and ulcerative colitis are summarized in Table 1.
Table 1. Baseline characteristics of Crohn's disease and ulcerative colitis patients initiating vedolizumab therapy.
| Crohn's disease | Total population (n=107) | MGH (n=46) | BWH (n=61) |
|---|---|---|---|
| Age – yrs | 39.7 (14.0) | 42.5 ± 14.5 | 37.6 ± 13.3 |
| Male sex - no (%) | 51 (47.7) | 25 (54.4) | 26 (42.6) |
| Duration of disease – yrs | 16.4 ± 10.6 | 16.0 ± 10.4 | 16.8 ± 10.8 |
| Current smoker - no. (%) | 13 (12.2) | 7 (15.2) | 6 (9.8) |
| Mean HBI score ‡ | 8.0 ± 6.9 | ||
| Mean C-reactive protein -mg/litre | 20.4 ± 28.4 | 17.3 ± 19.5 | 21.9 ± 33.2 |
| Disease location - no. (%) | |||
| Small bowel disease | 14 (13.1) | 9 (19.6) | 5 (8.2) |
| Colonic disease | 25 (23.4) | 11 (23.9) | 14 (23.0) |
| Small bowel and colonic disease | 68 (63.5) | 26 (56.5) | 42 (68.9) |
| Prior biologic therapy | |||
| Number of prior anti-TNFs | 2.2 ± 0.9 | 2.3 ± 0.8 | 2.1 ± 0.9 |
| Number of prior biologics | 2.6 ± 1.2 | 2.6 ± 1.1 | 2.6 ± 1.3 |
| ≥ 2 prior anti-TNF agents - no. (%) | 82 (76.7) | 37 (80.4) | 45 (73.8) |
| Prior surgery for Crohn's disease - no. (%) | 63 (58.8) | 29 (63.0) | 34 (55.7) |
| Stoma | 13 (12.1) | 6 (13.0) | 7 (11.5) |
| Ileoanal pouch | 5 (4.7) | 3 (6.5) | 2 (3.3) |
| Draining fistulae at baseline - no. (%) ‡ | 7 (15.2) | ||
| Corticosteroid at induction - no. (%) | 39 (39.4) | 22 (51.1) | 17 (30.4) |
| Prednisone equivalent dose at induction - mg | 11.3 ± 15.4 | 14.9 ± 16.0 | 8.5 (14.4) |
| Concomitant immunosuppressants | 34 (31.7) | 15 (35) | 19 (31.1) |
| Thiopurines | 16 (15.0) | 8 (18.2) | 8 (13.1) |
| Methotrexate | 18 (17.3) | 7 (15.9) | 11 (18.0) |
| Ulcerative colitis | Total population (n=65) | MGH (n=40) | BWH (n=25) |
| Age - yrs | 40.5 ± 13.7 | 40.7 ± 14.3 | 40.3 ± 12.9 |
| Male sex - no (%) | 32 (49.2) | 22 (55.0) | 10 (40.0) |
| Duration of disease - yrs | 10.1 ± 7.0 | 9.1 ± 7.1 | 11.6 ± 6.7 |
| Current smoker - no. (%) | 3 (4.6) | 1 (2.5) | 2 (8.0) |
| Mean SCCAI score ‡ | 6.5 ± 2.7 | ||
| Mean C-reactive protein -mg/litre | 9.8 ± 14.9 | 8.8 ± 12.8 | 11.4 ± 17.9 |
| Disease location - no. (%) | |||
| Rectum and sigmoid only | 8 (12.5) | 6 (15.4) | 2 (8.0) |
| Left colon | 21 (32.8) | 13 (33.3) | 8 (32.0) |
| Pancolitis | 35 (54.6) | 20 (51.3) | 15 (60.0) |
| Number of prior anti-TNFs | 1.9 ± 0.9 | 2.1 ± 0.9 | 1.7 (0.8) |
| ≥ 2 prior anti-TNF agents - no. (%) | 40 (61.5) | 27 (67.5) | 13 (52.0) |
| Corticosteroid at induction - no. (%) | 37 (57.8) | 27 (67.5) | 10 (41.7) |
| Prednisone equivalent dose at induction - mg | 15.9 ± 17.5 | 17.2 ± 16.2 | 13.8 ± 19.1 |
| Concomitant immunosuppressants - no. (%) | 17 (26.6) | 10 (25.6) | 7 (28.0) |
| Thiopurines | 11 (17.2) | 6 (15.4) | 5 (20.0) |
| Methotrexate | 6 (9.4) | 4 (10.3) | 2 (8.0) |
Plus–minus values are means ±SD. TNF denotes tumor necrosis factor.
HBI score, SCCAI score and draining fistulae at baseline available for MGH patients only.
Patients with a pouch (n=3) and stoma (n=6) were excluded.
Efficacy
Crohn's disease
Week 6 data was available in 42 patients (MGH only) of whom 25 (59.5%) achieved clinical response and 15 (35.7%) achieved clinical remission. Of the 88 patients (82.2%) with data available to week 14, 43 (48.9%) had a clinical response and 21 (23.9%) achieved clinical remission (Figure 1). Eighty-five patients had corticosteroid information available at week 14. Thirty-two of these patients were also on steroids at induction, of whom 25 (78.1%) were able to decrease the steroid dose by ≥ 50% and 14 (43.8%) were off steroids at week 14 (Table 2a). Sixteen patients (18.8%) were in steroid free clinical remission at week 14.
Figure 1. Crohn's disease.
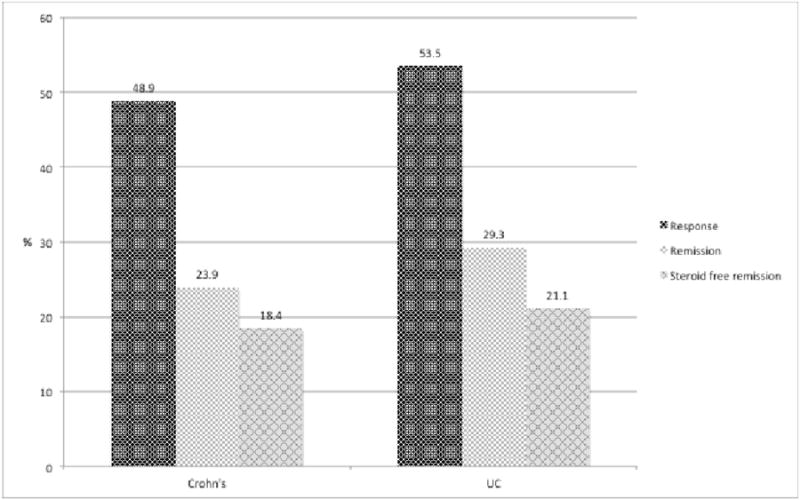
Week 6 data was available in 42 patients (MGH only) of whom 25 (59.5%) achieved clinical response and 15 (35.7%) achieved clinical remission. Of the 88 patients (82.2%) with data available to week 14, 43 (48.9%) had a clinical response and 21 (23.9%) achieved clinical remission.
Table 2. Outcome measures at week 6 and week 14 for vedolizumab as induction therapy for Crohn's disease and ulcerative colitis.
| Crohn's disease | Week 6¥ | Week 14 | ||
|---|---|---|---|---|
| Outcome | (n =42) | MGH (n=31) | BWH (n=57) | Total population (n=88) |
| Clinical response ‡ - no (%) | 25 (59.5) | 16 (51.6) | 27 (47.4) | 43 (48.9) |
| Clinical remission - no (%) | 15 (35.7) | 7 (22.6) | 14 (24.6) | 21 (23.9) |
| Corticosteroid free remission – no (%) | 16 (18.4) | |||
| Ulcerative colitis | Week 6¥ | Week 14 | ||
|---|---|---|---|---|
| Outcome | (n =40) | MGH (n=35) | BWH (n=23) | Total population (n=58) |
| Clinical response ‡ - no (%) | 18 (45.0) | 18 (51.4) | 13 (56.5) | 31 (53.5) |
| Clinical remission - no (%) | 6 (15.0) | 11 (31.4) | 6 (26.1) | 17 (29.3) |
| Corticosteroid free remission – no (%) | 12 (21.1) | |||
Week 6 information available for MGH patients only
Ulcerative colitis
Week 6 data was available in 40 patients (MGH only) of whom 18 (45.0%) achieved clinical response and 6 (15.0%) achieved clinical remission. Of the 58 patients (89.2%) with data available to week 14, 31 (53.5%) had a clinical response and 17 (29.3%) achieved clinical remission (Figure 1). Fifty-two patients had corticosteroid information available at week 14. Thirty-one of these patients were also on steroids at induction, of whom 22 (71.0%) were able to decrease the steroid dose by ≥ 50% and 15 (48.4%) were off steroids at week 14 (Table 2b). Twelve patients (23.1%) in steroid free clinical remission at week 14.
Predictors of response/remission at week 14
On univariate analysis, CRP at baseline was predictive of response/remission in both CD (p=0.04) and UC (p=0.05) with patients with an elevated CRP at baseline (>8.0mg/L) being less likely to respond. None of the other baseline characteristics including age, sex, disease duration or extent, concomitant immunomodulator or corticosteroid use at induction, prior surgery or prior anti-TNF use were found to be associated with response/remission. However, early response at week 6 was a statistically significant predictor of week 14 response/remission in patients with UC with a trend towards significance in CD. Only 12% of UC patients who were non-responders at week 6 achieved clinical response/remission at week 14 compared to 74% of early responders (p < 0.0001). In comparison, in CD, 42% of week 6 non-responders and 77% of week 6 responders achieved clinical response/remission at week 14 (p=0.12). Logistic regression was performed using variables felt a-priori to be related to response or remission (Table 3). The only variable predictive of week 14 response/remission was baseline CRP (OR = 0.33, 95% CI 0.15-0.95).
Table 3. Multivariate Analysis of predictors of response/remission at Week 14¥.
| Variable | OR | 95% CI` | p-value |
|---|---|---|---|
| Male | 0.74 | 0.25-2.12 | .57 |
| Active Smoker | 0.38 | 0.10-1.51 | .17 |
| Crohn's Disease | 0.69 | 0.16-2.89 | .61 |
| Perianal Disease | 0.32 | 0.08-1.26 | .10 |
| Number of prior anti-TNFs | 0.59 | 0.31-1.12 | .10 |
| Prednisone at induction | 0.34 | 0.10-1.18 | .08 |
| IMM at Induction | 0.56 | 0.19-1.66 | .30 |
| CRP > 8.0mg/L at Induction | 0.33 | 0.11-0.96 | .04 |
Week 14 response and remission was combined as a composite end point to classify patients as either week 14 responders or non-responders. IMM-immunomodulator.
Ostomy and pouch patients
There were 14 patients with a stoma (9 with an end ileostomy, 4 with a diverting ileostomy and 1 colostomy) enrolled. Twelve stoma patients had results to week 14, 5 (41.7%) of whom had a response and 2 (16.7%) who were in remission. There were 9 patients with an ileoanal pouch (4 refractory pouchitis and 5 CD of the pouch) enrolled. Eight ileoanal pouch patients had results to week 14, 6 (75.0%) of whom had a response and 1 (12.5%) who was in remission.
C-reactive protein
The CD patients had a non-significant improvement in mean CRP at week 14 compared with baseline (19.7 vs. 14.4 mg/L (p = 0.09) (Figure 2). Among the 52 patients with an elevated CRP at baseline and CRP information available at week 14, 25/52 (48.1%) normalised their CRP at week 14. Within the UC cohort there was also a non-significant, yet less marked improvement in mean CRP at week 14 compared to baseline (10.8 vs. 7.5, p=0.29) (Figure 2). Among the 21 patients with an elevated CRP at baseline, 10/21 (47.6%) normalised their CRP at week 14.
Figure 2. C-reactive protein.
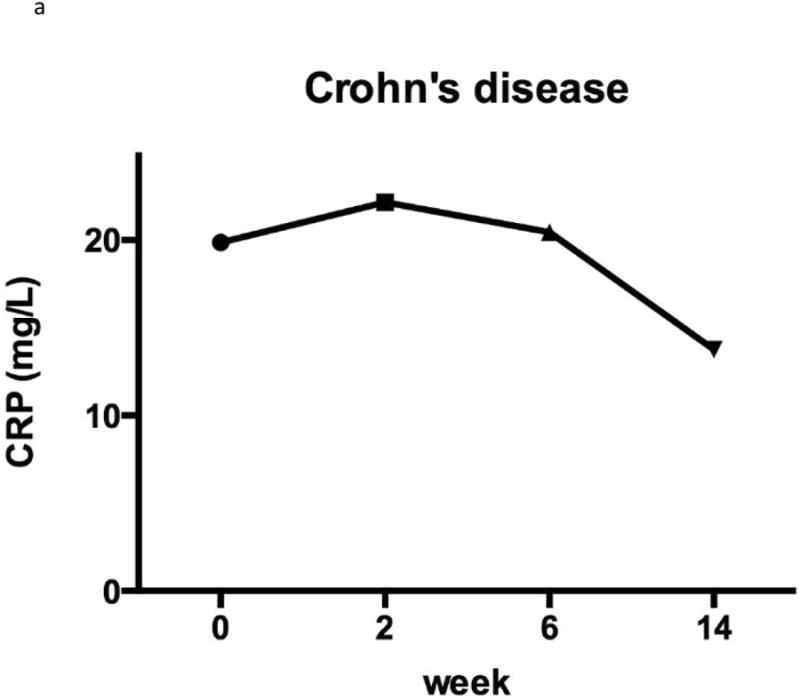
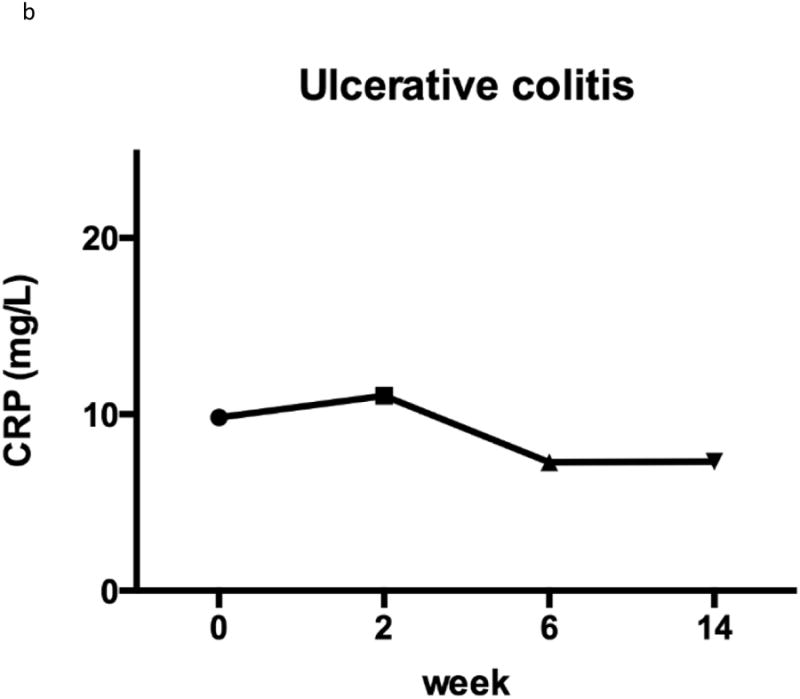
The CD patients had a non-significant improvement in mean CRP at week 14 compared with baseline (19.7 vs. 14.4 mg/L (p = 0.09) (Figure 2). Among the 52 patients with an elevated CRP at baseline and CRP information available at week 14, 25/52 (48.1%) normalised their CRP at week 14. Within the UC cohort there was also a non-significant, yet less marked improvement in mean CRP at week 14 compared to baseline (10.8 vs. 7.5, p=0.29) (Figure 2). Among the 21 patients with an elevated CRP at baseline, 10/21 (47.6%) normalised their CRP at week 14.
Safety
VDZ was generally well tolerated. Adverse events occurred in 18 patients (10.5%). These included hospitalisation in 4 patients (2 for intravenous steroids, 1 for nausea and abdominal pain, 1 for refractory disease requiring colectomy); 1 case of an infusion reaction at week 6 necessitating VDZ cessation, 8 patients with extraintestinal manifestations (4 with new arthralgia, 1 exacerbation of pre-existing enteropathic arthritis, 1 new peristomal pyoderma gangrenosum, and 2 with a butterfly rash); 3 had progressive perianal disease (1 developed complex fistulising disease and 2 developed a new perianal abscess); 1 infectious complication (salmonella gastroenteritis) and 1 retinal vein occlusion. No systemic infections or sepsis occurred.
Discussion
In this study of 172 refractory IBD patients treated with VDZ from two large academic referral clinics including 97% with prior anti-TNF failures, 14 with an ostomy and 9 with an ileoanal pouch, VDZ was effective and safe. Week 14 response and remission rates in CD were 48.9% and 23.9% respectively and in UC, 53.5% and 29.5% respectively.
In our study, week 14 was chosen as the time to assess VDZ efficacy, which is different from the phase 3 GEMINI trials where the primary end point was week 6 response.11,12 In the GEMINI-2 trial there was a lack of significant response in CD patients at week 6 versus placebo (31.4% versus 25.7% (p=0.23)) compared with the significant week 6 response in UC patients in the GEMINI-1 trial (47.1% versus 25.5% (p<0.001)). Additionally, week 6 non-responders in GEMINI-2, who continued VDZ had incremental increases in clinical remission and CDAI 100 response rates at weeks 10 and 14.13 Furthermore, in the GEMINI-3 study, among CD patients who had previously failed anti-TNF therapy, VDZ was superior to placebo for inducing clinical remission at week 10 but not 6.14 This has led to the belief that response takes longer in CD with the suggestion of an incremental benefit with ongoing VDZ therapy. One theory proposed to explain this delayed response in CD is that it is a transmural disease extending beyond the mucosa, whereas UC is confined to the superficial mucosal layers, which is the target site of VDZ, an α4β7 specific drug.15 As such, we decided upon week 14 to assess induction of response and remission to account for this delayed response in CD. Correspondingly our week 14 response and remission rates were higher than those seen in the GEMINI trials at week 6.
In the past two decades, treatment goals have become more ambitious driving towards mucosal healing. Despite the clear efficacy of anti-TNF therapy in moderate to severe IBD, a significant proportion of patients fail to respond to anti-TNF therapy or lose response over time. The GAIN trial demonstrated the benefit of a second anti-TNF, adalimumab, in infliximab failures and a retrospective study from MGH demonstrated the benefit of a third anti-TNF after two prior anti-TNF drug failures.3,16 However, it is a game of diminishing returns with decreased response with each subsequent anti-TNF. The introduction of VDZ with a novel mechanism of action, through gut selectivity, and favourable safety profile therefore presents a welcome addition to the treatment paradigm.
Our study represents the largest cohort of patients outside of the registry trials and hence can provide real world context to the use of VDZ in clinical practice. It also included a high percentage of patients (65.5%), who would have been excluded from the GEMINI studies. The high rates of response and remission seen in our treatment refractory population (average disease duration 14.0 years, 2 prior anti-TNFs in ≥ 70.9%) reinforces the benefit of VDZ for patients in whom prior anti-TNFs have failed. Reassuringly, despite the delayed onset of response of VDZ and the high number of patients on corticosteroids at baseline (46.0%), 71.8% were off steroids at week 14. We are also the first to demonstrate efficacy in treating patients with a pouch or stoma.
In contrast to the GEMINI trials in which UC patients had higher rates of remission, in our cohort there was no significant difference in the week 14 response/remission rates between CD and UC, which may be due to smaller numbers or the more uniformly refractory population. Baseline CRP elevation was found to be negatively associated with week 14 response/remission, which may relate to elevated CRP representing greater disease burden and more difficulty achieving early response, however the numbers were too small to make definitive conclusions. Week 6 response did predict week 14 response in UC and there was a trend toward a significant association in CD. There is increasing data, particularly in anti-TNF treated patients, to suggest that this early response either clinical or endoscopic predicts long-term outcomes. 17-21
Despite one-third of patients being on combination immunomodulator therapy, this study did not demonstrate a larger benefit for those on combination therapy, though our sample size may be too small to detect a difference. Similarly, 46.0% of patients were on systemic corticosteroids at induction and no increased benefit was found in this cohort either. VDZ is associated with a low rate of antibody formation (3.7-4.1% of patients in the GEMINI studies) and the presence of these antibodies is thought to be associated with decreased drug efficacy. 11,12,22 However, in a post hoc analysis of the GEMINI studies there was also no difference in efficacy demonstrated between VDZ monotherapy and combination therapy with corticosteroids and/or immunomodulators, although the small numbers make interpretation difficult. 23,24 In our study, antibody levels were not measured.
Whether interval shortening will be an effective technique in attaining or regaining response to VDZ remains unknown. Both treatment schedules in the GEMINI trials, 300mg every 4 or 8 weeks, fully saturated the target receptors in almost all patients, however, there was an association between higher VDZ concentrations and greater clinical efficacy, suggesting that the VDZ concentrations required for efficacy may exceed the concentrations required to achieve saturation of target receptors in peripheral blood.11,12,25
In our study, 10.5% of patients developed an adverse event. Five of these were worsening arthropathy and 1 a new pyoderma gangrenosum. There is no data to suggest VDZ has efficacy in extraintestinal manifestations, likely due to its gut selectivity. This raises the likelihood that some patients will require immunomodulator therapy for extraintestinal manifestations. Safety data in the GEMINI trials demonstrated an overall adverse event rate similar to placebo.26 A Cochrane review of VDZ in UC found no significant difference in safety between VDZ and placebo.27 Furthermore, the side effect profile is maintained in more elderly patients.28 The long term VDZ extension study (GEMINI-LTS) demonstrated similar adverse events between CD and UC but a higher incidence of new perianal abscess formation among patients with CD treated with VDZ (2%).29 We appreciated three cases of progressive perianal disease in our patients, including 2 cases with a new perianal abscess.
There are several limitations to our study, the most significant of which is that it is open label and some of the data was collected retrospectively. The patients were from two institutions and as such there was heterogeneity in the collection of data and the assessment of clinical response and remission. Despite this, there was consistency in clinical outcomes across both institutions in CD and UC. Furthermore, the collection of CRP data was prospective at both institutions. MGH and BWH are tertiary referral centers and this patient population is complex with the majority having multiple prior anti-TNF failures and long disease duration, so the applicability of this study to the broader patient population needs to be with caution.
VDZ may have an advantage to be used prior to an anti-TNF therapy in some patients but selection of those patients has not been clarified. Larger cohorts will be needed to identify safety signals and predictors of response, particularly if baseline characteristics including serologic markers, genetic parameters or fecal studies can predict response to anti-integrin versus anti-TNF therapy to allow more directed therapy. This study did not address the long-term durability of VDZ and whether, similar to anti-TNF therapy, there is a loss of response over time. Whether concomitant therapy with immune suppression may mitigate a possible loss of response is uncertain.
In a refractory IBD population VDZ is a safe, well-tolerated medication with efficacy in both UC and CD in a clinical setting, outside of a randomized controlled trial.
Abbreviations
- anti-TNF
antibodies to tumour necrosis factor α
- IBD
inflammatory bowel disease
- CD
Crohn's disease
- UC
ulcerative colitis
- PML
progressive multifocal leukoencephalopathy
- VDZ
vedolizumab
- MGH
Massachusetts General Hospital
- BWH
Brigham and Women's Hospital
- HBI
Harvey Bradshaw Index
- SCCAI
simple clinical colitis activity index
- CRP
C-reactive protein
- mPDAI
modified pouch disease activity index
- IBD-U
indeterminate colitis
Footnotes
Conflicts of interest: Ananthakrishnan has served on scientific advisory boards for Abbvie and Cubist. Yajnik has been a consultant for Janssen, UCB, Takeda and Abbvie. Sauk is employed by Seres Therapeutics. Khalili is supported by a career development award from the American Gastroenterological Association (AGA) and by National Institute of Diabetes and Digestive and Kidney Diseases (K23 DK099681). Korzenik is supported by funding from Takeda. None of the other authors have conflicts of interest to declare
References
- 1.Allez M, Karmiris K, Louis E, et al. Report of the ECCO pathogenesis workshop on anti-TNF therapy failures in inflammatory bowel diseases: Definitions, frequency and pharmacological aspects. Journal of Crohn's and Colitis. 2010;4:355–366. doi: 10.1016/j.crohns.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Allen P, Gilroy L. Is there a role for vedolizumab in the treatment of ulcerative colitis and Crohn's disease? CEG. 2014;163 doi: 10.2147/CEG.S45261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sandborn WJ, Rutgeerts P, Enns R, et al. Adalimumab induction therapy for Crohn disease previously treated with infliximab: a randomized trial. Ann Intern Med. 2007;146:829–838. doi: 10.7326/0003-4819-146-12-200706190-00159. [DOI] [PubMed] [Google Scholar]
- 4.Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious Infection and Mortality in Patients With Crohn's Disease: More Than 5 Years of Follow-Up in the TREAT™ Registry. AJG. 2012;107:1409–1422. doi: 10.1038/ajg.2012.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bongartz T, Sutton AJ, Sweeting MJ, et al. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295:2275–2285. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- 6.Clifford DB. Natalizumab and PML: a risky business? Gut. 2008;57:1347–1349. doi: 10.1136/gut.2008.155770. [DOI] [PubMed] [Google Scholar]
- 7.Ha C, Ullman TA, Siegel CA, et al. Patients Enrolled in Randomized Controlled Trials Do Not Represent the Inflammatory Bowel Disease Patient Population. CGH. 2012;10:1002–1007. doi: 10.1016/j.cgh.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Harvey RF, Bradshaw JM. A simple index of Crohn's-disease activity. The Lancet. 1980;1:514. doi: 10.1016/s0140-6736(80)92767-1. [DOI] [PubMed] [Google Scholar]
- 9.Walmsley RS, Ayres RC, Pounder RE, et al. A simple clinical colitis activity index. Gut. 1998;43:29–32. doi: 10.1136/gut.43.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen B, Achkar JP, Connor JT, et al. Modified pouchitis disease activity index: a simplified approach to the diagnosis of pouchitis. Diseases of the Colon & Rectum. 2003;46:748–753. doi: 10.1007/s10350-004-6652-8. [DOI] [PubMed] [Google Scholar]
- 11.Sandborn WJ, Feagan BG, Rutgeerts P, et al. Vedolizumab as Induction and Maintenance Therapy for Crohn's Disease. NEJM. 2013;369:711–721. doi: 10.1056/NEJMoa1215739. [DOI] [PubMed] [Google Scholar]
- 12.Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as Induction and Maintenance Therapy for Ulcerative Colitis. NEJM. 2013;369:699–710. doi: 10.1056/NEJMoa1215734. [DOI] [PubMed] [Google Scholar]
- 13.Sandborn WJ, Feagan B, Reinisch W, et al. P497 Efficacy of continued vedolizumab therapy in patients with Crohn's disease who did not respond to vedolizumab induction therapy at week 6. Journal of Crohn's and Colitis. 2014;8:S274–S275. [Google Scholar]
- 14.Sands BE, Feagan BG, Rutgeerts P, et al. Effects of Vedolizumab Induction Therapy for Patients With Crohn's Disease in Whom Tumor Necrosis Factor Antagonist Treatment Failed. Gastroenterology. 2014;147:618–627. doi: 10.1053/j.gastro.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 15.Cominelli F. Inhibition of Leukocyte Trafficking in Inflammatory Bowel Disease. NEJM. 2013;369:775–776. doi: 10.1056/NEJMe1307415. [DOI] [PubMed] [Google Scholar]
- 16.de Silva PSA, Nguyen DD, Sauk J, et al. Long-term outcome of a third anti-TNF monoclonal antibody after the failure of two prior anti-TNFs in inflammatory bowel disease. APT. 2012;36:459–466. doi: 10.1111/j.1365-2036.2012.05214.x. [DOI] [PubMed] [Google Scholar]
- 17.Colombel JF, Rutgeerts P, Reinisch W, et al. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology. 2011;141:1194–1201. doi: 10.1053/j.gastro.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Bosch O, Gisbert JP, Canas-Ventura A, et al. Observational study on the efficacy of adalimumab for the treatment of ulcerative colitis and predictors of outcome. Journal of Crohn's and Colitis. 2013;7:717–722. doi: 10.1016/j.crohns.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Ferrante M, Vermeire S, Fidder H, et al. Long-term outcome after infliximab for refractory ulcerative colitis. Journal of Crohn's and Colitis. 2008;2:219–225. doi: 10.1016/j.crohns.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Lee KM, Jeen YT, Cho JY, et al. Efficacy, safety, and predictors of response to infliximab therapy for ulcerative colitis: a Korean multicenter retrospective study. JGH. 2013;28:1829–1833. doi: 10.1111/jgh.12324. [DOI] [PubMed] [Google Scholar]
- 21.Taxonera C, Estelles J, Fernandez-Blanco I, et al. Adalimumab induction and maintenance therapy for patients with ulcerative colitis previously treated with infliximab. APT. 2011;33:340–348. doi: 10.1111/j.1365-2036.2010.04531.x. [DOI] [PubMed] [Google Scholar]
- 22.Bodger K, Gledhill New and emerging treatments for ulcerative colitis: a focus on vedolizumab. BTT. 2013;123 doi: 10.2147/BTT.S30416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colombel JF, Loftus EV, Siegel CA. Efficacy of vedolizumab with concomitant corticosteroid or immunomodulator use in patients with ulcerative colitis from GEMINI 1. Journal of Crohn's and Colitis. 2015:1–2. [Google Scholar]
- 24.Colombel JF, Loftus EV, Jr, Siegel CA. Efficacy of vedolizumab with concomitant corticosteroid or immunomodulator use in patients with Crohn's disease in GEMINI 2. Journal of Crohn's and Colitis. 2015:1–1. [Google Scholar]
- 25.Bryant R, Sandborn W, Travis S. Introducing Vedolizumab to Clinical Practice: Who, When, and How? Journal of Crohn's and Colitis. 2015;9:356–366. doi: 10.1093/ecco-jcc/jjv033. [DOI] [PubMed] [Google Scholar]
- 26.Colombel JF, Sands BE, Feagan BG, et al. Integrated Safety Analysis of Vedolizumab for the Treatment of Ulcerative Colitis or Crohn's Disease. Gastroenterology. 2015;144:S–113. [Google Scholar]
- 27.Mosli MH, MacDonald JK, Bickston SJ, et al. Vedolizumab for Induction and Maintenance of Remission in Ulcerative Colitis: A Cochrane Systematic Review and Meta-analysis. Inflammatory Bowel Diseases. 2015;21:1151–1159. doi: 10.1097/MIB.0000000000000396. [DOI] [PubMed] [Google Scholar]
- 28.Yajnik V, Khan N, D M. P566. Efficacy and safety of vedolizumab with advancing age in patients with ulcerative colitis: Results from the GEMINI 1 study. Journal of Crohn's and Colitis. 2015;9:S363–S364. [Google Scholar]
- 29.Colombel JF, Sands BE, Hanauer S. Long-term Safety of Vedolizumab for the Treatment of Ulcerative Colitis or Crohn's Disease. AJG. 2013;108:S500–S561. [Google Scholar]


