Abstract
Electron paramagnetic resonance imaging (EPRI) has been used to noninvasively provide 3D images of absolute oxygen concentration (pO2) in small animals. These oxygen images are well resolved both spatially (∼1mm) and in pO2 (1-3 torr). EPRI preclinical images of pO2 have demonstrated extremely promising results for various applications investigating oxygen related physiologic and biologic processes as well as the dependence of various disease states on pO2, such as the role of hypoxia in cancer.
Recent developments have been made that help to progress EPRI towards the eventual goal of human application. For example, a bimodal crossed-wire surface coil has been developed. Very preliminary tests demonstrated a 20 dB isolation between transmit and receive for this coil, with an anticipated additional 20dB achievable. This could potentially be used to image local pO2 in human subjects with superficial tumors with EPRI. Local excitation and detection will reduce the specific absorption rate limitations on images and eliminate any possible power deposition concerns. Additionally, a large 9 mT EPRI magnet has been constructed which can fit and provide static main and gradient fields for imaging local anatomy in an entire human. One potential obstacle that must be overcome in order to use EPRI to image humans is the approved use of the requisite EPRI spin probe imaging agent (trityl). While nontoxic, EPRI trityl spin probes have been injected intravenously when imaging small animals, which results in relatively high total body injection doses that would not be suitable for human imaging applications. Work has been done demonstrating the alternative use of intratumoral (IT) injections, which can reduce the amount of trityl required for imaging by a factor of 2000- relative to a whole body intravenous injection.
The development of a large magnet that can accommodate human subjects, the design of a surface coil for imaging of superficial pO2, and the reduction of required spin probe using IT injections all are crucial steps towards the eventual use of EPRI to image pO2 in human subjects. In the future this can help investigate the oxygenation status of superficial tumors (e.g., breast tumors). The ability to image pO2 in humans has many other potential applications to diseases such as peripheral vascular disease, heart disease, and stroke.
1 Introduction
EPR oxygen images have been shown to reproduce the ability of both the Eppendorf electrode and the more recent Oxylite quenching by molecular oxygen (O2) of the decay of fluorescence excited by a short optical pulse of light. 1 However, as images, they provide much more information. The images provide an inventory of locations within a tumor of the subregions where O2 is reduced: hypoxic subvolumes with fractions of its image voxels less than a threshold value of pO2 less than a certain value, e.g., 10 torr, in this case referred to as the hypoxic fraction (HF) less than 10 torr (HF10). This is accomplished by infusing intravenously (IV) in mice, a nontoxic spin probe carrying an unpaired electron prepared in a very low magnetic field, 9 milliTesla (mT) and subject to linear field gradients. The rate at which the longitudinal magnetization of an unpaired spin relaxes from an excitation provided by a short (50 ns) pulse of 250 MHz radiofrequency is nearly absolutely proportional to the local concentration of O2 through Heisenberg spin exchange with one of either of the unpaired O2 electron spins. 2 Small animal experiments provide a proof of principle that EPR O2 images can direct local therapies such as radiation to resistant portions of tumors, hypoxic subregions lacking O2, that can be a major source of therapeutic failure.3
The frequencies at which these experiments have been carried out are those used for a 6 T whole body MRI. This suggests that that EPR technology can be applied to human subjects to enhance local radiation therapy. In this paper we suggest that the initial investigation of EPR O2 imaging in the enhancement of radiation therapy will be in the derivation of local images, characterizing the oxygen physiology of localized tumors. Dealing with localized cancers with localized images is a natural starting point for the technology to minimize the dose of spin probe provided to human subjects and the applied specific (power) absorption rate (SAR).
2 Methods
Local EPR oxygen images provide near absolute measures of the pO2 in each of the approximately 1 mm3 voxels in the image. This is enabled by suffusing relevant tissue by the extracellular OX063d24 trityl, 2 whose spin lattice relaxation rates (R1) report the average local oxygen concentration. Preparation of the trityl electron spins is accomplished with a low main magnetic field, 9 mT, with an excitation frequency of 250 MHz.4 For the work at our center, EPR imaging is accomplished with fixed stepped magnetic field gradients, currently possible with our large imaging system (Fig. 1), capable of accommodating human size samples. At each gradient angle, the electron spins are subjected to the sequence of π − π/2 − π pulses (as shown in Fig. 2), referred to as Inversion Recovery with Electron Spin Echo (IRESE) readout. This allows acquisition of magnetization decay profiles representing the superposition of the decays of the trityl electrons shifted in planes perpendicular to the direction of the magnetic field gradient. The Fourier transform of these profiles in turn provides a frequency display of the projection which, with a complete set of gradient angles for each temporal decay, can provide an image of the magnetization amplitude in each voxel, reconstructed using filtered back projection. A sequence of eight T delays allows the reconstruction of the voxel spin lattice relaxation rate, calibrated absolutely proportional to voxel pO2. Confounding variation by trityl concentration or viscosity is less than the error of the voxel measurement, 2 torr. 2 To give anatomic meaning to the oxygen images, they must be registered with images that provide high anatomic resolution, such as T2-weighted MRI. A particular advantage of defining anatomy using MRI is the increase in free, slowly relaxing water within tumor tissue. Registration with EPR pO2 images allows definition of the tumor boundary within the pO2 image, providing a full inventory of the tumor pO2 values, many of which show steep gradients, as much as 50 torr/mm.
Figure 1.

Main magnet for EPR O2 imaging with a human subject.
Figure 2.
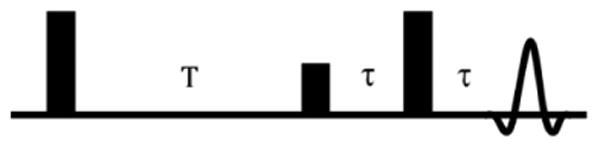
Inversion Recovery with Electron Spin Echo readout (IRESE) pulse sequence
2.1 Animal Imaging and Radiation Treatment
In mice, the entire animal is injected, IV, with the 1475 D spin probe OX63d24 trityl or a dose of approximately 50 mg/25 g animal, 2 g/kg. 2 Tumors are imaged with the IRESE sequence (Fig. 2) delivering approximately 0.06 W to a 2 mL tumor bearing leg (30 W/kg) in a 21 G animal, in a 19 mm diameter by 15 mm resistively Q-spoiled (from 100 to 14) loop gap resonator, with tumor temperature measurements showing no measurable increase within 0.5°C. This SAR with minimal temperature rise argues for the correct reduction in SAR limit for limited portions of the animal radiated. Other R1 pulse sequences have SAR reduced by three relative to that of the IRESE R1 sequences.
2.2 Mouse Intensity Modulated Radiation Therapy (IMRT)
IMRT to a mouse is enabled using an XRAD225 animal irradiator capable of delivering 5 Gy/minute to small radiation fields configured to deliver spherical radiation volumes to tumor regions of diameters from 4 mm to 10.25 mm in 1.25 mm increments to cover either hypoxic or well oxygenated tumor subregions of similar volumes. Larger fields are given to whole tumor regions as part of a treatment course. The diameter of the tumor volume to be boosted is determined by choosing that which minimizes the distance between the curve shown in Fig 4 B and the upper left value of the graph with 100% hypoxic voxels and 0% well oxygenated voxels. Tumors with dose boosts in this fashion are expected to be cured more frequently than those boosted with a shell of dose of similar volume to well oxygenated tumor voxels shown in the lower panel of Fig 3B. Data are currently being acquired to validate this hypothesis.
Figure 4.
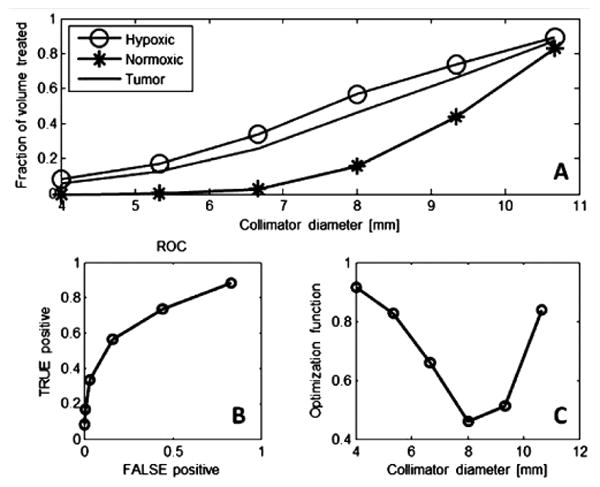
A. Plot of fraction of hypoxic or normoxic * cells vs spherical boost diameter. B. Plot of hypoxic cell fraction (True Positive) vs fraction of well oxygenated cells for each boost volume diameter. C. Distance to upper left corner in B. whose minimum is optimum.
Figure 3.
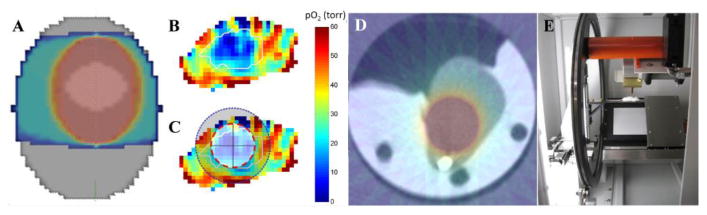
A. General tumor radiation B. a) Boost to hypoxic region b) boost to well oxygenated shell. C. CT scan of tumor with hypoxic boost. D. Mouse IMRT radiator.
3.1 Techniques for Oxygen Imaging to Guide Human IMRT
Although 381 mice have been treated with the trityl OX063 or its deuterated analog OX063d24, with no unexplained animal deaths (e.g. excessive anesthesia) initial approaches to O2 imaging for human application should involve two major modifications of our imaging protocol:
Reduction of the total trityl spin probe dose given to the subject, and
Reduction of the whole subject specific absorption rate (SAR) or radiofrequency power deposition.
Reduction of total trityl spin probe dose can be accomplished using intratumoral spin probe injection. As an example of this in mouse tumors, we show in Fig. 5 the pO2 distributions from the same tumors imaged via IV injection (Fig. 5A) and then, after a period of approximately one hour, when the IV trityl has substantially cleared, more trityl is injected via intratumoral (IT) injection. The apparent pO2 distribution from the IT injection is similar to the IV (Fig. 5B, red histograms), also seen in the additional half dozen animals in which this was tried. Early tumor stage for human tumors is often the case with maximum tumor linear dimension ≤ 2.0 cm (∼ 4 ml volume). The next tumor size categorized by the American Joint Commission (AJC) for tumor staging involves tumors with dimension >2.0 cm and ≤ 5.0 cm in most cases (up to ∼ 63 ml volume) so that an average tumor size can be taken as ∼ 15 ml. For such tumor size and IT injection, the reduction in the subject dose for a 70 kg human subject is a factor of 5,000. This translates to a subject dose of order 10s of nM rather than 100s of μM.
Figure 5.
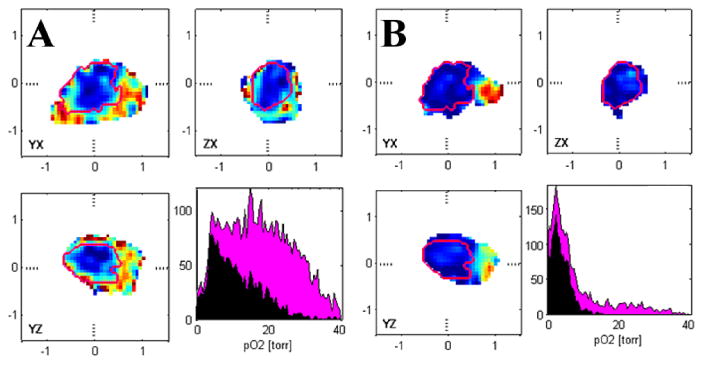
Images and histograms of pO2 in mouse leg (blue histogram) bearing tumor (red contour, red histogram)) A) after IV injection and B) after IT injection of trityl spin probe
Local tumor excitation and detection using surface coils reduces the whole subject SAR. This is recognized in the SAR specifications for MRI where the maximum local SAR is relaxed with reduced fraction of the subject radiated. https://www.aapm.org/meetings/02AM/pdf/8356-48054.pdf. The local excitation and detection system shown provides without any optimization isolation between the excitation power and detection signal of 23 dB. We have found that with greater than 40 dB isolation eliminates virtually all of the baseline signal that requires off resonance subtraction. This should be easily achievable. Modelling of the radiofrequency sensitivity of the bimodal excitation/detection surface coils indicates good B1 distributions to depths of 2-3 cm, making all T1 size tumors and many T2 size tumors accessible.
4 Conclusions
This combination of local trityl injection and local excitation and local detection reduces the risk of toxicity for the initial use of both spin probe and instrument power deposition to extremely low. Nanomolar concentrations of tri-acid spin probe, with extracellular distribution and rapid renal excretion and no murine toxicity argues that this substance approaches human tissue concentrations low enough to be below the threshold for concern.
Figure 6.
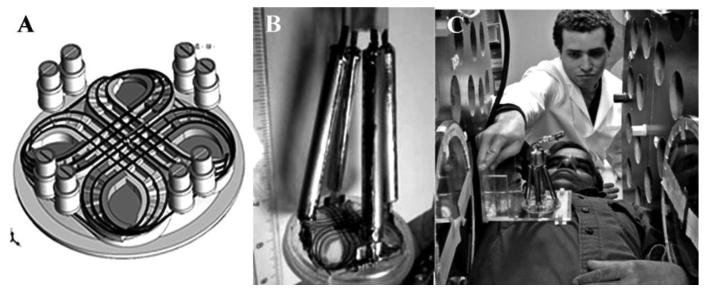
A) Scheme of bimodal surface coil with geometric mode decoupling; B) Construction of bimodal surface coil; C) placement of surface coil.
Acknowledgments
Supported by NIH grants P41 EB002034 and R01 CA98575..
References
- 1.Elas M, Bell R, Hleihel D, et al. Electron Paramagnetic Resonance Oxygen Image Hypoxic Fraction Plus Radiation Dose Strongly Correlates With Tumor Cure in FSa Fibrosarcomas. Int J Radiat Oncol Biol Phys. 2008;71:542–9. doi: 10.1016/j.ijrobp.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Epel B, Bowman MK, Mailer C, Halpern HJ. Absolute oxygen R imaging in vivo with pulse electron paramagnetic resonance. Magn Reson Med. 2013 doi: 10.1002/mrm.24926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Overgaard J. Hypoxic radiosensitization: adored and ignored. J Clin Oncol. 2007;25:4066–74. doi: 10.1200/JCO.2007.12.7878. [DOI] [PubMed] [Google Scholar]
- 4.Epel B, Sundramoorthy SV, Mailer C, Halpern HJ. A versatile high speed 250-MHz pulse imager for biomedical applications. Concepts in Magnetic Resonance Part B: Magnetic Resonance Engineering. 2008;33B:163–76. doi: 10.1002/cmr.b.20119. [DOI] [PMC free article] [PubMed] [Google Scholar]


