Abstract
Most commonly originating from breast malignancies, metastatic bone cancer causes bone destruction and severe pain. Although novel chemotherapeutic agents have increased life expectancy, patients are experiencing higher incidences of fracture, pain, and drug-induced side effects; furthermore, recent findings suggest that patients are severely undertreated for their cancer pain. Strong analgesics, namely opiates, are first-line therapy in alleviating cancer-related pain despite the severe side effects, including enhanced bone destruction with sustained administration. Bone resorption is primarily treated with bisphosphonates, which are associated with highly undesirable side effects, including nephrotoxicity and osteonecrosis of the jaw. In contrast, cannabinoid receptor 2 (CB2) receptor-specific agonists have been shown to reduce bone loss and stimulate bone formation in a model of osteoporosis. CB2 agonists produce analgesia in both inflammatory and neuropathic pain models. Notably, mixed CB1/CB2 agonists also demonstrate a reduction in ErbB2-driven breast cancer progression. Here we demonstrate for the first time that CB2 agonists reduce breast cancer–induced bone pain, bone loss, and breast cancer proliferation via cytokine/chemokine suppression. Studies used the spontaneously-occurring murine mammary cell line (66.1) implanted into the femur intramedullary space; measurements of spontaneous pain, bone loss, and cancer proliferation were made. The systemic administration of a CB2 agonist, JWH015, for 7 days significantly attenuated bone remodeling, assuaged spontaneous pain, and decreased primary tumor burden. CB2-mediated effects in vivo were reversed by concurrent treatment with a CB2 antagonist/inverse agonist but not with a CB1 antagonist/inverse agonist. In vitro, JWH015 reduced cancer cell proliferation and inflammatory mediators that have been shown to promote pain, bone loss, and proliferation. Taken together, these results suggest CB2 agonists as a novel treatment for breast cancer–induced bone pain, in which disease modifications include a reduction in bone loss, suppression of cancer growth, attenuation of severe bone pain, and increased survival without the major side effects of current therapeutic options.
Keywords: CANNABINOID 2 RECEPTOR, BONE, CANCER, PAIN, CYTOKINE, CHEMOKINE
Introduction
The World Health Organization estimates that the number of women living with breast cancer globally will rise to 15 million by the year 2020(1) with a significant portion of these patients in advanced stages of the disease. Breast cancer is the second leading cause of death in women,(1) with nearly 200,000 diagnoses each year in the United States alone. As a cancer that most commonly metastasizes to bone,(2) breast cancer cells in the bone microenvironment cause bone loss, fractures, anemia, and severe pain.(3) Despite marked advances in chemotherapeutics for early-stage breast cancers, few new therapies are effective in slowing disease progression and increasing survival in advanced disease. Recent findings with a serine-threonine protein kinase B-raf (B-RAF) inhibitor in advanced stages of melanoma emphasize the need for treatments that effectively reduce pain and increase survival time in terminally ill patients.(4) A recent study in cancer patients demonstrated a significant and severe deficit in treatment options for cancer pain, with high incidence in minority patients.(5) Reports also highlight the importance of patient quality of life in late stages of cancer, as well as the effects on surrounding family and physicians.(4)
The most commonly prescribed treatments for skeletal-related events(6) in advanced-stage breast cancer patients with bone metastasis and pain are bisphosphonates, radiation, nonsteroidal anti-inflammatory drugs (NSAIDs), and opioids.(6) Although these therapies reduce skeletal-related events (SREs) and/or pain, they bring about unwanted side effects and an overall decrease in quality of life without increasing patient survival or slowing disease progression.(6) The U.S. Food and Drug Administration (FDA) recently allowed a fast track review of denosumab (Xgeva), a human monoclonal antibody targeting receptor activator of NF-κ ligand (RANKL), as a result of the inadequacy of current therapies for treating advanced-stage breast cancer patients. Denosumab demonstrated a greater efficacy in preventing SREs in breast cancer patients with bone metastasis than the bisphosphonate zoledronic acid; however, neither bisphosphonates nor denosumab have shown a survival benefit.(5) Additionally, both treatments induce a small but significant subset of patients to develop serious adverse events including osteonecrosis of the jaw and disruption of normal serum calcium levels.(5) Cancer pain caused by bone metastases is inadequately managed by a combination of opiates and NSAIDS, as dictated by the guidelines set by the WHO Ladder Approach for Relief of Cancer Pain. NSAIDs, while effective at reducing inflammatory and musculoskeletal pains,(7) have been shown to aid in bone destruction and prevention of proper bone remodeling, thus decreasing bone strength in both animal models(8) and in human studies.(9) Recently, we demonstrated that sustained morphine not only intensifies tumor-induced pain but also accelerates tumor-induced bone destruction in a murine model of bone cancer.(10) Chronic opiate use results in a multitude of unbearable side effects including analgesic tolerance, somnolence, constipation, respiratory depression, and paradoxical states of hyperalgesia.(11) Neither palliative care nor therapeutics approved for use in advanced stages of breast cancer have improved in the last 30 years despite the persistence of the disease; for these reasons, alternative therapies must be developed.
One possible alternative includes the agonism of cannabinoid receptor 2 (CB2), which has previously shown analgesic activity in acute, chronic, inflammatory, and neuropathic pain without producing psychoactive or rewarding behavior.(12,13) Additionally, CB2 receptors are integral components of normal bone metabolism.(14–16) Activation of CB2 receptors improves bone integrity by stimulating the proliferation of osteoblasts and inhibiting the proliferation and activation of osteoclasts.(14,15) Recently, CB2 agonists have also been shown to have antitumor potential in the MMTV-neu mouse, ErbB2-driven breast cancer model in an protein kinase B (AKT) pathway-dependent fashion.(17) The specific mechanism(s) of antiproliferation by CB2 agonists is understudied and unclear. However, it is evident that CB2 agonists suppress the release of cytokines and chemokines from immune cells and some types of cancer cells,(18–20) molecules that are well known to promote breast cancer proliferation, migration, pain, and bone resorption.(21–27)
The present work evaluates the efficacy and mechanism of CB2 agonists in attenuating bone loss, pain, and cancer proliferation in a murine model of breast cancer–associated bone cancer and pain. Our data suggest that CB2 agonists may provide cancer patients with bone metastases a superior alternative to current available therapeutics.
Materials and Methods
In vitro
Cell culture
Murine mammary tumor line 66.1 cells were cultured in Eagle's minimum essential medium with 10% fetal bovine serum (FBS), 100 IU −1 penicillin, and 100 μg/mL streptomycin (P/S). The cell line was plated in 10-cm tissue culture dishes, allowed to grow exponentially, and housed in an incubator at 37°C and 5% CO2. For in vivo and in vitro assays, cells were centrifuged and counted using a gridded hemacytometer (Hausser Scientific, Horsham, PA, USA).
Immunoblotting
The 66.1 murine breast cancer cells were lysed, and protein extracts were separated electrophoretically and transferred to a polyvinylidene fluoride (PVDF) membrane as described.(28) Additionally, tumor cells were extruded from intramedullary space of the femur after 14 days implantation, and protein extracts were subsequently separated electrophoretically and transferred to a PVDF membrane. The membrane was incubated with polyclonal primary anti-CB2 antibody (Cell Signaling Technology, Danver, MA, USA) followed by horseradish peroxidase–conjugated secondary immunoglobulin G (IgG) antibody (Cell Signaling Technology) and developed using a chemiluminescent system (Amersham Biosciences). GAPDH or α-tubulin (Cell Signaling Technology) was used as loading control.
Sulforhodamine B assay
Cell proliferation in response to different treatments was measured as described.(29) Briefly ~1.6 × 104 66.1 cells per well were plated on six-well plates and allowed to grow overnight. On day 2, complete minimum essential medium (MEM) was replaced with Optimem (Invitrogen, Grand Island, NY, USA) serum-free medium and incubated for 16 hours. Cells were then stimulated with AM1241 or JWH015 on the morning of day 3 in varying concentrations. Cells receiving both agonist and antagonist were pretreated with antagonist 1 hour prior to agonist treatment. Then 48 hours later cells were fixed with ice-cold 50% trichloroacetic acid (TCA) (500 μL/well) for 1 hour at 4°C. Cells were then washed with deionized water and stained with sulforhodamine B (SRB) dye (2 mL/well) for 10 minutes at room temperature. Cells were washed with 1% acetic acid and the bound SRB dye was solubilized with 1 M unbuffered Tris for 10 minutes on a plate shaker. Optical density of each well was read at 540 nm using a plate reader (Biomek, Brea, CA, USA).
Bromodeoxyuridine assay
Bromodeoxyuridine (BrdU) cell proliferation assay (Millipore, Billerica, MA, USA) was performed according to the manufacturer's instructions after seeding ~10,000 66.1 cells per well in a 96-well plate. Cells were treated for 48 hours with vehicle, CB2 agonist, CB1 antagonist/inverse agonist, SR141716, CB2 antagonist/inverse agonists, AM630, and SR144528. Wells treated with both antagonist and agonist were pretreated with antagonist 1 hour prior to agonist treatment. After treatment, cells were incubated for 20 hours with BrdU for incorporation and then fixed to the plate. Plates were washed to remove excess BrdU. Plates were incubated with a peroxidase conjugated antibody against BrdU and read at 450 nm.
Cytokine and chemokine secretion
To evaluate secretion of inflammatory mediators by 66.1 cells, cells were plated at a density of 1 × 104 cells per well on a 96-well plate and grown overnight in serum-containing media. At 3 hours prior to assay, cells were switched to serum-free Opti-MEM (Invitrogen) containing drug or vehicle treatments. Cells receiving antagonist were pretreated for 1 hour prior to 3 hour incubation with agonist. Supernatant was collected following the 3-hour treatment incubation and centrifuged at 1000g for 5 minutes to remove cell debris. Concentrations of tumor necrosis factor a (TNFα), monocyte chemoattractant protein-1 (MCP-1), and interleukin 6 (IL-6) were determined using commercially available ELISA kits according to manufacturer specifications (Invitrogen; SABiosciences, Valencia, CA, USA; and eBiosciences, San Diego, CA, USA) with detection limits of 8.0, 25.5, and 0.21 pg/mL, respectively.
Cytokine proliferation response
In order to evaluate proliferative response of cells to cytokine stimulation, cells were plated at a density of 1 × 104 cells per well on a 96-well plate and grown overnight in serum-containing media. On day 2, cells were switched to serum-free Opti-MEM containing cytokine and drug or vehicle treatments. On day 3, cell proliferation was measured using SRB as described in 1990 by Skehan and colleagues29; briefly, cells were fixed with cold 10% TCA for 1 hour at 4°C. Cells were then washed with deionized water and stained with SRB dye for 10 minutes at room temperature. Cells were washed with 1% acetic acid to remove excess dye and the bound SRB dye was solubilized with 1 M unbuffered Tris for 10 minutes on a plate shaker. Optical density in wells was read at 540 nm.
In vivo
Animals
All procedures were approved by the University of Arizona Animal Care and Use Committee and conform to the Guidelines by the National Institutes of Health and the International Association for the Study of Pain. Female BALB/cfC3H mice (Harlan, IN, USA) were 15 to 20 g prior to initiation of study (n = 10–15 animals per treatment group). Mice were maintained in a climate-controlled room on a 12-hour light/dark cycle and allowed food and water ad libitum. Animals were weighed on days 0 (day of surgery), 7, 14, and 21. Animals were monitored for clinical signs of morbidity including paralysis and rapid weight loss (>20% in 1 week). Survival was expressed as a percentage of the number of animals remaining at day 28 to the number of total animals in each group on day 7.
Intramedullary implantation of 66.1 cells
Mice were anesthetized with ketamine/xylazine. An arthrotomy was performed. The condyles of the right distal femoris were exposed and a hole was drilled to create a space for injection of 1 × 105 66.1 cells in 5 μL complete MEM or 5 μL complete MEM without cells in control animals within the intramedullary space of the mouse femoris. Injections were made with an injection cannula affixed via plastic tubing to a 10-μL Hamilton syringe. Proper placement of the injector was confirmed through use of Faxitron X-ray imaging. Holes were sealed with bone cement.(30,31)
Drug treatment
Animals received CB2 receptor agonists, either AM1241 or JWH015 (Tocris, Minneapolis, MN, USA), dissolved in a vehicle solution of 10% dimethyl sulfoxide, 10% Tween-80, and 80% saline. All intraperitoneal (i.p.) injections were made at a volume of 10 mL/kg. Acute studies utilized one injection of JWH015 (6 mg/kg) 14 days after intramedullary cancer cell inoculation or medium. Chronic testing consisted of once daily CB2 agonist (6 mg/kg, 7–14 days). Designated animals were treated with CB1 antagonist/inverse agonist, SR141716 (1 mg/kg, every day [q.d.], 10 minutes prior to agonist), or CB2 antagonist/inverse agonist, SR144528 (1 mg/kg, q.d., 10 minutes prior to agonist). Antagonists were dissolved in the same vehicle as the agonist, based on previous publications.(13,32) Control groups included media only and vehicle-treated animals.
Analysis of acute pain
Spontaneous pain (flinching and guarding), and tactile allodynia were measured 0, 30, 60, 90, and 120 minutes after a single dose of drug was administrated in a blinded fashion. Breast cancer–induced hypersensitivity was shown to return to baseline levels 2 hours after drug administration.
Analysis of chronic pain
Animals were tested for spontaneous pain (flinching and guarding), and tactile allodynia before surgery (baseline) and at days 7 and 14 following surgery. All testing was performed during the day portion of the circadian cycle in a blinded fashion.
Spontaneous pain
Flinching and guarding were observed for duration of 2 minutes during a resting state. Flinching was characterized by the lifting and rapid flexing of the right hind paw when not associated with walking or movement. Flinches were recorded on a five-channel counter. Guarding was characterized by the lifting the right hind limb into a fully retracted position under the torso. Time spent guarding over the duration of 2 minutes was recorded and measurements were performed in blinded fashion.(31,32)
Tactile allodynia
The assessment of tactile allodynia consisted of measuring the withdrawal threshold of the paw ipsilateral to the site of tumor inoculation in response to probing with a series of calibrated von Frey filaments using the Chaplan up-down method(33) with the experimenter blinded to treatment groups. The 50% paw withdrawal threshold was determined by the nonparametric method of Dixon.(34)
Radiography
A digital Faxitron machine was used to acquire live radiographs on days 0, 7, and 14 of the intramedullary inoculation model. Bone loss was rated by a blinded third party expert in animal radiographs according to the following scale: 0 = normal, 1 = bone loss observed with no fracture, 2 = full thickness unicortical bone loss indicating unicortical bone fracture, and 3 = full thickness bicortical bone loss indicating bicortical bone fracture. From this rating, the incidence of fractures was reported and used to calculate the percent of animals with fractures. Before capturing images, mice were anesthetized with ketamine/xylazine.(35)
Ex vivo
Bone histology
Immediately following behavioral testing on day 14, mice were anesthetized (ketamine 80 mg/kg/xylazine 12 mg/kg, i.p.) and perfused transcardially with 0.1 M PBS followed by 10% neutral buffered formalin (Sigma, St. Louis, MO, USA). Femurs were collected and postfixed in picric acid with 4% formalin at 8°C overnight and decalcified in 10% EDTA (RDO-Apex, Aurora, IL, USA) for 14 days. Femurs were cut in the frontal plane into 5-μm sections and stained with hematoxylin and eosin (H&E) to visualize normal marrow elements and cancer cells under bright field microscopy on a Nikon E800 at 4× magnification. Tumor or marrow areas within the femur (six bones per treatment) were measured in square millimeters (mm2) between the epiphyseal plates using Metamorph imaging software by a blinded observer with the aid of a pathologist.
Micro–computed tomographic analysis of tumor-induced bone destruction
On day 14, mice were anesthetized (ketamine 80 mg/kg/xylazine 12 mg/kg, i.p.) and perfused transcardially with 0.1 M PBS followed by 10% neutral buffered formalin (Sigma). Femurs were collected and postfixed in picric acid with 4% formalin at 8°C overnight and stored in PBS until scanned. In order to characterize changes in breast cancer–induced bone remodeling at day 14 post–cell injection in animals treated with a cannabinoid 2 agonist (JWH015) or vehicle (10% dimethyl sulfoxide, 10% Tween 80, and 80% saline) and compared to control animals, femurs were analyzed with an eXplore Locus SP micro–computed tomography (μCT) scanner (GE Healthcare, Schenectady, NY, USA) to visualize densitometric and architectural parameters. This cone-beam μCT scanner uses a 2300 × 2300 CCD detector with current and voltage set at 80 μA and 80 kVp, respectively. Specimens were scanned in 810 views through 270 degrees with a 2100-ms integration time. Scans were then reconstructed at 16-μm3 resolution using Reconstruction Utility software (GE Healthcare). All 3D image manipulations and analyses were performed with the system's accompanying analysis software (Microview, Version 2.1, London, ON, Canada). The femoral reconstructions were reoriented along anatomical axes and regions of interest (ROIs) selected for quantification were selected in the following manner. For cortical analysis, a standard ROI measuring 2.5 mm wide × 2.5 mm long × 1 mm tall was generated and positioned such that the entire region of bone immediately 1 mm proximal of the distal growth plate was included in the ROI. Importantly, this area of bone, known as the distal metaphysis, was chosen for quantification because the majority of bone destruction observed in this model of breast cancer–induced bone remodeling occurred in this region. Using a threshold of 2222, the following parameters were gathered using the Cortical Analysis tool: cortical thickness (Ct.Th), total area (Tt.Ar), cortical area (Ct.Ar), Ct.Ar/Tt.Ar (tissue mineral density [TMD]), and periosteal perimeter (Ps.Pm). For trabecular analysis, an irregular ROI was generated 1 mm proximal of the distal growth plate using the spline tool to draw endocortical contours in five axial planes through 1 mm above the distal growth plate. The contours were then integrated and used to generate a three-dimensional (3D) ROI that filled the intramedullary space of the distal metaphysis. μCT parameters used to assess breast cancer–induced changes in trabecular bone microarchitecture were gathered at a threshold of 1337 mg HA/cm3 and included trabecular number (Tb.N), trabecular thickness (Tb.Th), trabecular spacing (Tb.Sp), and bone volume/total volume (BV/TV).(35,36)
Contralateral, non–tumor-bearing femurs were scanned with the above μCT protocol in order to determine whether the JWH015 compound was having osteogenic effects on naive bone. Centered along the midpoint of the femoral diaphysis, a rectangular ROI (2.5 mm long × 2.5 mm wide × 1 mm high) was used to evaluate μCT parameters for cortical bone (Ct.Th, Tt.Ar, Ct.Ar, Ct.Ar/Tt.Ar, TMD). Similar to the methods listed above, an irregular ROI was generated in the distal metaphysis for analysis of trabecular bone microarchitecture. In all cases, the μCT operator was blind to the experimental conditions of the specimens.
Serum biochemical assays
Animals were deeply anesthetized and whole blood was collected by transcardial puncture. Blood coagulated at room temperature for 1 hour, and was centrifuged to isolate serum. Serum was stored at −80°C until used for assays. Enzyme immunoassays were used to measure the serum concentrations of tartrate-resistant acid phosphatase form 5b (TRAP5b) for osteoclast number (Immunodiagnostic Systems, Fountain Hills, AZ, USA) and C-terminal telopeptide α1 chain of type I collagen (CTX) (Immunodiagnostic Systems, Fountain Hills, AZ, USA) for bone loss and osteocalcin (Biomedical Technologies, Stoughton, MA, USA). Assays were conducted according to the manufacturers’ instructions.
Bone marrow extrudate immunoassay
Animals were euthanized on day 14 postinoculation of cell-free media or 66.1 cells and 7 days drug treatment, and the ipsilateral and contralateral femurs were removed. For each femur, the proximal and distal ends were clipped and the intermedullary extrudate was rinsed into a vial; each femur was rinsed four times with phosphate-buffered saline containing protease inhibitor cocktail and EDTA (Pierce, Rockford, IL, USA). Four femurs from four animals were pooled per sample. Semiquantitative cytokine and chemokine array was used to determine the relative change of IL-1β, IL-4, IL-6, IL-12, IL-17a, IFNγ, TNFα, MCP-1, macrophage inflammatory protein 1a (MIP-1a), and MIP-1b due to cancer progression and effects of the CB2 agonist JWH015 (SABiosciences). From this array, MCP-1, MIP-1a, IL-6, and TNFα were selected and quantitatively assessed using commercially available ELISA kits according to manufacturers’ specifications (SABiosciences, eBiosciences, Invitrogen) with detection limits of 25.5, 26.3, 58.8, and 8.0 pg/mL, respectively.
Statistical analysis
Statistical comparisons between treatment groups were done using ANOVA. Pairwise comparisons were made with Student's t test, multiple comparisons between groups were done using Bonferroni's multiple comparison test. Dose-response effects were done with linear regression analysis of the linear portion of the log dose-response curve. Survival studies used the Kaplan Meier estimator with a log-rank Mantel-Cox test and Gehan-Breslow Wilcoxon test. Significance was set at p < 0.05. All data are presented as mean ± SEM and GraphPad Prism 5.0 (Graph Pad Inc., San Diego, CA, USA) was used to plot data.
Results
Acute or sustained CB2 agonist attenuates breast cancer–induced bone pain
Flinching and guarding behaviors were observed to determine the acute effects of JWH015 on bone cancer–induced spontaneous pain. Von Frey filaments were used to determine the withdrawal thresholds of the ipsilateral hind paw. Fourteen days after intrafemoral cancer (66.1) inoculation, mice displayed significant bone cancer–induced flinching, guarding, and decreases in hind paw withdrawal thresholds (Fig. 1A–C) indicating behavioral signs of pain. JWH015 resulted in a significant, time-related reduction in flinching and guarding with an increase in paw withdrawal thresholds when compared to vehicle-treated mice (Fig. 1A–C). Pain behaviors returned to a hypersensitive state in cancer treated animals 240 to 320 minutes after JWH015 administration. Control animals inoculated with media showed no significant flinching, guarding, or mechanical hypersensitivity when administered JWH015 or vehicle (data not shown).
Fig. 1.
Acute systemic administration of CB2 receptor agonist JWH015 attenuates breast (66.1) cancer–induced spontaneous and evoked pain on day 14 after femoral inoculation (time 0). Bone cancer–induced spontaneous (A) flinches (B) guarding, and (C) evoked paw withdrawal were all significantly attenuated in a time-related fashion in animals administered JWH015 (i.p.) compared to vehicle-treated animals (p ≤ 0.05; n = 15 mice per group).
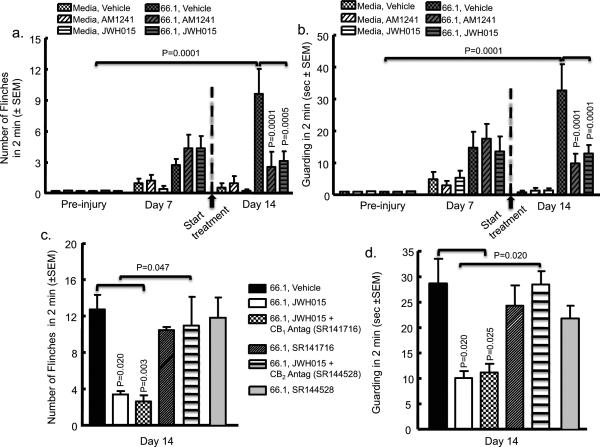
Mice receiving intrafemoral 66.1 cells and treated with vehicle displayed spontaneous flinching and guarding beginning at day 7 and increasing through day 14 (Fig. 2A, B). Sustained treatment of JWH015 or AM1241 (6 mg/kg, i.p., q.d., from day 7 to 14) in cancer-inoculated mice resulted in decreased guarding and flinching by day 14 in comparison to cancer-inoculated vehicle-treated animals (Fig. 2A, B).
Fig. 2.
Chronic systemic administration of CB2 receptor agonist JWH015 and AM1241 attenuates spontaneous pain, which is blocked by a CB2 antagonist/inverse agonist but not by a CB1 antagonist/inverse agonist. Animal femurs were injected with either breast cancer cells (66.1) or media only as a control after baseline (preinjury) behavioral measurements. On day 7 after femoral inoculation animals demonstrated bone cancer–induced (A) flinching and (B) guarding. CB2 agonists, JWH015 (6 mg/kg, i.p., q.d.) or AM1241 (6 mg/kg, i.p., q.d.) was administered after behavioral measurements and continued for 7 days. In A and B, spontaneous flinching and guarding, respectively, in cancer-bearing animals was significantly reduced by JWH015 (p = 0.0005; n = 16) and AM1241 (p = 0.0001; n = 12) compared to animals that received vehicle on day 14 (n = 20). No significant difference was observed in media-only control animals between vehicle-treated (n = 15), JWH015-treated (n = 12), or AM1241-treated (n = 12) animals. The attenuation of bone cancer–induced (C) flinching and (D) guarding by JWH015 (n = 15) on day 14 was blocked by pretreatment with the CB2 antagonist SR144528 (3 mg/kg, i.p., q.d., 30 minutes prior to agonist; n = 10;) but not by the CB1 antagonist SR141716 (3 mg/kg, i.p., q.d., 30 minutes prior to agonist; p = 0.003; n = 15). Neither CB2 antagonist/inverse agonist (n = 10) or CB1 antagonist/inverse agonist (n = 15) alone had a significant effect on the bone cancer–induced pain as compared to vehicle (n = 12).
Preadministration of the CB2 antagonist/inverse agonist, SR144528, but not the CB1 antagonist/inverse agonist, SR141716, inhibited the antinociceptive effect produced by the CB2 agonist (Fig. 2C, D). The treatment of either antagonist/inverse agonist alone had no effect on flinching or guarding. By day 7 postsurgery, cancer-inoculated animals began to display behavioral signs of tactile sensitivity (Supplemental Fig. S1), and media-treated animals displayed minor tactile sensitivity as a result of invasive surgery. On day 14, however, media control animals’ mechanical thresholds returned to baseline, whereas cancer-inoculated animals demonstrated significant mechanical hypersensitivity. Animals treated with sustained JWH015 or AM1241 demonstrated a significant attenuation of cancer-induced mechanical hypersensitivity compared to vehicle-treated controls (Supplemental Fig. S1).
CB2 agonist treatment reduces breast cancer–induced bone loss and fracture
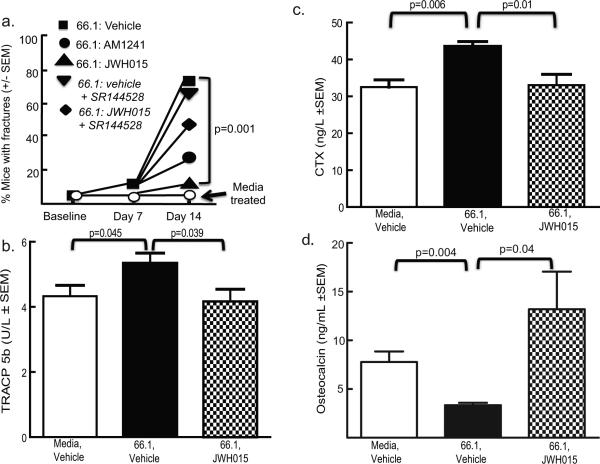
Radiographic images were taken following behavioral testing to determine the effect of sustained JWH015 on cancer-induced bone degradation. No bone loss or fractures were observed in animals injected with media and treated with vehicle (Fig. 3A). Cancer-induced bone loss (evidenced by the presence of radiolucent areas in the proximal and distal femoral heads) increased in tumor-bearing mice treated with vehicle. At day 14 post–cell injection, 68% of tumor-bearing mice treated with vehicle displayed femoral fractures, whereas no mice injected with media displayed femoral fractures (Figs. 3A, 4A). Sustained JWH015 treatment significantly reduced the amount of cancer-induced bone loss with a 40% reduction in the incidence of fractures (Figs. 3A, B, and 4A). Animals inoculated with media and treated with JWH015 had no observable changes in bone structure. The preadministration of the CB2 antagonist/inverse agonist significantly attenuated the ability of JWH015 to decrease cancer-induced fracture (Figs. 3A, B, and 4A). Similar findings were seen with AM1241 (Fig. 4A). Antagonist/inverse agonist alone in either cancer- or media-inoculated animals produced no significant change from controls (Figs. 3A, Fig. 4A).
Fig. 3.
(A) Radiographs of the femurs in the presence of either media (control) or breast cancer cells (66.1) on day 14 after inoculation. Mice received either vehicle or CB2 agonists (JWH015, 6 mg/kg, i.p., q.d.) in the absence or presence of the CB2 antagonist/inverse agonist (SR144528, 3 mg/kg, i.p., q.d. 30 minutes prior to agonist) from days 7 to 14 after femoral inoculation. Bone loss (hypodense at proximal and distal ends) identified in cancer (66.1)-treated animals as compared to media only (control) animals. JWH015 (6 mg/kg, i.p., q.d., days 7–14) attenuates breast cancer–induced bone loss compared to (B) cancer-inoculated, vehicle administration (10%-10%-80% DMSO, Tween 80, saline). The attenuation of bone cancer–induced bone loss by JWH015 on day 14 was inhibited by pretreatment with the CB2 antagonist/inverse agonist SR144528 (3 mg/kg, i.p., q.d., 30 minutes prior to agonist). SR144528 (3 mg/kg, i.p., q.d., 30 minutes prior to agonist) alone did not result in any differences. (B) and (C) show micro–computed tomographic analysis of tumor-induced bone destruction in the absence or presence of JWH015, respectively. Cortical bone loss is demonstrated in breast cancer (66.1)-inoculated animals as compared to control, naive, or media-treated animals. Radiographs in all panels are representative of images obtained of femurs obtained from each animal in Fig. 2.
Fig. 4.
Sustained CB2 agonist attenuates breast cancer–induced bone remodeling. (A) Based on radiographic images, the number of animals with a clear cortical bone fracture was counted from the different groups of mice at days 0, 7, and 14. Either CB2 agonists, AM1241 (n = 15) or JWH015 (n = 30) significantly reduced the number of animals with cancer-induced cortical fracture of the femoris by day 14 (p = 0.001) compared to vehicle (n = 30); this was blocked by the preadministration of the CB2 antagonist/inverse agonist, SR144528 (p = 0.001; n = 10). Antagonist alone (n = 10) had no significant effect. (B, C) TRACP 5b was measured in animals on day 14 as a marker of osteoclast activity. TRACP 5b levels were significantly higher in animals that received intrafemoral breast cancer cells (66.1) (p = 0.045) compared to intrafemoral media-treated animals. This increase in cancer-induced TRACP 5b levels was significantly reduced in JWH015-treated (6 mg/kg, i.p., q.d., from day 7 to 14) animals (p = 0.039; n = 8). In C, CTX was measured in animals on day 14 as a marker of bone resorption. The amount of CTX was significantly higher in animals that received intrafemoral breast cancer cells (66.1) (p = 0.006; n = 5) compared to intrafemoral media-treated animals. This increase in cancer-induced CTX levels was significantly reduced in JWH015-treated (6 mg/kg, i.p., q.d., from day 7 to 14) animals (p = 0.01; n = 5). (D) Osteocalcin was measured in animals on day 14 as a marker of bone resorption. The amount of OC was significantly decreased in animals that received intrafemoral breast cancer cells (66.1) (p = 0.004; n = 5) compared to intrafemoral media-treated animals. This decrease in cancer-induced OC levels was significantly attenuated in JWH015-treated (6 mg/kg, i.p., q.d., from day 7 to 14) animals (p = 0.04; n = 5).
At day 14, femurs were analyzed using μCT (Fig. 3B, C). In this cancer model, we observe osteolytic and osteoblastic characteristics, resulting in ectopic periosteal bone remodeling. This is consistent with human breast cancers in which 70% of breast lesions in bone are osteolytic, 15% are osteoblastic, and 15% take on a mixed growth pattern.(37) In cancer-inoculated (66.1) vehicle-treated animals (Table 1) there is a significant increase in the trabecular bone parameters BV/TV, Tb.N, and Tb.Sp compared to control animals. Cancer-inoculated animals treated with JWH-015 showed a significant increase in BV/TV, Tb.N, and Tb.Sp compared to cancer- inoculated vehicle-treated animals (Table 1). Additionally, cancer-inoculated animals show a significant change in cortical bone parameters, Ct.Ar, Ct.Ar/Tt.Ar, TMD, and Ps,Pm compared to control animals (Table 3). Cancer-inoculated animals treated with JWH015 show a significant change in TMD and Ps.Pm compared to cancer-inoculated animals treated with cancer (Table 3). For the contralateral femurs in which surgery was not performed, significant changes were not observed across treatment groups (Tables 2 and 4). These data indicate that in this breast cancer model, abnormal bone remodeling occurs locally and is attenuated by administration with the CB2 agonist JWH015.
Table 1.
Trabecular Bone Parameters
| Ipsilateral femur | Media, vehicle | Cancer, vehicle | Cancer, JWH015 |
|---|---|---|---|
| BV/TV (% ± SEM) | 0.17 ± 0.02 | 0.32 ± 0.03* | 0.24 ± 0.01** |
| Tb.N (1/mm ± SEM) | 4.16 ± 0.4 | 5.6 ± 0.16* | 5.06 ± 0.38** |
| Tb.Th (mm ± SEM) | 0.04 ± 0.002 | 0.05 ± 0.003* | 0.04 ± 0.001** |
| Tb.Sp (mm ± SEM) | 0.16 ± 0.01 | 0.16 ± 0.02 | 0.14 ± 0.01 |
BV/TV = bone volume fraction; Tb.N = trabecular number; Tb.Th = trabecular thickness; Tb.Sp = trabecular separation.
p < 0.05; indicates significant change from control animals (media, vehicle).
p < 0.05; indicates significant change from cancer, vehicle animals.
Table 3.
Cortical Bone Parameters
| Ipsilateral femur | Media, vehicle | Cancer, vehicle | Cancer, JWH015 |
|---|---|---|---|
| Tt.Ar (mm2 ± SEM) | 1.29 ± 0.09 | 1.51 ± 0.02 | 1.4 ± 0.06 |
| Ct.Ar (mm2 ± SEM) | 0.68 ± 0.06 | 0.80 ± 0.03* | 0.85 ± 0.06* |
| Ct.Ar/Tt.Ar (% ± SEM) | 0.19 ± 0.02 | 0.53 ± 0.02* | 0.61 ± 0.01* |
| Ct.Th (mm ± SEM) | 0.17 ± 0.01 | 0.14 ± 0.01* | 0.16 ± 0.01** |
| TMD (mg/cm3 ± SEM) | 1136.72 ± 18.12 | 1081.23 ± 2.65* | 1096.17 ± 7.89** |
| Ps.Pm (mm ± SEM) | 4.03 ± 0.25 | 4.97 ± 0.12* | 4.51 ± 0.04** |
Tt.Ar = total cross sectional area inside the periosteal envelope; Ct.Ar = cortical bone area (cortical volume/number of slices × slice thickness); Ct.Ar/Tt.Ar = cortical area fraction; Ct.Th = average cortical thickness; TMD = tissue mineral density; Ps.Pm= periosteal perimeter.
p < 0.05; indicates significant change from control animals (media, vehicle).
p < 0.05; indicates significant change from cancer, vehicle animals.
Table 2.
Trabecular Bone Parameters
| Contralateral femur | Media, vehicle | Cancer, vehicle | Cancer, JWH015 |
|---|---|---|---|
| BV/TV (% ± SEM) | 0.13 ± 0.02 | 0.10 ± 0.01 | 0.13 ± 0.02 |
| Tb.N (1/mm ± SEM) | 3.63 ± 0.41 | 2.99 ± 0.38 | 3.59 ± 0.41 |
| Tb.Th (mm ± SEM) | 0.03 ± 0.001 | 0.03 ± 0.001 | 0.03 ± 0.002 |
| Tb.Sp (mm ± SEM) | 0.22 ± 0.02 | 0.28 ± 0.04 | 0.24 ± 0.03 |
BV/TV = bone volume fraction; Tb.N = trabecular number; Tb.Th = trabecular thickness; Tb.Sp = trabecular separation.
Table 4.
Cortical Bone Parameters
| Contralateral femur | Media, vehicle | Cancer, vehicle | Cancer, JWH015 |
|---|---|---|---|
| Tt.Ar (mm2 ± SEM) | 1.43 ± 0.03 | 1.3 ± 0.03 | 1.18 ± 0.08 |
| Ct.Ar (mm2 ± SEM) | 0.76 ± 0.02 | 0.66 ± 0.02 | 0.64 ± 0.05 |
| Ct.Ar/Tt.Ar (% ± SEM) | 0.53 ± 0.01 | 0.51 ± 0.01 | 0.54 ± 0.01 |
| Ct.Th (mm ± SEM) | 0.21 ± 0.004 | 0.19 ± 0.01 | 0.19 ± 0.01 |
| TMD (mg/cm3 ± SEM) | 1190.72 ± 4.03 | 1129.79 ± 12.36 | 1132.47 ± 81.8 |
| PsPm (mm ± SEM) | 4.37 ± 0.05 | 4.97 ± 0.12 | 3.74 ± 0.36 |
Tt.Ar = total cross-sectional area inside the periosteal envelope; Ct.Ar = cortical bone area (cortical volume/number of slices × slice thickness); Ct.Ar/Tt.Ar = cortical area fraction; Ct.Th = average cortical thickness; TMD = tissue mineral density; Ps.Pm = periosteal perimeter.
To confirm CB2 agonist attenuation of cancer-induced bone remodeling, the bone resorption markers, TRACP 5b and CTX, and the bone formation marker, osteocalcin, were measured in serum. Additionally, the levels of TRACP 5b were significantly increased in cancer-inoculated animals treated with vehicle when compared to media-inoculated control animals (Fig. 4B). Sustained JWH015 treatment attenuated cancer-induced elevations in serum TRACP 5b (Fig. 4B). CTX concentration in cancer-inoculated mice treated with vehicle significantly increased compared to control animals injected with media (Fig. 4C). Cancer-inoculated mice treated with JWH015 showed attenuation of CTX elevations in serum (Fig. 4C). Osteocalcin concentration in cancer-inoculated mice treated with vehicle significantly decreased compared to control animals injected with media (Fig. 4D). Cancer-inoculated mice treated with JWH015 showed a significant increase in osteocalcin levels compared to cancer-inoculated vehicle-treated animals attenuation of CTX elevations in serum (Fig. 4D).
CB2 agonists inhibit cancer cell growth in vivo and in vitro
The murine breast cancer cells used in this study, 66.1, were found to express the CB2 receptor (Supplemental Fig. 4A). Breast cancer cells were treated in vitro with varying concentrations of JWH015 or AM1241. In a concentration-dependent manner, both JWH015 and AM1241 (Fig. 5A, C) significantly decreased the percent of cell viability compared to vehicle-treated cells. Additionally, JWH015 significantly decreased cellular incorporation of BrdU, as a direct measure of proliferation (Fig. 5D). Preincubation of JWH015-treated cells with the CB2 antagonist/inverse agonists SR144528 and AM630 reversed the antiproliferative effect of JWH015; preincubation of JWH015 with CB1 antagonist/inverse agonist, SR141716 had no effect on JWH015-mediated antiproliferation. SR144528, AM630, and SR141716 had no independent effect on BrdU incorporation at the concentrations used (Fig. 5D). These data suggest that JWH015 exerts a CB2-mediated suppression of cell proliferation in the 66.1 breast cancer cells.
Fig. 5.
CB2 agonist decreases 66.1 breast cancer proliferation in vitro and in vivo. (A, C) SRB assay indicates breast cancer cells (66.1) treated with JWH015 or AM1241 demonstrate a concentration-related decrease in their viability over a 48-hour period in a six-well plate compared to vehicle-treated cells (p ≤ 0.001; n = 18 per group). (B) Tumor burden using H&E staining of a femoris from intrafemoral cancer animals administered either vehicle or JWH015. JWH015 reduces the number of 66.1 cells within the intramedullary space compared to vehicle-treated animals (p = 0.01; n = 4 per group). (D) BrDU assay demonstrates breast cancer cells (66.1) treated with JWH015 decrease their ability to proliferate in a 96-well plate compared to vehicle treated cells; this effect can be reversed by the CB2 antagonist/inverse agonist SR144528 but not CB1 antagonist/inverse agonist SR141716 (p = 0.0001; n = 6 per group).
In order to determine the relevance of in vitro findings, bone marrow extrudates were collected from experimental day 14 animals and analyzed for tumor burden. Intramedullary tumors were shown to express the CB2 receptor (Supplemental Fig. 4B). Morphological analysis showed that breast cancer cells occupied 70% of the medullary cavity on day 14 in animals treated with vehicle, whereas breast cancer cells occupied 40% of the medullary cavity on day 14 after JWH015 treatment (Fig. 5B).
Antinociceptive effect of CB2 agonists in breast cancer–induced bone cancer in vivo is associated with suppression of the cytokines, TNFα and IL6, and the chemokines, MIP-1A and MCP-1.
Intramedullary bone extrudates were collected from breast cancer– or media-inoculated mice treated with either vehicle or JWH015 for 7 days. A multiantibody ELISA array was used to semiquantitatively assesses alterations in inflammatory mediators that occurred with cancer progression, and to determine whether changes were sensitive to JWH015 treatment.
Inflammatory mediators (TNFα, IL6, MIP-1A, and MCP-1) showed the most appreciable alterations from control and sensitivity to JWH015 treatment, and were therefore selected for further quantitative ELISA analysis (Supplemental Fig. 2). Concentrations of TNFα, IL6, MIP-1A, and MCP-1 in bone marrow extrudate were elevated in cancer-inoculated animals when compared to media-inoculated controls on day 14 (Fig. 6A–D). Cancer-inoculated animals that received chronic CB2 agonist (6 mg/kg, i.p., q.d., from day 7 to 14) showed a significant reduction in intramedullary TNFα, IL-6, MIP-1A, and MCP-1 when compared to vehicle-treated, cancer-inoculated animals (Fig. 6A–D).
Fig. 6.
Quantitative ELISA was used to measure amounts of each inflammatory mediator, reported as relative expression compared to control (animals inoculated with cell-free media and treated with vehicle). (A) Increased levels of IL-6 from extrudates from intramedullary space of femur of animals inoculated with 66.1 breast cancer cells and treated with vehicle (day 14; p < 0.0001; n = 4) as compared to animals inoculated with media. Cancer-inoculated animals treated JWH015 significantly decreased 66.1-induced levels of IL-6 (p < 0.0001; n = 4). (B) Increased levels of MCP-1 from extrudates from intramedullary space of femur of animals inoculated with 66.1 breast cancer cells and treated with vehicle (day 14; p < 0.0001; n = 4) as compared to animals inoculated with media. Cancer-inoculated animals treated JWH015 significantly decreased 66.1-induced levels of IL-6 (p < 0.0001; n = 4). (C) Increased levels of TNFα from extrudates from intramedullary space of femur of animals inoculated with 66.1 breast cancer cells and treated with vehicle (day 14; p < 0.05; n = 4) as compared to animals inoculated with media. Cancer-inoculated animals treated with AM1241 or JWH015 significantly decreased 66.1-induced levels of TNFα (p < 0.05; n = 4). (D) Increased levels of MIP-1A from extrudates from intramedullary space of femur of animals inoculated with 66.1 breast cancer cells and treated with vehicle (day 14; p < 0.0001; n = 4) as compared to animals inoculated with media. Cancer-inoculated animals treated with JWH015 significantly decreased 66.1-induced levels of MIP-1A (p < 0.0001; n = 4).
To determine whether inflammatory mediators could be secreted and affected at the level of the 66.1 tumor, cell supernatant from treated or nontreated 66.1 cells was assayed via ELISA for secreted levels of MCP-1, TNFα, and IL-6. Cultured 66.1 cells alone secreted appreciable levels of the cytokines and chemokines assayed (Fig. 7A–C). JWH015 treatment (1 μM, 3 hours) significantly decreased supernatant values for MCP-1 (Fig. 7A), IL6 (Fig. 7B), and TNFα (Fig. 7C), and suppression was partially reversed by pretreatment with the CB2 inverse agonist, SR144528 (100 nM, 1 hour). SR144528 (100 nM) alone had no effect on the release of MCP-1, TNFα, or IL-6. These data suggest that JWH015 suppresses secretion of inflammatory mediators from 66.1 cells in a CB2-dependent manner.
Fig. 7.
Cells from murine mammary cell line (66.1) were treated acutely for 3 hours with JWH015 (1000 ng) and/or SR144528 (100 ng) (n = 6 wells per treatment group) Quantitative ELISA was used to measure amount of each inflammatory mediator secreted by cells, reported as % relative expression of secretion compared to control (vehicle-treated cells). (A) JWH015 treatment alone significantly reduces expression of MCP-1 (p = 0.02) but not in the presence of SR144528 (p < 0.0001). (B) JWH015 treatment alone significantly reduces expression of IL6 (p = 0.02) but this effect is not seen with SR144528 treatment. (C) JWH015 treatment significantly reduces expression of TNFα but not in the presence of SR144528. (D) Twenty-four–hour IL-6 treatment (1 ng) significantly increases viability (p < 0.00001). Twenty-four–hour JWH015 treatment attenuates IL6-induced effect on viability (p < 0.00001). One-hour SR144528 pretreatment inhibits 24-hour JWH015 attenuation of IL6-induced increase in viability (p < 0.00001), as evidenced by SBR assay.
To demonstrate that cytokines elicited from 66.1 tumors could contribute to proliferation, 66.1 cells were treated with log concentrations of either IL-6 or TNFα. At 1 ng/mL, both IL-6 and TNFα promoted cell proliferation as measured by SRB assay (Supplemental Fig. 3A, B). Additionally, JWH015 (1 μM) attenuated IL-6–induced proliferation in a manner that was partially reversible by SR144528 pretreatment (100 nM, 1 hour) (Fig. 6D) but not by SR141716 (100 nM, 1 hour) (data not shown).
CB2 agonists maintain body weight and increase survival
Animal weights were significantly decreased from baseline (17.21 ± 0.25 g) by day 21 in cancer-inoculated, vehicle-treated animals (14.30 ± 1.17 g) compared to cancer-treated animals that received sustained JWH015 or AM1241 (16.87 ± 0.43 g and 17.03 ± 0.15 g, respectively) (Supplemental Fig. 5A). Animals with cancer-inoculated femurs and sustained vehicle resulted in a significant decrease in survival by day 21 compared to a nonsignificant change in survival from control in the JWH015- or AM1241-treated group (Supplemental Fig. 5B).
Discussion
Epidemiological studies show that one out of seven women in the United States will develop breast cancer in their lifetime.(1) The National Institutes of Health estimates overall costs of cancer in the United States at $206 billion, with breast cancer being the most frequent malignant tumor.(1) Up to 80% of patients with advanced breast cancer develop bone metastases associated with bone loss and fracture, which contribute to incapacitating pain and limited or total loss of mobility.(23–25,37) Pain, the first symptom in many cancer patients, substantially decreases the quality of life(23–25,37) and is poorly addressed by the current first-line therapies.(5) Skeletal-related events (SREs) and pain due to breast cancer metastases are treated using radiation therapy, opiates, and bisphosphonates.(26,38,39) Bisphosphonate treatment is associated with an array of off-target effects including nephrotoxicity, osteonecrosis of the jaw, hypocalcemia, and flulike symptoms(40,41,42); furthermore, the unwanted side effects of opioids and NSAIDs are well-documented.(7–9) Chronic pain treatments for advanced stage breast cancer have not been developed, and the failure of current pain therapies has generated a need for innovative and tailored approaches to managing cancer-pain.
Here we show that either a single injection of a CB2 agonist or sustained administration significantly attenuates spontaneous and evoked pain behaviors in an animal model of breast cancer–induced bone cancer pain. Inhibition of pain behaviors by the CB2 agonist are similar in efficacy to a single injection of morphine, suggesting that CB2 agonists have the ability to directly inhibit pain activity. Our antagonist studies support the assertion that CB2 agonist effects occur via CB2 receptors and not off-target CB1 receptors. Based on studies both in vivo and in vitro, we speculate that antinociceptive effects by CB2 agonists in our bone cancer model occur via anti-cytokine/anti-inflammatory activity localized to the tumor-bone microenvironment. Cancer metastasis to the bone initiates an immune response within the bone and the nervous system.(3) This immune response activates nociceptors and subsequently generates pain.(43) A mechanism of pain inhibition by CB2 agonists is proposed by decreasing pronociceptive cytokines such as IL-6, TNFα, and IL-1β released from infiltrating immune cells(18–20) and from the cancer cells themselves(44,45); we have further validated the ability of cancer cells to release pronociceptive cytokines in our cancer model. Studies have demonstrated that IL-1β, TNFα, and IL-6 are released from macrophages, monocytes, breast cancer, and glial cells to promote nociception.(46–48) IL-1β and IL-6 have been shown to increase the expression of other pronociceptive factors such as prostaglandins via upregulation of COX-2(49,50) and nerve growth factor (NGF)(51) while increasing the production and release of other cytokines to promote nociception. TNFα acts directly at its receptor, TNFR1, to produce nociception and indirectly via increasing prostanoids and sympathetic amines.(43,52) Activation of CB2 receptors on immune cells has been shown to inhibit the release of a number of cytokines from monocytes and macrophages in animal(18,27,53,54) and human studies.(18,55,56) In addition to cytokine activity, it is well known that certain chemokines can result in nociception (for review see Verri and colleagues(44)). The chemokine, macrophage inflammatory protein-1 (MCP-1 also known as CCL2) and macrophage inflammatory protein-1a (MIP-1A also known as CCL3), in mice have been reported as producing time- and dose-related hypernociception via their receptors CCR2 and CCR5 (GPCRs) on nociceptive fibers.(57,58) A significant decrease in neuropathic pain response was demonstrated in CCR2 −/− mice.(59) In addition to directly stimulating nociceptive fibers, it has been shown that MCP-1/CCL2 can increase pronociceptive sympathetic amines and IL-1β production.(60) MIP-1A/CCL3 has been shown to sensitize primary afferents to capsaicin and the in vivo administration of MIP-1A/CCL3 resulted in thermal and mechanical hypernociception.(44,61) In the end, these pronociceptive cytokines and chemokines are released from cancer-related infiltrating immune cells, as well as from the tumor cells, in order to promote pain and continual tumor proliferation, resulting in a “feed-forward” process that we show to be inhibited by CB2 receptor activation. Additional explanations of pain inhibition by CB2 agonists may include the inhibition of cancer-induced activation of spinal microglia that contribute to central sensitization,(54) as well as their effects on bone remodeling.
Another major component of bone cancer pain that results in decreased quality of life and survival is osteolysis and bone fracture.(62) When metastatic breast cancer invades the bone, the balance between bone building and bone destruction (normal bone metabolism) is disrupted in favor of net bone loss.(58) Tumor-induced bone remodeling is associated with an acidic environment created by the tumor itself, local acidosis associated with tissue injury, and bone resorption by osteoclasts that directly sensitize and excite local primary afferent fibers within the bone.(63) Both osteolytic activity and osteoblastic activity result in weakening of the normal healthy bone and predispose the patients to skeletal complications including bone pain from loading stress, as well as from damage to sensory nerve endings in the intramedullary space, impaired mobility, pathological fracture, spinal cord compression, and symptomatic hypercalcemia.(43) CB2 receptors are expressed on osteoclasts and have the ability to affect bone homeostasis and structure.(15) CB2 agonists attenuate ovariectomy-induced bone loss in mice.(15) Our radiographic analysis indicated that implanted breast cancer cells induced significant bone remodeling, and that 7-day treatment with JWH015 in cancer-bearing mice reduced the incidence of fractures. Further μCT analysis indicated a cancer-induced modification of both cortical and trabecular bone parameters, which were attenuated by JWH015 treatment. Finally, JWH015 treatment also significantly reduced serum markers of bone degradation in cancer animals, including CTX and TRACP 5b, and increased the serum bone formation marker, osteocalcin. Our studies suggest that treatment with a CB2 agonist attenuates breast cancer–induced bone loss and helps stabilize cancer-inflicted bone activity. Recent studies demonstrating that a selective CB2 agonist significantly inhibits osteoclast activity and osteoclastogenesis,(15,64) combined with our findings of a CB2-dependent reversal of cancer-related bone loss evidenced via markers relating to bone resorption, we believe that CB2 agonists in breast cancer–induced bone malignancy are reducing osteoclast activity and number, therefore decreasing bone loss and related pain.
Recent studies,(13,18,61) as well as findings reported here suggest that CB2 agonists may have antiproliferative effects, yet further explanation of how such compounds may alter proliferation are needed. Bone cancer including breast metastasis results in a marked influx of hematological and inflammatory cells into the medullary portion of the bone, resulting in not only activation of nociceptors,(65–67) but also secretion of RANKL and initiation of NF-κB signaling, which stimulates osteoclastogenesis. Osteolytic processes further promote the proliferation of the metastases,(3) resulting in further invasion of the bone, advanced bone degradation, and pursuing pain. The cancer-driven influx of hematological and inflammatory cells increases the production of cytokines and chemokines,(68) including IL-6, TNFα, MCP-1, and MIP-1A, that accelerate cancer proliferation.(3) IL-6 levels are significantly elevated in breast cancer patients and IL-6 via its major intracellular effector, STAT3, has been reported as protumorigenic in breast cancer cells.(69) Immune and inflammatory cells in close proximity with breast cancer cells are capable of producing prodigious amounts of “startup” IL-6, required for early tumor promotion.(70) Furthermore, IL-6 has been shown to be released from breast cancer cells and act in an autocrine and paracrine fashion through IL-6R/gp130 receptors expressed by the breast cancer cells contributing to cellular transformation and growth.(71) TNFα, which is released from macrophages as well as from primary breast cancer cell lines,(49) regulates epithelial invasion through activation of downstream signaling cascades including JNK and NFκB.(72) Breast cancer cell release of TNFα demonstrates an autocrine and/or a paracrine secretion that results in breast cancer self-proliferation and invasion.(73) Likewise, chemokines such as MCP-1/CCL2 and MIP-1A/CCL3 were recently shown to have trophic qualities, known to attract inflammatory cells to tumors and to inhibit the generation of tumor reactive T cells and natural killer cells.(74,75)
Here we demonstrate that 66.1 breast cancer cells express the CB2 receptor and release cytokines and chemokines that promote self-proliferation, which can be inhibited by a CB2 agonist and reversed by the CB2 antagonist/inverse agonist. The application of cytokines IL-6 and TNFα directly onto 66.1 cells results in a significant increase in proliferation that was attenuated by a CB2 agonist, suggesting that CB2 agonists act by inhibiting cytokines that promote proliferation. Using our murine model, we showed a significant cancer-induced increase in IL-6, TNFα, MCP-1, and MIP-1A within the intramedullary space that was significantly attenuated by the administration of a CB2 agonist. These data support a mechanism of CB2 receptor-mediated inhibition of factors that have been shown both in vitro and in vivo to promote the proliferation and invasion of breast cancer cells. Using H&E staining we found that sustained CB2 agonist treatment in breast cancer–inoculated animals significantly reduced the percentage of tumor burden within the intramedullary cavity of the femoris, supporting the presence of an antiproliferative effect in vivo. Further studies will highlight the importance of each of the cytokines and chemokines using antagonists or antibodies toward the cytokines and chemokines to determine whether the inhibition of one individual factor has similar antiproliferative properties to the broad inhibition seen by the CB2 agonist. In addition, further studies are needed to elucidate the molecular intracellular pathway of CB2 receptor coupling and cytokine/chemokine release in breast cancer cell lines. Previous studies in MMTV-neu mice with ErbB2-driven metastatic breast cancer show that the expression of the CB2 receptor, and a selective CB2 agonist, JWH133, reduced tumor growth, tumor number, and lung metastases by inhibiting active Akt.(17) Some groups have postulated that cannabinoid-mediated inhibition of tumor growth may induce apoptosis through modulation of the Ras-MAPK/ERK and PI3K-AKT pathways.(17,76) These observations may be in line with our data; we have already observed the antitumoral effects of other selective CB2 compounds, JWH015 and AM1241, in vivo and in vitro. However, recent findings also suggest alternative receptor coupling of the CB2 receptor or unidentified cannabinoid receptors and splice variants.(77) The difficulty of determining that effects are in fact specifically cannabinoid receptor–mediated is complicated by the constitutive activity of both cannabinoid receptors that may sustain biological processes, and a lack of neutral antagonists for both receptors. Future studies are likely to determine the molecular pathway in which CB2 agonists successfully inhibit breast cancer proliferation and cytokine-chemokine secretion.
Unlike existing treatments for advanced-stage cancers, we found that sustained treatment of breast cancer–induced bone malignancy with CB2 agonists significantly increased subject survival and helped maintain animal body weight. Patients who develop metastatic breast cancer have an average survival of 1.5 to 3 years.(78) A predictor of a poor prognosis is linked to the lack of treatment available once tumor cells have migrated to the bone.(79) Although advances have been made in breast cancer detection and early stage treatment, there have been relatively few advances in late-stage drug development for cancer proliferation, SREs, and pain.(79) Recent preclinical trials with a B-RAF inhibitor in advanced skin cancer patients resulted in an increased average survival rate of 8 to 12 months, with a significant reduction in tumors and pain. Although the cancer returned, the extended months without pain were beneficial and meaningful to both the patient and family members.(80) Here we have shown, using an established, advanced cancer model, that CB2 agonists, unlike drugs approved for late-stage breast cancer, may yield increased patient survival times.
In conclusion, advanced-stage cancer demands novel drugs for the treatment of bone metastasis. Our most recent data suggests that nonpsychotropic CB2 agonists may serve as a disease-modifying treatment for metastatic breast cancer patients, with the potential to increase the survival rate, relieve pain, improve bone structure, and inhibit tumor cell growth. As demonstrated by the recent discovery of the B-RAF inhibitor for advanced melanoma, drugs that increase the survival time and quality of life greatly benefit patients, family, doctors, and the community.
Supplementary Material
Acknowledgments
This work was supported by NIH grants R01 CA142115-01, the AZCC Better Than Ever grant, and the Maine Cancer Foundation.
Footnotes
Additional Supporting Information may be found in the online version of this article.
Disclosures
All authors state that they have no conflicts of interest.
Authors’ roles: ANL-O: experimental design, data collection and interpretation, manuscript draft/revision; accepts responsibility for integrity of data analysis. KEH: experimental design, data collection and interpretation, manuscript draft/revision. AMS: experimental design, data collection and interpretation, manuscript draft/revision. TML-M: experimental design, data collection, manuscript revision. JJH: data collection, manuscript revision. HLF III: data collection, manuscript revision. AC: experimental design, data collection, manuscript revision. MO-A: data collection, manuscript revision. TN-Z: data collection, manuscript revision. APB: data collection, manuscript draft/revision. JMJ-A: experimental design, data collection, manuscript draft/revision. TK: experimental design, data collection and analysis, manuscript draft. FP: experimental design, manuscript draft. MAN: experimental design, manuscript draft. PWM: experimental design, manuscript draft. TWV: experimental design, data analysis and interpretation, manuscript draft/revision; accepts responsibility for integrity of data analysis.
References
- 1.American Cancer Society . Cancer Facts & Figures, 2010 [Internet] American Cancer Society; Atlanta, GA, USA: 2010. [2012 Aug 5]. Available from: http://www.cancer.org/acs/groups/content/@nho/documents/document/acspc-024113.pdf. [Google Scholar]
- 2.Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006 Oct 15;12(20 Pt 2):6243s–9s. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- 3.DeNardo DG, Johansson M, Coussens LM. Immune cells as mediators of solid tumor metastasis. Cancer Metastasis Rev. 2008;27(1):Mar, 11–8. doi: 10.1007/s10555-007-9100-0. [DOI] [PubMed] [Google Scholar]
- 4.Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, O'Dwyer PJ, Lee RJ, Grippo JF, Nolop K, Chapman PB. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010 Aug 26;363(9):809–19. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisch MJ, Lee JW, Weiss M, Wagner LI, Chang VT, Cella D, Manola JB, Minasian LM, McCaskill-Stevens W, Mendoza TR, Cleeland CS. Prospective, observational study of pain and analgesic prescribing in medical oncology outpatients with breast, colorectal, lung, or prostate cancer. J Clin Oncol. 2012 Jun 1;30(16):1980–8. doi: 10.1200/JCO.2011.39.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stopeck AT, Lipton A, Body JJ, Steger GG, Tonkin K, de Boer RH, Lichinitser M, Fujiwara Y, Yardley DA, Viniegra M, Fan M, Jiang Q, Dansey R, Jun S, Braun A. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol. 2010 Dec 10;28(35):5132–9. doi: 10.1200/JCO.2010.29.7101. [DOI] [PubMed] [Google Scholar]
- 7.Barkin RL, Beckerman M, Blum SL, Clark FM, Koh EK, Wu DS. Should nonsteroidal anti-inflammatory drugs (NSAIDs) be prescribed to the older adult? Drugs Aging. 2010 Oct 1;27(10):775–89. doi: 10.2165/11539430-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 8.Altman RD, Latta LL, Keer R, Renfree K, Hornicek FJ, Banovac K. Effect of nonsteroidal antiinflammatory drugs on fracture healing: a laboratory study in rats. J Orthop Trauma. 1995;9(5):392–400. doi: 10.1097/00005131-199505000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Wilkinson AN, Viola R, Brundage MD. Managing skeletal related events resulting from bone metastases. BMJ. 2008;337:a2041. doi: 10.1136/bmj.a2041. [DOI] [PubMed] [Google Scholar]
- 10.King T, Vardanyan A, Majuta L, Melemedjian O, Nagle R, Cress AE, Vanderah TW, Lai J, Porreca F. Morphine treatment accelerates sarcoma-induced bone pain, bone loss, and spontaneous fracture in a murine model of bone cancer. Pain. 2007 Nov;132(1–2):154–68. doi: 10.1016/j.pain.2007.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vanderah TW, Gardell LR, Burgess SE, Ibrahim M, Dogrul A, Zhong CM, Zhang ET, Malan TP, Jr, Ossipov MH, Lai J, Porreca F. Dynorphin promotes abnormal pain and spinal opioid antinociceptive tolerance. J Neurosci. 2000 Sep 15;20(18):7074–9. doi: 10.1523/JNEUROSCI.20-18-07074.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malan TP, Jr, Ibrahim MM, Lai J, Vanderah TW, Makriyannis A, Porreca F. CB2 cannabinoid receptor agonists: pain relief without psychoactive effects?. Curr Opin Pharmacol. 2003 Feb;3(1):62–7. doi: 10.1016/s1471-4892(02)00004-8. [DOI] [PubMed] [Google Scholar]
- 13.Ibrahim MM, Deng H, Zvonok A, Cockayne DA, Kwan J, Mata HP, Vanderah TW, Lai J, Porreca F, Makriyannis A, Malan TP., Jr Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: pain inhibition by receptors not present in the CNS. Proc Natl Acad Sci U S A. 2003 Sep 2;100(18):10529–33. doi: 10.1073/pnas.1834309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ofek O, Attar-Namdar M, Kram V, Dvir-Ginzberg M, Mechoulam R, Zimmer A, Frenkel B, Shohami E, Bab I. CB2 cannabinoid receptor targets mitogenic Gi protein-cyclin D1 axis in osteoblasts. J Bone Miner Res. 2011 Feb;26(2):308–16. doi: 10.1002/jbmr.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ofek O, Karsak M, Leclerc N, Fogel M, Frenkel B, Wright K, Tam J, Attar-Namdar M, Kram V, Shohami E, Mechoulam R, immer A, Bab I. Peripheral cannabinoid receptor, CB2, regulates bone mass. Proc Natl Acad Sci U S A. 2006 Jan 17;103(3):696–701. doi: 10.1073/pnas.0504187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karsak M, Cohen-Solal M, Freudenberg J, Ostertag A, Morieux C, Kornak U, Essig J, Erxlebe E, Bab I, Kubisch C, de Vernejoul MC, Zimmer A. Cannabinoid receptor type 2 gene is associated with human osteoporosis. Hum Mol Genet. 2005 Nov 15;14(22):3389–96. doi: 10.1093/hmg/ddi370. [DOI] [PubMed] [Google Scholar]
- 17.Caffarel MM, Andradas C, Mira E, Perez-Gomez E, Cerutti C, Moreno-Bueno G, Flores JM, Garcia-Real I, Palacios J, Manes S, Guzman M, Sanchez C. Cannabinoids reduce ErbB2-driven breast cancer progression through Akt inhibition. Mol Cancer. 2010;9:196. doi: 10.1186/1476-4598-9-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rajesh M, Mukhopadhyay P, Batkai S, Hasko G, Liaudet L, Huffman JW, Csiszar A, Ungvari Z, Mackie K, Chatterjee S, Pacher P. CB2-receptor stimulation attenuates TNF-alpha-induced human endothelial cell activation, transendothelial migration of monocytes, and monocyteendothelial adhesion. Am J Physiol Heart Circ Physiol. 2007 Oct;293(4):H2210–8. doi: 10.1152/ajpheart.00688.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klein TW, Newton C, Larsen K, Lu L, Perkins I, Nong L, Friedman H. The cannabinoid system and immune modulation. J Leukoc Biol. 2003 Oct;74(4):486–96. doi: 10.1189/jlb.0303101. [DOI] [PubMed] [Google Scholar]
- 20.Massi P, Vaccani A, Parolaro D. Cannabinoids, immune system and cytokine network. Curr Pharm Des. 2006;12(24):3135–46. doi: 10.2174/138161206777947425. [DOI] [PubMed] [Google Scholar]
- 21.Coussens LM, Werb Z. Inflammatory cells and cancer: think different!. J Exp Med. 2001 Mar 19;193(6):F23–6. doi: 10.1084/jem.193.6.f23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003 Jun;3(6):453–8. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 23.Costa L, Badia X, Chow E, Lipton A, Wardley A. Impact of skeletal complications on patients' quality of life, mobility, and functional independence. Support Care Cancer. 2008 Aug;16(8):879–89. doi: 10.1007/s00520-008-0418-0. [DOI] [PubMed] [Google Scholar]
- 24.Costa L, Major PP. Effect of bisphosphonates on pain and quality of life in patients with bone metastases. Nat Clin Pract Oncol. 2009 Mar;6(3):163–74. doi: 10.1038/ncponc1323. [DOI] [PubMed] [Google Scholar]
- 25.Portenoy RK, Lesage P. Management of cancer pain. Lancet. 1999 May 15;353(9165):1695–700. doi: 10.1016/S0140-6736(99)01310-0. [DOI] [PubMed] [Google Scholar]
- 26.Pockett RD, Castellano D, McEwan P, Oglesby A, Barber BL, Chung K. The hospital burden of disease associated with bone metastases and skeletal-related events in patients with breast cancer, lung cancer, or prostate cancer in Spain. Eur J Cancer Care (Engl) 2010 Nov;19(6):755–60. doi: 10.1111/j.1365-2354.2009.01135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohtori S, Takahashi K, Moriya H, Myers RR. TNF-alpha and TNF-alpha receptor type 1 upregulation in glia and neurons after peripheral nerve injury: studies in murine DRG and spinal cord. Spine (PhilaPa 1976) 2004 May 15;29(10):1082–8. doi: 10.1097/00007632-200405150-00006. [DOI] [PubMed] [Google Scholar]
- 28.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990 Jul 4;82(13):1107–12. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 30.Schwei MJ, Honore P, Rogers SD, Salak-Johnson JL, Finke MP, Ramnaraine ML, Clohisy DR, Mantyh PW. Neurochemical and cellular reorganization of the spinal cord in a murine model of bone cancer pain. J Neurosci. 1999 Dec 15;19(24):10886–97. doi: 10.1523/JNEUROSCI.19-24-10886.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sukhtankar D, Okun A, Chandramouli A, Nelson MA, Vanderah TW, Cress AE, Porreca F, King T. Inhibition of p38-MAPK signaling pathway attenuates breast cancer induced bone pain and disease progression in a murine model of cancer-induced bone pain. Mol Pain. 2011;7:81–96. doi: 10.1186/1744-8069-7-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lozano-Ondoua AN, Wright C, Vardanyan A, King T, Largent-Milnes TM, Nelson M, Jimenez-Andrade JM, Mantyh PW, Vanderah TW. A cannabinoid 2 receptor agonist attenuates bone cancer-induced pain and bone loss. Life Sci. 2010 Apr 24;86(17–18):646–53. doi: 10.1016/j.lfs.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994 Jul;53(1):55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 34.Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980;20:441–62. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- 35.Luger NM, Honore P, Sabino MA, Schwei MJ, Rogers SD, Mach DB, Clohisy DR, Mantyh PW. Osteoprotegerin diminishes advanced bone cancer pain. Cancer Res. 2001 May 15;61(10):4038–47. [PubMed] [Google Scholar]
- 36.Koewler NJ, Freeman KT, Buus RJ, Herrera MB, Jimenez-Andrade JM, Ghilardi JR, Peters CM, Sullivan LJ, Kuskowski MA, Lewis JL, Mantyh PW. Effects of a monoclonal antibody raised against nerve growth factor on skeletal pain and bone healing after fracture of the C57BL/ 6J mouse femur. J Bone Miner Res. 2007 Nov;22(11):1732–42. doi: 10.1359/jbmr.070711. [DOI] [PubMed] [Google Scholar]
- 37.Tang P, Hicks DG. The histopathology of skeletal metastases. In: Heyman D, editor. Bone cancer: progression and therapeutic approaches. Elsevier; New York: 2010. pp. 243–50. [Google Scholar]
- 38.Coleman RE. Skeletal complications of malignancy. Cancer. 1997 Oct 15;80(8 Suppl):1588–94. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1588::aid-cncr9>3.3.co;2-z. [DOI] [PubMed] [Google Scholar]
- 39.Lipton A, Theriault RL, Hortobagyi GN, Simeone J, Knight RD, Mellars K, Reitsma DJ, Heffernan M, Seaman JJ. Pamidronate prevents skeletal complications and is effective palliative treatment in women with breast carcinoma and osteolytic bone metastases: long term follow-up of two randomized, placebo-controlled trials. Cancer. 2000 Mar 1;88(5):1082–90. doi: 10.1002/(sici)1097-0142(20000301)88:5<1082::aid-cncr20>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 40.Abrahm JL, Banffy MB, Harris MB. Spinal cord compression in patients with advanced metastatic cancer: “all I care about is walking and living my life”. JAMA. 2008 Feb 27;299(8):937–46. doi: 10.1001/jama.299.8.937. [DOI] [PubMed] [Google Scholar]
- 41.Chang JT, Green L, Beitz J. Renal failure with the use of zoledronic acid. N Engl J Med. 2003 Oct 23;349(17):1676–9. doi: 10.1056/NEJM200310233491721. discussion 1676–9. [DOI] [PubMed] [Google Scholar]
- 42.Oh WK, Proctor K, Nakabayashi M, Evan C, Tormey LK, Daskivich T, Antras L, Smith M, Neary MP, Duh MS. The risk of renal impairment in hormone-refractory prostate cancer patients with bone metastases treated with zoledronic acid. Cancer. 2007 Mar 15;109(6):1090–6. doi: 10.1002/cncr.22504. [DOI] [PubMed] [Google Scholar]
- 43.Luger NM, Mach DB, Sevcik MA, Mantyh PW. Bone cancer pain: from model to mechanism to therapy. J Pain Symptom Manage. 2005 May;29(5 Suppl):S32–46. doi: 10.1016/j.jpainsymman.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 44.Verri WA, Jr, Cunha TM, Parada CA, Poole S, Cunha FQ, Ferreira SH. Hypernociceptive role of cytokines and chemokines: targets for anal-gesic drug development?. Pharmacol Ther. 2006 Oct;112(1):116–38. doi: 10.1016/j.pharmthera.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 45.Balkwill F, Coussens LM. Cancer: an inflammatory link. Nature. 2004 Sep 23;431(7007):405–6. doi: 10.1038/431405a. [DOI] [PubMed] [Google Scholar]
- 46.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002 Dec 19-26;420(6917):860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cunha FQ, Poole S, Lorenzetti BB, Ferreira SH. The pivotal role of tumour necrosis factor alpha in the development of inflammatory hyperalgesia. Br J Pharmacol. 1992 Nov;107(3):660–4. doi: 10.1111/j.1476-5381.1992.tb14503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cunha JM, Cunha FQ, Poole S, Ferreira SH. Cytokine-mediated inflammatory hyperalgesia limited by interleukin-1 receptor antagonist. Br J Pharmacol. 2000 Jul;130(6):1418–24. doi: 10.1038/sj.bjp.0703434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hiratsuka S, Watanabe A, Aburatani H, Maru Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol. 2006 Dec;8(12):1369–75. doi: 10.1038/ncb1507. [DOI] [PubMed] [Google Scholar]
- 50.Samad TA, Moore KA, Sapirstein A, Billet S, Allchorne A, Poole S, Bonventre JV, Woolf CJ. Interleukin-1beta-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature. 2001 Mar 22;410(6827):471–5. doi: 10.1038/35068566. [DOI] [PubMed] [Google Scholar]
- 51.Cunha TM, Verri WA, Jr, Silva JS, Poole S, Cunha FQ, Ferreira SH. A cascade of cytokines mediates mechanical inflammatory hypernociception in mice. Proc Natl Acad Sci U S A. 2005 Feb 1;102(5):1755–60. doi: 10.1073/pnas.0409225102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Woolf CJ, Allchorne A, Safieh-Garabedian B, Poole S. Cytokines, nerve growth factor and inflammatory hyperalgesia: the contribution of tumour necrosis factor alpha. Br J Pharmacol. 1997 Jun;121(3):417–24. doi: 10.1038/sj.bjp.0701148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lunn CA, Fine J, Rojas-Triana A, Jackson JV, Lavey B, Kozlowski JA, Hipkin RW, Lundell DJ, Bober L. Cannabinoid CB(2)-selective inverse agonist protects against antigen-induced bone loss. Immunopharmacol Immunotoxicol. 2007;29(3–4):387–401. doi: 10.1080/08923970701674997. [DOI] [PubMed] [Google Scholar]
- 54.Romero-Sandoval A, Nutile-McMenemy N, DeLeo JA. Spinal micro-glial and perivascular cell cannabinoid receptor type 2 activation reduces behavioral hypersensitivity without tolerance after peripheral nerve injury. Anesthesiology. 2008 Apr;108(4):722–34. doi: 10.1097/ALN.0b013e318167af74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cravatt BF, Lichtman AH. The endogenous cannabinoid system and its role in nociceptive behavior. J Neurobiol. 2004 Oct;61(1):149–60. doi: 10.1002/neu.20080. [DOI] [PubMed] [Google Scholar]
- 56.Gkoumassi E, Dekkers BG, Droge MJ, Elzinga CR, Schmidt M, Meurs H, Zaagsma J, Nelemans SA. Virodhamine and CP55,940 modulate cAMP production and IL-8 release in human bronchial epithelial cells. Br J Pharmacol. 2007 Aug;151(7):1041–8. doi: 10.1038/sj.bjp.0707320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.White FA, Sun J, Waters SM, Ma C, Ren D, Ripsch M, Steflik J, Cortright DN, Lamotte RH, Miller RJ. Excitatory monocyte chemoattractant protein-1 signaling is up-regulated in sensory neurons after chronic compression of the dorsal root ganglion. Proc Natl Acad Sci U S A. 2005 Sep 27;102(39):14092–7. doi: 10.1073/pnas.0503496102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kiguchi N, Kobayashi Y, Kishioka S. Chemokines and cytokines in neuroinflammation leading to neuropathic pain. Curr Opin Pharmacol. 2012 Feb;12(1):55–61. doi: 10.1016/j.coph.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 59.Abbadie C, Lindia JA, Cumiskey AM, Peterson LB, Mudgett JS, Bayne EK, DeMartino JA, MacIntyre DE, Forrest MJ. Impaired neuropathic pain responses in mice lacking the chemokine receptor CCR2. Proc Natl Acad Sci U S A. 2003 Jun 24;100(13):7947–52. doi: 10.1073/pnas.1331358100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cunha FQ, Lorenzetti BB, Poole S, Ferreira SH. Interleukin-8 as a mediator of sympathetic pain. Br J Pharmacol. 1991 Nov;104(3):765–7. doi: 10.1111/j.1476-5381.1991.tb12502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang N, Inan S, Cowan A, Sun R, Wang JM, Rogers TJ, Caterina M, Oppenheim JJ. A proinflammatory chemokine, CCL3, sensitizes the heat-and capsaicin-gated ion channel TRPV1. Proc Natl Acad Sci U S A. 2005 Mar 22;102(12):4536–41. doi: 10.1073/pnas.0406030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mundy GR, Yoneda T. Facilitation and suppression of bone metastasis. Clin Orthop Relat Res. 1995 Mar;(312):34–44. [PubMed] [Google Scholar]
- 63.Mantyh PW, Clohisy DR, Koltzenburg M, Hunt SP. Molecular mechanisms of cancer pain. Nat Rev Cancer. 2002 Mar;2(3):201–9. doi: 10.1038/nrc747. [DOI] [PubMed] [Google Scholar]
- 64.George KL, Saltman LH, Stein GS, Lian JB, Zurier RB. Ajulemic acid, a nonpsychoactive cannabinoid acid, suppresses osteoclastogenesis in mononuclear precursor cells and induces apoptosis in mature osteoclast-like cells. J Cell Physiol. 2008 Mar;214(3):714–20. doi: 10.1002/jcp.21263. [DOI] [PubMed] [Google Scholar]
- 65.Poonawala T, Levay-Young BK, Hebbel RP, Gupta K. Opioids heal ischemic wounds in the rat. Wound Repair Regen. 2005 Mar-Apr;13(2):165–74. doi: 10.1111/j.1067-1927.2005.130207.x. [DOI] [PubMed] [Google Scholar]
- 66.Scadding JW. The permanent anatomical effects of neonatal capsaicin on somatosensory nerves. J Anat. 1980 Oct;131(Pt 3):471–82. [PMC free article] [PubMed] [Google Scholar]
- 67.Hill EL, Turner R, Elde R. Effects of neonatal sympathectomy and capsaicin treatment on bone remodeling in rats. Neuroscience. 1991;44(3):747–55. doi: 10.1016/0306-4522(91)90094-5. [DOI] [PubMed] [Google Scholar]
- 68.Ben-Baruch A. The multifaceted roles of chemokines in malignancy. Cancer Metastasis Rev. 2006 Sep;25(3):357–71. doi: 10.1007/s10555-006-9003-5. [DOI] [PubMed] [Google Scholar]
- 69.Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer. 2005 Nov;41(16):2502–12. doi: 10.1016/j.ejca.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 70.Karin M, Lawrence T, Nizet V. Innate immunity gone awry: linking microbial infections to chronic inflammation and cancer. Cell. 2006 Feb 24;124(4):823–35. doi: 10.1016/j.cell.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 71.Grivennikov S, Karin M. Autocrine IL-6 signaling: a key event in tumorigenesis?. Cancer Cell. 2008 Jan;13(1):7–9. doi: 10.1016/j.ccr.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 72.Hagemann T, Wilson J, Kulbe H, Li NF, Leinster DA, Charles K, Klemm F, Pukrop T, Binder C, Balkwill FR. Macrophages induce invasiveness of epithelial cancer cells via NF-kappa B and JNK. J Immunol. 2005 Jul 15;175(2):1197–205. doi: 10.4049/jimmunol.175.2.1197. [DOI] [PubMed] [Google Scholar]
- 73.Szlosarek P, Charles KA, Balkwill FR. Tumour necrosis factor-alpha as a tumour promoter. Eur J Cancer. 2006 Apr;42(6):745–50. doi: 10.1016/j.ejca.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 74.Baay M, Brouwer A, Pauwels P, Peeters M, Lardon F. Tumor cells and tumor-associated macrophages: secreted proteins as potential targets for therapy. Clin Dev Immunol. 2011;2011:5651–87. doi: 10.1155/2011/565187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maghazachi AA. Role of chemokines in the biology of natural killer cells. Curr Top Microbiol Immunol. 2010;341:37–58. doi: 10.1007/82_2010_20. [DOI] [PubMed] [Google Scholar]
- 76.Ellert-Miklaszewska A, Kaminska B, Konarska L. Cannabinoids down-regulate PI3K/Akt and Erk signalling pathways and activate proapoptotic function of Bad protein. Cell Signal. 2005 Jan;17(1):25–37. doi: 10.1016/j.cellsig.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 77.Liu QR, Pan CH, Hishimoto A, Li CY, Xi ZX, Liorente-Berzal A, Viveros MP, Ishiguro H, Arinami T, Onaivi ES, Uhl GR. Species differences in cannabinoid receptor 2 (CNR2 gene): Identification of novel human and rodent CB2 isoforms, differential tissue expression and regulation by cannabinoid receptor ligands. Genes Brain Behav. 2009;8(5):519–30. doi: 10.1111/j.1601-183X.2009.00498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Allen JM. Economic/societal burden of metastatic breast cancer: a US perspective. Am J Manag Care. 2010 Sep;16(9):697–704. [PubMed] [Google Scholar]
- 79.Mercadante S. Malignant bone pain: pathophysiology and treatment. Pain. 1997 Jan;69(1–2):1–18. doi: 10.1016/s0304-3959(96)03267-8. [DOI] [PubMed] [Google Scholar]
- 80.Harmon A. Target cancer. New drugs stir debate on rules of clinical trials [Internet] New York Times (New York edition); New York (NY): Sep 19, 2010. [2012 Sep 5]. p. A1. Available from: http://www.nytimes.com/2010/09/19/health/research/19trial.html?pagewanted=all#. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.