Abstract
Background
Difficulty turning during gait is a major contributor to mobility disability, falls and reduced quality of life in patients with Parkinson’s disease (PD). Unfortunately, the assessment of mobility in the clinic may not adequately reflect typical mobility function or its variability during daily life. We hypothesized that quality of turning mobility, rather than overall quantity of activity, would be impaired in people with PD over 7 days of continuous recording.
Methods
13 subjects with PD and 8 healthy control subjects of similar age wore 3 Opal inertial sensors (on their belt and on each foot) throughout 7 consecutive days during normal daily activities. Turning metrics included average and coefficient of variation (CV) of: 1) number of turns per hour, 2) turn angle amplitude, 3) turn duration, 4) turn mean velocity, and 5) number of steps per turn. Turning characteristics during continuous monitoring were compared with turning 90 and 180 degrees in a observed gait task.
Results
No differences were found between PD and control groups for observed turns. In contrast, subjects with PD showed impaired quality of turning compared to healthy control subjects (Turn Mean Velocity: 43.3±4.8°/s versus 38±5.7°/s, mean number of steps 1.7±1.1 versus 3.2±0.8). In addition, PD patients showed higher variability within the day and across days compared to controls. However, no differences were seen between PD and control subjects in the overall activity (number of steps per day or percent of the day walking) during the 7 days.
Conclusions
We show that continuous monitoring of natural turning during daily activities inside or outside the home is feasible for patients with PD and the elderly. This is the first study showing that continuous monitoring of turning was more sensitive to PD than observed turns. In addition, the quality of turning characteristics was more sensitive to PD than quantity of turns. Characterizing functional turning during daily activities will address a critical barrier to rehabilitation practice and clinical trials: objective measures of mobility characteristics in real-life environments.
Keywords: Parkinson’s disease, functional mobility, continuous monitoring
Introduction
The assessment of mobility function during activities of daily living is now technically possible (Horak, King, & Mancini, 2014). Body-worn sensors can measure mobility, similarly to how a Heart Rate Holter Monitor characterizes cardiac function over days and weeks. In fact, a critical barrier to effective physical therapy is the need for measuring mobility in natural, functional settings across long periods of time. Continuous monitoring of mobility allows characterization of fluctuations across the day and week, response to medications and other interventions and influence of real-world distractions and complex environments (Horak et al., 2014). Moreover, mobility assessment in the home and community provides important information about disease progression, fall risk and effectiveness of rehabilitation.
Studies have suggested that the assessment of mobility in the clinic or laboratory in patients with Parkinson’s disease does not adequately reflect typical mobility function during daily life (Lidstone, 2014; Zampieri, Salarian, Carlson-Kuhta, Nutt, & Horak, 2011). In addition, increased attentional control, alertness, effort to impress the examiner during clinical or laboratory testing may enhance motor performance. A recent pilot study from our group reported worse mobility performance, assessed with an Instrumented Timed-Up and Go test, in the home compared to the laboratory in a group of patients with mild PD (Zampieri et al., 2011). In addition, single, sparsely-spaced measures cannot assess within-day, day-to-day or other clinically relevant windows of change such as medication-induced motor fluctuations or fatigue.
Currently, activity monitors reflect the quantity, but not the quality of mobility. Activity monitors use accelerations to measure relative daily activity and/or the percent of the day a subject is standing, walking or sitting/lying (Ford et al., 2010; Skidmore et al., 2008; Zwartjes, Heida, van Vugt, Geelen, & Veltink, 2010). More informative measures include total activity duration, total number of steps taken, the time spent in each activity, and time spent engaging in different intensity levels of activity (Cavanaugh et al., 2012; Lord et al., 2013; Rochester, Chastin, Lord, Baker, & Burn, 2012). Whilst informative, these measures do not characterize specific gait impairments, features of postural control, or the patterns of daily activity.
Recent studies (Weiss, Herman, Giladi, & Hausdorff, 2014a, 2014b; Weiss et al., 2011) have focused attention on quality of gait mobility in PD measured at home using wearable, light-weight inertial sensors placed on different parts of the body. However, turning, defined as changing of walking direction, is an aspect of mobility that has been overlooked. Turning can be even more frequent than straight-ahead walking in older people confined to small homes. Turning performance is compromised in PD, leading to a significant disability, freezing of gait, falls, and loss of function (E. Stack & Ashburn, 2008). Laboratory studies reported abnormal spatial and temporal turning strategies, as well as increased number of steps to turn and turn duration (Hong, Perlmutter, & Earhart, 2009; Huxham, Baker, Morris, & Iansek, 2008; Mak, Patla, & Hui-Chan, 2008; E. L. Stack, Ashburn, & Jupp, 2006).
However, no studies have yet attempted to measure natural turning continuously during the day in the home and community environments. We recently introduced and validated a novel method to measure turning mobility over a week of continuous recording calculated from both accelerometers and gyroscopes (El-Gohary et al., 2013) in healthy older subjects and subjects with PD.
The objective of the present study was to determine the feasibility and potential usefulness of continuous monitoring of turning during spontaneous, daily activity in people with PD and age-matched elderly subjects.
Methods
Subjects
We examined turning in 13 subjects with PD, 65 ± 6.0 years, 24.5 ± 7.5 Unified Parkinson’s Disease Rating Scale (UPDRS Part III tested ON medication), mean±STD Levodopa Equivalent Dose: 886.8±318.8mg (range from 506mg to 1448mg); and 19 control subjects of similar age (67 ± 9.0 years). Inclusion criteria for PD were diagnosis of idiopathic Parkinson’s disease treated with levodopa (Hoehn and Yahr scores of II-IV). Exclusion criteria for all the participants were dementia, others factors affecting gait, like hip replacement, musculoskeletal disorders, uncorrected vision or vestibular problems, or inability to stand and walk in the home without an assistive device.
Data collection and processing
Subjects wore 3 Opal inertial sensors (APDM, Inc., Portland, OR, USA) for an average of ten hours, every day for seven days. On the morning of the first day, a study coordinator met subjects at their homes and instructed them on how to wear the sensors and charge them at the end of each day. The 3 Opal sensors were worn, with elastic bands, on the pelvis at the lumbar 5, vertebral level and one on top of each foot. In addition, with the study coordinator the subjects performed an observed, short walk back and forth through a doorway, with 5 repetitions of 90 degree and 180 degree turns. The study coordinator also administered the UPDRS Motor Part III while ON antiparkinsonian medication. Participants wore the Opal sensors during the observed task and UPDRS and all day for seven days, and recharged them each night. Data were stored in the internal memory of the Opal and downloaded to a laptop at the end of the 7 days. An Opal is lightweight (22 g), has a battery life of 16 h, and includes 8 GB of storage, which can record over 30 days of data. The Opals use patented, wireless, synchronization technology to ensure multiple units collect data with a precision of better than ±1ms.
Data analysis and extracted parameters
The algorithm for detecting and characterizing turning was detailed previously (El-Gohary et al., 2013). In summary, periods of walking were first detected and the walking period of 10 seconds or longer were defined as gait bouts, and were used by the algorithm to search for potential turns. We defined a turn as a trunk rotation about the transverse plane with a minimum of 45 degrees, accompanied with at least one right and one left foot stepping. We used the rotational rate of the lumbar sensor to detect turning events during bouts. Turns were detected from segments in which the maxima of the vertical rotational rate exceed a threshold of 15 degrees/s. Only turns with durations between .5 and 10 seconds, and turn angles of 45 degrees or more were considered. Relative turn angles were obtained by integrating the angular rate of the lumbar sensor about the vertical axis. This turning algorithm was validated with Motion Analysis System (Santa Rosa, CA) in a previous study in the Balance Disorders Laboratory at the Oregon Health and Science University (OHSU) in 15 subjects with PD and 19 age-matched control subjects. Compared to Motion Analysis, the algorithm maintained a sensitivity of 0.90 and a specificity of 0.75 for detecting turns.
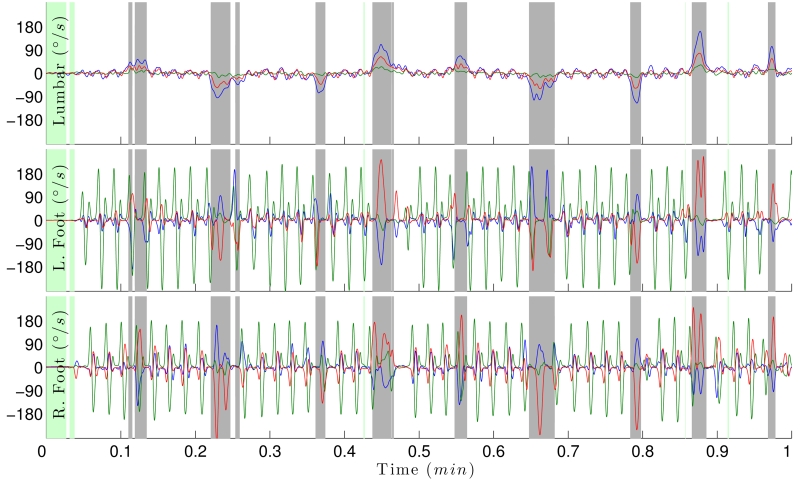
Figure 1 shows rotational rate of the gyroscope sensors placed on the lumbar, right and left foot. The figure shows periods of turning (gray) during walking and when a subject is sedentary (green).
Figure 1.
Rotational rate of the lumbar (top), left and right foot (bottom). Blue, green and red traces are the gyroscope x, y and z-axes. Green areas represent periods in which the subject is sedentary; gray represents periods of turning, and white represent periods of walking in this 30 second-segment.
The analysis of the rotational rate and acceleration of the lumbar and feet sensors provided the following metrics: hourly frequency of turning, duration of each turn, number of steps needed to complete a turn, peak and average rotational turning rate. Furthermore, we used the coefficient of variation (CV) of each metric to analyze the variability of turns characteristics throughout the day and week. Activity rate was also calculated as the percent of time when subjects were walking or turning, compared to the total monitoring time per day.
Statistical Analysis
Normality of data was checked with the Shapiro-Wilk test, One-way Analysis of Variance was used to compare selected metrics between PD and control subjects. Lastly, Pearson correlation coefficients were used to assess the relationships between turning metrics, and UPDRS III.
Results
Turning in an observed test is not as sensitive to PD as turning during daily activities
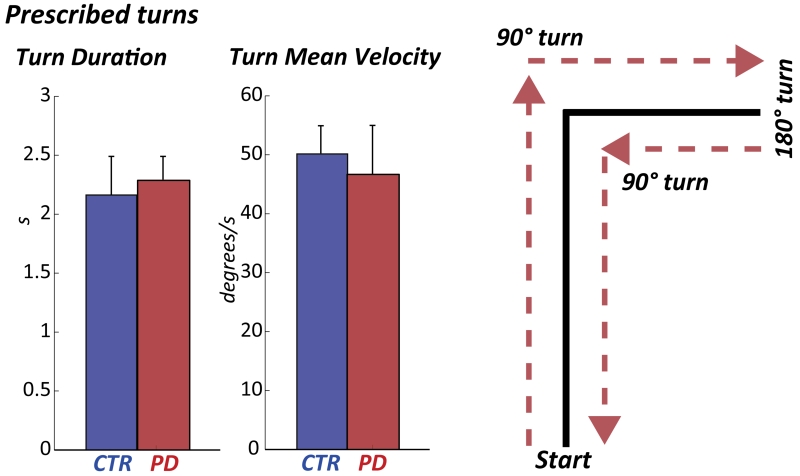
Interestingly, turning performances assessed with the observed test, and quantified by the Turn Mean Velocity and Turn Duration, were similar between healthy and PD subjects (p=0.34 and p=0.33), see Figure 2. In contrast, the Turn Mean Velocity from daily activities was significantly slower in PD compared to healthy subjects (p=0.04, Figure 3).
Figure 2.
Turn Duration and Mean Velocity during the prescribed task at home (mean±STD). The prescribed task is depicted on the right panel.
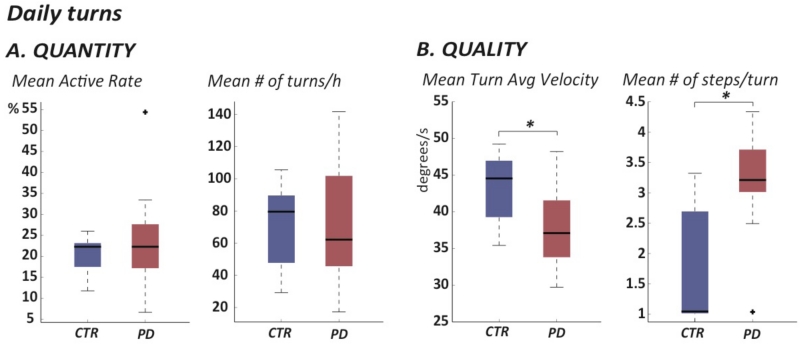
Figure 3.
Box-plot of Quantity (A) and Quality (B) of mobility measured across the 7 days of continuous monitoring.
Quality, but not quantity, of turning differs between PD and control groups
PD group turned an average of 70 times per hour and the control group turned an average of 71 times per hour. Quantity of activity, measured by the Active Rate and the mean number of turns/hour across the seven days was similar between PD and healthy subjects, indicating a similar level of activity in both groups. See details in Table 1 and Figure 3.
Table 1.
Mean and STD in healthy subjects (CTR) and PD subjects of turning metrics calculated during daily activity across 7 days of continuous monitoring. The table also shows the F-value and p-value for the statistical test.
|
CTR
|
PD
|
|||||
|---|---|---|---|---|---|---|
| Mean | STD | Mean | STD | F-value | p-value | |
| Active Rate (%) | 20.5 | 4.6 | 23.6 | 11.7 | 0.5 | 0.50 |
|
| ||||||
| Number of turns /hour | 71.1 | 26.6 | 70.7 | 35.2 | 0.0 | 0.98 |
|
| ||||||
| Turn Angle (degrees) | 95.8 | 4.7 | 92.0 | 6.8 | 1.9 | 0.18 |
|
| ||||||
| CV Turn Angle | 0.17 | 0.09 | 0.14 | 0.08 | 0.50 | 0.50 |
|
| ||||||
| Turn Duration (s) | 1.91 | 0.24 | 2.01 | 0.29 | 0.60 | 0.40 |
|
| ||||||
| CV Turn Duration | 0.16 | 0.07 | 0.25 | 0.22 | 1.27 | 0.20 |
|
| ||||||
| Number of steps /turn | 1.7 | 1.1 | 3.2 | 0.8 | 13.0 | 0.002 |
|
| ||||||
| CV Number of steps /turn | 0.28 | 0.15 | 0.22 | 0.08 | 1.34 | 0.26 |
|
| ||||||
| Turn Mean Velocity (degrees/s) | 43.3 | 4.8 | 38.0 | 5.7 | 4.8 | 0.04 |
|
| ||||||
| CV Turn Mean Velocity | 0.16 | 0.08 | 0.18 | 0.09 | 0.24 | 0.60 |
In contrast, quality of turning was significantly compromised in PD compared to healthy subjects. Specifically, besides turn mean velocity, the mean number of steps to complete a turn was larger in PD than control subjects (3.2 versus 1.7 steps).
Furthermore, PD subjects tended to complete shorter turns with smaller turn angles (92.0 degrees), compared to the control group (96 degrees). Table 1 also shows that variability of turning metrics, measured by coefficient of variation (CV), was consistently larger in the PD than control subjects.
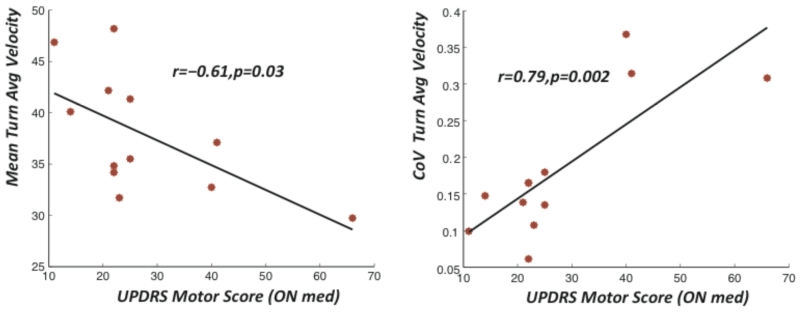
Disease severity is related to continuous measures of turning mobility
The coefficient of variation of turn velocity showed a high correlation with the UPDRS motor score (r=0.79, p=0.01). Similarly, the correlation between the number of steps per turn (r=0.61 and p=0.03) and turn velocity (r=0.61, p=0.03) with the UPDRS motor score were statistically significant.
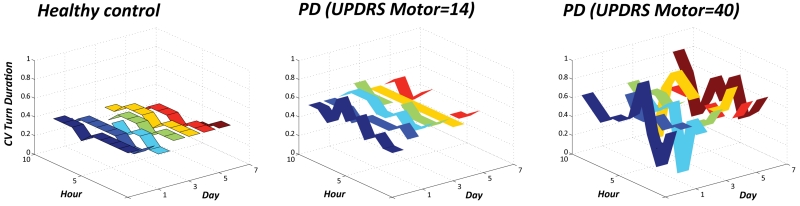
Subjects with PD show larger variability of turning compared to healthy subjects
Figure 5 clearly shows the trend towards increase in variability in PD subjects for turn duration within the same day and across day compared to healthy subjects. Interestingly, variability seems to increase with disease severity (measured by UPDRS III) which may reflect motor fluctuations which tend to be larger in more severely affected PD subjects.
Figure 5.
The CV of Turn Duration is plotted over the 7 days and every hour of the day for a representative healthy subjects, a mild PD and a moderate PD.
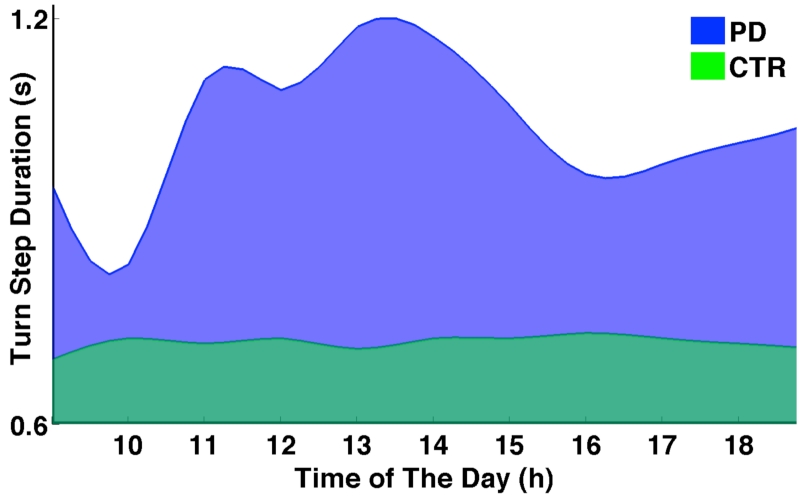
Figure 6 shows the average step duration during turning for a PD and a control subject throughout a 10-hour period of each day, averaged across seven days. Compared to the consistent step duration of the control subject, the PD subject not only has slower steps, but also exhibits greater turn step variability.
Figure 6.
PD subjects exhibit more motor fluctuations during the day. Blue waves show the hourly fluctuation in step duration during turns of a patient with PD (blue), compared to a control subject (green), averaged across seven days.
Discussion
This is the first study characterizing turning during a week of daily activities using body-worn inertial sensors. The elderly subjects in our study and subjects with PD turned approximately 700 times per day while walking, which allowed averaging of turning metrics over many trials. Our study demonstrates that continuous monitoring of natural turning during daily activities inside or outside the home is feasible and useful for elderly people with or without Parkinson’s disease. These findings suggest that: 1) continuous monitoring of turning may be more useful than an observed test in revealing differences between PD and healthy subjects, 2) a decline in the quality of turning, but not quantity, is present in PD, and that such decline may be associated with disease severity, and 3) measures of turning variability may be indicative of disease- and medication-induced motor fluctuations.
Decision-making by physical therapists or physicians could benefit by prescribing a week of continuous monitoring of quality of mobility in their patients. The present study showed that turning characteristics of people with PD during an observed gait task for a clinician are more normal than turning during daily life. In addition, continuous monitoring of movement with small, body-worn inertial sensors allows clinicians to aseess the effect of disease, rehabilitation intervention or medications on patient’s real-life, functional mobility. Objective measures of mobility can replace subjective diaries and observed tests in the clinic that do not reflect actual functional performance.
The decline in quality, but not quantity, of turning activity suggests that people with PD do not alter how often they turn but they do alter how they turn. Turning may be more vulnerable to functional impairments than straight-ahead, linear gait. Compared to walking straight, turning involves more inter-limb coordination, more coupling between posture and gait (Patla, Adkin, & Ballard, 1999), and modifications of locomotor patterns requiring frontal lobe cognitive and executive functional that control postural transitions (Herman, Giladi, & Hausdorff, 2011; King et al., 2012). In fact, it is possible that speed of turning will predict functional limitations and fall risk even better than gait speed.
As PD advances, patients increasingly experience motor fluctuations. These fluctuations are one of the most common and troublesome problems in the management of PD and are the major reason for surgical intervention (Obeso et al., 2000; Olanow & Obeso, 2000). Neurologists currently rely on anecdotal patient interviews and subjective diaries for medical decision-making regarding motor fluctuations (Hauser, Deckers, & Lehert, 2004; Hauser et al., 2006). Movement disorder specialists spend much of their effort with patients who have PD to reduce motor fluctuations by adjusting medication schedules and dosage, and referring patients to neurosurgery for deep brain stimulation (DBS). Precise measures of mobility, objectively characterizing motor fluctuations could provide neurologists with a screening tool for motor fluctuations, and will potentially help reduce the extensive, costly visits necessary to adjust stimulation settings after DBS surgery.
The present study demonstrates that it is simple and feasible for patients to wear 3 sensors and obtain continuous measures of mobility in the home and community. The results from this study suggest that adding measures of mobility that are objective and continuous extends the usefulness of home evaluations and provides a useful and realistic idea of how patients are functioning during their daily lives. All the subjects applied and charged the sensors every day without problems. It only took clinicians about 10 minutes to teach patients how to use the sensors. The data can be collected up to a month and uploaded to a laptop or database for analysis. Rehabilitation assessment and decision-making will benefit from new technologies that unobtrusively quantify mobility impairments during daily functional activities. There are several limitations to this study: i) limited sample size, ii) the prescribed test was carried out only in the ON state, iii) the time of medication intake was not tracked, therefore motor fluctuation could not be directly related to medication cycles.
Figure 4.
Relationship between disease severity (measured by UPDRS Motor Score) and turning metrics during daily activities.
Acknowlegments
This research was supported by the National Institute of Neurological Disorders and Stroke (NINDS) of the National Institutes of Health under award number R41NS076088-02, Oregon Center for Aging and Technology (ORCATECH) pilot grant and the Italian Ministry for Foreign Affairs-Direzione Generale per la Promozione del Sistema Paese. The authors would like to thank Courtney Bowman and Ryan Meyer for helping with data collection and storing.
Footnotes
Declaration of Interest
OHSU, Drs. El-Gohary, McNames and Horak have a significant financial interest in APDM, a company that may have a commercial interest in the results of this research and technology. This potential institutional and individual conflict has been reviewed and managed by OHSU.
References
- Cavanaugh JT, Ellis TD, Earhart GM, Ford MP, Foreman KB, Dibble LE. Capturing ambulatory activity decline in Parkinson’s disease. J Neurol Phys Ther. 2012;36(2):51–57. doi: 10.1097/NPT.0b013e318254ba7a. doi: 10.1097/NPT.0b013e318254ba7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Gohary M, Pearson S, McNames J, Mancini M, Horak F, Mellone S, Chiari L. Continuous monitoring of turning in patients with movement disability. Sensors (Basel) 2013;14(1):356–369. doi: 10.3390/s140100356. doi: 10.3390/s140100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MP, Malone LA, Walker HC, Nyikos I, Yelisetty R, Bickel CS. Step activity in persons with Parkinson’s disease. J Phys Act Health. 2010;7(6):724–729. doi: 10.1123/jpah.7.6.724. [DOI] [PubMed] [Google Scholar]
- Hauser RA, Deckers F, Lehert P. Parkinson’s disease home diary: further validation and implications for clinical trials. Mov Disord. 2004;19(12):1409–1413. doi: 10.1002/mds.20248. doi: 10.1002/mds.20248. [DOI] [PubMed] [Google Scholar]
- Hauser RA, Russ H, Haeger DA, Bruguiere-Fontenille M, Muller T, Wenning GK. Patient evaluation of a home diary to assess duration and severity of dyskinesia in Parkinson disease. Clin Neuropharmacol. 2006;29(6):322–330. doi: 10.1097/01.WNF.0000229546.81245.7F. doi: 10.1097/01.WNF.0000229546.81245.7F. [DOI] [PubMed] [Google Scholar]
- Herman T, Giladi N, Hausdorff JM. Properties of the ‘timed up and go’ test: more than meets the eye. Gerontology. 2011;57(3):203–210. doi: 10.1159/000314963. doi: 10.1159/000314963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong M, Perlmutter JS, Earhart GM. A kinematic and electromyographic analysis of turning in people with Parkinson disease. Neurorehabil Neural Repair. 2009;23(2):166–176. doi: 10.1177/1545968308320639. doi: 10.1177/1545968308320639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horak F, King L, Mancini M. Role of Body-Worn Movement Monitor Technology for Balance and Gait Rehabilitation. Phys Ther. 2014 doi: 10.2522/ptj.20140253. doi: 10.2522/ptj.20140253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxham F, Baker R, Morris ME, Iansek R. Footstep adjustments used to turn during walking in Parkinson’s disease. Mov Disord. 2008;23(6):817–823. doi: 10.1002/mds.21932. doi: 10.1002/mds.21932. [DOI] [PubMed] [Google Scholar]
- King LA, Mancini M, Priest K, Salarian A, Rodrigues-de-Paula F, Horak F. Do clinical scales of balance reflect turning abnormalities in people with Parkinson’s disease? J Neurol Phys Ther. 2012;36(1):25–31. doi: 10.1097/NPT.0b013e31824620d1. doi: 10.1097/NPT.0b013e31824620d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidstone SC. Great expectations: the placebo effect in Parkinson’s disease. Handb Exp Pharmacol. 2014;225:139–147. doi: 10.1007/978-3-662-44519-8_8. doi: 10.1007/978-3-662-44519-8_8. [DOI] [PubMed] [Google Scholar]
- Lord S, Godfrey A, Galna B, Mhiripiri D, Burn D, Rochester L. Ambulatory activity in incident Parkinson’s: more than meets the eye? J Neurol. 2013;260(12):2964–2972. doi: 10.1007/s00415-013-7037-5. doi: 10.1007/s00415-013-7037-5. [DOI] [PubMed] [Google Scholar]
- Mak MK, Patla A, Hui-Chan C. Sudden turn during walking is impaired in people with Parkinson’s disease. Exp Brain Res. 2008;190(1):43–51. doi: 10.1007/s00221-008-1446-1. doi: 10.1007/s00221-008-1446-1. [DOI] [PubMed] [Google Scholar]
- Obeso JA, Rodriguez-Oroz MC, Chana P, Lera G, Rodriguez M, Olanow CW. The evolution and origin of motor complications in Parkinson’s disease. Neurology. 2000;55(11 Suppl 4):S13–20. discussion S21-13. [PubMed] [Google Scholar]
- Olanow CW, Obeso JA. Preventing levodopa-induced dyskinesias. Ann Neurol. 2000;47(4 Suppl 1):S167–176. discussion S176-168. [PubMed] [Google Scholar]
- Patla AE, Adkin A, Ballard T. Online steering: coordination and control of body center of mass, head and body reorientation. Exp Brain Res. 1999;129(4):629–634. doi: 10.1007/s002210050932. [DOI] [PubMed] [Google Scholar]
- Rochester L, Chastin SF, Lord S, Baker K, Burn DJ. Understanding the impact of deep brain stimulation on ambulatory activity in advanced Parkinson’s disease. J Neurol. 2012;259(6):1081–1086. doi: 10.1007/s00415-011-6301-9. doi: 10.1007/s00415-011-6301-9. [DOI] [PubMed] [Google Scholar]
- Skidmore FM, Mackman CA, Pav B, Shulman LM, Garvan C, Macko RF, Heilman KM. Daily ambulatory activity levels in idiopathic Parkinson disease. J Rehabil Res Dev. 2008;45(9):1343–1348. [PubMed] [Google Scholar]
- Stack E, Ashburn A. Dysfunctional turning in Parkinson’s disease. Disabil Rehabil. 2008;30(16):1222–1229. doi: 10.1080/09638280701829938. doi: 10.1080/09638280701829938. [DOI] [PubMed] [Google Scholar]
- Stack EL, Ashburn AM, Jupp KE. Strategies used by people with Parkinson’s disease who report difficulty turning. Parkinsonism Relat Disord. 2006;12(2):87–92. doi: 10.1016/j.parkreldis.2005.08.008. doi: 10.1016/j.parkreldis.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Weiss A, Herman T, Giladi N, Hausdorff JM. New evidence for gait abnormalities among Parkinson’s disease patients who suffer from freezing of gait: insights using a body-fixed sensor worn for 3 days. J Neural Transm. 2014a doi: 10.1007/s00702-014-1279-y. doi: 10.1007/s00702-014-1279-y. [DOI] [PubMed] [Google Scholar]
- Weiss A, Herman T, Giladi N, Hausdorff JM. Objective assessment of fall risk in Parkinson’s disease using a body-fixed sensor worn for 3 days. PLoS One. 2014b;9(5):e96675. doi: 10.1371/journal.pone.0096675. doi: 10.1371/journal.pone.0096675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A, Sharifi S, Plotnik M, van Vugt JP, Giladi N, Hausdorff JM. Toward automated, at-home assessment of mobility among patients with Parkinson disease, using a body-worn accelerometer. Neurorehabil Neural Repair. 2011;25(9):810–818. doi: 10.1177/1545968311424869. doi: 10.1177/1545968311424869. [DOI] [PubMed] [Google Scholar]
- Zampieri C, Salarian A, Carlson-Kuhta P, Nutt JG, Horak FB. Assessing mobility at home in people with early Parkinson’s disease using an instrumented Timed Up and Go test. Parkinsonism Relat Disord. 2011;17(4):277–280. doi: 10.1016/j.parkreldis.2010.08.001. doi: 10.1016/j.parkreldis.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwartjes DG, Heida T, van Vugt JP, Geelen JA, Veltink PH. Ambulatory monitoring of activities and motor symptoms in Parkinson’s disease. IEEE Trans Biomed Eng. 2010;57(11) doi: 10.1109/TBME.2010.2049573. doi: 10.1109/TBME.2010.2049573. [DOI] [PubMed] [Google Scholar]