Abstract
Background
Peritoneal adhesion formation is a well-recognized consequence of abdominal and pelvic surgery, causing infertility, chronic pelvic pain and intestinal obstruction. We hypothesized that ghrelin, a 28 amino acid peptide predominantly found in the stomach, plays an important role in preventing post-operative surgical adhesions. The purpose of this study is to develop a new surgical peritoneal adhesion model to define the role that ghrelin plays in wound healing and adhesion formation.
Materials and Methods
C57BL/6 wild type mice (n=40) and growth hormone secretagogue receptor-knockout (GHSR KO) mice (n=20) underwent a midline laparotomy to establish a peritoneal adhesion model characterized by the combination of two different techniques: ischemic peritoneal buttons and cecal multiple abrasion. All mice received intraperitoneal injections with ghrelin (0.16 mg/kg) or saline twice daily for 20 days post-surgery. Peritoneal ischemic buttons were harvested to determine protein expression of collagen (Masson’s trichrome, Picrosirius red stain and Western blot).
Results
The novel mouse model demonstrated consistent and easily reproducible formation of intra-abdominal adhesions. Ghrelin administration significantly reduced post-operative adhesion formation (p-value < 0.001) in wild type mice. The anti-fibrotic effect of ghrelin in wild type mice was confirmed by measuring collagen I protein levels via Western blot analysis. The anti-adhesion effect of ghrelin seen in wild type mice was not detected in GHSR KO mice demonstrating that this effect is mediated by the GHSR-1a receptor.
Conclusions
Ghrelin administration may improve surgical outcome by reducing peritoneal adhesion formation and fibrotic response in a mouse model.
Keywords: Ghrelin, Post-Operative Adhesion, Collagen I, Fibrosis, GHSR-1a receptor
Introduction
Intraperitoneal adhesion formation is a well-recognized consequence of abdominal and pelvic surgery. Postoperative intra-abdominal and pelvic adhesions are a leading cause of infertility, chronic pelvic pain, and intestinal obstruction [1]. The incidence of adhesion-related re-admissions according to the surgical procedure ranges between 0.1% and 24%, with the highest incidences occurring after pelvic surgery [2]. In 2011, adhesiolysis was reported to be responsible for 351,777 hospitalizations, 976,332 days of inpatient care, and $2.3 billion in hospitalization and surgeon expenditures [3, 4]. Beyond the economic impact, these post-surgical adhesions are responsible for hospital readmissions and delays in patient recovery. The presence of adhesions may increase the risk of inadvertent visceral injuries and lengthen the operation time during subsequent laparotomies [5, 6]. The risk of needing repeat abdominal surgery is relatively high and is expected to increase in the Western world with the increase in life expectancy and developments in surgical technique [7, 8].
The pathophysiology of adhesions originates from an inflammatory reaction stimulated by tissue trauma with increased vessel permeability, extravasation of immune cells and deposition of fibrin. In ischemic conditions, such as after surgery, physiological fibrinolysis is insufficient leading to the formation of fibrin bridges between the areas of traumatized tissue and unaffected tissue. Subsequent organization of fibrin matrix with neovascularization and invasion of fibroblasts and myofibroblasts under the influence of specific growth factors ultimately causes the formation of fibrous adhesions [9].
Numerous interventions have been developed to reduce or prevent post-surgical adhesion formation, including fibrinolytic agents [10], antibiotics, anti-inflammatory agents [11], surgical lysis [12], anticoagulants [13], and chemical and physical barriers [14, 15]. Unfortunately, none of these approaches has proven to be reliable and effective at preventing the formation of postoperative adhesions and subsequent complications. Currently, the only adhesion prevention strategy approved by Food and Drug Administration is Seprafilm (Genzyme Biosurgery Corp., Cambridge, MA) [16, 17]. Although Seprafilm, a bioresorbable physical barrier composed of hyaluronic acid and carboxymethylcellulose (HA/CMC), reduces adhesion formation by preventing apposition of injured tissue where placed, there are significant limitations to its use including difficulties with application and their effectiveness appears to be limited to the site of application [18, 19]. The anti-adhesion mechanism of the Seprafilm barrier is thought to act in vivo as a physical barrier to separate peritoneal tissue surfaces during the early phases of wound healing [20].
Based on data from preliminary studies, we hypothesized that acylated ghrelin, a 28-amino acid gastric peptide, would reduce post-operative adhesion formation. Ghrelin was first isolated and characterized from the rat stomach and also identified as an endogenous ligand for the growth hormone secretagogue receptor (GHSR) [21]. The endocrine activities of ghrelin are mediated by the GHSR, and while primarily studied as a gastric hormone, it is also expressed in many other tissues where it works in a paracrine manner [22]. Ghrelin circulates in an acylated (AG) and des-acylated form (dAG) [21]. The presence of the acyl side chain attached to the serine 3 residue of the ghrelin peptide is required for binding to the receptor GHSR-a1 [21]. The majority of ghrelin (> 90%) circulates in human plasma as dAG form, although the exact ratio of circulating AG to dAG varies depending on metabolic status. [23]. This effect is due to the shorter half-life of AG compared to the dAG. The AG plasma level rapidly disappears from circulation due to binding to the GHSR-1a in the systemic tissue [24] or deacylation by circulating butyrylcolinesterase or carboxylesterase [25]. Previous studies suggest that the majority of AG circulates bound to lipoproteins while dAG flows as a free peptide [26]. Two GHSRs subtypes, GHSR-1a and GHSR-1b, have been identified [21]. GHSR-1a mediates ghrelin’s effects on growth hormone secretion; while the function of the GHSR-1b is still unclear [27]. GHSR-1a is a G protein-coupled receptor expressed in the pituitary gland to mediate growth hormone release and in the hypothalamus to stimulate food intake and appetite [21, 28]. The GHSR-1a receptor is also expressed in several peripheral organs such as bowel, pancreas, liver, heart, lungs, kidneys and testes, facilitating the multiple paracrine, autocrine and endocrine actions of ghrelin [28].
Ghrelin exercises a large range of biological functions, including anti-inflammatory effects [29] and cardio-protective effects [30]. It also alleviates anorexia, cachexia, chronic diseases [31–33] and renal damage [34]. Moreover, Ghrelin improves renal function after ischemia/reperfusion injury and is shown to be involved in the healing of colonic anastomoses [35]. Recently ghrelin was suggested to possess anti-fibrotic properties [34, 36] and based on these observations we hypothesized that this endogenous ligand may play an important role in reducing post-operative adhesion formation. This study developed a new experimental mouse model for the consistent induction of post-operative adhesions, which allows for analysis and evaluation of the effectiveness of ghrelin in reducing the formation of intraperitoneal adhesions. We demonstrated the capability of ghrelin administration to reduce post-surgical adhesion formation in a GHSR-dependent manner.
Materials and Methods
Chemicals
Rat lyophilized acylated ghrelin was obtained from Tocris Bioscience (Bristol, UK). Ghrelin was dissolved in sterile saline (0.9 % sodium chloride, Baxter Healthcare Corporation, IL) before injection.
Animals
GHSR KO mice, C57BL/6 mice with a deletion of the growth hormone secretagogue receptor (Ghsr−/−), were developed at Baylor College of Medicine. Ghsr −/− mice were backcrossed at least 10 generations to C57/BL6 mice to create an isogenic line. Male C57BL/6 wild type mice were purchased from Charles River Laboratories (Wilmington, MA). A total of 40 male wild type mice and 20 male GHSR KO, 50–55 days of age and weighing between 19–21 g, were used in the study. All animals were allowed free access to Purina Rodent Chow 5001 (Farmer’s Exchange, Framingham, MA) and filtered tap water ad libitum. Mice were housed in the Brown University Animal Care Facility and maintained in a temperature (25–28 °C) and humidity (30–70%) controlled environment with a 12 hr alternating dark-light cycle. Body weights of the experimental animals were recorded immediately before the surgical procedure. All investigations were conducted in accordance with Guide for the Care and Use of Laboratory Animals and were approved by the Brown University Institutional Animal Care and Use Committee.
Adhesion creation
Anesthesia was induced in a chamber with isoflurane (Baxter Healthcare Corp.) gas and maintained with 2–3% isoflurane via a nose cone throughout the entire procedure. All experimental animals underwent laparotomy through a 3 cm midline incision. Peritoneal adhesions were created using a combination of two different techniques: ischemic peritoneal buttons and cecal multiple abrasion.
Ischemic button
Two ischemic peritoneal buttons, spaced 0.5 cm apart, are created on the parietal peritoneum by grasping 3 mm of peritoneum with a hemostat and ligating the base of the segment with 6–0 silk suture.
Cecal multiple abrasion
The cecum was abraded with 10 strokes of a folded 2×2cm gauze.
The laparotomy incision was closed in two layers with continuous 5–0 Vicryl suture. The same surgeon performed all the procedures. Two mice (one from each group of wild type mice) were excluded from the study due to the presence of intraperitoneal abscesses.
Ghrelin and Saline Treatment
The mice were divided into two groups. In the control group (wild type mice =20, GHSR KO mice =10), mice were injected intraperitoneally with saline (0.1 ml), while in the ghrelin group (wild type mice =20, GHSR KO mice =10), mice received intraperitoneal injections of ghrelin (0.16 mg/kg) diluted in 0.1 ml of saline twice daily for 2 days before surgery and 20 days post-surgery. Ghrelin was given for two days before surgery to ensure that there were systemic effects of ghrelin before the “trauma” occurred. Mice were euthanized by Isoflurane overdose.
Serum collection
Blood was collected from wild type mice via cardiac puncture at the time of euthanasia. Serum samples were used for the measurement of the pro-inflammatory marker tumor necrosis factor-alpha (TNF-α) by ELISA (Abcam, Cambridge, MA).
Macroscopic and histological evaluation
The mice were euthanized 20 days post-surgery, and the adhesions were scored by a single surgeon, blinded to animal group using a scoring system described by Mazuji MK et al, 1964 [37]. Image analysis was performed by taking gross photos of the abdominal cavity. Half of the peritoneal buttons were collected and fixed in 10% neutral buffered formalin, dehydrated through a series of graded ethanols, and embedded in paraffin. The other half were frozen in liquid nitrogen and stored at − 80 °C for RNA extraction and protein isolation. Paraffin sections were cut at 5 μm, and morphologic changes were assessed in the peritoneal button tissue with Hematoxylin & Eosin, Masson’s trichrome and Picrosirius red staining. Slides were scanned into an Aperio Scanscope CS microscope (Aperio Technologies, Vista, CA) for evaluation.
Picrosirius red stain
Thick sections (5 μm) were deparaffinized in xylene and hydrated through decreasing grades of alcohol. Sections were stained with Weigert’s Hematoxylin for 8 minutes and followed by rinsing with tap water. The sections were incubated in 0.1% Sirius red F3B in saturated Picric acid solution for 1 hour at room temperature (rt), followed by rinsing with acidified water, dehydration and mounting. When Picrosirius red slides were viewed under a polarized light microscope the collagenous stroma of fibrotic tissue showed the spectrum of colors ranging from yellow-orange to green. The color of the collagen fibers was noted by two independent observers to eliminate any subjective bias.
Western blot
Protein lysates were prepared by homogenizing the peritoneal button with an electric homogenizer in radioimmunoprecipitation assay buffer (RIPA buffer:50 mM Tris-HCl (pH 8), 150 mM NaCl, 0.1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS) with 1% phenylmethylsulfonyl fluoride (PMSF) and 1% protease and phosphatase inhibitor cocktail (Thermo Scientific, Rockford, IL). After the samples were centrifuged for 20 minutes at 12,000 rpm, supernatants were aspirated and placed in new tubes. The protein concentration was determined for each sample using Protein Assay kit (Bio-Rad, CA). The samples were denatured but non-reduced with a loading buffer containing 4% anionic denaturing detergent sodium dodecyl sulfate (SDS), 20% glycerol, 0.004% bromophenol blue and 0.125 m Tris-HCl (pH 6.8) and boiled at 95 °C for 10 minutes. Proteins were separated by SDS-PAGE and electrotransferred to ImmunoBlot PVDF membrane (Bio-Rad, Hercules, CA). PVDF membranes were blocked with 5% non-fat milk for 1 hour at rt and under agitation were incubated with primary antibody (anti-collagen I, ab21286 Abcam, Cambridge, MA and anti-tubulin, T6557 Sigma, St. Louis, MO) for 3 hours at rt. After washing the membranes with 0.01% TBS buffer, the membranes were incubated with the secondary antibody (HRP-conjugated anti-rabbit 7076S and HRP-conjugated anti-mouse 7074S antibodies, Cell Signaling Technology, Boston, MA) for 1 hour at rt. Immunodetection was determined using ECL-plus Kit (21106 Thermo Scientific, Waltham, MA) and autoradiography. Relative intensities of the bands were quantified by densitometric analysis using Image-J software. The anti-tubulin antibody was used as an internal control.
Statistical analysis
All data are presented as mean ± standard error of the mean (SEM). Statistical analysis was performed using GraphPad Prism software. Student’s t-test was used to determine statistical difference between control and ghrelin-treated groups. Multiple comparisons between ghrelin and saline-treated wild type and GHSR KO mice groups are obtained using a one-way analysis of variance (ANOVA) and Bonferroni post-hoc test. Values were considered to be significant at p < 0.05. A z-score transformation, known also as Fisher transformation, was performed to combine sets of data coming from different Western blots.
Results
Intraperitoneal adhesion mouse model
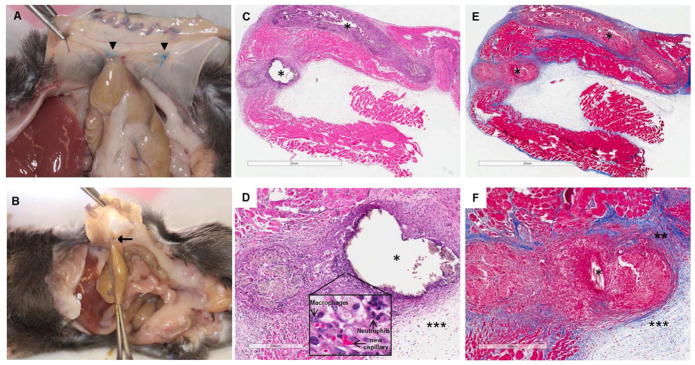
Cecal multiple abrasion and peritoneal ischemic buttons were created in mice as a consistent method to facilitate the formation of intra-abdominal adhesions. Visible adhesions were detected in all the mice on day 20 post-surgery. This animal model was reliable and easily reproducible in stimulating formation of intra-abdominal adhesions between the cecum and the peritoneal ischemic button (Fig. 1A, B). This combination adhesion model technique led to an increased number of adhesions, compared to the single model approach, and significantly improved the quality of the data. To characterize the nature of the surgically-induced fibrosis, histological examination of ischemic buttons was performed. The peritoneal tissue around the suture was markedly thickened by the proliferation of fibroblasts and deposition of collagen fibers. The thickened area around the suture contained macrophages, neutrophils and new capillary formation, confirming that the principal processes involved in the development of peritoneal fibrosis were inflammation, angiogenesis and fibrosis (Fig. 1C, D, E, F).
Figure 1. Combination adhesion induction model is effective in producing consistent adhesions.
Necropsy pictures from control group animal showing abdominal adhesion (arrow) between cecum and the intraperitoneal ischemic buttons (arrow heads) created on one side of the parietal peritoneum in male mice (A, B). Photomicrographs of the peritoneal ischemic buttons tissue in the saline-treated wild type mouse. H&E (C, D) and Masson’s trichrome staining (E, F). Suture (*), fibrotic thickening (**), exudate (***). Bars, 2 mm (C, E), 300 μm (D) and 400 μm (F).
Effect of ghrelin on adhesion formation post-surgery
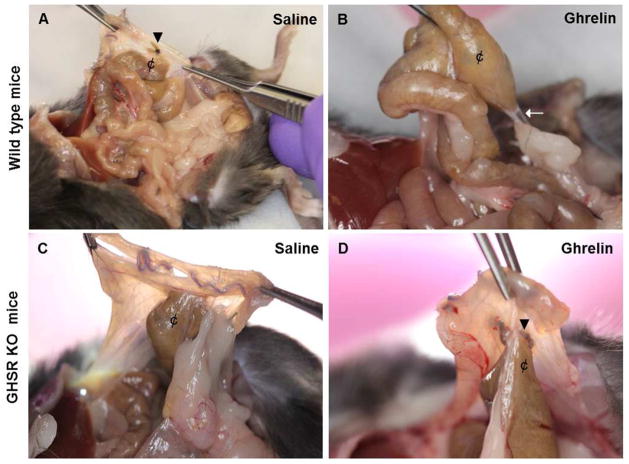
Ghrelin treatment significantly (p<0.001) reduced the adhesion score in ghrelin treated wild type mice compared to saline treated mice (Fig. 2A, B, 3B). The anti-adhesion effect of ghrelin seen in wild type mice was not detected in GHSR KO mice (Fig. 2C, D). The adhesion scores (mean ± SEM) for the ghrelin and saline treated wild type mice were 1.45 ± 0.13 and 2.5 ± 0.21, respectively, a treatment-related reduction of 42.11% (Fig. 3B). We detected a reduction of fibrotic thickening and inflammatory cells around the suture in the ghrelin treated samples in comparison with the saline treated tissues (Fig. 4). The reduction in collagen deposition in the peritoneal ischemic button isolated from ghrelin-treated wild type mice was confirmed by Picrosirius red staining. The collagen I expression (yellow and orange fibers) after surgery was lower in ghrelin-treated compared to the saline-treated ischemic buttons (Fig. 4). However, in both treatments, collagen fibers around the suture in the peritoneal button consisted predominately of collagen I, rather than collagen type III (green fibers), indicating there was no qualitative difference in the type of collagen fibers between the ghrelin and saline-treated samples.
Figure 2. Comparison of ghrelin’s effect on post-surgical adhesion formation in wild type and GHSR KO mice.
Necropsy pictures from saline-treated wild type mice, saline and ghrelin-treated GHSR KO mice showing abdominal adhesions between cecum (¢) and the intraperitoneal ischemic buttons (arrow heads) (A, C, D). Adhesion very thin in ghrelin-treated wild type animal group (arrow) (B).
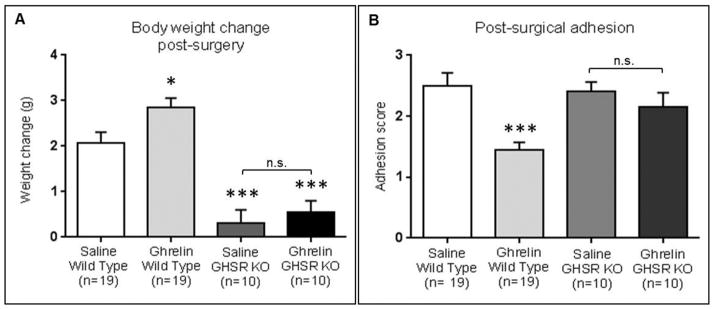
Figure 3. Ghrelin administration significantly stimulates weight gain and reduces postoperative adhesion formation in wild type mice.
The body weight gain in the ghrelin-treated group (n=19) was significantly higher than in the saline-treated group (n= 19). No significant difference in body weight gain between saline (n=10) and ghrelin -treated GHSR KO mice (n=10) (A). Post-surgical adhesions were objectively scored using a scoring system described by Mazuji (Mazuji MK et al, 1964). Ghrelin administration (n=19) reduced significantly post-operative adhesion formations compared to controls (n=19) in wild type mice. No significant difference were detected between saline (n=10) and ghrelin (n=10)-treated GHSR KO mice (B). Data are analyzed by Student’s t-test and One way ANOVA followed by Bonferroni post hoc analysis (*** p-value < 0.001, ** p-value <0.01, * p-value < 0.05).
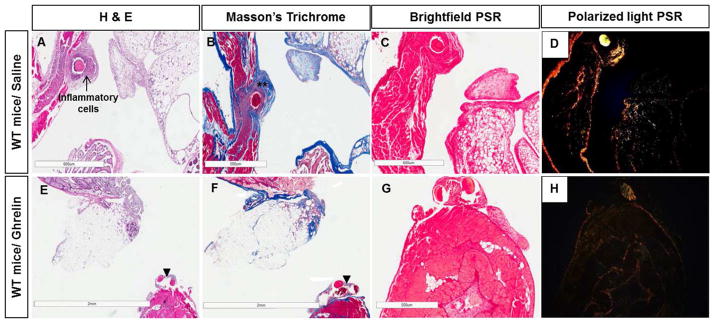
Figure 4. Ghrelin reduces inflammatory infiltrate and fibrotic thickening in peritoneal ischemic buttons.
Photomicrographs of surgical adhesion model of saline (A, B, C, D) and ghrelin-treated wild type mice (E, F, G, H). H&E (A, E), Masson’s trichrome (B, F) and Picrosirius red (PSR) (C, D, G, H) staining. Saline-treated mice showed peritoneal wall thickness with thickened collagen bundles (**) and infiltration of inflammatory cells (arrow) around the suture (A, B). In comparison, ghrelin-treated animals showed reduction of fibrotic thickening and collagen deposition around the sutures (arrow heads) (F). Photomicrographs of Picrosirius red-stained sections with polarized light used to distinguish collagen type I (yellow and orange fibers) from Collagen type III (green fibers) (D, H). The amount of collagen I deposition after surgery was strongly higher in the saline-treated samples (D) than in the ghrelin-treated ones (H). Bars, 600 μm (A, C, D, H), 500 μm (B, G) and 2 mm (E, F).
Ghrelin treatment increased mouse body weight after surgery
Ghrelin administered intraperitoneally twice daily, dosing from preoperative day 2 to postoperative day 20, significantly (p<0.05) increased body weight gain in wild type mice compared to controls (2.75 g ± 0.23 g versus 2.08 g ± 0.23 g). No significant differences were detected between saline and ghrelin-treated GHSR KO mice. The body weight change was significantly (p<0.001) lower in saline and ghrelin-treated GHSR KO mice compared to control wild type mice (Fig. 3A).
Effect of Ghrelin administration on serum level of TNF-α
Serum levels of TNF-α were not significantly different between ghrelin-treated (99.19 ± 4.65 pg/ml) and saline-treated (107.91 ± 14.69 pg/ml) wild type mice (Fig. S1), and were similar to the basal level of TNF-α (2–80 pg/ml).
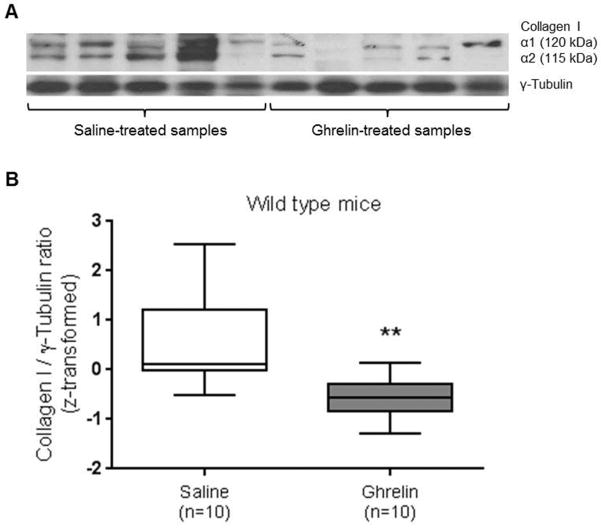
Effect of ghrelin on peritoneal collagen I protein levels in wild type mice
To confirm that ghrelin has anti-fibrotic effects on post-surgical adhesion formation, collagen I protein levels were determined by immunoblotting. Two different gels were run to allow for a wider spectrum of ghrelin and saline – treated wild type mice samples to be analyzed. Each experimental set of data showed that the amount of collagen I was reduced in the ghrelin-treated samples by 62.56% and 55.91% compared to the control samples. Collagen I is usually observed as two bands of different sizes, 115 kDa and 120 kDa, that represent collagen I α1 and collagen I α2 respectively (Fig. 5A). As shown in the Fig. 5B, we detected a significant (p-value = 0.0054 paired t-test) reduction in collagen I protein levels in ghrelin-treated tissue compared to controls (Fig. 5B).
Figure 5. Ghrelin reduces expression of collagen I protein in peritoneal ischemic buttons excised from wild type mice.
Western blot analysis of collagen I protein expression in saline (n= 5) and ghrelin-treated samples (n= 5) (A). Densiometric analysis showed that ghrelin decreased collagen I protein levels. The collagen I protein levels were significantly reduced in ghrelin-treated samples (n=10) compared to the controls (n=10). The statistical analysis was conducted using the Fisher transformation (z-score transformation), that combined sets of data obtained by two immunoblots (B). Data are analyzed by Student’s t-test (** p-value <0.01).
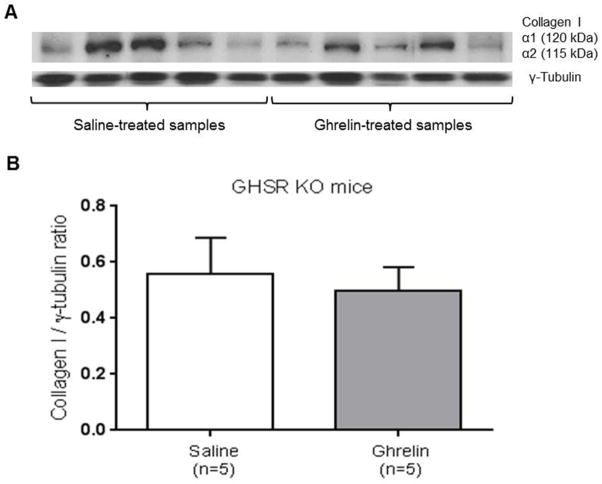
Effect of ghrelin on peritoneal collagen I protein levels in GHSR KO mice
To further test the hypothesis that ghrelin anti-fibrotic effects on post-surgical adhesion formation are mediated by GHSR-1a receptor, collagen I protein levels were quantified in GHSR KO mice by immunoblotting. Collagen I / γ-tubulin ratio protein levels were not significantly different between saline and ghrelin-treated GHSR KO mice (Fig. 6A, B).
Figure 6. Ghrelin reduces expression of collagen I protein in GHSR-1a receptor dependent manner.
Western blot analysis of collagen I protein expression in saline (n=5) and ghrelin-treated GHSR KO mice samples (n= 5). Data are from the same blots after removal of intervening lanes (A). The collagen I protein levels were not significantly reduced in ghrelin-treated samples demonstrating that the antifibrotic effect of ghrelin is GHSR-1a receptor mediated (B). Data are analyzed by Student’s t-test.
Discussion
Ghrelin administered intraperitoneally decreases post-operative intra-abdominal adhesion formation. A more profound understanding of the molecular and cellular mechanisms involved in post-operative adhesion formation and the role of ghrelin may lead to an efficient strategy for adhesion prevention. Multiple pathways are involved in adhesiogenesis, and many are also important in normal wound healing [38]. Surgery causes tissue injury and ischemia, stimulating an acute inflammatory response. Subsequently, there is production of fibrinous exudate, which in association with down-regulation of the peritoneal fibrinolytic activity causes adhesion formation [39]. Despite many clinical studies and animal experiments dedicated to establishing methods to reduce the frequency and severity of post-operative intra-abdominal adhesions, there is still no clear therapy to prevent its onset. Previously targeted pathways include inhibition of the inflammatory response and release of exudate [11], blockade of the coagulation of the exudate [13], stimulation of fibrinolysis [10], barrier techniques via physical separation of fibrin-coated surfaces [14, 15].
Our limitation was the difficulty to test ghrelin effect in reducing peritoneal adhesion formation in surgical animal models that have been developed previously. Although several animal models have been developed previously to assess formation of the peritoneal adhesions, very few have produced consistent or quantifiable results [40]. These experimental animal models show heterogeneous grades of adhesions, which make it difficult to reproduce the same methods or to compare the pathophysiological events. A reliable animal model that allows for objective quantification of adhesions is a key component in elucidating the authenticity of various anti-adhesion strategies. While both the cecal abrasion and peritoneal ischemic button models are individually effective at creating abdominal adhesions, we discovered that the adhesions were unpredictable with a wide variety of different organs adherent to the cecum and/or the peritoneal button respectively preventing any level of consistency. We developed a new technique to facilitate adhesion formation by combining the two previous models of cecal multiple abrasion and peritoneal ischemic buttons. This new surgical animal model significantly improves the consistency of the development of intra-abdominal adhesions between the cecum and the ischemic button every time. In the present study, we evaluated peritoneal adhesions at time point of 20 days post-surgically to fully characterize the surgical adhesion model. By post-surgical day 20, the adhesions were fully formed, and more distinctive allowing for full evaluation of the combined animal adhesion model in comparison to earlier time points.
Ghrelin is defined as a ‘hunger hormone’ because it increases food intake, and promotes positive energy balance and adiposity [41]. The body weight gain endpoint provided a positive control to indicate the central action of ghrelin intraperitoneally administered for twenty days after surgery. Our data showed an increase of body weight 20 days after surgery of the ghrelin-treated wild type mice compared to the controls and GHSR KO mice confirming the involvement of ghrelin in feeding stimulation in a GHSR-dependent manner. Intraperitoneal administration of ghrelin from 2 days before surgery to 20 days post-surgery was effective in reducing adhesion formation after laparotomy in wild type mice. Pre-operative serum ghrelin levels prior to surgery were not measured because of the instability and the short half-life of acylated ghrelin in the serum [25, 42]. Ghrelin was given pre-operatively to ensure that there were systemic effects of ghrelin in progress and not necessarily circulating levels before surgery. Our findings showed that ghrelin treatment reduces post-surgical adhesion formation in wild type mice but not in GHSR KO mice, demonstrating that the anti-fibrotic effect of ghrelin is mediated by GHSR-1a receptor. Ghrelin treatment in GHSR KO mice denoted that the GHSR-1a signaling pathway is required for ghrelin-mediated protection from post-surgical adhesion formation.
The effectiveness of ghrelin in decreasing post-operative adhesions was confirmed by macroscopic evaluation, histological staining and Western blot results. Fibrotic thickening, inflammatory infiltrates in connective tissue and deposition of collagen I around the sutures were reduced in ghrelin-treated peritoneal ischemic tissues compared to controls.
Inflammatory, coagulatory and fibrinolytic pathways seem to play an important role in the pathogenesis of peritoneal adhesions, as has been previously reported in humans and animal models. Down-regulation of the fibrinolytic activity results in increased adhesions, while up-regulation results in a reduction of adhesions [10, 43, 44].
To evaluate the potential for ghrelin-induced anti-inflammatory effects, levels of TNF-α in blood serum were analyzed. However, TNF-α was not found to be systemically upregulated in any group.
Although it is not yet clear through which precise molecular pathways ghrelin and its receptor GHSR-1a mediate the anti-fibrotic response, our data strongly demonstrate the effectiveness of ghrelin in preventing post-operative adhesion formation. Therefore, the evaluation of the effect of ghrelin at different early time-points may contribute to a better understanding of the molecular mechanism by which ghrelin impacts fibrinous adhesion formation.
In conclusion, our data provide direct evidence that ghrelin reduces postoperative adhesions via blockage of fibrotic activities and that the hormone-receptor signaling pathway is essential for the ghrelin’s protective effect. Ghrelin may be a candidate treatment for human surgical patients to reduce hospitalization time, and improve overall surgical outcomes by avoiding the common consequences of postoperative adhesions, such as chronic pelvic and abdominal pain, intestinal obstruction and infertility.
Supplementary Material
No significant difference in serum TNF-α levels between saline and ghrelin-treated post-operative mice (ELISA). Data are analyzed by Student’s t-test.
Acknowledgments
This work was supported by funds from ASRM 2012 Research Grant and Lifespan Grant. This study was supported in part by the 1K12DK083014 Multidisciplinary K12 Urology Research Career Development Program at Baylor to DJL and KH from the National Institute of Kidney and Digestive Diseases.
Footnotes
Authors Contributions: conceived and designed the experiments: EB, KB, KH. Performed the experiments: EB, KH. Analyzed the data: EB, KB, KH. Contributed reagents/materials/analysis tools: EB, MS, DJL, SJH and KH. Wrote the paper: EB, KH.
Disclosure: Kim Boekelheide is an occasional expert consultant for chemical and pharmaceutical companies. Kim Boekelheide and Susan Hall both own stock in CytoSolv,Inc., an early stage biotechnology companies developing a wound healing therapeutic. The authors have no additional financial interests. The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vrijland WW, Jeekel J, van Geldorp HJ, Swank DJ, Bonjer HJ. Abdominal adhesions: intestinal obstruction, pain, and infertility. Surg Endosc. 2003;17:1017–22. doi: 10.1007/s00464-002-9208-9. [DOI] [PubMed] [Google Scholar]
- 2.Schnuriger B, Barmparas G, Branco BC, Lustenberger T, Inaba K, Demetriades D. Prevention of postoperative peritoneal adhesions: a review of the literature. Am J Surg. 2011;201:111–21. doi: 10.1016/j.amjsurg.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 3.Sikirica V, Bapat B, Candrilli SD, Davis KL, Wilson M, Johns A. The inpatient burden of abdominal and gynecological adhesiolysis in the US. BMC Surg. 2011;11:13. doi: 10.1186/1471-2482-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ellis H, Moran BJ, Thompson JN, Parker MC, Wilson MS, Menzies D, et al. Adhesion-related hospital readmissions after abdominal and pelvic surgery: a retrospective cohort study. Lancet. 1999;353:1476–80. doi: 10.1016/S0140-6736(98)09337-4. [DOI] [PubMed] [Google Scholar]
- 5.Coleman MG, McLain AD, Moran BJ. Impact of previous surgery on time taken for incision and division of adhesions during laparotomy. Dis Colon Rectum. 2000;43:1297–9. doi: 10.1007/BF02237441. [DOI] [PubMed] [Google Scholar]
- 6.van Goor H. Consequences and complications of peritoneal adhesions. Colorectal Dis. 2007;9(Suppl 2):25–34. doi: 10.1111/j.1463-1318.2007.01358.x. [DOI] [PubMed] [Google Scholar]
- 7.Grant HW, Parker MC, Wilson MS, Menzies D, Sunderland G, Thompson JN, et al. Population-based analysis of the risk of adhesion-related readmissions after abdominal surgery in children. J Pediatr Surg. 2006;41:1453–6. doi: 10.1016/j.jpedsurg.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 8.Kwok AC, Semel ME, Lipsitz SR, Bader AM, Barnato AE, Gawande AA, et al. The intensity and variation of surgical care at the end of life: a retrospective cohort study. Lancet. 2011;378:1408–13. doi: 10.1016/S0140-6736(11)61268-3. [DOI] [PubMed] [Google Scholar]
- 9.Rajab TK, Wauschkuhn CA, Smaxwil L, Kraemer B, Wallwiener M, Wallwiener CW. An improved model for the induction of experimental adhesions. J Invest Surg. 2010;23:35–9. doi: 10.3109/08941930903469474. [DOI] [PubMed] [Google Scholar]
- 10.Menzies D, Ellis H. The role of plasminogen activator in adhesion prevention. Surg Gynecol Obstet. 1991;172:362–6. [PubMed] [Google Scholar]
- 11.De Wilde RL, Brolmann H, Koninckx PR, Lundorff P, Lower AM, Wattiez A, et al. Prevention of adhesions in gynaecological surgery: the 2012 European field guideline. Gynecol Surg. 2012;9:365–368. doi: 10.1007/s10397-012-0764-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menzies D. Peritoneal adhesions. Incidence, cause, and prevention. Surg Annu. 1992;24(Pt 1):27–45. [PubMed] [Google Scholar]
- 13.Jansen RP. Failure of peritoneal irrigation with heparin during pelvic operations upon young women to reduce adhesions. Surg Gynecol Obstet. 1988;166:154–60. [PubMed] [Google Scholar]
- 14.Diamond MP. Reduction of adhesions after uterine myomectomy by Seprafilm membrane (HAL-F): a blinded, prospective, randomized, multicenter clinical study. Seprafilm Adhesion Study Group. Fertil Steril. 1996;66:904–10. [PubMed] [Google Scholar]
- 15.Becker JM, Dayton MT, Fazio VW, Beck DE, Stryker SJ, Wexner SD, et al. Prevention of postoperative abdominal adhesions by a sodium hyaluronate-based bioresorbable membrane: a prospective, randomized, double-blind multicenter study. J Am Coll Surg. 1996;183:297–306. [PubMed] [Google Scholar]
- 16.Ward BC, Panitch A. Abdominal adhesions: current and novel therapies. J Surg Res. 2011;165:91–111. doi: 10.1016/j.jss.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 17.Brochhausen C, Schmitt VH, Planck CN, Rajab TK, Hollemann D, Tapprich C, et al. Current strategies and future perspectives for intraperitoneal adhesion prevention. J Gastrointest Surg. 2012;16:1256–74. doi: 10.1007/s11605-011-1819-9. [DOI] [PubMed] [Google Scholar]
- 18.Takeuchi H, Kitade M, Kikuchi I, Shimanuki H, Kinoshita K. A novel instrument and technique for using Seprafilm hyaluronic acid/carboxymethylcellulose membrane during laparoscopic myomectomy. J Laparoendosc Adv Surg Tech A. 2006;16:497–502. doi: 10.1089/lap.2006.16.497. [DOI] [PubMed] [Google Scholar]
- 19.Lim R, Morrill JM, Lynch RC, Reed KL, Gower AC, Leeman SE, et al. Practical limitations of bioresorbable membranes in the prevention of intra-abdominal adhesions. J Gastrointest Surg. 2009;13:35–41. doi: 10.1007/s11605-008-0724-3. discussion 41–2. [DOI] [PubMed] [Google Scholar]
- 20.Gago LA, Saed GM, Chauhan S, Elhammady EF, Diamond MP. Seprafilm (modified hyaluronic acid and carboxymethylcellulose) acts as a physical barrier. Fertil Steril. 2003;80:612–6. doi: 10.1016/s0015-0282(03)00767-2. [DOI] [PubMed] [Google Scholar]
- 21.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–60. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 22.Gnanapavan S, Kola B, Bustin SA, Morris DG, McGee P, Fairclough P, et al. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J Clin Endocrinol Metab. 2002;87:2988. doi: 10.1210/jcem.87.6.8739. [DOI] [PubMed] [Google Scholar]
- 23.Patterson M, Murphy KG, le Roux CW, Ghatei MA, Bloom SR. Characterization of ghrelin-like immunoreactivity in human plasma. J Clin Endocrinol Metab. 2005;90:2205–11. doi: 10.1210/jc.2004-1641. [DOI] [PubMed] [Google Scholar]
- 24.Akamizu T, Shinomiya T, Irako T, Fukunaga M, Nakai Y, Nakai Y, et al. Separate measurement of plasma levels of acylated and desacyl ghrelin in healthy subjects using a new direct ELISA assay. J Clin Endocrinol Metab. 2005;90:6–9. doi: 10.1210/jc.2004-1640. [DOI] [PubMed] [Google Scholar]
- 25.Hosoda H, Doi K, Nagaya N, Okumura H, Nakagawa E, Enomoto M, et al. Optimum collection and storage conditions for ghrelin measurements: octanoyl modification of ghrelin is rapidly hydrolyzed to desacyl ghrelin in blood samples. Clin Chem. 2004;50:1077–80. doi: 10.1373/clinchem.2003.025841. [DOI] [PubMed] [Google Scholar]
- 26.De Vriese C, Hacquebard M, Gregoire F, Carpentier Y, Delporte C. Ghrelin interacts with human plasma lipoproteins. Endocrinology. 2007;148:2355–62. doi: 10.1210/en.2006-1281. [DOI] [PubMed] [Google Scholar]
- 27.Smith RG, Leonard R, Bailey AR, Palyha O, Feighner S, Tan C, et al. Growth hormone secretagogue receptor family members and ligands. Endocrine. 2001;14:9–14. doi: 10.1385/ENDO:14:1:009. [DOI] [PubMed] [Google Scholar]
- 28.Delporte C. Structure and physiological actions of ghrelin. Scientifica (Cairo) 2013;2013:518909. doi: 10.1155/2013/518909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu R, Zhou M, Das P, Dong W, Ji Y, Yang D, et al. Ghrelin inhibits sympathetic nervous activity in sepsis. Am J Physiol Endocrinol Metab. 2007;293:E1697–702. doi: 10.1152/ajpendo.00098.2007. [DOI] [PubMed] [Google Scholar]
- 30.Chang L, Ren Y, Liu X, Li WG, Yang J, Geng B, et al. Protective effects of ghrelin on ischemia/reperfusion injury in the isolated rat heart. J Cardiovasc Pharmacol. 2004;43:165–70. doi: 10.1097/00005344-200402000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Neary NM, Small CJ, Wren AM, Lee JL, Druce MR, Palmieri C, et al. Ghrelin increases energy intake in cancer patients with impaired appetite: acute, randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2004;89:2832–6. doi: 10.1210/jc.2003-031768. [DOI] [PubMed] [Google Scholar]
- 32.Wynne K, Giannitsopoulou K, Small CJ, Patterson M, Frost G, Ghatei MA, et al. Subcutaneous ghrelin enhances acute food intake in malnourished patients who receive maintenance peritoneal dialysis: a randomized, placebo-controlled trial. J Am Soc Nephrol. 2005;16:2111–8. doi: 10.1681/ASN.2005010039. [DOI] [PubMed] [Google Scholar]
- 33.Binn M, Albert C, Gougeon A, Maerki H, Coulie B, Lemoyne M, et al. Ghrelin gastrokinetic action in patients with neurogenic gastroparesis. Peptides. 2006;27:1603–6. doi: 10.1016/j.peptides.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 34.Sun GX, Ding R, Li M, Guo Y, Fan LP, Yue LS, et al. Ghrelin attenuates renal fibrosis and inflammation of obstructive nephropathy. J Urol. 2014;193:2107–15. doi: 10.1016/j.juro.2014.11.098. [DOI] [PubMed] [Google Scholar]
- 35.Ceran C, Aksoy RT, Gulbahar O, Ozturk F. The effects of ghrelin on colonic anastomosis healing in rats. Clinics (Sao Paulo) 2013;68:239–44. doi: 10.6061/clinics/2013(02)OA19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moreno M, Chaves JF, Sancho-Bru P, Ramalho F, Ramalho LN, Mansego ML, et al. Ghrelin attenuates hepatocellular injury and liver fibrogenesis in rodents and influences fibrosis progression in humans. Hepatology. 2010;51:974–85. doi: 10.1002/hep.23421. [DOI] [PubMed] [Google Scholar]
- 37.Mazuji MK, Kalambaheti K, Pawar B. Prevention of Adhesions with Polyvinylpyrrolidone. Preliminary Report. Arch Surg. 1964;89:1011–5. doi: 10.1001/archsurg.1964.01320060079015. [DOI] [PubMed] [Google Scholar]
- 38.Hellebrekers BW, Kooistra T. Pathogenesis of postoperative adhesion formation. Br J Surg. 2011;98:1503–16. doi: 10.1002/bjs.7657. [DOI] [PubMed] [Google Scholar]
- 39.Reed KL, Fruin AB, Gower AC, Stucchi AF, Leeman SE, Becker JM. A neurokinin 1 receptor antagonist decreases postoperative peritoneal adhesion formation and increases peritoneal fibrinolytic activity. Proc Natl Acad Sci U S A. 2004;101:9115–20. doi: 10.1073/pnas.0403210101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ozel H, Avsar FM, Topaloglu S, Sahin M. Induction and assessment methods used in experimental adhesion studies. Wound Repair Regen. 2005;13:358–64. doi: 10.1111/j.1067-1927.2005.130402.x. [DOI] [PubMed] [Google Scholar]
- 41.Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, et al. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. 2001;86:5992. doi: 10.1210/jcem.86.12.8111. [DOI] [PubMed] [Google Scholar]
- 42.Hosoda H, Kangawa K. Standard sample collections for blood ghrelin measurements. Methods Enzymol. 2012;514:113–26. doi: 10.1016/B978-0-12-381272-8.00008-8. [DOI] [PubMed] [Google Scholar]
- 43.Montz FJ, Fowler JM, Wolff AJ, Lacey SM, Mohler M. The ability of recombinant tissue plasminogen activator to inhibit post-radical pelvic surgery adhesions in the dog model. Am J Obstet Gynecol. 1991;165:1539–42. doi: 10.1016/0002-9378(91)90402-d. [DOI] [PubMed] [Google Scholar]
- 44.Sulaiman H, Dawson L, Laurent GJ, Bellingan GJ, Herrick SE. Role of plasminogen activators in peritoneal adhesion formation. Biochem Soc Trans. 2002;30:126–31. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
No significant difference in serum TNF-α levels between saline and ghrelin-treated post-operative mice (ELISA). Data are analyzed by Student’s t-test.