Abstract
Systemic hypertension is a risk factor for many diseases affecting the heart, brain, and kidneys. It has long been thought that hypertension leads to a thickening and stiffening of central arteries (i.e., stiffness is a consequence) while more recent evidence suggests that stiffening precedes hypertension (i.e., stiffness is a cause). We submit, however, that consideration of the wall biomechanics and hemodynamics reveals an insidious positive feedback loop that may render it irrelevant whether hypertension causes or is caused by central arterial stiffening. A progressive worsening can ensue in either case, thus any onset of stiffening merits early intervention.
Keywords: Arterial stiffness, pulse pressure, pulse wave velocity, hypertension, high blood pressure
Mechanical Foundations
Understanding arterial function requires integration of biological and mechanical information1–3. Stress (a force intensity) is a key concept in biomechanics; it enables one to calculate the stiffness of a material and assess its strength. Mean circumferential stresses in arteries can be estimated using Laplace’s equation:
| (1) |
where P is pressure, a the pressurized luminal radius, and h the whole wall thickness. In vitro experiments reveal nonlinear pressure-radius relations, P = P̂(a), hence acute increases in blood pressure increase both wall stress (with a increasing due to wall elasticity and h decreasing due to the near incompressibility) and material stiffness (essentially the slope of the stress-stretch relation). Such pressure-induced increases in material stiffness increase most clinical measures of arterial stiffness, thus it is important to delineate acute and chronic (remodeling) changes. The latter can arise from mechanobiological responses (e.g., altered gene expression) by endothelial cells, smooth muscle cells, and fibroblasts to changes in hemodynamically-induced loads, with an apparent goal of preserving homeostatic values of stress and/or material stiffness3, though often at the expense of increasing structural stiffness (essentially wall thickness times material stiffness).
Different metrics are used clinically to assess structural stiffness of central arteries, with carotid-to-femoral pulse wave velocity (cfPWV) the current gold standard1,2. It is thought that an increased cfPWV causes the reflected pressure wave to return to the proximal aorta earlier in the cardiac cycle, which augments central pulse pressure. Albeit not strictly applicable, the Moens-Korteweg equation provides some intuition:
| (2) |
where PWV denotes the speed at which the pressure wave propagates, E is a material stiffness, h and a are thickness and inner radius, and ρ is the density of (assumed inviscid) blood that flows within a long vessel of uniform geometry and properties. With Eh the structural stiffness, equation 2 shows that increases in either material stiffness or wall thickness can impact the hemodynamics equally.
Radius a tends to increase in central arteries in hypertension and aging1,2, which can be beneficial hemodynamically (lower PWV) but problematic mechanobiologically (higher stress σθ and lower mean wall shear stress τw =4μQ/πa3, where μ is viscosity and Q volumetric flowrate). Increased circumferential stress promotes matrix synthesis, often via local production of angiotensin-II, and associated wall thickening3; decreased wall shear stress downregulates endothelial nitric oxide, a vasodilator and anti-inflammatory mediator3. Inflammation is an important contributor to arterial stiffening in hypertension and aging4,5, hence stress-mediated changes in angiotensin-II and nitric oxide can exacerbate stiffening.
The ratio h/a also affects local (equation 1) and global (equation 2) biomechanics. It would need to increase to restore σθ toward normal in response to a chronic increase in pressure, which would be mechanobiologically favorable. Yet, such a change could increase PWV, which would be hemodynamically unfavorable since it could augment central pulse pressure. Hence, local and global mechanics could again be at odds unless a decrease in material stiffness (E in equation 2) offsets effects of an increased h/a on PWV. Most data suggest, however, that material stiffness remains nearly the same or increases in hypertension and aging2.
Stiffening as a Consequence?
Because of the complexity and progressive nature of hypertension and its effects, animal models remain essential for collecting longitudinal information on biological and mechanical changes. Early work, in the 1950s-1970s, suggested that sustained increases in blood pressure stimulate matrix synthesis and thus vascular thickness and structural stiffness6,7. These findings seem to be supported by many subsequent animal studies even though most do not delineate cause and consequence because of imprecise comparisons of evolving pressure and wall properties. Nevertheless, in vivo aortic banding studies confirm that the aorta stiffens structurally in response to increased pressure8, consistent with in vitro cell and ex vivo organ culture studies wherein mechanosensitive cells respond to increased stresses by producing matrix9,10. Thus, induced hypertension leads to stiffening (i.e., stiffening is a consequence), typically via an increase in structural stiffness that adversely affects hemodynamics despite possibly being initially favorable mechanobiologically.
Stiffening as a Cause?
Seminal work in the late 1990s suggested that11 “impaired elasticity [increased structural stiffness] of larger arteries is an antecedent factor in the natural history of BP [blood pressure] elevation at the population level.” This initial clinical finding has been supported by more recent population-based studies12,13 as well as by multiple animal studies14,15. For example, structural stiffness is higher in aortas of young spontaneously hypertensive rats, due in part to a greater wall thickness, despite blood pressure being normal; pressure subsequently increases, however, despite differences in structural stiffness becoming less compared with controls14. As noted earlier, this structural stiffening seems to occur without material stiffening, implying that intramural cells attempt to preserve material stiffness while offsetting increased pressure-induced stresses by thickening the wall. Although a definite proof of causality remains wanting, these animal and population-based clinical studies suggest that stiffening can precede hypertension (stiffening is a cause), again via an increased structural stiffness that adversely affects the hemodynamics while possibly being favorable mechanobiologically.
Cause and Consequence
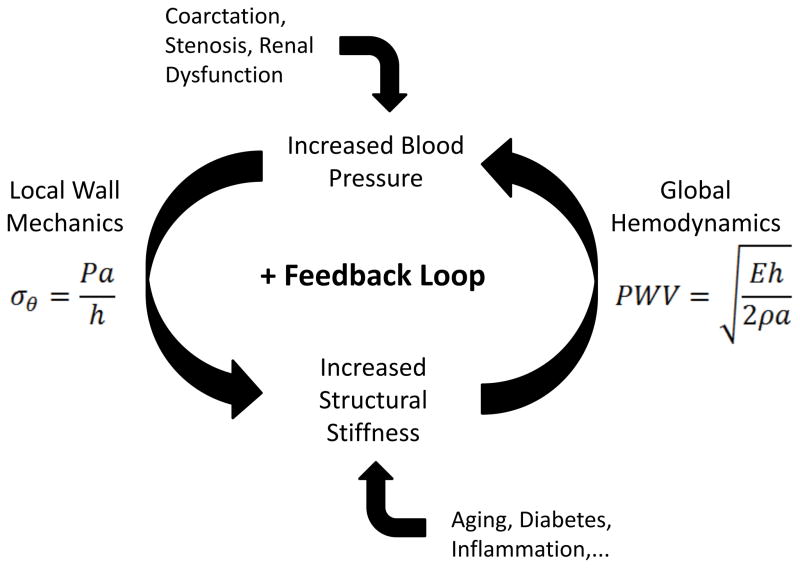
The tendency in science and medicine is to seek simplicity. Hence, based on the preponderance of recent evidence we now find suggestions that, “vascular stiffness is a precursor rather than a result of hypertension”13 or “…support the hypothesis that arterial stiffness is a cause rather than a consequence of hypertension”15. Nevertheless, the totality of clinical and experimental findings suggest that (i) induced hypertension can lead to stiffening and (ii) de novo stiffening can lead to hypertension. The former is consistent with local mechanobiological responses to increases in pressure-induced wall stress; in humans, this may underlie many types of secondary hypertension. In contrast, the latter appears to initiate as a global hemodynamic response to diffuse antecedent structural stiffening; in humans, this may underlie many types of essential hypertension and aging. Notwithstanding the importance of understanding and controlling essential hypertension, whether central arterial stiffening is the cause or consequence of developing hypertension, progressive local mechanobiological responses and adverse global hemodynamic changes are expected in both cases. That is, a potentially insidious feedback loop could exacerbate both central artery stiffening and increasing blood pressure (Figure 1). The real problem, therefore, may be that changes in arterial properties are both cause and consequence, which is ultimately worse because of the possible positive feedback. We should thus be careful not to underestimate the clinical challenge.
Figure 1.
Possible positive feedback loop in central arteries that links local wall mechanics and global hemodynamics and renders it is irrelevant whether the initiator is (i) an increase in blood pressure that increases the structural stiffness of the wall or (ii) an increase in the structural stiffness of the wall that increases pulse wave velocity and thereby augments central pulse pressure. Clearly, the local mechanics that affects cell mechanobiology and the global hemodynamics that controls systemic physiology are linked strongly, but they need not work together to promote overall health. A similar positive feedback loop likely exists in resistance vessels7 and between small-and-large vessels2.
Indeed, a similarly vicious feedback loop appears to link large and small arteries2. In contrast to large arteries, small (resistance) vessels tend to increase the ratio h/a in hypertension via an inward remodeling process (i.e., decreased radius a), likely due to a mechanobiological myogenic response that is distinct to arterioles7. This decrease in radius could help protect the microcirculation from increased pulse pressure-induced damage, yet it increases peripheral resistance to flow (R~8μL/πa4, where L is the length over which the pressure drops) and thereby increases mean arterial pressure. Again, local wall mechanics/mechanobiology and global hemodynamics/physiology can be at odds. Finally, roles of the initially stiffer medium-sized (muscular) arteries in hypertension and aging are less clear; these vessels tend not to change in caliber or stiffen further, which may also be favorable locally but detrimental globally. As central arteries stiffen, the normal gradient in stiffness from elastic-to-muscular arteries decreases and pressure waves propagate farther distally where they can damage the microcirculation of end-organs despite inward remodeling of the resistance vessels1,2.
Closure
Research over the past 15 years reveals that biomechanical properties of central arteries play fundamental roles in both the health of and the development and progression of disease in end-organs1–4. Hence, despite controversy over the best metric to employ, central artery stiffness is an important diagnostic metric and a therapeutic target. Our interpretations based on physical-mathematical-biological concepts support these prior conclusions, but emphasize a greater concern. An insidious positive feedback loop between local mechanobiological responses and global hemodynamics may render central artery stiffening both a cause and a consequence of hypertension. Moreover, this situation can be exacerbated by a similarly vicious cycle between large and small vessel remodeling2, particularly when microvessels are damaged in the kidneys, which are fundamental to long-term blood pressure control16.
The need for early intervention is thus acute, as is the need to identify strategies to prevent entry into these feedback loops prior to the elevation of blood pressure or pulse wave velocity. Clinically, arterial stiffness should be a mandatory measurement in any trial of lifestyle change or anti-hypertensive drug efficacy2,4. Fundamentally, we must understand better the genetic basis of stiffness and early vascular aging, mechanisms of cellular sensing and regulation of the extracellular matrix that endows the wall with its biomechanical functionality and structural integrity, interactions between the mechanobiology and inflammation, and inter-relations among large and small arteries, particularly those of the kidney. Toward this end, physical-mathematical-biological approaches promise to yield increased insight.
Supplementary Material
Acknowledgments
Funding
NIH RO1-HL105294 and RO1-HL105297.
References
- 1.Safar M. Arterial aging–hemodynamic changes and therapeutic options. Nat Rev Cardiol. 2010;7:442–449. doi: 10.1038/nrcardio.2010.96. [DOI] [PubMed] [Google Scholar]
- 2.Laurent S, Boutouyrie P. The structural factor of hypertension: Large and small artery alterations. Circ Res. 2015;116:1007–1021. doi: 10.1161/CIRCRESAHA.116.303596. [DOI] [PubMed] [Google Scholar]
- 3.Humphrey JD. Cardiovascular Solid Mechanics. Springer; NY: 2002. [Google Scholar]; McMaster WG, Kirabo A, Madhur MS, Harrison DG. Inflammation, immunity, and hypertensive end-organ damage. Circ Res. 2015;116:1022–1033. doi: 10.1161/CIRCRESAHA.116.303697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Townsend RR, Wilkinson IB, Schriffin EL, et al. Recommendations for improving and standardizing research on arterial stiffness. Hypertension. 2015;66:698–722. doi: 10.1161/HYP.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolinsky H. Long-term effects of hypertension on the rat aortic wall and their relation to concurrent aging changes. Circ Res. 1972;30:301–309. doi: 10.1161/01.res.30.3.301. [DOI] [PubMed] [Google Scholar]
- 6.Folkow B. “Structural factor” in primary and secondary hypertension. Hypertension. 1990;16:89–101. doi: 10.1161/01.hyp.16.1.89. [DOI] [PubMed] [Google Scholar]
- 7.Fridez P, Makino A, Kakoi D, Miyazaki H, Meister JJ, Hayashi K, Stergiopulos N. Adaptation of conduit artery vascular smooth muscle tone to induced hypertension. Annl Biomed Engr. 2002;30:905–916. doi: 10.1114/1.1507326. [DOI] [PubMed] [Google Scholar]
- 8.Bardy N, Merval R, Benessiano J, Samuel JL, Tedgui A. Pressure and angiotensin-II synergistically induce aortic fibronectin expression in organ culture model of rabbit aorta. Circ Res. 1996;79:70–78. doi: 10.1161/01.res.79.1.70. [DOI] [PubMed] [Google Scholar]
- 9.Humphrey JD, Dufrense E, Schwartz MA. Mechanotransduction and extracellular matrix homeostasis. Nat Rev Molc Cell Biol. 2014;15:802–812. doi: 10.1038/nrm3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liao D, Arnett DK, Tyroler HA, Riley WA, Chambless LE, Szklo M, Heiss G. Arterial stiffness and the development of hypertension. Hypertension. 1999;34:201–206. doi: 10.1161/01.hyp.34.2.201. [DOI] [PubMed] [Google Scholar]
- 11.Dernellis J, Panaretou M. Aortic stiffness is an independent predictor of progression to hypertension in nonhypertensive subjects. Hypertension. 2005;45:426–431. doi: 10.1161/01.HYP.0000157818.58878.93. [DOI] [PubMed] [Google Scholar]
- 12.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. 2012;308:875–881. doi: 10.1001/2012.jama.10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Gorp AW, van Ingen Schenau DS, Hoeks APG, Struijker Boudier HAJ, de Mey JGR, Reneman RS. In spontaneously hypertensive rats alterations in rat aortic wall properties precede development of hypertension. Am J Physiol. 2000;78:H1241–1247. doi: 10.1152/ajpheart.2000.278.4.H1241. [DOI] [PubMed] [Google Scholar]
- 14.Weisbrod RM, Shiang T, Al Sayah L, Fry JL, Bajpai S, Reinhart-King CA, Lob HE, Santhanam L, Mitchell G, Cohen RA, Seta F. Arterial stiffening precedes systolic hypertension in diet-induced obesity. Hypertension. 2013;62:1105–1110. doi: 10.1161/HYPERTENSIONAHA.113.01744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guyton AC. Blood pressure control–special role of the kidney and body fluids. Science. 191;252:1813–1816. doi: 10.1126/science.2063193. [DOI] [PubMed] [Google Scholar]
- 16.Nilsson PM, Boutouyrie P, Cunha P, Kotsis V, Narkiewicz K, Parati G, Rietzschel E, Scuteri A, Laurent S. Early vascular ageing in translation: from laboratory investigations to clinical applications in cardiovascular prevention. J Hyperten. 2013;31:1517–1526. doi: 10.1097/HJH.0b013e328361e4bd. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.