Abstract
Background
This therapeutic drug monitoring (TDM) study aimed to determine the role of olanzapine (OLZ) and N-desmethyl-OLZ (DMO) levels in the therapeutic efficacy of OLZ in patients with schizophrenia.
Method
Plasma concentrations of OLZ (COLZ) and DMO (CDMO) in schizophrenic patients 12 hours post-dose were assessed. The correlations of COLZ and CDMO with the various scores of the Positive and Negative Syndrome Scale (PANSS) were evaluated. A receiver operating characteristic curve (ROC) was utilized to identify the threshold COLZ and COLZ/CDMO ratio for maintenance of satisfactory efficacy.
Results
A total of 151 samples from patients with schizophrenia were analyzed for individual COLZ and CDMO levels. The mean COLZ and CDMO levels were 37.0 ± 25.6 and 6.9 ± 4.7 ng/mL, respectively, and COLZ was ~50% higher in female or nonsmokers (p<0.01). In all patients, the daily dose of OLZ was positively correlated with COLZ and CDMO. Linear relationships between COLZ and OLZ dose were observed in both nonsmokers and smokers (rs = 0.306, 0.426, p<0.01), although CDMO was only correlated with OLZ dose in smokers (rs = 0.485, p<0.01) and not nonsmokers. In all patients, COLZ was marginally negatively correlated with the total PANSS score. The total PANSS score was significantly negatively correlated with the COLZ/CDMO ratio (p<0.005), except in smokers. The ROC analysis identified a COLZ/CDMO ratio ≥2.99 or COLZ ≥22.77 ng/mL as a predictor of maintenance of an at least mildly ill status (PANSS score ≤58) of schizophrenia in all patients.
Conclusions
A significantly negative correlation between the steady-state COLZ/CDMO ratio and total PANSS score was observed in Taiwanese schizophrenic patients. TDM of both OLZ and DMO levels could assist clinical practice when individualizing OLZ dosage adjustments for patients with schizophrenia.
Introduction
Schizophrenia is a chronic and disabling mental disease [1]. Two generations of antipsychotic drugs are used for schizophrenia symptom management. The CATIE (Clinical Antipsychotic Trials for Intervention Effectiveness) Schizophrenia Trial examined fundamental issues about second-generation antipsychotic medications. Atypical antipsychotics, such as clozapine, olanzapine (OLZ), and quetiapine, have fewer extrapyramidal side effects but are associated with weight gain and metabolic problems [2]. The World Federation of Societies of Biological Psychiatry suggests OLZ, quetiapine, and risperidone as first-line medications of first-episode schizophrenia patients [3]. Moreover, Hatta reported that OLZ and risperidone are superior to quetiapine and aripiprazole for the acute treatment of psychosis in hospitalized patients experiencing their first episode [4]. A more recent study demonstrated that OLZ was more likely to be reserved for patients with more severe schizophrenia symptoms, whereas OLZ was less likely to be prescribed to patients with heavier body weight and those with a higher BMI [5]. However, the CATIE Schizophrenia Trial found that olanzapine is relatively the most effective antipsychotics, as measured by treatment discontinuation [2]. The olanzapine’s superiority in efficacy needs to be weighed against weight gain and metabolic abnormalities than other second generation antipsychotics [6].
OLZ is a thienobenzodiazepine (2-methyl-4-(4-methyl-1-piperazinyl) -10H-thieno[2,3-b][1,5]benzodiazepine) with high affinity for various receptors [7,8]. Indications for OLZ include schizophrenia, mania and maintenance of bipolar disorders [9]. The pharmacokinetic parameters of OLZ indicate that OLZ levels are linearly correlated with dose, with 60% bioavailability and ~93% protein binding in blood [10]. The main components present in blood are OLZ-10-N-glucuronide and 4’-N-desmethyl-olanzapine (DMO), which is formed by the metabolism of OLZ by cytochrome P450 (CYP) 1A2 [8,10,11]. Smoking status, sex, and race accounted for 26%, 12%, and 7% of the variability of olanzapine clearance, respectively [12]. The plasma concentration of OLZ (COLZ) increases linearly with increasing daily oral doses and is correlated with improvements in the clinical symptoms of schizophrenia patients [13–15]. According to the guidelines for therapeutic drug monitoring (TDM) in psychiatry, COLZ is suggested to be within 20–80 ng/mL at 12 hours after dosing for patients with schizophrenia [16]. Perry et al. reported that patients with COLZ higher than 23.2 ng/mL at 12 hours after dosing exhibit clinical responses to OLZ therapy [14]. A minimum effective concentration of 9.3 ng/mL (24 hours post-dose) has also been reported [17]. The Maudsley prescribing guidelines [18] note that toxicity can be induced at COLZ higher than 100 ng/mL and that there is a risk of death when levels reach 160 ng/mL.
To ensure effectiveness and minimize the side effects of OLZ, the AGNP Consensus Guidelines suggest that patients receiving OLZ treatment may benefit from TDM [16] because COLZ exhibits inter-individual variations of up to 25-fold [19]. Several non-genetic factors, such as age, gender, smoking, co-medication or disease states, may influence OLZ levels [20]. Information about the role of DMO concentrations (CDMO) in the clinical efficacy of OLZ is scare.
Therefore, this study aimed to investigate the roles of COLZ and CDMO in OLZ effectiveness.
Materials and Methods
This study used modified high-performance liquid chromatography (HPLC) coupled with electrochemical detector as described in our previous study [21], except that 80 mM phosphate buffer (NaH2PO4) was used to enhance system stability and LC-MS-grade acetonitrile was used to minimize noise. Patients meeting the inclusion criteria (aged 18–60 years, stable OLZ dose for at least three months, and full capacity to consent) were recruited according to the methodology of Lu et al. [21]. This study, including its procedures, was approved by the institutional review board and the ethics committee of Taipei Medical University (Approval No. F950206), and all clinical investigations were conducted according to the principles expressed in the Declaration of Helsinki. The participants were included in the study only if they had full capacity by themselves to provide written consent to participate in the study. The understanding of the patients of all procedures and their capacity to provide consent was assessed by direct examination of the participants by a clinician experienced in the evaluation of mental illness. Patients with addictions and who were pregnant, lactating, or had disease conditions that might interfere with monitoring were excluded. Samples from patients prescribed medications with evidence for interactions with OLZ were excluded. Samples were drawn in the morning approximately 12 hours after the last dose of OLZ and analyzed to determine COLZ and CDMO.
During the study period, the symptom severity of the recruited patients was also routinely assessed by a psychiatric physician using the Positive and Negative Syndrome Scale (PANSS). PANSS is clinically used to assess schizophrenic symptoms, including positive, negative and general psychopathology scales [22]. The clinical efficacy of olanzapine was determined using the PANSS [23,24]. PANSS scores ≤58 were defined as mildly ill, as established by Leucht and coworkers[23].
Descriptive statistics are presented as the mean ± standard deviation (SD). The drug concentration normalized by the administered dose is expressed as the C/D ratio. Recruited patients were subgrouped by smoking status and sex, which are known to influence variations of OLZ levels. Intergroup comparisons were performed by Mann-Whitney U test. To quantify the ability of drug-level indicators (COLZ, CDMO or ratio of COLZ/CDMO) to identify schizophrenic symptomatic status in terms of various PANSS scores, Spearman’s rank order correlation method (rs) analysis was conducted using SigmaPlot 12.0. The false discovery rate (FDR) was applied for multiple testing corrections [25]. To determine the cut-off values to indicate that the patients’ clinical symptoms were maintained at an at least mildly ill status (PANSS total score ≤58) [23], the ROC curve was plotted using SPSS 20, and the area under the curve (AUC) was used for accuracy comparison. The indicators with the highest sensitivity and specificity were considered the thresholds for each test. A p value <0.05 was considered significant.
Results
Demographic Characteristics and Subgroup Analysis of Drug Concentration Indicators
This study included a total of 151 samples from patients with schizophrenia. No patients had previously been administered clozapine, and therefore the use of clozapine as an internal standard did not interfere with the reliability of the bioanalysis. Demographic characteristics and steady-state drug level indicators are presented in Table 1. There were no significant gender differences in demographic characteristics except body weight. Because smoking status and gender can influence the variation in drug levels, patients were subgrouped for comparison. There were no differences in DMO levels or C/D ratio between males and females. Compared with male patients, females had higher OLZ levels and C/D ratios (p<0.001), whereas the mean administered doses were similar. OLZ levels, OLZ C/D ratios, and COLZ/CDMO ratios were lower in smokers than in nonsmokers (p<0.05), but OLZ doses were similar in the two groups. Smokers represented 46.48% of the male and 8.75% of the female patients.
Table 1. Demographic characteristics.
| Parameters | All | Smoker | Non-smoker | p value * | Male | Female | p value $ |
|---|---|---|---|---|---|---|---|
| (n = 151) | (n = 40) | (n = 111$) | (n = 71) | (n = 80) | |||
| Age (y/o) | 41.3±12.1 | 40.8±12.6 | 41.5±12.0 | 0.914 | 40.1±11.9 | 42.4±12.1 | 0.350 |
| OLZ dose (mg) | 14.2±5.4 | 15.5±5.4 | 13.7±5.4 | 0.063 | 14.5±5.7 | 13.9±5.2 | 0.454 |
| Weight (kg) | 68.1±15.1 | 71.6±14.5 | 66.8±15.1 | 0.050 | 72.9±15.8 | 63.8±13.0 | <0.001 |
| BMI (kg/m2) | 25.9±6.4 | 26.0±9.0 | 25.9±5.2 | 0.372 | 26.1±7.7 | 25.8±4.9 | 0.729 |
| PANSS, Total | 57.2±16.4 | 58.4±15.7 | 56.8±16.7 | 0.467 | 57.6±15.8 | 56.8±17.0 | 0.645 |
| Positive | 15.2±5.3 | 15.3±4.3 | 15.1±5.6 | 0.775 | 15±5.3 | 15.3±5.4 | 0.551 |
| Negative | 15.0±5.8 | 15.7±6.0 | 14.8±5.7 | 0.297 | 15.7±5.9 | 14.3±5.6 | 0.098 |
| General | 27.0±8.2 | 27.4±8.2 | 26.9±8.2 | 0.707 | 26.9±7.6 | 27.2±8.7 | 0.982 |
| DMO level (ng/mL) | 6.9±4.7 | 7.6±6.3 | 6.6±4.0 | 0.763 | 7.4±5.7 | 6.4±3.7 | 0.505 |
| OLZ level (ng/mL) | 37.0±25.6 | 27.0±23.1 | 40.5±25.6 | 0.004 | 29.3±24.0 | 43.8±25.1 | <0.001 |
| DMO C/D (ng/mL/mg) | 0.6±0.4 | 0.5±0.4 | 0.6±0.4 | 0.559 | 0.6±0.4 | 0.5±0.4 | 0.376 |
| OLZ C/D (ng/mL/mg) | 2.9±2.3 | 1.7±1.2 | 3.3±2.5 | <0.001 | 2.1±1.7 | 3.5±2.6 | <0.001 |
| Ratio of OLZ/DMO | 7.0±6.16 | 4.8±4.1 | 7.85±6.4 | 0.005 | 5.4±4.9 | 8.6±6.6 | <0.001 |
Abbreviations: OLZ, olanzapine; DMO, N-desmethyl-olanzapine; BMI, body mass index
*smoker vs. nonsmoker;
$female vs. male;
p value was set at 0.05; an independent t-test was used for age, and a Mann-Whitney U-test was used for all other comparisons.
Correlation Analysis of Drug-Level Indicators and OLZ Dose
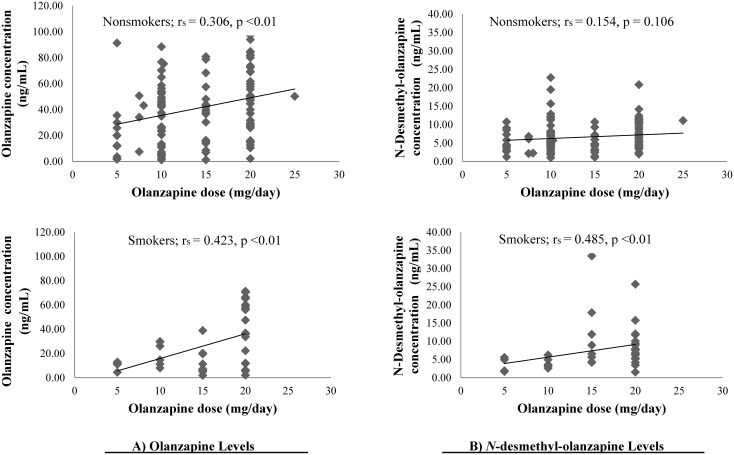
Daily doses of OLZ in all patients were positively correlated with COLZ (rs = +0.300, p<0.001) and CDMO (rs = +0.254, p<0.001). Significant dose-concentration correlations for OLZ were observed in nonsmokers (rs = +0.306, p<0.01) and smokers (rs = +0.423, p<0.01) (Fig 1A). No correlation was observed between OLZ dose and DMO levels in nonsmokers, but OLZ dose and CDMO were significantly correlated in smokers (rs = +0.485, p<0.01) (Fig 1B). The COLZ/CDMO ratio was not correlated with OLZ dose.
Fig 1. Relationships between drug levels and olanzapine dose among nonsmokers and smokers.
A) olanzapine; B) N-desmethyl-olanzapine.
Correlation Analysis of Drug Level Indicators and PANSS Scores
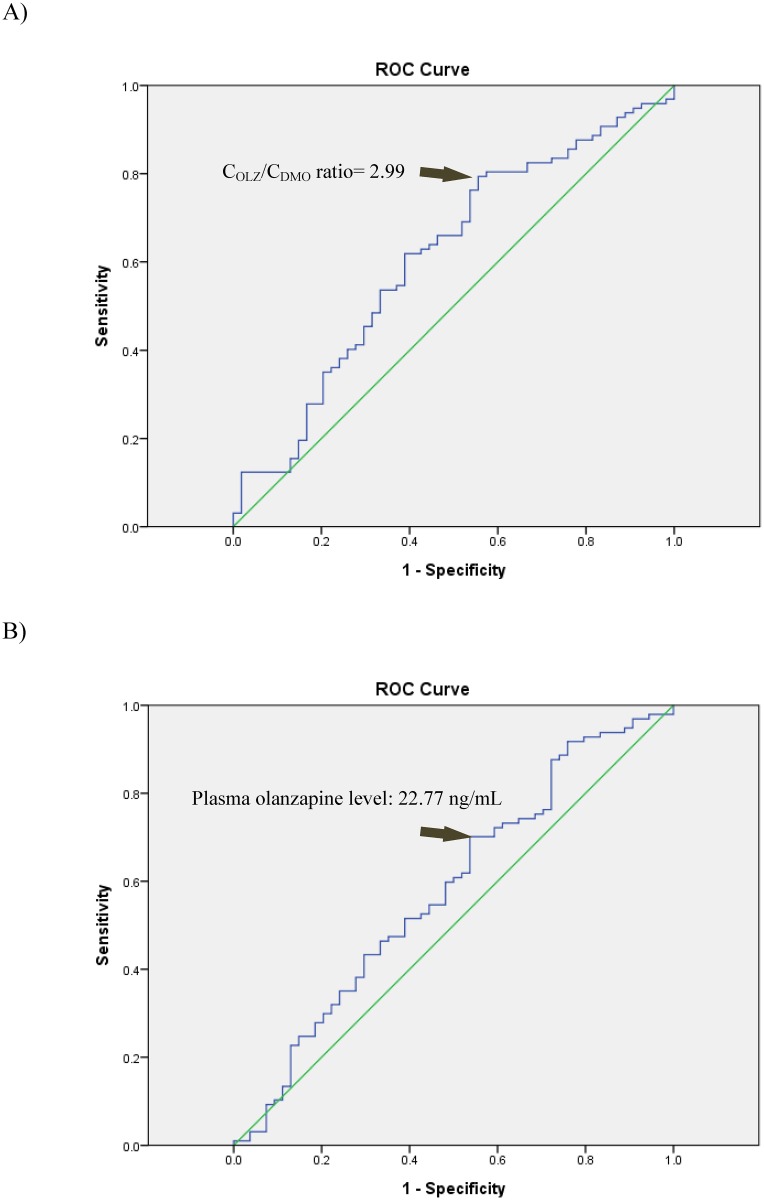
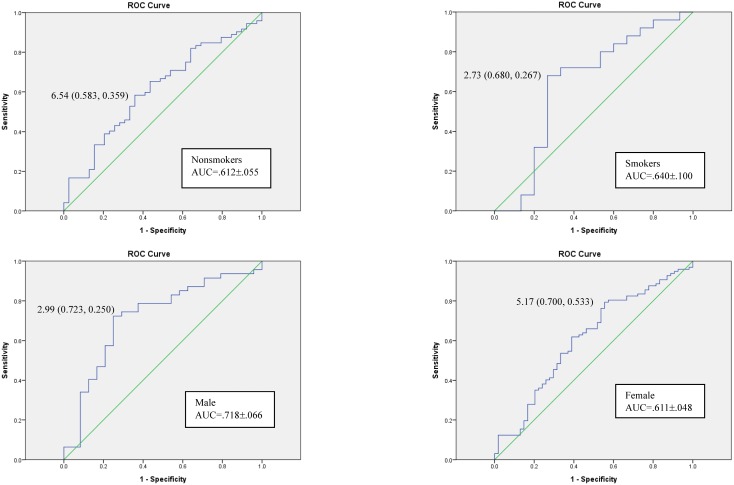
Correlation analysis was performed to determine if COLZ, CDMO, or the COLZ/CDMO ratio was more reliable as a predictor of the PANSS score (Table 2). The total PANSS score was significantly negatively correlated with the COLZ/CDMO ratio (p<0.01) in the whole sample and in non-smokers but not in smokers. To determine the cut-off values of drug levels to indicate that the patients’ symptoms were maintained at an at least mildly ill status (PANSS score ≤58) and to determine if the COLZ/CDMO ratio can be used to predict symptomatic status beyond correlational analysis, ROC analysis was performed. A COLZ/CDMO ratio greater than 2.99 (AUC = 0.611±0.048; sensitivity: 0.794; specificity: 0.444; accuracy: 66.9%) and COLZ greater than 22.77 ng/mL (AUC = 0.579±0.049; sensitivity: 0.701; specificity: 0.463; accuracy: 61.6%) were identified as predictors of symptomatic status in all patients (Fig 2). The ROC subgroup analyses for nonsmokers, smokers, and male or female patients are presented in Fig 3.
Table 2. Correlation tests for PANSS scores and levels of olanzapine and its metabolite DMO.
| All (n = 151) | DMO | OLZ | OLZ/DMO |
|---|---|---|---|
| PANSS, total | |||
| rs = | 0.125 | -0.174* | -0.250*¶ |
| p = | 0.127 | 0.0328 | 0.00201 |
| PANSS, positive | |||
| rs = | 0.0369 | -0.152 | -0.188* |
| p = | 0.652 | 0.0623 | 0.0211 |
| PANSS, negative | |||
| rs = | 0.178* | -0.0206 | -0.148 |
| p = | 0.0285 | 0.802 | 0.0696 |
| PANSS, general | |||
| rs = | 0.0651 | -0.240*¶ | -0.244*¶ |
| p = | 0.427 | 0.00301 | 0.00256 |
| Nonsmokers (n = 111) | |||
| PANSS, total | |||
| rs = | 0.175 | -0.143 | -0.289*¶ |
| p = | 0.0658 | 0.135 | 0.00219 |
| PANSS, positive | |||
| rs = | 0.113 | -0.0861 | -0.184 |
| p = | 0.236 | 0.368 | 0.0527 |
| PANSS, negative | |||
| rs = | 0.195* | 0.012 | -0.190* |
| p = | 0.04 | 0.901 | 0.0456 |
| PANSS, general | |||
| rs = | 0.116 | -0.197* | -0.254* |
| p = | 0.227 | 0.038 | 0.00737 |
| Smokers (n = 40) | |||
| PANSS, total | |||
| rs = | -0.0434 | -0.286 | -0.162 |
| p = | 0.789 | 0.0732 | 0.315 |
| PANSS, positive | |||
| rs = | -0.251 | -0.407* | -0.202 |
| p = | 0.117 | 0.00945 | 0.209 |
| PANSS, negative | |||
| rs = | 0.127 | -0.0924 | -0.0689 |
| p = | 0.433 | 0.568 | 0.671 |
| PANSS, general | |||
| rs = | -0.103 | -0.399* | -0.239 |
| p = | 0.523 | 0.011 | 0.137 |
*p value <0.05; Spearman’s rank order correlation method.
¶rs remained significant after FDR correction.
Abbreviations: OLZ, olanzapine; DMO, N-desmethyl-olanzapine; C/D ratio, concentration-dose ratio; PANSS, Positive and Negative Syndrome Scale
Fig 2. Receiver operating characteristic curves for A) the COLZ/CDMO ratio and B) plasma olanzapine levels as predictors of clinical symptoms maintained at an at least mildly ill status (PANSS score ≤58) among the recruited patients (n = 151).
Fig 3. Receiver operating characteristic curves for the COLZ/CDMO ratio cut-off values as predictors of maintaining an at least mildly ill status (PANSS score ≤58) for the various subgroups of patients.
Discussion
Among all samples, the OLZ dose was linearly correlated with COLZ and CDMO, similar to Skogh’s results [19]. The present study primarily explored the application of the COLZ/CDMO ratio in monitoring clinical symptomatic status as indicated by PANSS scores in patients with schizophrenia. The COLZ/CDMO ratio was significantly negatively correlated with PANSS scores (total and general psychopathology) after FDR correction. The role of the COLZ/CDMO ratio in predicting clinical symptomatic status was then assessed using ROC analysis. The COLZ/CDMO ratio threshold was 2.99 and yielded superior diagnostic accuracy compared to COLZ.
According to the AGNP-TDM guidelines [16], COLZ is suggested to be within the therapeutic range (20~80 ng/mL) for symptom improvement because intra-individual differences can reach 29.7-fold, and levels vary by gender and smoking behavior [26]. The mean COLZ values of the current samples or subgroups fall within the suggested therapeutic ranges. As shown in Fig 2B, overall, 70% (68/97) of samples with OLZ levels higher than 22.77 ng/mL met the criteria for mildly ill status following OLZ therapy. However, COLZ failed to predict the clinical symptomatic status of female patients (AUC of the ROC analysis was less than 0.5).
The cut-off level of OLZ (22.77 ng/mL) identified in this study is similar to previously reported response levels (23.2 ng/mL) for acutely ill patients or stably treated patients [14,27]. However, an improved total Brief Psychiatric Rating Scale (BPRS) score is not correlated with the plasma OLZ concentration, although individual BPRS scores related to improvement of suspiciousness, hallucinations, and blunted affect are significantly correlated with plasma OLZ concentration [15]. In a trial in a Western population, changes in PANSS scores during periods of OLZ therapy were not correlated with COLZ [28]. Similarly, in this study in a Taiwanese population, OLZ levels were not correlated with PANSS scores (except the general psychopathology score) in all samples or subgroup analyses of nonsmokers/smokers after FDR correction. By contrast, the COLZ/CDMO ratio was correlated with total and general PANSS scores for all patients, and the COLZ/CDMO ratio in nonsmokers was also correlated with the total PANSS score (p<0.005).
COLZ or its C/D ratio was higher in females, consistent with the previous literature [19,20,28]. These gender differences may be related to higher CYP1A2 activity in males [19]. Moreover, lean body mass also causes gender differences because females commonly have more fatty tissue in which OLZ may accumulate, resulting in increased drug levels [29]. No differences in CDMO or CDMO/D ratios were observed between genders, consistent with Skogh’s results [19]. However, female nonsmokers had significantly higher CDMO/D ratios than female smokers (0.56 vs. 0.29). The absence of differences in the various drug level indicators between male smokers and nonsmokers may have occurred because the male smokers were light cigarette smokers who smoked fewer than 4 cigarettes per day [30].
OLZ levels may have been higher in nonsmokers (Table 1) due to gender effects because 65.8% of the samples from nonsmokers were obtained from females. OLZ levels were nearly 1.42 times higher in female nonsmokers than in male nonsmokers, whereas DMO levels were similar. Accordingly, the threshold ratio for satisfactory efficacy was nearly two times higher for nonsmokers or females (8.8% smokers) than for smokers or males (82.5% smokers). Females may have lower metabolic enzyme activity [15], and smoking cigarettes results in greater induction of CYP1A2 activity, thus resulting in larger differences between female nonsmoker vs. female smoker in the COLZ/D ratio (3.66 vs. 1.9). Similar observations are also reported by a previous study [31] in children and adolescents which reported that male patients displayed a lower COLZ/CDMO ratio than females.
The drug levels in this study were measured at the steady-state 12-hr post-dose. The DMO and OLZ levels can both be considered averages of the steady-state plasma concentrations as described in a pharmacokinetic study [32]. In the subgroup analysis (Fig 3), the cut-off values of the COLZ/CDMO ratio for the subgroups of males or smokers had higher sensitivity and specificity than the values for the other subgroups and thus may be more reliable. However, these results were simply resolved from cross-sectional clinical scores; future studies should utilize changes in symptomatic scores from baseline in a larger population to provide a good guide for clinical response.
In conclusion, a significantly negative correlation between the steady-state COLZ/CDMO ratio and total PANSS score was observed in Taiwanese schizophrenic patients. The COLZ/CDMO ratio can assist in determining individual metabolic differences due to smoking status and gender. Measuring 12-hr post-dose levels of DMO and OLZ in clinical practice may be employed to optimize treatment outcomes that are less than satisfactory. Our findings suggest that TDM of both OLZ and DMO is useful to assess efficacy in patients treated with OLZ.
Data Availability
All relevant data are within the paper files.
Funding Statement
The Ministry of Science and Technology (NSC-96-2314-B-038-031-MY3; MOST104-2320-B-038-018), Department of Health, Executive Yuan (DOH96-TD-D-113-033), National Health Research Institute (NHRI-EX99-9741PI), and Taipei Medical University-Wan Fang Hospital (103TMU-WFH-13), Taipei, Taiwan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.McGrath J, Saha S, Chant D, Welham J. Schizophrenia: A concise overview of incidence, prevalence, and mortality. Epidemiol Rev. 2008;30: 67–76. 10.1093/epirev/mxn001 [DOI] [PubMed] [Google Scholar]
- 2.Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353: 1209–1223. [DOI] [PubMed] [Google Scholar]
- 3.Hasan A, Falkai P, Wobrock T, Lieberman J, Glenthoj B, Gattaz WF, et al. World federation of societies of biological psychiatry (wfsbp) guidelines for biological treatment of schizophrenia, part 1: Update 2012 on the acute treatment of schizophrenia and the management of treatment resistance. World J Biol Psychiatry. 2012;13: 318–378. [DOI] [PubMed] [Google Scholar]
- 4.Hatta K, Sato K, Hamakawa H, Takebayashi H, Kimura N, Ochi S, et al. Effectiveness of second-generation antipsychotics with acute-phase schizophrenia. Schizophr Res. 2009;113: 49–55. 10.1016/j.schres.2009.05.030 [DOI] [PubMed] [Google Scholar]
- 5.Takahashi M, Fujikoshi S, Funai J, Alev L, Iyo M. Differences in baseline characteristics of patients treated with olanzapine or other antipsychotics in japanese patients with acute schizophrenia: A 1-year observational study under routine clinical practice in japan. Open J Psychiatry. 2015;5: 301–309. [Google Scholar]
- 6.Komossa K, Rummel-Kluge C, Hunger H, Schmid F, Schwarz S, et al. Olanzapine versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev 2010: CD006654 10.1002/14651858.CD006654.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fulton B, Goa KL. Olanzapine. A review of its pharmacological properties and therapeutic efficacy in the management of schizophrenia and related psychoses. Drugs. 1997;53: 281–298. [DOI] [PubMed] [Google Scholar]
- 8.Maloney AE, Sikich L. Olanzapine approved for the acute treatment of schizophrenia or manic/mixed episodes associated with bipolar i disorder in adolescent patients. Neuropsychiatr Dis Treat. 2010;6: 749–766. 10.2147/NDT.S6614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bishara D, Olofinjana O, Sparshatt A, Kapur S, Taylor D, Patel MX. Olanzapine: A systematic review and meta-regression of the relationships between dose, plasma concentration, receptor occupancy, and response. J Clin Psychopharmacol. 2013;33: 329–335. 10.1097/JCP.0b013e31828b28d5 [DOI] [PubMed] [Google Scholar]
- 10.Callaghan JT, Bergstrom RF, Ptak LR, Beasley CM. Olanzapine. Pharmacokinetic and pharmacodynamic profile. Clin Pharmacokinet. 1999;37: 177–193. [DOI] [PubMed] [Google Scholar]
- 11.Kassahun K, Mattiuz E, Nyhart E Jr., Obermeyer B, Gillespie T, Murphy A, et al. Disposition and biotransformation of the antipsychotic agent olanzapine in humans. Drug Metab Dispos. 1997;25: 81–93. [PubMed] [Google Scholar]
- 12.Bigos KL, Pollock BG, Coley KC, Miller DD, Marder SR, Aravagiri M, et al. Sex, race, and smoking impact olanzapine exposure. J Clin Pharmacol. 2008;48: 157–165. 10.1177/0091270007310385 [DOI] [PubMed] [Google Scholar]
- 13.Mauri MC, Steinhilber CP, Marino R, Invernizzi E, Fiorentini A, Cerveri G, et al. Clinical outcome and olanzapine plasma levels in acute schizophrenia. Eur Psychiatry. 2005;20: 55–60. [DOI] [PubMed] [Google Scholar]
- 14.Perry PJ, Lund BC, Sanger T, Beasley C. Olanzapine plasma concentrations and clinical response: Acute phase results of the north american olanzapine trial. J Clin Psychopharmacol. 2001;21: 14–20. [DOI] [PubMed] [Google Scholar]
- 15.Nozawa M, Ohnuma T, Matsubara Y, Sakai Y, Hatano T, Hanzawa R, et al. The relationship between the response of clinical symptoms and plasma olanzapine concentration, based on pharmacogenetics: Juntendo university schizophrenia projects (jusp). Ther Drug Monit. 2008;30: 35–40. 10.1097/FTD.0b013e31816336fd [DOI] [PubMed] [Google Scholar]
- 16.Hiemke C, Baumann P, Bergemann N, Conca A, Dietmaier O, Egberts K, et al. Agnp consensus guidelines for therapeutic drug monitoring in psychiatry: Update 2011. Pharmacopsychiatry. 2011;44: 195–235. [DOI] [PubMed] [Google Scholar]
- 17.Perry PJ, Sanger T, Beasley C. Olanzapine plasma concentrations and clinical response in acutely ill schizophrenic patients. J Clin Psychopharmacol. 1997;17: 472–477. [DOI] [PubMed] [Google Scholar]
- 18.Taylor D, Paton C. and Kapur S. The Maudsley Prescribing Guidelines, Tenth Edition London, UK: CRC Press; 2009. [Google Scholar]
- 19.Skogh E, Reis M, Dahl ML, Lundmark J, Bengtsson F. Therapeutic drug monitoring data on olanzapine and its n-demethyl metabolite in the naturalistic clinical setting. Ther Drug Monit. 2002;24: 518–526. [DOI] [PubMed] [Google Scholar]
- 20.Gex-Fabry M, Balant-Gorgia AE, Balant LP. Therapeutic drug monitoring of olanzapine: The combined effect of age, gender, smoking, and comedication. Ther Drug Monit. 2003;25: 46–53. [DOI] [PubMed] [Google Scholar]
- 21.Lu ML, Lin CH, Chen YC, Yang HC, Wu TH. Determination of olanzapine and n-desmethyl-olanzapine in plasma using a reversed-phase hplc coupled with coulochemical detection: Correlation of olanzapine or n-desmethyl-olanzapine concentration with metabolic parameters. PLoS One. 2013;8: e65719 10.1371/journal.pone.0065719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (panss) for schizophrenia. Schizophr Bull. 1987;13: 261–276. [DOI] [PubMed] [Google Scholar]
- 23.Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR. What does the panss mean? Schizophr Res. 2005;79: 231–238. [DOI] [PubMed] [Google Scholar]
- 24.Opler MGA, Yang LH, Caleo S, Alberti P. Statistical validation of the criteria for symptom remission in schizophrenia: Preliminary findings. BMC psychiatry. 2007;7: 35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann Stat. 2001;29: 1165–1188. [Google Scholar]
- 26.Skogh E, Sjodin I, Josefsson M, Dahl ML. High correlation between serum and cerebrospinal fluid olanzapine concentrations in patients with schizophrenia or schizoaffective disorder medicating with oral olanzapine as the only antipsychotic drug. J Clin Psychopharmacol. 2011;31: 4–9. 10.1097/JCP.0b013e318204d9e2 [DOI] [PubMed] [Google Scholar]
- 27.Fellows L, Ahmad F, Castle DJ, Dusci LJ, Bulsara MK, Ilett KF. Investigation of target plasma concentration-effect relationships for olanzapine in schizophrenia. Ther Drug Monit. 2003;25: 682–689. [DOI] [PubMed] [Google Scholar]
- 28.Citrome L, Stauffer VL, Chen L, Kinon BJ, Kurtz DL, Jacobson JG, et al. Olanzapine plasma concentrations after treatment with 10, 20, and 40 mg/d in patients with schizophrenia: An analysis of correlations with efficacy, weight gain, and prolactin concentration. J Clin Psychopharmacol. 2009;29: 278–283. 10.1097/JCP.0b013e3181a289cb [DOI] [PubMed] [Google Scholar]
- 29.Weiss U, Marksteiner J, Kemmler G, Saria A, Aichhorn W. Effects of age and sex on olanzapine plasma concentrations. J Clin Psychopharmacol. 2005;25: 570–574. [DOI] [PubMed] [Google Scholar]
- 30.Wu TH, Chiu CC, Shen WW, Lin FW, Wang LH, Chen HY, et al. Pharmacokinetics of olanzapine in chinese male schizophrenic patients with various smoking behaviors. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32: 1889–1893. 10.1016/j.pnpbp.2008.08.022 [DOI] [PubMed] [Google Scholar]
- 31.Theisen FM, Haberhausen M, Schulz E, Fleischhaker C, Clement HW, Heinzel-Gutenbrunner M, et al. Serum levels of olanzapine and its n-desmethyl and 2-hydroxymethyl metabolites in child and adolescent psychiatric disorders: Effects of dose, diagnosis, age, sex, smoking, and comedication. Ther Drug Monit. 2006;28: 750–759. [DOI] [PubMed] [Google Scholar]
- 32.Wakamatsu A, Aoki K, Sakiyama Y, Ohnishi T, Sugita M. Predicting pharmacokinetic stability by multiple oral administration of atypical antipsychotics. Innov Clin Neurosci. 2013;10: 23–30. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper files.