The present report reviews the normal function of the immune system, how cancers escape the normal immune system, and how these new therapies improve immune system reactions against cancers.
Keywords: Reversal of cancer-associated immune suppression, T-cell function, B-cell function, Dendritic cell function, Immunotherapy actions, Basic immunology
Abstract
With the Food and Drug Administration and other worldwide regulatory authorities’ approval of ipilimumab (Yervoy), sipuleucel-T (Provenge), nivolumab (Opdivo), and pembrolizumab (Keytruda), oncologic therapy has now moved into noncancer cell targets within the immune system. For many nonimmunologists, understanding how these vastly different therapies work to improve survival, like no other therapies have in the past, is a challenge. The present report reviews the normal function of the immune system, how cancers escape the normal immune system, and how these new therapies improve immune system reactions against cancers.
Implications for Practice:
Oncologists have tremendous experience with therapies that target the cancer cells. New biologic agents have been rapidly introduced recently that target not cancer cells, but the patient’s immune cells. The mechanisms of action of these immune-based biologic agents are within the host immune system. To understand these new biologic therapies, basic knowledge of normal and abnormal immune function is essential. The present report explains the up-to-date basic immune normal and abnormal function and prepares the oncologist to understand how the new drugs work, why they work, and why there are associated adverse events.
Introduction
In the past few years, regulatory agencies worldwide have approved several new therapeutic immune system targeting products that have demonstrated important anticancer activity. However, the drugs do not directly target the cancer itself. Sipuleucel-T (approved April 29, 2010; Provenge; Dendreon Corp., Seattle, WA, http://www.dendreon.com), an antigen-presenting cell (APC) activation therapy, is the first autologous cellular therapy to improve survival in men with metastatic prostate cancer [1]. Ipilimumab (approved May 25, 2011; Yervoy; Bristol-Myers Squibb, New York, NY, http://www.bms.com), an anti-cytotoxic T-lymphocyte associated antigen-4 (CTLA-4) monoclonal antibody therapy, is the first drug ever to result in prolonged survival in patients with advanced melanoma [2]. Nivolumab (Opdivo; Bristol-Myers Squibb), a human monoclonal anti-programmed cell death receptor 1 (PD-1) antibody, has been shown to improve the overall survival of patients with melanoma (approved December 22, 2014) and squamous cell and nonsquamous cell non-small-cell lung cancer (approved March 4, 2015 and October 9, 2015, respectively) [3–5]. Pembrolizumab, another anti-PD-1 antibody, results in increased progression-free survival and overall survival in patients with advanced melanoma (approved indication September 1, 2015; Keytruda; Merck & Co., Kenilworth, NJ, http://www.merck.com) with less toxicity than ipilimumab [6, 7] and demonstrated efficacy in non-small cell lung cancer (approved October 5, 2015) [8, 9]. The biologic interactions behind these therapies and newer immune active (noncancer cell-targeted) drugs are unclear to many nonimmunologists who might already be applying or soon will be applying these therapies. Physicians who prescribe these newer drugs might observe their profound therapeutic effects but might not have had easy access to the immunological progress made during the past decade that has led to an understanding of the mechanisms of action and how that progress has been translated into anticancer activity.
Although a complete elucidation of the human immune system function has not yet been accomplished, a dramatic increase has occurred in the appreciation of the immune system’s normal and abnormal functioning during the past decade. The cellular interactions in this system are very complex, involving many cell types and various chemical mediators. However, basic knowledge is now fairly well established. We review the newer basic concepts and knowledge for those who are not full-time immunologists but who want to understand the immune therapies that have been approved.
The concept of the immune system and its interface with cancer has existed for more than 150 years. Paul Ehrlich's first paper as a medical student provided a description of the initial tissues and cells in the body’s self-defense mechanisms known today as the immune system. Ehrlich was able to develop an antitoxin that, in 1892, was used to treat diphtheria. In 1908, Ehrlich and the Russian scientist Elie Metchnikoff were awarded the Nobel Prize in Medicine and Physiology for their work on serum therapy and immunity [10].
During this period, one of the first physicians to propose the concept of stimulating a person’s own immune system to attack cancer was Dr. William Coley, an American orthopedic surgeon and cancer researcher. He noted that some cancer patients with postoperative bacterial infections had a longer lifespan than those without postoperative infection [11]. In 1893, Coley hypothesized that a curative process stimulated by the infection led to activation of some type of immune phenomenon that could recognize and fight the cancer cells.
Spontaneous Regression of Tumors
Researchers have been working for decades to develop effective treatments for cancers of all types. Interestingly, some cancers have been noted to spontaneously regress, leading the patient to improved health. Although such spontaneous regressions of cancer are rare, they have been reported, most commonly in neuroblastoma, renal cell carcinoma, lymphomas, leukemia, and malignant melanoma [12]. One hypothesis as to why these cancers suddenly disappear is that the individual’s immune system becomes activated in some unexpected manner that leads to the recognition of non-self proteins and the destruction of cancer cells. Cases of spontaneous regression provide an opportunity to research and develop treatments for use in future patients [13].
Spontaneous antibody responses to autologous cancers have been documented in some patients [14, 15]. Additionally, spontaneous T-cell responses have been reported [16]. In the cases of spontaneous regression of cancer that have been reported [17], the strongest evidence connecting an immune response has been T-cell responses associated with spontaneous regression of melanoma [18, 19].
The natural capacity for spontaneous regression suggests that the normal immune system has the capacity to recognize some tumor cells as abnormal and destroy them. In the middle of the 20th century, the immunologists Lewis Thomas and Macfarlane Burnet termed the concept “immune surveillance,” in which normal immune cells could recognize and block cancerous cell development [20, 21]. Conceptualizing how incipient cancer cells could progress beyond immune surveillance to outright cancer, Dunn et al. suggested a process called “immunoediting,” which has three distinct phases [22]. The first phase is immune surveillance, which is marked by an efficient elimination of abnormal cells. However, progression to the next phase of immunoediting occurs if an abnormal cell in the population somehow evades immune destruction. The progeny of such rare cells might allow progression to the second phase called “immune equilibrium.” This phase of immune editing is conceptually similar to a clinical dormancy (i.e., the presence of an occult, dormant tumor). During this phase, a standoff exists, in which the cancer cells cling to survival and the immune cells fight back, such that the tumor, as a whole, neither grows nor shrinks in size significantly. With the evolution of further mutation and metabolic changes within the cancer cells of such a dormant tumor, one or more significant changes can evolve to further restrict or evade the host’s immune reactions. In this third phase, called “immune escape,” the cancer cell progeny will more efficiently evade the immune system’s imposed equilibrium, and the tumor as a whole will begin to grow, invade, and, ultimately, metastasize. When this situation occurs, the immune system has in essence been defeated, because it is no longer effective in blocking the outgrowth of a malignant tumor. In the program of immunoediting, which represents the current state-of-the-art of understanding in cancer immunology, mutated cells that have achieved “immune escape” characterize most human cancers that have reached a clinical stage that has attracted the attention of the patient and/or physician [23]. Immune escape in a tumor is coincident with its observed malignant conversion.
Review of Normal Immune Function for Nonimmunologists
In healthy people, the immune system is designed to protect the whole organism from infectious agents such as bacteria, fungi, and viruses; however, the system has several mechanisms to eliminate host cells that have internal and external abnormalities. The first line of defense is a physical barrier, such as the skin and mucous membranes, that guards the body against a possible pathogen invasion. The second line of defense is the innate immune system, which consists of groups of cells (e.g., phagocytes/macrophages, dendritic cells, and natural killer [NK] cells) that are present beneath the physical barriers (see below). These cells can phagocytize and eliminate bacteria and other infectious agents that are able to breach the physical barrier. The primary function of these two lines of defense is to stop invaders from further entry into the host and, if access occurs, to eliminate pathogens that pass through the physical barriers. The third and final tier is the adaptive immune system. These immune cells, when exposed to specific antigens that are phagocytized, processed and presented by APCs, such as macrophages and dendritic cells, are activated to proliferate and kill the invader pathogen (see below).
The innate immune system is primarily composed of phagocytic cells derived from the multipotent stem cells within the bone marrow. Macrophages, monocytes, neutrophils, and dendritic cells are primary players, although mast cells, NK cells, and NK T cells are also significant within the function of the innate immune system. All cells present “self” protein antigens on their cell surface in complexes with molecules called major histocompatibility complex class I (MHC-I), helping the immune system to discriminate “self” from “non-self.” However, there are also specialized immune cells, antigen-presenting cells, that can present protein antigens to adaptive immune cells in conjunction with major histocompatibility complex class I and II (MHC-II). B cells and “professional” APCs called dendritic cells express MHC-I and MHC-II, using it to present antigens they have accumulated from anywhere in the body. The MHC-I and -II raise, hold, and expose pieces of the engulfed materials on their surface for naïve T cells to examine and bind if a match occurs (Fig. 1).
Figure 1.
MHC presentation of processed antigen. A cartoon demonstrating the process of shed cancer cell (red spiky shape) antigen being processed within the dendritic cell and then expressed on the cell surface attached to MCH-class I and II holding the processed cancer cell antigen (downward pointing spike) available for recognition by the adaptive immune system cells.
Abbreviation: MHC, major histocompatibility complex.
In the early development of both T and B cells, significant random gene rearrangements occur in the DNA of the T-cell receptor (gene coding for a membrane-bound heterodimeric protein that binds specific antigens) and B-cell receptor (gene coding for immunoglobulin heavy and light chains) loci, within the thymus for T cells and the bone marrow for B cells. Random gene rearrangements within these cells produce millions of different sequences, one per cell, that then are transcribed to produce the specific T-cell receptor surface complex (TCR) in the specific pre-T cell or the immunoglobulin (Ig) produced by the specific pre-B cell. Because the rearrangement process is random, some gene rearrangements will produce Igs or TCRs that recognize normal host proteins/sequences. Cells that randomly produce Igs or TCRs to “self” sequences are regulated through a process known as self-tolerance, either by deletion (central tolerance) or by suppressing their activation in the periphery (peripheral tolerance).
Specificity is critical for T cells. Effector T cells are capable of killing normal and cancer cells if they are recognized as non-self. Most peripheral T cells never encounter a foreign antigen to which they can respond specifically. However, all T cells must be able to recognize self-peptides and self-MHC (abbreviated as self-pMHC) and demonstrate only low-affinity interactions to avoid autoimmune reactions and maintenance of homeostasis once they leave the thymus and enter the peripheral circulations for their lifespan.
The TCR has α- and β-dimeric subunits. Within the thymus, the TCRα genes and TCRβ genes are rearranged. Diversity is increased further by adding and subtracting nucleotides from the junctions of the gene segments. These rearrangements allow the T cell to potentially bind millions of foreign peptides specifically. Some of these rearrangements will bind self-peptides, and these must be identified and eliminated to maintain homeostasis.
Thymic Positive and Negative Selection of T cells
During development in the thymus, early T cells (thymocytes) are exposed to the thymic epithelium, which expresses self-proteins via the MHC. The binding strength of the developing thymocytes appears to significantly determine both positive and negative selection. Positive selection requires interactions of the TCR with the self-MHC. If these TCR-self-MHC interactions are of low affinity, they lead to positive selection and retention of this T cell. Self-peptides that might be associated with the MHC must not only evoke very-low- or low-affinity binding but must demonstrate high recognition of the peptide to be positively selected within the thymus and to gain access to the peripheral circulation [24].
Negative selection occurs when T-cell receptors recognize self-proteins presented by self-MHCs and elicit a higher affinity binding and T-cell activation response. Signaling then occurs to induce these higher binding (to self) T cells to start the process of self-apoptosis [25].
Conservation of germline-specific sequences within the variable regions are critical to MHC binding and peptide recognition. MHC binding is the integral first step in TCR binding to a presented antigen. The CD3 region of the TCR then contacts the presented peptide and must be recognized as self by these conserved sequences. If the location of the conserved sequences are recognized, no distortion in the CD3 will result, and the TCR and the T cell will be positively selected.
The newly rearranged positively selected T cells then exit the thymus and circulate via the blood and lymphatic vessel system. Thymocyte gene rearrangements, followed by positive and negative selection of these rearranged TCRs, results in approximately 2.5 × 108 (250 billion) different TCRs in the periphery of humans. Through constant recirculation, these lymphocytes continually search the human organism. Most of the time, they do not encounter their antigen and continue to move throughout the body looking for a match (Fig. 1) [26].
In both the developing thymocyte and the naïve peripheral T cell, the low-affinity interactions with self-MHC-presenting self proteins are perhaps the most important influence on the composition of the peripheral T-cell subtypes and representation. Extremely low-strength consistent signals via TCR interactions with self-pMHC do not induce effector cell function but rather more homeostatic nonreactivity function.
High-affinity peripheral interactions between TCRs and APCs presented with foreign agonist ligand peptide-MHCs result in more efficient activation of naïve T cells. The activation of the naïve T cell consists of four steps: (a) proximal TCR component phosphorylation of CD3, (b) signaling by the Ras-Erk pathway, (c) activation of the transcription factor nuclear factor-κB by protein kinase C-θ, and (d) signaling by transmembrane Ca2+ flux. Studies of various strength peptides and interactions have demonstrated that a relationship exists, showing that the higher the affinity of the interaction, the stronger the activation of the T cell. Low-affinity interactions result in incomplete T-cell activation and, subsequently, little or no reactivity [27].
B cells are derived from bone marrow [27]. On activation by binding to their specific protein-rearranged sequence in the B-cell receptor (BCR) immunoglobulin molecule, B cells become activated and differentiate into antibody-secreting cells (plasma cells) [28]. For full activation and differentiation, B cells require an additional costimulatory signal from either T cells or T-cell-independent factors such as Toll-like receptor ligands. For T-cell-dependent responses, B cells internalize their specific protein via the BCR and present processed parts of the protein (peptides) on their surface via their MHC-class II molecules. The helper T cell (Th-cell) that specifically recognizes the presented peptide binds to it in a complex with an MHC-II molecule, generating both soluble and membrane-bound factors that trigger changes in the B cell, activating its conversion into an antibody-producing plasma cell or a memory B cell (Fig. 2).
Figure 2.
Activation of B cells. CD4+ cells are T-helper cells. They assist B cells that recognize the processed antigen activate and progress to plasma cells. This T to B cell interaction requires the MHC-class II presenting a processed antigen, plus two additional recognition connections via CD4, specifically recognizing the MHC-II complex and the CD40 binding to CD40 ligand.
Abbreviation: MHC, major histocompatibility complex.
Specific antibodies produced in this manner then circulate within the blood plasma and interstitial and lymph fluids. When these antibodies come into contact with the specific protein sequence they recognize, called an antigen, they facilitate its elimination, along with any attached organism, through innate immune cells that absorb and destroy the antibody-antigen complex.
When the invader protein is no longer present (i.e., the infection is cleared), the plasma cells that are no longer needed will internally trigger a cell suicide process called apoptosis, leaving behind only the memory cells [29, 30]. Later, if the memory cells are re-exposed to their specific protein sequence, they will be able to quickly differentiate, without any T-helper cell assistance, into plasma cells that can rapidly produce large quantities of the specific antibody.
Unlike B cells, T cells recognize their specific antigens as processed peptide sequences presented on the surface of antigen-presenting cells in the context of only MHC molecules (MHC-class I—cytotoxic T cells; MHC-class II—helper T cells). These MHC-antigen complexes are presented such that roaming T cells with specific TCRs bind to the presented processed antigen, which triggers, depending on the strength of the interaction, bidirectional communication with the dendritic cell. Cross-presentation allows dendritic cells to take exogenous materials and present peptides using MHC-class I to CD8+ T cells, and MHC-class II to present peptides to CD4+ Th-cells. This can be one of several types of T cells, either cytotoxic or helper T cells, as described further below.
APCs can acquire antigens in several ways. Abnormal proteins can be shed from cells or generated as a result of their destruction by innate immune cells. One special feature of APCs is that they can perform a process known as cross-presentation, which allows exogenous antigens acquired from other cells to be processed and presented on MHC-class I and II complexes to stimulate diverse immune responses. When a peptide antigen is presented on an APC surface in an MHC, brushing up against a specific T cell that recognizes the antigen can start the process of T-cell activation that triggers a subsequent immune response [31, 32].
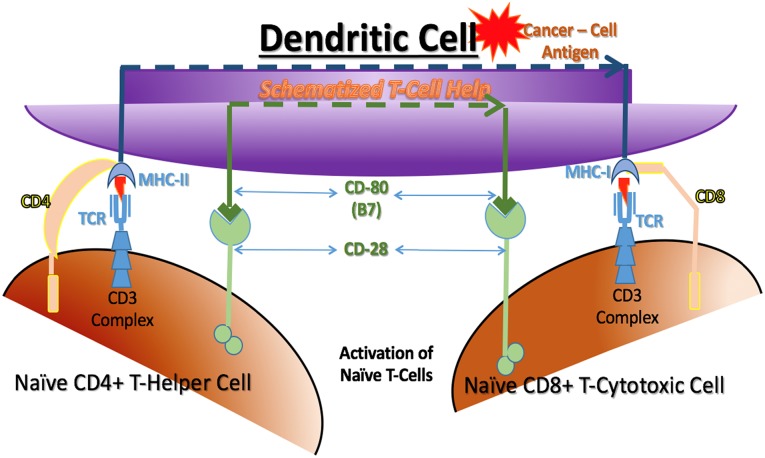
T cells are defined by the presence of a cell surface marker called CD3. They are further subdivided by two other cell surface markers, CD4 and CD8. In general, T cells that are CD8+ are cytotoxic T lymphocytes (or effector cells), and T cells that are CD4+ are helper or regulatory T lymphocytes, which can be subdivided further into Th1, Th2, Th17, regulatory T (Treg) cell, and other types of helper or regulatory cells, respectively. The control of T-cell activation is complex, and knowledge has recently been accumulating rapidly on how Treg cells and other regulatory factors limit immune responses. This regulation is extremely important in the control of normal immune reactions. It is crucial to turn off immune reactions that are no longer needed to protect the host. The normal regulation function of the immune system is hijacked in the case of cancer immunoediting, which has been found to turn off cancer cell destruction and thereby protect malignant cancer cells. A key point to understand is that the function of any T cell, whether cytotoxic, helper, or regulatory, will be activated when it binds to its specific antigen when presented on an APC with associated T-cell help (Fig. 3) [33, 34].
Figure 3.
Activation of cytotoxic T cells. Cytotoxic T cells can only be activated by binding to dendritic cells. Cytotoxic T cells must recognize the processed cancer cell antigen presented only by MHC-class I. Cytotoxic T-cell full activation is enhanced by helper T cells binding to processed antigen on the same dendritic cell as the MHC-class II presenting a processed cancer cell antigen. Each T-cell receptor must bind the processed antigen presented by the appropriate MHC. CD4 and CD8 bind to the respective MHC molecules and CD28 binds to CD80 (also known as B7). These three couplings are required; however, many other bindings are also known to be possible to modify the activation process.
Abbreviations: MHC, major histocompatibility complex; TCR, T-cell receptor surface complex.
For an antigen to activate a T cell that specifically recognizes the antigen, three connections to the APC are required. First, the TCR must bind to the specific antigen displayed by the MHC. Next, the MHC class I or II molecules on the APC must be properly engaged with accessory CD8 or CD4 molecules on the T cell, respectively. Finally, a coregulatory signal must be delivered by yet another set of cell surface receptors and receptor ligands on the T cell and the APC, respectively. This coregulatory signal can be either positive (activating) or negative (regulatory) for the T cell, determining its action once activated by its antigen. Multiple coregulatory receptors and ligands can be expressed by T cells and APCs as a result of their previous experience, and those on their cell surface matter also. For example, a positive signal will be created if CD80 (B7) on the APC binds to CD28 on the T cell, telling it to kill or help kill when its antigen is bound. In contrast, a negative signal will be created if CD80 (B7) on the APC binds to CTLA-4 on the T cell, telling it to stop the T cell from killing. Coregulatory signaling is vital with antigen binding for proper control of the T-cell response [35, 36] (Fig. 3).
All cells in the human body (except red blood cells) express MHC-I molecules on their surface, thereby constantly displaying pieces of their normal or abnormal internally produced proteins to roving immune cells. During early development, the immune system learns not to respond to normal protein fragments (“self” peptides); thus, this presentation does not produce any immune activity (although this can go awry in autoimmune diseases). If the cell begins to manufacture a foreign protein, for example as a result of viral infection, the internal manufacturing process will lead to the display of foreign peptide sequences on the cell surface via MHC-I molecules [37]. The non-self proteins are then available for recognition by roving immune cells, which should kill those infected somatic cells, degrading their contents for APC accumulation and display. Because cancer cells undergo mutation, they tend to manufacture abnormal proteins that can be presented on their surface via MHC-I molecules similarly to viral peptides. This presentation might be a chief basis for immune surveillance involving innate immune cells and T cells, in the manner described above.
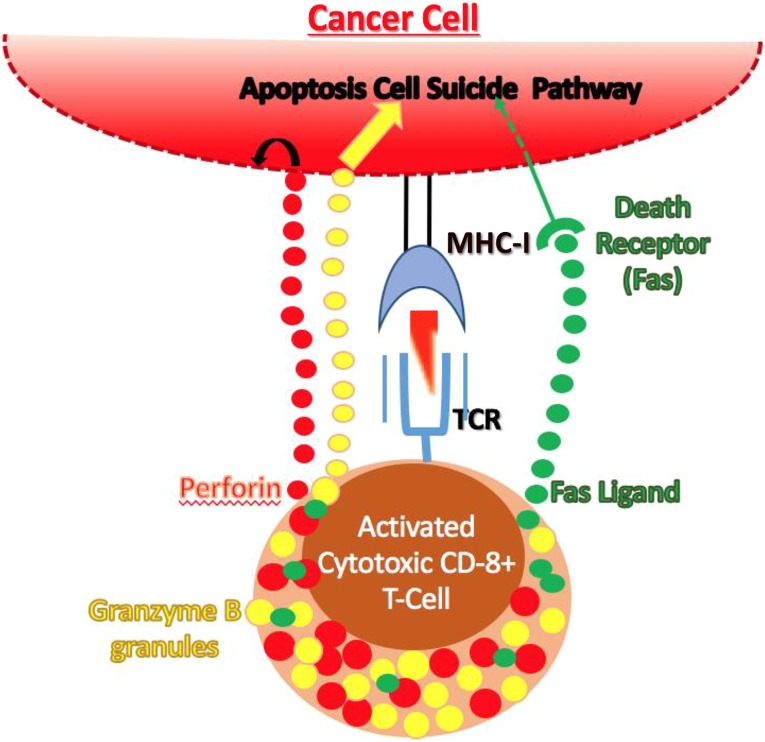
T-cell-mediated target recognition and killing involves several steps. APCs that accumulate “non-self” antigens will present these antigens to T cells present in tissue-draining lymph nodes. T cells that recognize the antigen will be activated and exit the lymph node; during transit to the site of the abnormal cell, the activated T cell manufactures several new proteins for release (Fig. 4). One protein, perforin, causes fenestrations, or holes, in the cell membrane of the target cell on its recognition by the T cell [38–41]. Another protein, granzyme B, can then enter the cell and trigger cell suicide by apoptosis. Finally, a third protein termed Fas ligand (FasL) can be displayed on the T cell or secreted by it, binding to a cell suicide-inducing receptor called Fas that is expressed by all cell types, including many cancer cells. The combination of fenestration with signals leading to complementary mechanisms of apoptosis triggering will effectively kill the target cell (Fig. 4).
Figure 4.
Maturation of a cytotoxic T cell. Cytotoxic T cells are activated as outlined in Figure 3. Once activated, the T cells leave the lymph node and are attracted by cytokines and chemokines released by the innate immune cells at the tumor site. During the transit time, specific series of protein production are started. These proteins (perforin, granzyme B, and Fas ligand) are available to kill the cancer cell. Perforin causes fenestrations (windows) within the cancer cell membrane, allowing entry of granzyme B and Fas ligand. Granzyme B and Fas ligand induce apoptosis via two different mechanisms leading to cancer cell death.
Abbreviations: MHC, major histocompatibility complex; TCR, T-cell receptor surface complex.
Helper T cells support this process by secreting a variety of different cytokines, especially interferon-γ and interleukin-2, that help stimulate cytotoxic T-cell function and other elements of the local immune reaction. This process can be balanced by the action of Treg cells, which suppress cytotoxic reactions and thereby limit immune responses to the target cell. Treg cell function is often overactivated in cancer, in which it can be a dominant driver of immune escape.
After the clearance of the antigenic stimulus on altered host somatic cells, these expanded specific and activated B and T cells begin to regress, returning the clone size to a few dedicated memory cells. In the event of re-exposure to the same antigens, the immune system will react quickly, and often the host will not experience symptoms; the goal of generating immune memory is the reason for booster vaccination injections. Some cancer immunologists have argued that the generation of adaptive immune memory might be essential for effective long-term immune surveillance, not only in normal individuals, but also in patients with cancer who have achieved long-term remission after therapy.
For the immune system to attack cancer cells, the production of abnormal proteins in the cancer cell must be recognized by binding to innate immune cells and/or the specific rearranged B- and T-cell receptors. Before their neoplastic transformation, low-grade cancer cells had been considered “self” by the host immune system. Thus, at an early point in cancer evolution, specific abnormalities in protein synthesis must be recognized to provoke an immune response. These altered proteins are often oncogenes that can unleash unrestrained growth [42]. For example, mutations in receptor tyrosine kinase pathways have been identified to drive continued cell growth. These abnormal proteins (mutated enzymes within the cancer cells) will be expressed on the cancer cell surface via MHC-I molecules. However, because their structural alteration could be subtle—perhaps affecting only a single amino acid—they might or might not be sufficiently different from normal proteins or peptides to be recognized as abnormal by the roving immune cells.
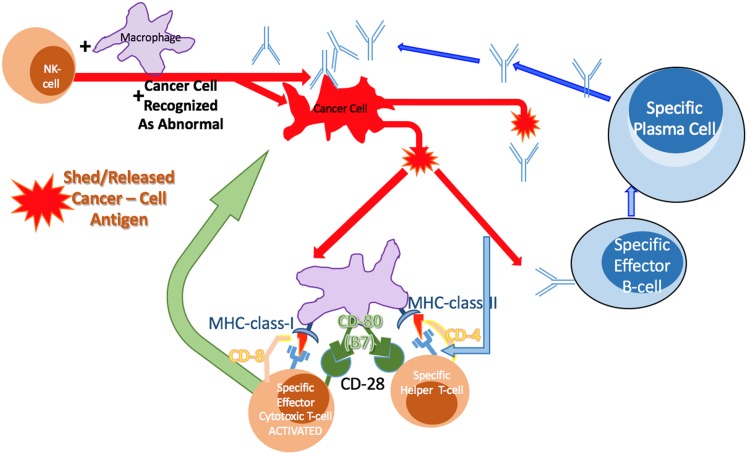
In principle, the action of an immune cell in lysing a single cancer cell and distributing its contents systemically within the host could be enough to allow the cancer antigens to become available to B and T cells within a local tissue-draining lymph node. Antibodies produced by specifically activated B cells can attach to the cancer cells, killing them directly via complement-mediated lysis or indirectly by antibody-dependent cellular cytotoxicity. Cancer cell antigens can be cross-presented by APCs to T cells, which also become activated in the lymph nodes. Previous release of cytokines and chemokines by the innate immune system cells, which had previously arrived, draws the T cells toward tumor cells. Thus, by this sequence of events, abnormal proteins presented by a cancer cell via the MHC-I could effectively signal its immune eradication (Fig. 5).
Figure 5.
The innate and adaptive immune system coordinated functions to kill cancer cells. The innate immune system can first respond to abnormal proteins recognized on the cancer cell surface. These cells can kill the cancer cells, allowing many cancer cell antigens to be shed into the environment, blood, and lymphatic fluid. This can then result in B-cell activation (Fig. 2) and T-cell activation (Figs. 3, 4). B cells produce antibody specific to the processed antigen activated against and shed from the cancer cells. T cells are activated and then release cytotoxic proteins in the vicinity of the cancer cells. The activated T cells can again bind to the MHC-class I if expressed on the cancer cells and presenting the antigen recognized by the T-cell receptor to localize the release of the cytotoxic proteins.
Abbreviations: MHC, major histocompatibility complex; NK, natural killer.
Cancer Revolution Not Evolution
Charles Darwin first proposed the evolution of species [43]. These same principles appear to hold true for cancer cells, except more in the manner of a revolt (revolution) than an evolution. A prevailing model for cancer etiology is that errors in DNA replication lead to mistakes in gene sequences (mutations) that read out into the transcription and translation processes, changing cell function and character. At least some of these errors will be presented on the cancer cell surface via MHC class I molecules and lead to the cancer cell being identified as abnormal by the immune system, leading to the cell’s death; therefore, the mutated trait will not be passed on. In contrast, cells that sustain gene mutations that can elude the immune system might not be recognized as foreign and would be able to evolve in more insidious directions. The application of evolutionary principles to cancer research could have special relevance for the considerations of immunoediting, in which the nature of the parry and thrust of the tumor with the immune system presents a dynamic battle, one that can play out over many years in occult, dormant lesions that might or might not ever progress through immune escape to frank cancer.
Immune Escape Mechanisms and Therapeutic Intervention
If one cancer cell has genetic changes that allow it to avoid or withstand the killing processes by normal immune cells, it can survive, and its progeny, with this inherited survival trait in the mutated DNA, will continue to grow and thrive. The ability to signal the immune system to slow down immune reactions or stop them completely has been recognized as a common development in cancer [43]. Such uncanny processes of immune escape might represent the revolution of one cell in acquiring one or more mechanisms that can switch off or suppress the immune reaction in its vicinity. This characteristic of slowing or stopping immune cell reactions might be one of the most important characteristics for a cancer to develop, grow, metastasize, and eventually kill patients. Understanding these immune inhibitory processes is crucial to understanding how cancers escape the normal immune system. To unleash the latent power of the immune system in a patient with cancer, it will be important to both boost natural cancer-killing capabilities but also degrade immune inhibitory processes. The first four immune therapies to be approved by the Food and Drug Administration (FDA), described in more detail below, represent the initial steps to boost T-cell cancer-killing capabilities.
T-Cell Maturation and Receptor Activation
Chemical substances elaborated directly from the cancer cells or stimulated from the cancer through the surrounding tissues can cause dendritic and cytotoxic T cells to become slow-reacting or nonreactive against the tumor cells within the vicinity. As described above, the mechanism through which cytotoxic T cells become activated involves the recognition of cancer cell antigens presented on the dendritic cell, requiring three essential attachments: specific antigen peptide binding by the TCR in the context of the MHC; engagement of MHC molecules by CD8 or CD4 molecules on the T cell; and generation of a positive coregulatory signal provided by binding of CD80 (B7) to CD28. The strength of this affinity is essential to no, partial, or near-full activation of the Teffector cell. Full activation of cytolytic T-cell function also involves T-cell help through binding the same dendritic cell via MHC-class II (Fig. 3).
Once activated, the T cell exits the lymph node and moves to the site of the abnormal cancerous cell by surveillance or is attracted by the innate immune cells and elaboration of cytokines, chemokines, and other molecules. During this transit from the lymph node to the cancer site, the T cell begins to transcribe and produce CTLA-4 and PD-1, which are then expressed on the T-cell surface. These are accumulated on the activated T cells as the population expands and moves to the immune reaction site. By the time a normal infection has been controlled by the immune system, millions of T cells with these receptors are present. Importantly, these negative-acting molecules can begin to suppress the immune response normally by turning off or suppressing any immune reaction.
Sipuleucel-T Mechanism of Action
As noted above, for cytotoxic T cells to recognize a cancerous cell as abnormal, the cancer cell must shed abnormal proteins, or the innate immune system must first locally destroy one or more of these cells. The abnormal proteins are released and reach professional APCs, most effectively completed by dendritic cells, which can internalize the proteins, process them, and express the segments of protein on their surface using the MHC-class I and class II molecules. Only then can naïve cytotoxic T cells be activated when the specific rearranged T-cell receptor recognizes the presented protein fragments on the MHC-class I molecules and become activated by T-cell help binding onto the MHC-class II molecules (Fig. 3). The dendritic-T-cell association and communication is critical to the initial formation of specific cytotoxic T cells against the tumor cells.
Dendritic cells need to have access to the abnormal proteins so they can activate cytotoxic T cells. In many cancers, this dendritic cell presentation of cancer proteins might not be sufficient to activate T cells against the cancer. This can be performed pharmacologically using the patient’s own mononuclear cells, including APCs. In brief, the patient’s cells are cultured in vitro and treated with prostatic acid phosphatase (PAP), an antigen present on ∼95% of prostate cancers. The PAP antigen used is a recombinant fusion protein, which includes the PAP protein fused to granulocyte-macrophage colony stimulating factor, a powerful innate immune cell stimulator. The patient’s APCs will internalize the PAP protein conjugant, process it as usual, and express it via the MHC-I and -II [44]. After this exposure and dendritic cell activation, the dendritic cells activate the T cells present and are all reintroduced into the patient, where they will travel to the lymph nodes and interact with T helper cells that recognize the PAP peptides presented via MHC-class II and with cytotoxic T cells via MHC-class I. These additionally activated T cells are then primed to search for the specific PAP sequences presented by the indigenous cancer cells in the patient. When discovered, the T cells will release perforin, granzyme B, and FasL, killing the cancer cells. This “cellular therapy” is the basis for activated APCs known as sipuleucel-T (Provenge; Dendreon Corp.).
In a double-blind, placebo-controlled, multicenter phase III trial, 512 patients were randomly assigned in a 2:1 ratio to receive either sipuleucel-T (341 patients) or placebo (171 patients) administered intravenously every 2 weeks for a total of three infusions. In the sipuleucel-T group, the cellular therapy was well tolerated but demonstrated no significant declines in PSA level nor improvements in progression-free survival. However, a significant relative reduction of 22% was found in the risk of death (overall survival) compared with the placebo group (hazard ratio, 0.78; 95% confidence interval [CI], 0.61–0.98; p = .03). This reduction represented a 4.1-month improvement in median overall survival (25.8 months in the sipuleucel-T group vs. 21.7 months in the placebo group). The 36-month survival probability was 31.7% in the sipuleucel-T group versus 23.0% in the placebo group [1].
Ipilimumab Mechanism of Action
Pharmaceutical antibodies that can bind CTLA-4 can neutralize the function of CTLA-4 and inhibit normal T-cell binding to a dendritic cell and result in lower suppression or complete suppression of that cytotoxic T-cell reaction. Thus, the reactivity will continue to progress as more and more specific cytotoxic T cells are activated by dendritic cells, locate the tumor, and express their cell killing molecules in larger and larger numbers.
Ipilimumab (approved by the U.S. FDA in March 2011) has been associated with detrimental and potentially fatal adverse effects owing to T-cell activation and proliferation in the absence of controlled braking [45, 46]. The anti-CTLA-4 therapy releases the CTLA-4 brake, not only on T cells that recognize and destroy the cancer cells, but also on possibly many or all T cells, including those held in peripheral tolerance by CTLA-4 against tissue somatic cells. In relieving the inhibition of these T cells, ipilimumab can, in essence, produce a graft-versus-host-like disease that causes significant adverse events in some treated patients.
Patients prescribed ipilimumab can experience a wide range of adverse events resulting from T-cell overactivity among or against normal somatic cells. If cross-reactive adverse events become clinically evident, the medical scenario might resemble the bone marrow transplant syndrome of graft-versus-host disease. In fact, a risk evaluation and mitigation strategy has been created to inform prescribers of the potential risks associated with ipilimumab. However, despite its potential for severe side effects, ipilimumab has prolonged the lives of many patients with melanoma. According to a melanoma study published in 2010, the median overall survival was 10.0 months among the patients receiving ipilimumab plus glycoprotein 100 (GP100), compared with 6.4 months among the patients receiving control GP100 therapy alone (hazard ratio for death, 0.68; p < .001). The 1-year survival was 45.6% among patients receiving ipilimumab compared with 25.3% among patients receiving GP100 alone, and at 2 years, it was 23.5% versus 13.7% [2]. Recently presented data of ipilimumab have for the first time demonstrated that 22% of patients were still alive after 3 years. No patients who survived beyond 7 years died of their disease, at which time the overall survival rate was 17%. The longest overall survival follow-up in the database was reported as 9.9+ years. The data suggest a survival plateau starting near the 3-year follow-up point, and it appears that the plateau might continue with a follow-up period reported near 10 years [47]. By relieving a powerful inhibitory brake on T cells, ipilimumab has made a clinically significant advance in the therapy and survival of patients with melanoma.
Pharmacological blockade of the so-called immune checkpoint mechanisms has proved to be a successful strategy to reverse the inhibitory immune reactions engendered by tumors, which have achieved immune escape. As the first drug to bind to CTLA-4 and block the immune checkpoint mechanism, the humanized monoclonal antibody ipilimumab was approved by the FDA and is now marketed as Yervoy. Cytotoxic T-lymphocytes (CTLs) have the ability to destroy cancerous cells. Ipilimumab blocks the CTLA-4 inhibitory mechanism (Fig. 6), effectively removing a natural T-cell-braking mechanism that frees CTLs to destroy cancer cells [2]. In addition to its first use in melanoma, ipilimumab is currently undergoing clinical trials for treatment of many other solid tumors [48, 49].
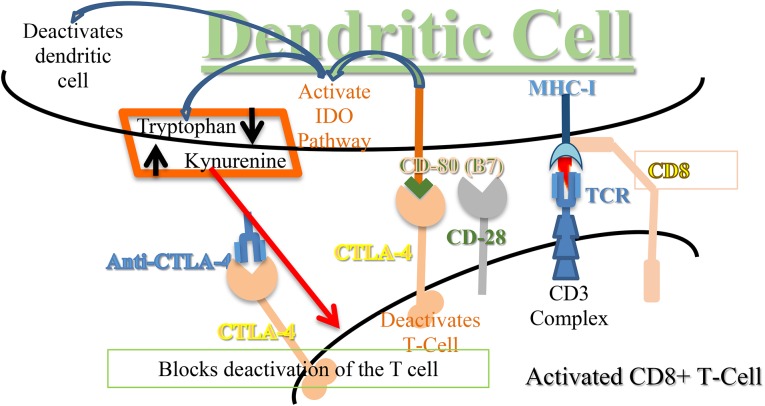
Figure 6.
The IDO pathway effects to slow the immune response, anti-CTLA-4 blocks CTLA-4 from binding CD80. When CTLA-4 binds to CD80 (B7), it starts a series of pathway steps. One of these is activation of the IDO pathway, which converts tryptophan to kynurenine. The lowering of available tryptophan and elevation of kynurenine levels slow the immune reaction of the dendritic cell, T cells, and perhaps other immune system cells. If a cancer cell increases its internal IDO pathway, by “Darwinian revolution,” it will produce similar environmental levels of tryptophan and kynurenine; thus, the environment will tell the immune system to slow or stop, allowing the cancer cell to grow unchecked. Treatment with anti-CTLA-4 will stop the binding to CD80 and allows the T cell to remain activated.
Abbreviations: CTLA-4, cytotoxic T-lymphocyte associated antigen-4; IDO, indoleamine 2,3-dioxygenase; MHC, major histocompatibility complex; TCR, T-cell receptor surface complex.
Anti-PD-1 Antibody Mechanism of Action
Since the approval of the first T-cell-activating antibody against CTLA-4, two new anti-PD-1 products, nivolumab (Opdivo, Bristol-Myers Squibb) and pembrolizumab (Keytruda), have been approved. These therapies have expanded the therapeutic options of what has been called “immune checkpoint” drugs, because of their ability to target an undesired check (blockade) to a productive immune response. The drugs are all monoclonal antibodies against PD-1 on the T cell.
PD-1 is expressed on activated T cells and its ligands (PD-L1/PD-L2) are expressed on the surface of dendritic cells and some mutated cancer cells [50–53]. The ligands of PD-1, PD-L1, and PD-L2, have also been recently shown to appear on endothelial and epithelial cells [54]. When PD-L1 binds to the PD-1 (CD279) receptor on T cells, an inhibition of T-cell growth occurs that includes slowing of activation signals in addition to suppressing cytokine release.
The immunosuppressive function of PD-1 has been elucidated as a viable means of intervention within a tumor environment. Although similar in outcome, the precise mechanism of relief by PD-1 blocking antibodies is distinct from CTLA-4 targeted antibodies in terms of the T cell [55–57]. Anti-PD-1 blocks the PD1 receptor from binding PD-L1, such that the T-cell suppression pathway is not available.
In recent months, nivolumab, a PD-1 targeting monoclonal antibody, has increased overall survival in patients with metastatic melanoma and advanced squamous cell non-small-cell lung cancer by 3.2 months compared with docetaxel [3]. Additionally, the risk of death was 41% lower for patients treated with nivolumab versus docetaxel. The 1-year survival rate (42%), response rate (20%), and median progression-free survival (3.5 months) were all increased with nivolumab compared with docetaxel (24%, 9%, and 2.8 months, respectively; hazard ratio, 0.59; 95% CI, 0.44–0.79; p < .001). Furthermore, nivolumab has significantly decreased grade 3 and 4 treatment-related adverse events from 55% with docetaxel to 9% with nivolumab. Nivolumab works as a human IgG4 PD-1 inhibitory antibody that disrupts signaling and continuously activates while administering antitumor activity [3].
In a phase III study of pembrolizumab, which also targets PD-1 and promotes normal T-cell function without immunosuppression, the estimated 6-month progression-free survival rates increased from 26.5% with ipilimumab to 47.3% with pembrolizumab given every 2 weeks and 46.4% when given every 3 weeks (hazard ratio, 0.58; 95% CI, 0.46–0.72 and 0.47–0.72, respectively). The estimated 12-month survival rate also increased to 74.2% with pembrolizumab given every 2 weeks compared with ipilimumab, 58.2% (hazard ratio for pembrolizumab every 2 weeks, 0.63; 95% CI, 0.47–0.83; p = .005; hazard ratio for pembrolizumab every 3 weeks, 0.69; 95% CI, 0.52–0.90; p = .0036). Furthermore, the response rate increased from 11.9% with ipilimumab to 33.7% with pembrolizumab given every 2 weeks. Concurrently, the rates of treatment-related adverse events (grade 3–5) decreased with administration of pembrolizumab dosed every 2 weeks (13.3%) compared with ipilimumab (19.9%) [8]. Recently, pembrolizumab was granted accelerated approval (October 2, 2015) to treat patients with advanced (metastatic) non-small-cell lung cancer whose disease has progressed after other treatments and those with tumors that express PD-L1 [9, 10]. Additionally, nivolumab was recent demonstrated to be superior in overall survival versus docetaxel in a randomized clinical trial [58] and was approved by the FDA on October 9, 2015 [5].
By ectopic expression of PD-L1, many types of cancer cells evolve the ability to shroud themselves from the host immune system [59–65]. These studies have also demonstrated that patients with PD-L1 tumor expression have a poorer prognosis than PD-L1-negative subjects. This expression is also more pronounced in advanced-stage cancer than in early-stage disease, and it might be a marker of tumor aggressiveness (or a lack of immune killing of the cancer) that confers a poor prognosis. In a study of patients with breast cancer, PD-L1 expression on tumor cells was significantly associated with grade 3 histologic type, estrogen and progesterone receptor-negative tumors, larger tumor size, and HER2-positive status. Blockade of PD-L1 is sufficient for CD8+ T cells to infiltrate tumors and induce local immune activation. Additionally, tumor cell death can occur when the PD-L1/PD-1 interaction is blocked [66, 67].
Combined Anti-CTLA-4 and Anti-PD-1 Summary
In addition to studies of these agents as monotherapy, for example, in studies of anti-CTLA-4 [2], clinical trials have also combined these modalities (e.g., anti-CTLA-4 plus anti-PD-1) [68]. Work to date has established that these therapeutic monoclonal antibodies bind CTLA-4 or PD-1 and block their natural ligand from binding, thereby relieving an extant immune inhibitory process that permits restoration of the latent T-cell activation state characteristic of immune surveillance [69, 70].
The Indoleamine 2,3-Dioxygenase Pathway Mechanism of Immune Escape
The activation of a specific biochemical pathway has now been demonstrated in dendritic cells when CTLA-4 binds to CD80 (B7). The immune suppressive pathway activated is mediated by the tryptophan catabolic enzyme indoleamine 2,3-dioxygenase (IDO) [71, 72].
By CTLA-4 binding to CD80 (B7), T cells signal the dendritic cell to enter a suppressed state. IDO controls the rate-limiting step in degradation of the essential amino acid tryptophan in catalyzing the first step of the biosynthesis of the central metabolic cofactor nicotinamide adenine dinucleotide. Evidence has demonstrated that IDO and the tryptophan pathway are linked to immune tolerance. More specifically, recent findings have suggested that IDO is, in fact, overexpressed in many types of cancers and possibly in APCs within tumor-draining lymph nodes. Notably, CTLA-4 and IDO appear to act coordinately in turning off an activated immune reaction, with IDO mediating an essential downstream signal for CTLA-4 function.
In its normal state, IDO appears to act to protect against an overactive immune system. The pathway was first discovered to induce local immune suppression to avoid destruction of the fetus in utero, based on immune recognition of “foreign” paternal antigens [73]. With higher enzymatic activity in the IDO pathway, tryptophan is decreased and its product, kynurenine, is increased (Fig. 6) [74]. In a small initial study, patients with ovarian cancers that did not display increased IDO levels in the tumor experienced a very high survival rate at 5 years, and those displaying increased IDO levels by tissue staining appeared to experience much shorter survival [54].
Three IDO pathway inhibitors are presently in clinical study. Two of these drugs are specific inhibitors of the IDO enzyme activity itself. The third drug is a simple derivative of tryptophan (1-methyl-d-tryptophan [1DMT, 1MT] or indoximod) with a distinct mechanism of action that remains under investigation. Indoximod was the first drug to enter clinical trials and is currently in phase II studies of breast, prostate, and other cancers. The reported experiences with indoximod to date suggest that therapeutic levels can be attained safely and that reactivation of the immune system can be documented with an associated tumor response [75, 76].
Furthermore, recent research has suggested that a close relative of IDO (IDO1), IDO2 (which lies just downstream of IDO1 on chromosome 8) might be the crucial element to immune behavior with regard to cancer immune escape. In a rheumatoid arthritis inducible mouse model (KRN g7 B6), our institute has shown that deletion of the IDO2 gene alleviates arthritis [77]. IDO2 knockout (ko) KRN g7 mice were shown to have a significant delay in the onset of arthritis and an overall reduction in the amount of ankle swelling compared with KRN g7 and IDO1 ko KRN g7 mice. In the same study, deletion of the IDO2 gene resulted in decreased autoantibody production but did not affect total antibody titers and antibody response in arthritic mice. It has also been shown that the reduction of autoantibodies in IDO2 ko KRN g7 mice is caused by a decreased Th cell response. The study concluded that it is IDO2, not IDO1, that is crucial to the production of autoantibodies and autoimmune disease [81]. These data are crucial to understanding how tumor cells are able to escape the immune response. By further understanding the mechanisms of self-recognition and immune evasion, researchers and clinicians can work together to prolong the lives of patients.
Conclusion
Killing tumor cells is within the natural repertoire of the normal human immune system’s NK and T cells and chemical elaborations. However, aggressive human cancers, through a “Darwinian revolution,” are selected by the killing of sensitive tumor cells via the host immune system and survival of immune-resistant cancer cells that have undergone the Darwinian revolt. The ability of these cells to escape “normal” immune killing activities might be a major cause of progressive malignancy in humans, leading to clonal expansion, metastases, and eventual patient death. In re-engaging the immune system to stage a counter-revolution of sorts, sipuleucel-T, ipilimumab, nivolumab, and pembrolizumab have demonstrated significant statistical and clinical improvement in overall survival of patients with prostate cancer, melanoma, and lung cancer. Specific IDO and general IDO pathway inhibitors might offer small molecule strategies to further leverage efforts to relieve immune inhibition in the tumor, reviving latent T-cell activity that might restore a semblance of immune surveillance, particularly in combination with chemotherapy, radiotherapy, and immunostimulatory immunotherapies. As these immune checkpoint therapies move toward further approvals and wider applications, physicians might see new options for patients with otherwise terminal cancers. Although new classes of immunotherapy to enliven T cells in patients with cancer will not offer a new method to directly attack tumor cells, they might prove to be highly effective through their ability to restore the patient’s intrinsic immune capacities, further opening a new therapeutic front in the clinical management of cancer.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Author Contributions
Conception/design: Jonathan L. Messerschmidt, Gerald L. Messerschmidt
Provision of study material or patients: Jonathan L. Messerschmidt
Collection and/or assembly of data: Jonathan L. Messerschmidt, Gerald L. Messerschmidt
Data analysis and interpretation: Jonathan L. Messerschmidt
Manuscript writing: Jonathan L. Messerschmidt, George C. Prendergast, Gerald L. Messerschmidt
Final approval of manuscript: Jonathan L. Messerschmidt, George C. Prendergast, Gerald L. Messerschmidt
Disclosures
George C. Prendergast: New Link Genetics Inc., Meditope Biosciences Inc. (C/A), New Link Genetics Inc. (RF, IP), New Link Genetics Inc., Meditope Biosciences Inc., Man’s Best Friend Therapeutics Inc. (OI). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 2.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 4.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.FDA News Release FDA expands approved use of Opdivo in advanced lung cancer. Opdivo demonstrates survival benefit in squamous and non-squamous non-small cell lung cancer. N Engl J Med. 2015 [Google Scholar]
- 6.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 7.FDA News Release. Pembrolizumab. Available at: http://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm412861.htm.
- 8.FDA News Release. FDA approves Keytruda for advanced non-small cell lung cancer. First drug approved in lung cancer for patients whose tumors express PD-L1. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm465444.htm.
- 9.Brahmer JR, Kim ES, Zhang J, et al. KEYNOTE-024: Phase III trial of pembrolizumab (MK-3475) vs platinum-based chemotherapy as first-line therapy for patients with metastatic non-small cell lung cancer (NSCLC) that expresses programmed cell death ligand 1 (PD-L1) J Clin Oncol. 2015;33(suppl):TPS8103a. [Google Scholar]
- 10.Ehrlich P. Beiträge zur Kenntniss der Anilinfärbungen und ihrer Verwendung in der mikroskopischen Technik. Arch Mikroskopische Anat. 1877;13:263–277. [Google Scholar]
- 11.Coley WB. The treatment of malignant tumors by repeated inoculations of erysipelas: With a report of ten original cases. Am J Med Sci. 1893;105:487–511. [PubMed] [Google Scholar]
- 12.Papac RJ. Spontaneous regression of cancer. Cancer Treat Rev. 1996;22:395–423. doi: 10.1016/s0305-7372(96)90023-7. [DOI] [PubMed] [Google Scholar]
- 13.Papac RJ. Spontaneous regression of cancer: Possible mechanisms. In Vivo. 1998;12:571–578. [PubMed] [Google Scholar]
- 14.Carey TE, Takahashi T, Resnick LA, et al. Cell surface antigens of human malignant melanoma: Mixed hemadsorption assays for humoral immunity to cultured autologous melanoma cells. Proc Natl Acad Sci USA. 1976;73:3278–3282. doi: 10.1073/pnas.73.9.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ueda R, Shiku H, Pfreundschuh M, et al. Cell surface antigens of human renal cancer defined by autologous typing. J Exp Med. 1979;150:564–579. doi: 10.1084/jem.150.3.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knuth A, Danowski B, Oettgen HF, et al. T-cell-mediated cytotoxicity against autologous malignant melanoma: Analysis with interleukin 2-dependent T-cell cultures. Proc Natl Acad Sci USA. 1984;81:3511–3515. doi: 10.1073/pnas.81.11.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cole WH, Everson TC. Spontaneous regression of cancer: Preliminary report. Ann Surg. 1956;144:366–383. doi: 10.1097/00000658-195609000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferradini L, Mackensen A, Genevée C, et al. Analysis of T cell receptor variability in tumor-infiltrating lymphocytes from a human regressive melanoma: Evidence for in situ T cell clonal expansion. J Clin Invest. 1993;91:1183–1190. doi: 10.1172/JCI116278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zorn E, Hercend T. A MAGE-6-encoded peptide is recognized by expanded lymphocytes infiltrating a spontaneously regressing human primary melanoma lesion. Eur J Immunol. 1999;29:602–607. doi: 10.1002/(SICI)1521-4141(199902)29:02<602::AID-IMMU602>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 20.Thomas L. Reactions to homologous tissue antigens in relation to hypersensitivity. In: Lawrence HS, ed. Cellular and Humoral Aspects of the Hypersensitive States: A Symposium Held at the New York Academy of Medicine. New York Academy of Medicine: Symposia of the Section on Microbiology, No. 9. New York, NY: Hoeber-Harper, 1959:529–532. [Google Scholar]
- 21.Burnet M. Cancer: A biological approach. I. The processes of control. BMJ. 1957;1:779–786. doi: 10.1136/bmj.1.5022.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dunn GP, Bruce AT, Ikeda H, et al. Cancer immunoediting: From immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 23.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 24.Alam SM, Travers PJ, Wung JL, et al. T-cell-receptor affinity and thymocyte positive selection. Nature. 1996;381:616–620. doi: 10.1038/381616a0. [DOI] [PubMed] [Google Scholar]
- 25.Hogquist KA, Jameson SC, Heath WR, et al. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 26.Morris GPO, Allen PM. How the TCR balances sensitivity and specificity for the recognition of self and pathogens. Nat Immunol. 2012;13:121–128. doi: 10.1038/ni.2190. [DOI] [PubMed] [Google Scholar]
- 27.Nagasawa T. Microenvironmental niches in the bone marrow required for B-cell development. Nat Rev Immunol. 2006;6:107–116. doi: 10.1038/nri1780. [DOI] [PubMed] [Google Scholar]
- 28.Vos Q, Lees A, Wu ZQ, et al. B-cell activation by T-cell-independent type 2 antigens as an integral part of the humoral immune response to pathogenic microorganisms. Immunol Rev. 2000;176:154–170. doi: 10.1034/j.1600-065x.2000.00607.x. [DOI] [PubMed] [Google Scholar]
- 29.Fairfax KA, Kallies A, Nutt SL, et al. Plasma cell development: From B-cell subsets to long-term survival niches. Semin Immunol. 2008;20:49–58. doi: 10.1016/j.smim.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 30.Shapiro-Shelef M, Calame K. Regulation of plasma-cell development. Nat Rev Immunol. 2005;5:230–242. doi: 10.1038/nri1572. [DOI] [PubMed] [Google Scholar]
- 31.Bourgeois C, Tanchot C. Mini-review CD4 T cells are required for CD8 T cell memory generation. Eur J Immunol. 2003;33:3225–3231. doi: 10.1002/eji.200324576. [DOI] [PubMed] [Google Scholar]
- 32.Jenkins MR, Griffiths GM. The synapse and cytolytic machinery of cytotoxic T cells. Curr Opin Immunol. 2010;22:308–313. doi: 10.1016/j.coi.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bevan MJ. Helping the CD8(+) T-cell response. Nat Rev Immunol. 2004;4:595–602. doi: 10.1038/nri1413. [DOI] [PubMed] [Google Scholar]
- 34.Ahmed R, Bevan MJ, Reiner SL, et al. The precursors of memory: Models and controversies. Nat Rev Immunol. 2009;9:662–668. doi: 10.1038/nri2619. [DOI] [PubMed] [Google Scholar]
- 35.Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol. 2003;3:984–993. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- 36.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trambas CM, Griffiths GM. Delivering the kiss of death. Nat Immunol. 2003;4:399–403. doi: 10.1038/ni0503-399. [DOI] [PubMed] [Google Scholar]
- 38.Smyth MJ, Thia KY, Street SE, et al. Perforin-mediated cytotoxicity is critical for surveillance of spontaneous lymphoma. J Exp Med. 2000;192:755–760. doi: 10.1084/jem.192.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berke G. The binding and lysis of target cells by cytotoxic lymphocytes: Molecular and cellular aspects. Annu Rev Immunol. 1994;12:735–773. doi: 10.1146/annurev.iy.12.040194.003511. [DOI] [PubMed] [Google Scholar]
- 40.Nagata S, Suda T. Fas and Fas ligand: LPR and GLD mutations. Immunol Today. 1995;16:39–43. doi: 10.1016/0167-5699(95)80069-7. [DOI] [PubMed] [Google Scholar]
- 41.Berke G. The CTL’s kiss of death. Cell. 1995;81:9–12. doi: 10.1016/0092-8674(95)90365-8. [DOI] [PubMed] [Google Scholar]
- 42.Prendergast GC. Immune escape as a fundamental trait of cancer: Focus on IDO. Oncogene. 2008;27:3889–3900. doi: 10.1038/onc.2008.35. [DOI] [PubMed] [Google Scholar]
- 43.Darwin C. On the Origin of Species by Means of Natural Selection, or the Preservation of Favoured Races in the Struggle for Life. London, UK: John Murray; 1859; modern reprint in Darwin C, Huxley J. On the Origin of Species. New York, NY: Signet Classics, 2003. [Google Scholar]
- 44.Di Lorenzo G, Buonerba C, Kantoff PW. Immunotherapy for the treatment of prostate cancer. Nat Rev Clin Oncol. 2011;8:551–561. doi: 10.1038/nrclinonc.2011.72. [DOI] [PubMed] [Google Scholar]
- 45.Jefferson E. (2011-03-25) FDA approves new treatment for a type of late-stage skin cancer (press release). U.S. Food and Drug Administration (FDA). Available at http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm1193237.htm. Accessed April 9, 2014.
- 46.Drugs.com. Yervoy side effects. Available at http://www.drugs.com/sfx/yervoy-side-effects.html. Last accessed October 15, 2015.
- 47.Schadendorf D, Hodi FS, Robert C et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in metastatic or locally advanced, unresectable melanoma. European Cancer Congress (ECCO) Amsterdam 2013, abstract 24. Available at http://www.esmo.org/Conferences/Past-Conferences/European-Cancer-Congress-2013/News/ECC-2013-Press-Release-Longest-Follow-Up-of-Largest-Number-of-Melanoma-Patients-Treated-with-Ipilimumab-Shows-Some-Survive-up-to-Ten-Years. Accessed October 15, 2015.
- 48.ClinicalTrials.gov. NCT00527735: Phase II Study for Previously Untreated Subjects With Non Small Cell Lung Cancer (NSCLC) or Small Cell Lung Cancer (SCLC). Available at http://www.clinicaltrials.gov/ct2/show/NCT00527735?term=NCT00527735+Phase+II+Study+for+Previously+Untreated+Subjects+With+Non+Small+Cell+Lung+Cancer+%28NSCLC%29+or+Small+Cell+Lung+Cancer+%28SCLC%29&rank=1. Accessed October 15, 2015.
- 49.ClinicalTrials.gov. NCT00323882: Phase I/II Study of MDX-010 in Patients With Metastatic Hormone-Refractory Prostate Cancer (MDX010-21). Available at http://www.clinicaltrials.gov/ct2/show/NCT00323882?term=NCT00323882+Phase+I%2FII+Study+of+MDX-010+in+Patients+With+Metastatic+Hormone-Refractory+Prostate+Cancer+%28MDX010-21%29&rank=1. Accessed October 15, 2015.
- 50.Sharpe AH, Wherry EJ, Ahmed R, et al. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 51.Agata Y, Kawasaki A, Nishimura H, et al. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8:765–772. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- 52.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 53.Okamoto A, Nikaido T, Ochiai K, et al. Indoleamine 2,3-dioxygenase serves as a marker of poor prognosis in gene expression profiles of serous ovarian cancer cells. Clin Cancer Res. 2005;11:6030–6039. doi: 10.1158/1078-0432.CCR-04-2671. [DOI] [PubMed] [Google Scholar]
- 54.Pico de Coaña Y, Choudhury A, Kiessling R. Checkpoint blockade for cancer therapy: Revitalizing a suppressed immune system. Trends Mol Med. 2015;21:482–491. doi: 10.1016/j.molmed.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 55.Parry RV, Chemnitz JM, Frauwirth KA, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sheppard KA, Fitz LJ, Lee JM, et al. PD-1 inhibits T-cell receptor induced phosphorylation of the ZAP70/CD3zeta signalosome and downstream signaling to PKCtheta. FEBS Lett. 2004;574:37–41. doi: 10.1016/j.febslet.2004.07.083. [DOI] [PubMed] [Google Scholar]
- 57.Yokosuka T, Takamatsu M, Kobayashi-Imanishi W, et al. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J Exp Med. 2012;209:1201–1217. doi: 10.1084/jem.20112741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ohigashi Y, Sho M, Yamada Y, et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res. 2005;11:2947–2953. doi: 10.1158/1078-0432.CCR-04-1469. [DOI] [PubMed] [Google Scholar]
- 60.Konishi J, Yamazaki K, Azuma M, et al. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res. 2004;10:5094–5100. doi: 10.1158/1078-0432.CCR-04-0428. [DOI] [PubMed] [Google Scholar]
- 61.Nomi T, Sho M, Akahori T, et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res. 2007;13:2151–2157. doi: 10.1158/1078-0432.CCR-06-2746. [DOI] [PubMed] [Google Scholar]
- 62.Thompson RH, Gillett MD, Cheville JC, et al. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci USA. 2004;101:17174–17179. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ghebeh H, Mohammed S, Al-Omair A, et al. The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: Correlation with important high-risk prognostic factors. Neoplasia. 2006;8:190–198. doi: 10.1593/neo.05733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thompson RH, Kuntz SM, Leibovich BC, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66:3381–3385. doi: 10.1158/0008-5472.CAN-05-4303. [DOI] [PubMed] [Google Scholar]
- 65.Okazaki T, Honjo T. PD-1 and PD-1 ligands: From discovery to clinical application. Int Immunol. 2007;19:813–824. doi: 10.1093/intimm/dxm057. [DOI] [PubMed] [Google Scholar]
- 66.Curiel TJ, Wei S, Dong H, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9:562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 67.Strome SE, Dong H, Tamura H, et al. B7-H1 blockade augments adoptive T-cell immunotherapy for squamous cell carcinoma. Cancer Res. 2003;63:6501–6505. [PubMed] [Google Scholar]
- 68.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Waterhouse P, Penninger JM, Timms E, et al. Lymphoproliferative disorders with early lethality in mice deficient in CTLA-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 70.Magistrelli G, Jeannin P, Herbault N, et al. A soluble form of CTLA-4 generated by alternative splicing is expressed by nonstimulated human T cells. Eur J Immunol. 1999;29:3596–3602. doi: 10.1002/(SICI)1521-4141(199911)29:11<3596::AID-IMMU3596>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 71.Grohmann U, Orabona C, Fallarino F, et al. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3:1097–1101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- 72.Munn DH, Sharma MD, Mellor AL. Ligation of B7-1/B7-2 by human CD4+ T cells triggers indoleamine 2,3-dioxygenase activity in dendritic cells. J Immunol. 2004;172:4100–4110. doi: 10.4049/jimmunol.172.7.4100. [DOI] [PubMed] [Google Scholar]
- 73.Munn DH, Zhou M, Attwood JT, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 74.Katz JB, Muller A, Metz R, et al. Immune escape: Role of indoleamine 2,3-dioxygenase in tumor tolerance. In: Lustgarten J, Cui Y, Li S, eds. Targeted Cancer Immune Therapy. Dordrecht, The Netherlands: Springer, 2009:257–284. [Google Scholar]
- 75.Soliman HH, Minton SE, Han HS, et al. A phase I study of ad.p53 DC vaccine in combination with indoximod in metastatic solid tumors. J Clin Oncol. 2013;31(suppl):3069a. [Google Scholar]
- 76.Jackson E, Dees EC, Kauh JS, et al. A phase I study of Indoximod in combination with docetaxel in metastatic solid tumors. J Clin Oncol. 2013;31(suppl):3026a. [Google Scholar]
- 77.Merlo LM, Pigott E, DuHadaway JB, et al. IDO2 is a critical mediator of autoantibody production and inflammatory pathogenesis in a mouse model of autoimmune arthritis. J Immunol. 2014;192:2082–2090. doi: 10.4049/jimmunol.1303012. [DOI] [PMC free article] [PubMed] [Google Scholar]