In this study, serum albumin was measured in 1,070 patients with active cancer. Decreased serum albumin levels were significantly associated with increased risk of venous thromboembolism and mortality.
Keywords: Cancer, Mortality, Risk, Serum albumin, Venous thromboembolism
Abstract
Background.
In cancer patients, reduced serum albumin has been described as a marker for global declining health and poor prognosis. Our aim was to investigate the association of albumin concentrations with the occurrence of venous thromboembolism (VTE) and mortality in patients with cancer.
Methods.
This investigation was performed in the framework of the Vienna Cancer and Thrombosis Study (CATS), a prospective observational cohort study. We included 1,070 patients with active cancer and assayed serum albumin from venous blood taken at study inclusion. Risk for occurrence of VTE was calculated in a proportional subdistribution hazard regression model with respect to competing risk of death and adjusted for cancer site, leukocyte count, estimated glomerular filtration rate, and cholinesterase.
Results.
Patients (630 males [58.9%] and 440 females [41.1%]) were observed for a median of 723 days. During follow-up, 90 VTE events (8.4%) and 396 deaths (37.0%) occurred. The median albumin was 41.3 g/L (25th–75th percentile, 37.6–44.2). Patients with albumin levels below the 75th percentile had a 2.2-fold increased risk of VTE (95% confidence interval [CI] 1.09–4.32), as well as a 2.3-fold increased risk of death (95% CI 1.68–3.20) compared with patients with albumin above the 75th percentile.
Conclusion.
Decreased serum albumin levels in cancer patients were significantly associated with increased risk of VTE and mortality. Serum albumin, a marker of a cancer patient’s overall prognosis, could be considered for risk assessment of important clinical outcomes such as VTE and mortality.
Implications for Practice:
Cancer patients are at increased risk of venous thromboembolism (VTE). In this prospective cohort study of 1,070 cancer patients, decreased serum albumin was a marker for risk of VTE and mortality, independent of kidney or liver function and inflammation markers. The study identified a group of patients with high risk of cancer-associated VTE and a reduced prognosis who may benefit from supportive therapy such as primary VTE prophylaxis.
Introduction
Cancer patients are at increased risk of venous thromboembolism (VTE), a disease entity that includes deep vein thrombosis and pulmonary embolism. The individual risk of VTE in cancer patients can be assessed by the presence of specific risk factors [1].
In the general population, reduced serum albumin levels are associated with a moderately increased risk of VTE occurrence [2]. Recently, serum albumin has been suggested as a novel risk factor for VTE occurrence in cancer patients [3]. The underlying causality of VTE risk has been considered to lie in renal loss of albumin in nephrotic syndrome [4] or albumin decrease caused by inflammatory processes [5]. However, cancer patients are a special population with an inherently high risk of VTE, warranting a detailed investigation of the association between albumin levels and VTE occurrence. A decrease in serum albumin in cancer patients has further been recognized as an expression of the consuming nature of the neoplasm and is associated with poor prognosis in cohorts of different malignancies [6–9]. It is currently not clear whether serum albumin is actively involved in mechanisms that lead to the occurrence of VTE and death or whether the observed association is a manifestation of underlying inflammatory processes [5], a renal loss of albumin in kidney disease [4], or an unknown process.
For the current investigation, we aimed to examine the association of decreased serum albumin with increased risk of VTE and mortality in a relatively large cohort of cancer patients with a wide range of malignancies and whether it interacts with or is confounded by markers of inflammation and liver or kidney disease.
Patients and Methods
This investigation was performed within the framework of the Vienna Cancer and Thrombosis Study (CATS), a prospective observational cohort study conducted with approval of the local ethics committee and in accordance with the Declaration of Helsinki. Patients with active cancer, histologically confirmed malignancy, age of at least 18 years, willingness to participate, and documented informed consent were eligible for inclusion. A breach of a single exclusion criterion resulted in study exclusion. Exclusion criteria were (a) acute infection with bacterial or viral pathogens within the preceding 2 weeks, (b) continuous anticoagulation therapy during the observation time, (c) chemotherapy within the last 2 months, (d) radiation therapy or surgery within the last 2 weeks, or (e) VTE or arterial thromboembolism within the last 3 months. There were no restrictions on the use short-term anticoagulation treatment during hospital stays or antiplatelet agents. Upon study inclusion, a clinical investigator took the patient’s medical history and sampled venous blood. Serum albumin concentration was assayed from venous blood taken at study baseline by the bromocresol green method with an accredited routine process, along with serum creatinine, leukocyte count, and cholinesterase level.
Patients received mail questionnaires approximately every 3 months over a maximum period of 2 years regarding the occurrence of VTE events. The time to occurrence of VTE events was the primary endpoint of the study. An independent committee of medical experts adjudicated all VTE events, based on objective imaging evidence. Of the total study population of 1,636 patients enrolled between October 2003 and May 2011, for the current investigation we had to exclude 202 patients who did not meet inclusion criteria after reevaluation and 364 patients because albumin levels were unavailable.
Statistical Analysis
We describe all continuous variables using medians and 25th–75th percentiles, and all categorical variables using absolute and relative frequencies. The correlation between continuous variables was assessed by the Spearman correlation coefficient. We calculated the median follow-up time with the reverse Kaplan–Meier method according to Schemper and Smith [10]. Albumin concentration, as the variable of interest, was analyzed continuously, grouped into quartiles, and with a cutoff at the 75th percentile.
The risk for occurrence of VTE was calculated in the proportional subdistribution hazard regression model according to Fine and Gray [11] with respect to competing risk of death from any cause and adjusted for categories of cancer sites with recognized risk of VTE, estimated glomerular filtration rate (eGFR) (CKD-EPI equation), leukocyte count, and cholinesterase level. According to the Khorana score [1], brain, pancreas, and stomach cancers were defined as having very high risk of VTE; lung, kidney, and genitourinary cancers and myeloma and lymphoma as high risk of VTE; and breast, colon, prostate, and all other included cancers as low risk of VTE (brain and renal cancers were not included in the initial Khorana score publication). The 29 patients with the highest leukocyte counts (>45 × 103 g/L), all of whom had leukemia, were excluded from the multivariable subdistribution hazards models. Cumulative incidence functions of the cohort grouped into patients with high and low serum albumin levels were compared using Gray’s test.
Multivariable Cox regression analysis was applied to calculate the association between albumin and risk of mortality, adjusted for cancer site, eGFR, leukocyte count, and cholinesterase level. The cumulative probability of death was assessed using the Kaplan–Meier method comparing low and high serum albumin. Interaction terms were created for the product of albumin with eGFR, leukocyte count, and cholinesterase and were considered to be significant if the interaction resulted in a statistically significant change of the hazard ratio with p < .05 in the multivariable models for VTE occurrence and mortality, respectively. As a sensitivity analysis, we modeled the risk of VTE occurrence in competing risk regression with the covariates serum albumin, age, body mass index (BMI), gender, cholinesterase, eGFR, cancer site and C-reactive protein (CRP) levels. We performed competing risk calculations with STATA (Windows version 13.0, Stata Corp., Houston, TX, http://www.stata.com) and further calculations with SPSS (Windows Version 22.0; IBM Corp., Armonk, NY, http://www.ibm.com).
Results
Population
The current investigation included 1,070 patients, with a median observation time of 723 days (25th–75th percentile, 244–731). The study cohort had a median age of 62 years (53–68) and a median BMI of 25.2 kg/m2 (22.4–28.3) and included 440 women (41.1%). All patients had a confirmed diagnosis of active cancer; 764 (71.4%) were newly diagnosed cancers and 306 (28.6%) were cancer progressions after partial or complete remission. The frequency of patients and respective cancer sites are reported in Table 1.
Table 1.
Study population and baseline patient characteristics
Serum albumin had a median level of 41.3 g/L (37.6–44.2) in the full cohort. Women and men had median albumin levels of 41.2 g/L (37.9–44.1) and 41.3 g/L (37.5–44.3), respectively. The highest median albumin level was found in patients with breast cancer (44.0 g/L [41.0–46.2]), and the lowest median albumin levels were found in colon cancer (39.0 g/L [34.9–42.0]), gastric cancer (39.3 g/L [33.9–43.4]), and pancreatic cancer (39.3 g/L [36.1–41.8]). The median albumin level in patients with newly diagnosed cancer was 40.7 g/L (37.2–43.9) and 42.2 g/L (38.6–44.7) in patients with progression of disease at time of study inclusion. Albumin level was weakly correlated with age (correlation coefficient −0.23, p < .001), BMI (0.098, p = .001), eGFR (−0.07, p = .021), and leukocyte count (0.072, p = .019) and moderately correlated with cholinesterase level (0.606, p < .001).
Risk of Venous Thromboembolism
Ninety VTE events (8.4%) occurred during the observation period. The cumulative probabilities of VTE occurrence were 6.2% (95% CI 4.9%–7.8%) at 6 months, 7.6% (6.1%–9.3%) at 12 months, and 8.5% (6.9%–10.2%) at 24 months.
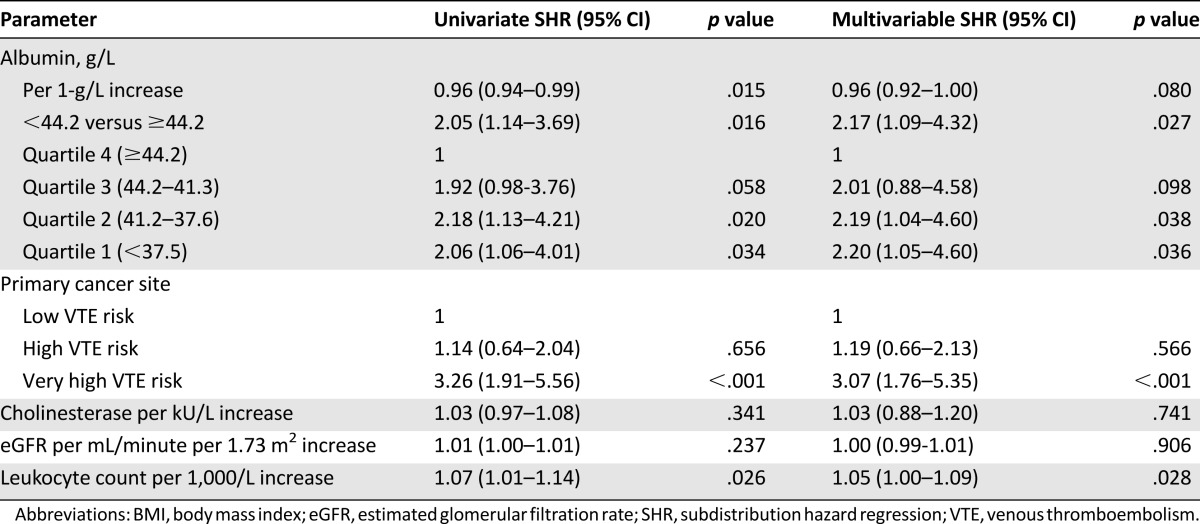
In the univariate subdistribution hazards model with competing risk of death, an albumin level below the 75th percentile (<44.2 g/L) was significantly associated with increased risk of VTE occurrence (subdistribution hazard ratio [SHR] 2.05, 95% confidence interval [CI] 1.14–3.69) compared with patients with albumin levels above the 75th percentile (Table 2). Per 1-g/L increase in albumin, the risk of VTE decreased by 3.7% (SHR 0.96, 95% CI 0.94–0.99). Compared with patients in the highest quartile of albumin levels, patients with albumin levels in the second highest quartile had a SHR of 1.9 (95% CI 0.98–3.76) for occurrence of VTE. Patients in the third and fourth highest quartiles of albumin levels had SHRs of 2.2 (95% CI 1.13–4.21) and 2.1 (CI 1.06–4.01), respectively, compared with the highest quartile (Table 2).
Table 2.
Risk of VTE occurrence calculated in univariate and multivariable SHR models of risk for VTE occurrence with competing risk of death.
In the multivariable competing risk model adjusted for cancer site risk category, eGFR, cholinesterase, and leukocyte count, patients with decreased serum albumin levels below the 75th percentile (<44.2 g/L) were at 2.2-fold increased risk of VTE occurrence (SHR 2.17, 95% CI 1.09–4.32) (Table 2; Fig. 1). Patients with serum levels in the third and fourth highest quartiles had SHRs of 2.19 (p = .038, 95% CI 1.04–4.60) and 2.20 (p = .036, 95% CI 1.05–4.60) compared with those with the highest serum albumin (Table 2). Interactions in the risk of VTE occurrence between albumin levels and eGFR, cholinesterase, and leukocyte count were ruled out with the interaction terms SHR 0.999 (p = .511), 0.992 (p = .445), and 1.004 (p = .445), respectively. In the sensitivity analysis, the risk of VTE occurrence, with borderline significance, remained associated with albumin levels below the 75th percentile (<44.2 g/L) in the multivariable competing risk regression model (SHR 2.04, 95% CI 0.96–4.33, p = .064).
Figure 1.
Cumulative incidence of venous thromboembolism (VTE) and Gray’s test. We compared the cumulative incidence of VTE in patients with a serum albumin level below the 75th percentile with that of patients above the 75th percentile.
Abbreviation: Alb, serum albumin.
Risk of Mortality
Death was observed in 396 patients (37.0%) during follow-up. The cumulative probabilities of mortality occurrence were 11.4% (9.4%–13.4%) at 6 months, 23.0% (20.5%–25.5%) at 12 months, and 39.5% (36.4%–42.6%) at 24 months.
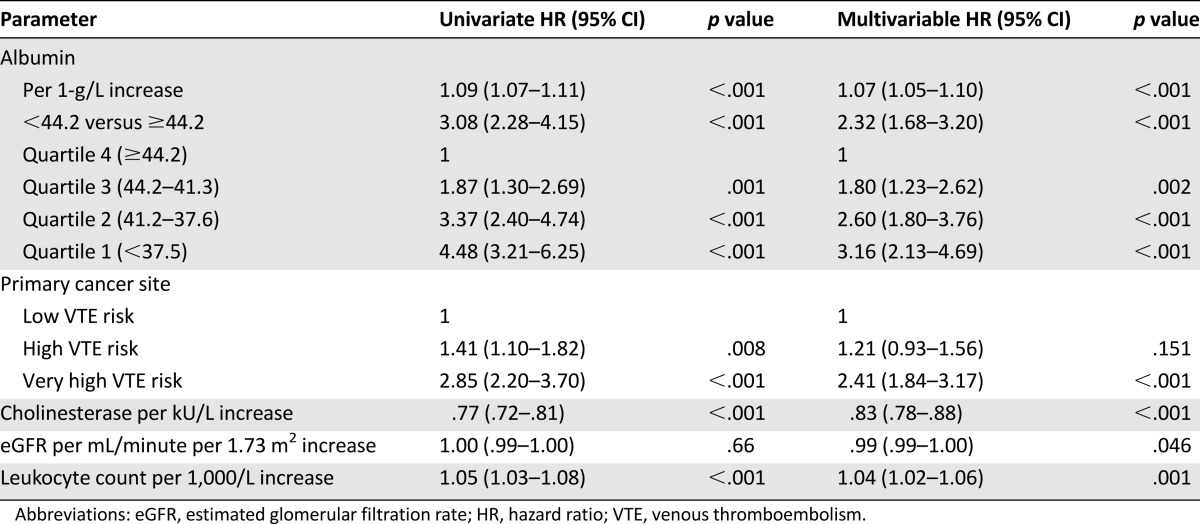
In the univariate Cox proportional hazards model, patients with albumin levels below the 75th percentile were at threefold (95% CI 2.28–4.15) increased risk of death compared with patients above 75th percentile (Table 3). In the multivariable model adjusted for cancer site, eGFR, cholinesterase, and leukocyte count, the risk of mortality in patients with albumin below the 75th percentile was reduced to a hazard ratio (HR) of 2.3 (95% CI 1.69–3.20) compared with those with the highest albumin levels (Table 3; Fig. 2). Divided into quartiles, the risk of mortality increased stepwise, from an HR of 1.80 (1.23–2.62) for patients in the second highest quartile, to 2.60 (1.80–3.76) in the third highest quartile, and to 3.16 (2.13–4.69) in the lowest quartile of albumin levels compared with the highest quartile of albumin level (Table 3). Interactions on the risk of mortality between albumin levels and eGFR, cholinesterase levels, and leukocyte count were ruled out with the interaction terms HR 1.00 (p = .14), HR 0.99 (p = .06), and HR 1.00 (p = .46), respectively.
Table 3.
Risk of mortality calculated in univariate and multivariable Cox regression models for risk of death.
Figure 2.
Cumulative incidence of death and log-rank test. We compared the cumulative incidence of death in patients with serum albumin level below the 75th percentile with that of patients above the 75th percentile.
Abbreviation: Alb, serum albumin.
Discussion
In our investigation, we were able to show that patients with cancer and a lower serum albumin level were at increased risk of VTE occurrence and mortality compared with cancer patients with high serum albumin. Even the slight decrement of serum albumin below 44.2 g/L was independently associated with an approximately twofold increased risk of VTE compared with serum albumin level >44.2 g/L. Beyond a dichotomous cutoff at the 75th percentile (44.2 g/L), we were also able to show that the risk of VTE increased continuously with decreasing levels of serum albumin.
Previously, Shah et al. approached the association between albumin and VTE occurrence in cancer patients by emphasizing that albumin levels >40 g/L in cancer patients were associated with a 39% cumulative risk reduction in VTE risk (HR 0.61, 95% CI 0.39–0.94) [3]. Instead, we chose to look at VTE as a preventable event by identifying patients at high risk of VTE who could benefit from prophylactic anticoagulation treatment [12].
We were also able to confirm the association of albumin and mortality in cancer patients. Previous studies have established reduced serum albumin as a marker of poor prognosis in cancer patients [6, 7, 9, 13–15] and also of a patient’s capacity to endure aggressive cancer therapy [16, 17]. A reduced albumin level has previously been attributed to the consuming nature of the cancer [3, 7]. However, in our cohort, serum albumin levels were not significantly lower in those patients with progressive disease after complete or partial remission at the time of study inclusion than in those with newly diagnosed cancer. Serum albumin was, however, the lowest in pancreatic cancers, which have among the worst prognosis. We adjusted for categories of cancer sites according to their VTE risk in our multivariable risk models for both VTE occurrence and mortality and ruled out cancer site as a confounder.
In the general noncancer population, a decreased serum albumin level is also associated with the occurrence of VTE. In the prospective Reasons for Geographic and Racial Differences in Stroke (REGARDS) study, a 25% (95% CI 1.06–1.48) increased risk of VTE was found for every standard deviation drop in serum albumin [5]. The authors reconfirmed previous findings that the drop in serum albumin is attributed to a hypercoagulable or low-level inflammatory state, which might be the underlying cause of VTE risk [2, 5]. We therefore examined whether the association between albumin level and the risk of VTE occurrence in our cohort was confounded by the leukocyte count as a marker of inflammation. Although leukocyte count is also a risk factor for VTE occurrence in our cohort, its inclusion in the multivariable model did not diminish the association of albumin level and VTE occurrence. In a previous investigation in a subset of solid tumor patients of the CATS cohort, Kanz et al. showed that the VTE risk increased by 20% (HR 1.2, 95% CI 1.0–3.8, p = .037) per double increase in CRP levels [18]. We substituted leukocyte counts for CRP levels in the sensitivity analysis of this investigation and obtained similar results. The exclusion criteria in our study ensured that acute bacterial or viral infections did not influence albumin levels. The association of albumin with VTE occurrence remained independent of leukocyte count and was unmodified by an interaction.
As there is no perceivable direct causal relationship between albumin level and VTE occurrence or mortality, we considered further potential confounders and interactions. We were able to show that the association is not confounded by kidney function, as assessed by eGFR. We were not able to prove that decreased serum albumin was caused by impaired kidney function in this cohort of cancer patients. Although no relevant correlation of serum albumin with renal function, assessed by eGFR, was found (correlation coefficient −0.07, p = .021), a considerable urinary loss of albumin cannot be excluded, because albuminuria may not correlate with eGFR, and microalbuminuria is common in cancer patients [19]. Patients with severe kidney failure in Kidney Disease: Improving Global Outcomes stage 4 and 5 with eGFR <30 mL/minute per 1.73 m2 were also not present in this cohort. In a previous investigation, we were able to show that kidney function itself is not associated with occurrence of VTE in cancer patients [20]. Further, there was no modification of VTE risk or mortality risk caused by an interaction between eGFR and albumin. We further investigated whether the liver’s capacity to synthesize proteins was a confounder in our analysis. The association between serum albumin and VTE occurrence was independent of cholinesterase levels as a measure of liver synthesis capacity within the range of cholinesterase levels of the cohort, which did not include patients with severe liver failure. We also found that there was no increase in VTE risk in patients with both reduced albumin and cholinesterase levels in the interaction analysis. Admittedly, liver synthesis capacity was not significantly reduced overall; therefore, our findings may not apply to patients with severe liver failure.
Conclusion
Although we cannot establish a causal relationship between albumin levels and VTE occurrence in this study design, we were able to show that reduced serum albumin levels are associated with increased risk of VTE independently from kidney function, inflammatory markers, and liver synthesis capacity. Reduced serum albumin is further associated with less favorable prognosis in cancer patients. Serum albumin may therefore be considered as a marker for VTE risk and mortality in cancer patients.
Acknowledgments
We thank the members of the adjudication committee: Renate Koppensteiner and Andrea Willfort-Ehringer (Department of Angiology, Medical University of Vienna), Sylvia Metz-Schimmerl (Department of Diagnostic Radiology, Medical University of Vienna), and Robert Dudczak (Department of Nuclear Medicine, Medical University of Vienna). This study was supported by funds of the Oesterreichische Nationalbank (Anniversary Fund, project 14744) and a grant from the Hochschuljubiläumsstiftung der Stadt Wien.
Author Contributions
Conception/Design: Ingrid Pabinger, Cihan Ay
Provision of study material or patients: Julia Riedl, Eva-Maria Reitter
Collection and/or assembly of data: Oliver Königsbrügge, Florian Posch, Julia Riedl
Data analysis and interpretation: Oliver Königsbrügge, Florian Posch, Ingrid Pabinger, Cihan Ay
Manuscript writing: Oliver Königsbrügge
Final approval of manuscript: Oliver Königsbrügge, Florian Posch, Julia Riedl, Eva-Maria Reitter, Christoph Zielinski, Ingrid Pabinger, Cihan Ay
Disclosures
The authors indicated no financial relationships.
References
- 1.Khorana AA, Kuderer NM, Culakova E, et al. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902–4907. doi: 10.1182/blood-2007-10-116327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Folsom AR, Lutsey PL, Heckbert SR, et al. Serum albumin and risk of venous thromboembolism. Thromb Haemost. 2010;104:100–104. doi: 10.1160/TH09-12-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah MA, Capanu M, Soff G, et al. Risk factors for developing a new venous thromboembolism in ambulatory patients with non-hematologic malignancies and impact on survival for gastroesophageal malignancies. J Thromb Haemost. 2010;8:1702–1709. doi: 10.1111/j.1538-7836.2010.03948.x. [DOI] [PubMed] [Google Scholar]
- 4.Mahmoodi BK, Gansevoort RT, Næss IA, et al. Association of mild to moderate chronic kidney disease with venous thromboembolism: Pooled analysis of five prospective general population cohorts. Circulation. 2012;126:1964–1971. doi: 10.1161/CIRCULATIONAHA.112.113944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olson NC, Cushman M, Lutsey PL, et al. Inflammation markers and incident venous thromboembolism: The REasons for Geographic And Racial Differences in Stroke (REGARDS) cohort. J Thromb Haemost. 2014;12:1993–2001. doi: 10.1111/jth.12742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asher V, Lee J, Bali A. Preoperative serum albumin is an independent prognostic predictor of survival in ovarian cancer. Med Oncol. 2012;29:2005–2009. doi: 10.1007/s12032-011-0019-5. [DOI] [PubMed] [Google Scholar]
- 7.Douglas E, McMillan DC. Towards a simple objective framework for the investigation and treatment of cancer cachexia: The Glasgow Prognostic Score. Cancer Treat Rev. 2014;40:685–691. doi: 10.1016/j.ctrv.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Proctor MJ, McMillan DC, Horgan PG, et al. Systemic inflammation predicts all-cause mortality: A Glasgow inflammation outcome study. PLoS One. 2015;10:e0116206. doi: 10.1371/journal.pone.0116206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seebacher V, Grimm C, Reinthaller A, et al. The value of serum albumin as a novel independent marker for prognosis in patients with endometrial cancer. Eur J Obstet Gynecol Reprod Biol. 2013;171:101–106. doi: 10.1016/j.ejogrb.2013.07.044. [DOI] [PubMed] [Google Scholar]
- 10.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17:343–346. doi: 10.1016/0197-2456(96)00075-x. [DOI] [PubMed] [Google Scholar]
- 11.Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 12.Lyman GH, Bohlke K, Khorana AA, et al. American Society of Clinical Oncology Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update 2014. J Clin Oncol. 2015;33:654–656. doi: 10.1200/JCO.2014.59.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ulas A, Turkoz FP, Silay K, et al. A laboratory prognostic index model for patients with advanced non-small cell lung cancer. PLoS One. 2014;9:e114471. doi: 10.1371/journal.pone.0114471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du X-J, Tang L-L, Mao Y-P, et al. The pretreatment albumin to globulin ratio has predictive value for long-term mortality in nasopharyngeal carcinoma. PLoS One. 2014;9:e94473. doi: 10.1371/journal.pone.0094473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yi JH, Lee J, Park SH, et al. A prognostic model to predict clinical outcomes with first-line gemcitabine-based chemotherapy in advanced pancreatic cancer. Oncology. 2011;80:175–180. doi: 10.1159/000328449. [DOI] [PubMed] [Google Scholar]
- 16.Ayuk F, Bussmann L, Zabelina T, et al. Serum albumin level predicts survival of patients with gastrointestinal acute graft-versus-host disease after allogeneic stem cell transplantation. Ann Hematol. 2014;93:855–861. doi: 10.1007/s00277-013-1957-0. [DOI] [PubMed] [Google Scholar]
- 17.Pant S, Martin LK, Geyer S, et al. Baseline serum albumin is a predictive biomarker for patients with advanced pancreatic cancer treated with bevacizumab: A pooled analysis of 7 prospective trials of gemcitabine-based therapy with or without bevacizumab. Cancer. 2014;120:1780–1786. doi: 10.1002/cncr.28648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanz R, Vukovich T, Vormittag R, et al. Thrombosis risk and survival in cancer patients with elevated C-reactive protein. J Thromb Haemost. 2011;9:57–63. doi: 10.1111/j.1538-7836.2010.04069.x. [DOI] [PubMed] [Google Scholar]
- 19.Pedersen LM, Sørensen PG. Mediators of inflammation correlate with microalbuminuria in patients with non-Hodgkin’s lymphoma. Br J Haematol. 2003;121:275–279. doi: 10.1046/j.1365-2141.2003.04285.x. [DOI] [PubMed] [Google Scholar]
- 20.Königsbrügge O, Lötsch F, Zielinski C, et al. Chronic kidney disease in patients with cancer and its association with occurrence of venous thromboembolism and mortality. Thromb Res. 2014;134:44–49. doi: 10.1016/j.thromres.2014.04.002. [DOI] [PubMed] [Google Scholar]