Stereotactic surgery (SRS) has revolutionized local treatment of brain metastases. Targeted therapies, including small-molecule inhibitors and monoclonal antibodies that target cancer cell metabolism or angiogenesis, have transformed managing systemic disease. This article discusses reported and ongoing clinical trials assessing the safety and efficacy of targeted therapy during SRS.
Keywords: Targeted therapy, Stereotactic radiosurgery, Brain metastasis
Abstract
Brain metastases are the most common intracranial malignancy. Many approaches, including radiation therapy, surgery, and cytotoxic chemotherapy, have been used to treat patients with brain metastases depending on the patient’s disease burden and symptoms. However, stereotactic surgery (SRS) has revolutionized local treatment of brain metastases. Likewise, targeted therapies, including small-molecule inhibitors and monoclonal antibodies that target cancer cell metabolism or angiogenesis, have transformed managing systemic disease. Prospective data on combining these treatments for synergistic effect are limited, but early data show favorable safety and efficacy profiles. The combination of SRS and targeted therapy will further individualize treatment, potentially obviating the need for cytotoxic chemotherapy or whole-brain radiation. There is a great need to pursue research into these exciting modalities and novel combinations to further improve the treatment of patients with brain metastases. This article discusses reported and ongoing clinical trials assessing the safety and efficacy of targeted therapy during SRS.
Implications for Practice:
Treatment of patients with brain metastases requires a multidisciplinary approach. Stereotactic radiosurgery is increasingly used in the upfront setting to treat new brain metastasis. Targeted therapies have revolutionized systemic treatment of many malignancies and may sometimes be used as initial treatment in metastatic patients. There is sparse literature regarding safety and efficacy of combining these two treatment modalities. This article summarizes the supporting literature and highlights ongoing clinical trials in combining radiosurgery with targeted therapy.
Introduction
Brain metastasis (BM) is the most common intracranial malignancy in adults, occurring in 20%–40% of all patients with malignant tumors [1]. The incidence of BM may be rising because of improvement in local therapy, systemic therapy, and better control of extracranial metastatic disease. In addition, detection of metastatic lesions has improved because of increased use of magnetic resonance imaging. Patients with BM often have poor prognosis and survival [2–4].
Advances in radiation oncology technology have permitted precise delivery of an ultrahigh definitive dose of radiation to brain lesions. Conventionally fractionated radiation therapy (RT) typically delivers treatment in 1.8–2.0 Gy fractions over as long as a 6-week course, but stereotactic radiosurgery (SRS) provides ablative treatment often in a single treatment. SRS offers the ability to tightly conform radiation to the target volume and spare substantial amounts of normal tissue. For properly selected patients, usually those with a limited number of lesions and controlled extracranial disease, many studies show that SRS is as effective as surgical resection for the management of BM, with local control rates as high as 94% [5–7].
Targeted medical therapies in oncology interfere with the progression and dissemination of cancer by directly modulating cancer metabolism and progression. Targeted therapies have revolutionized cancer treatment, potentially offering an improved therapeutic ratio. They are broadly classified into the major categories of small-molecule inhibitors (SMIs) and monoclonal antibodies. Targeted agents can modulate angiogenesis, signal transduction, the immune system, and cellular apoptosis.
Multiple medications have already entered into routine clinical use for several different cancers. These include v-raf murine sarcoma viral oncogene homolog B (BRAF) inhibitors in combination with MEK inhibitors for melanoma, epidermal growth factor receptor (EGFR) inhibitors in colon cancer, and anaplastic lymphoma kinase (ALK) inhibitors for non-small cell lung cancer.
As the indications for targeted agents were refined, it became possible to integrate them into clinical trials in arrangement with established therapeutic modalities, such as radiotherapy [8]. As a result, an enormous opportunity exists to combine ablative SRS treatment with targeted therapy to improve local control, systemic control, and overall survival.
The aim of this article is to review the current status of targeted therapies combined with stereotactic radiosurgery for patients with brain metastases.
Materials and Methods
Three major components were used in the search strategy, linked with the "AND" operator, in each database to find clinical trials or reports of combined SRS and targeted agents. The first component consisted of search terms related to targeted therapies, including “targeted therapy,” or >60 individual targeted agents selected for search (generic and brand names). The second comprised search terms related to SRS, including “stereotactic radiosurgery,” “SRS,” “radiosurgery,” or “Gamma Knife.” The third was composed of terms related to brain metastasis including “brain metastasis” or “brain metastases.”
The MEDLINE (via PubMed), Cochrane Library, Embase, and ClinicalTrials.gov databases were searched for reports of clinical studies that were published between 1990 and 2014,were written in English, and assessed safety or efficacy of combined SRS and targeted therapies. All results at each stage of the literature search were combined in an electronic bibliography management program with duplicates removed electronically and manually. After combining these search strategies in the above databases, the investigators (F.Y.M. and G.N.M.) used manual exclusion or filters when available to limit studies to English-language clinical reports of studies in human participants.
Two investigators (F.Y.M. and G.N.M.) independently reviewed all returned articles using a 3-stage selection process. During stage 1, 219 records were returned in the initial database searches. During stage 2, all studies were manually reviewed to ensure studies included only human patients receiving a combination of SRS and targeted therapy for brain metastases; 182 studies were excluded during stage 2. During stage 3, editorials, case reports, or trials with unknown or unreported endpoints were excluded. Thirteen published studies ultimately were selected for review, as well as four ongoing clinical trials.
SRS in the Treatment of Intracranial Disease
SRS plays a valuable role in the management of patients with BM as a stand-alone treatment or combined with surgical resection or whole-brain RT (WBRT) [9].
SRS is a suitable modality for patients who have 1–4 brain lesions, generally up to 4 cm in maximum diameter. Independent of histologic features, SRS is effective even for diseases that have been traditionally thought to be relatively radioresistant, such as renal cell carcinoma or colon cancer [10, 11]. The Radiation Therapy Oncology Group (RTOG) used recursive partitioning analysis (RPA) to stratify patients into prognostic groups. Key determinants to determine RPA class include Karnofsky performance status (KPS), age, and extracranial disease control. The median duration of survival for patients in RPA class I is 7.1 months, whereas those with poor KPS (RPA class III) have a median survival duration of 2.3 months [12]. Patients with an excellent performance status, solitary or few intracranial metastases, and controlled extracranial disease are the best candidates for SRS [13]. More recently, some preliminary studies have shown that SRS without WBRT can lead to good clinical outcomes when performed even in patients with 5 or more brain metastases [14, 15].
Patients with an excellent performance status, solitary or few intracranial metastases, and controlled extracranial disease are the best candidates for SRS.
The Radiation Therapy Oncology Group evaluated the maximum tolerated dose (MTD) of SRS in relation to tumor size. Lesions 2 cm or less can receive 24 Gy (MTD was not reached); for lesions 2.1–3.0 cm, up to 18 Gy; and for lesions 3.1–4.0 cm, up to 15 Gy [16].
The exact radiobiology of tumor destruction from SRS is incompletely understood. Conventionally fractionated radiation mediates DNA damage, leading to eventual mitotic catastrophe. In contrast, SRS is probably mediated through damage, with substantial endovascular disruption leading to microvascular damage. An exciting and emerging field of study in SRS is the potential enhanced antitumor immunologic response that ablative treatments may offer [17, 18]. Refined understanding of these pathways may potentially allow for the improvement of radioprotectors or radiosensitizers that may enhance the therapeutic ratio.
SRS and the Tumor Microenvironment
The effect of fractionated RT on the tumor microenvironment has been extensively studied. Used for decades, fractionated RT is designed to maximize tumor cell kill with acceptable damage to normal tissue. The primary mediator of tumor cell kill in fractionated RT is loss of reproductive ability caused by DNA double-strand breaks. The effect of RT has been established by the “4 Rs” of radiobiology: repair of sublethal damage, repopulation of cells after exposure, redistribution of cells within the cell cycle, and reoxygenation of the surviving population. A fifth R was later added: intrinsic radiosensitivity of the tumor [18]. The balance of these effects determines net cell kill. For example, reoxygenation and redistribution places cells into a more radiosensitive state, whereas repopulation and repair of sublethal damage allow for tumor recovery.
The linear quadratic (LQ) model of radiation therapy is a satisfactory model to predict the effects of modest fraction size, generally 1–5 Gy. However, the LQ model may be unsatisfactory to model the effects of high-dose RT given additional effects of high dose, such as increased antitumor immune response and ablative vascular and stromal damage. Fuks and Kolesnick have described that the radiation sensitivity of tumors above 10 Gy may instead be governed by the radiosensitivity of tumor endothelial cells [19, 20]. In addition, the LQ model balances the differences in α/β ratio between tumor and normal tissue. SRS uses image guidance and precision within millimeters to limit dose beyond the tumor, potentially obviating the intrinsic radiosensitivity between tumor and normal tissue. Other models, such as the universal survival curve, hybridize the linear quadratic model and the multitarget model to better predict RT effects at both modest and ablative high doses [21].
Fractionated radiation primarily mediates tumor kill from DNA double-strand breaks by reactive oxygen species. In high-dose, single-fraction treatment, vascular damage and tumor antigen release play an important role in SRS. Tumor vasculature is often tortuous and dilated, has incomplete or inadequate basement membranes or supporting stroma, and is leaky and branched. Although tumor vasculature may incorporate native normal vasculature, the structure is different and susceptible to multiple stressors. The ablative nature of SRS may also increase antitumor immune response given increased antigen release [22].
We understand that the radiobiology of high-dose radiation is likely different from that of fractionated radiation therapy. SRS generally treats intracranial lesions with 15–25 Gy in 1–2 fractions. This modality supplants, and even replaces as appropriate, the use of whole-brain radiation therapy for palliation of brain metastases. SRS is possible with technological advances, such as tumor imaging improvements, on-board immediate pretreatment imaging, patient immobilization improvements, and intensity-modulated radiation therapy.
The substantial improvement in tumor localization and fractionation may have reached a temporary plateau, so the need to potentiate radiation via exploiting tumor biology will be critical [23]. Targeted therapies have already been used in combination with radiation in multiple phase III trials. These drugs are characterized by being rationally designed to target an aberrant cellular process or molecular characteristic of the tumor that may be critical to tumor progression, as opposed to cell division in general with cytotoxic chemotherapy. Targets include EGFR, human epidermal growth factor receptor 2 (HER2/neu), vascular endothelial growth factor, CD20, BRAF, and histone deacetylase. For example, a phase III trial of RT with or without cetuximab in locally advanced squamous cell carcinoma of the head and neck showed 10% improved survival at 3 years, with improved locoregional control [24]. However, active studies are still limited as combination trials represent less than 1% of all phase III oncology trials [8].
The exact mechanism of how the tumor microenvironment changes with SRS and targeted agents is not well understood and is highly individualized to each targeted therapy. In the 1970s, George Steel proposed a framework to describe possible interactions of radiation and chemotherapy, including spatial cooperation, temporal modulation, cytotoxic enhancement, and normal tissue protection [25]. Drug can radiosensitize or be synergistic with radiation, thereby increasing antitumor effect or normal tissue damage. Drug can also be radioprotective if it is able to selectively protect normal cells. Because of targeted therapy’s improved specificity for disease, there is potential for synergy with improved toxicity profile compared with cytotoxic chemotherapy. Morris and Harari expanded the Steel framework to include biologic cooperation with targeted therapy, suggesting that targeted therapy may kill a cell population resistant to radiation [8]. However, microenvironment change may not be a substantial contributor to cell kill considering the ablative nature of SRS treatments.
Prospective Assessment of SRS and Targeted Therapies in Brain Metastases
The application of targeted therapy has already resulted in impressive tumor responses in several histologic cancer types, particularly with EGFR-mutant non-small cell lung cancer or HER2/neu-positive breast cancer. However, its specific role in the management of patients with brain metastasis is not well-defined [26, 27]. The initial management of these lesions is often with resection, SRS, WBRT, or some combination of these modalities, as indicated. Following this, the treatment approach should rely on a multidisciplinary approach to rationally combine targeted therapy when standard initial options have failed or the patient has progressed.
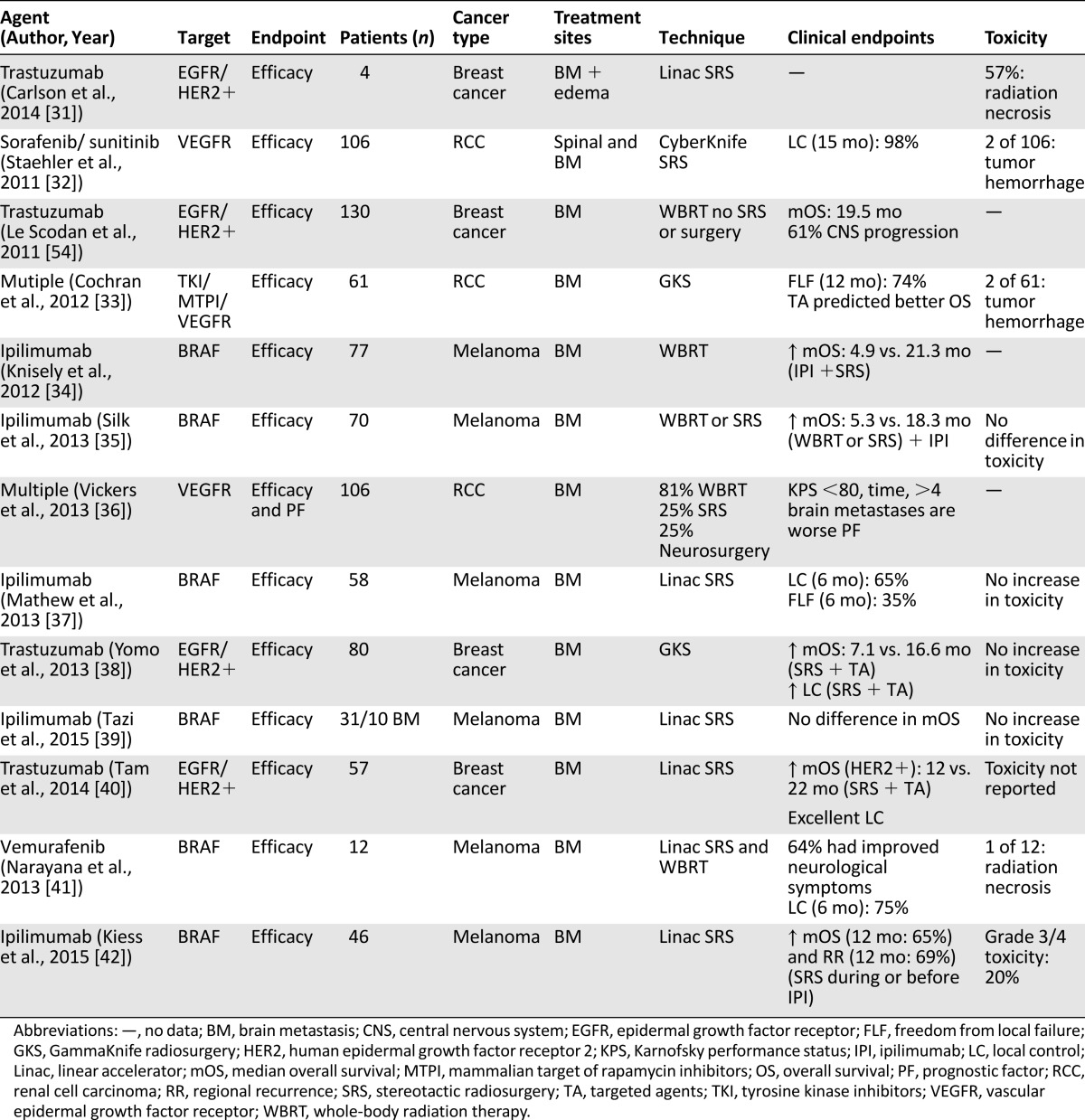
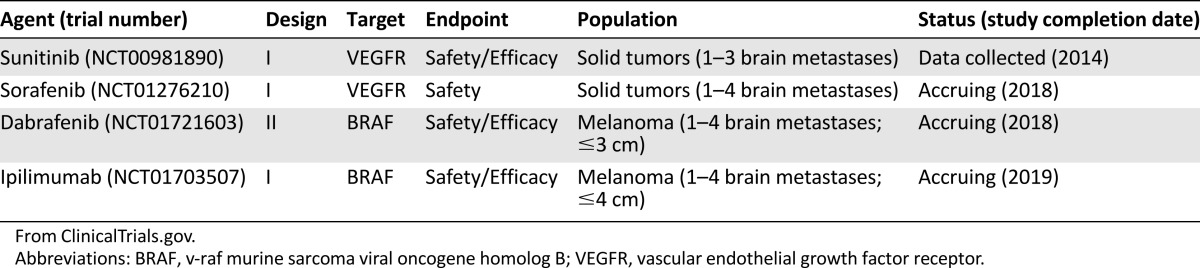
The preponderance of clinical evidence combining SRS with targeted therapy in brain metastasis comes from retrospective studies and case reports [28–30]. These reports are summarized in Table 1 [31–42]. Several ongoing prospective trials are evaluating this novel combination of treatments (Table 2) [43–46].
Table 1.
Published studies of targeted therapies and stereotactic radiosurgery for brain metastasis (via MEDLINE)
Table 2.
Ongoing or completed clinical trials of targeted therapies and stereotactic radiosurgery for brain metastasis
RTOG 0320 was a phase III clinical trial that assessed 3 different treatment groups: (a) WBRT (37.5 Gy in 15 fractions) and SRS, (b) WBRT plus SRS with temozolomide, and (c) WBRT plus SRS with erlotinib. Patients included those with non-small cell lung cancer who had 1–3 metastatic brain lesions. Temozolomide or erlotinib was offered in the adjuvant setting up to 6 months after completion of radiation. A total of 126 patients were enrolled, and the primary endpoint was overall survival. The median survival durations for the 3 treatments groups were as follows: WBRT + SRS, 13.4 months; WBRT +SRS + temozolomide, 6.3 months; and WBRT + SRS + erlotinib, 6.1 months. Despite an absolute survival improvement in the WBRT + SRS group, the survival difference among the 3 groups did not reach statistical significance. In each of the radiation with targeted therapy groups, grade 3–5 toxicities were significantly higher than in the radiation-only group. The study suggests that the addition of temozolomide or erlotinib to radiation therapy had no survival benefit, and the additional toxicity may have contributed to the poorer survival [47]. The temozolomide and erlotinib groups may have compromised further chemotherapy in these patients, which may have affected the primary endpoint. In addition, the study was underpowered for its final analysis given that it reached approximately one third of its target accrual goal.
A prospective trial assessed the use of CyberKnife-based (Accuray Inc., Sunnyvale, CA; https://www.cyberknife.com/) radiosurgery followed by early adjuvant bevacizumab for metastatic tumors in patients with a solitary brain lesion and symptomatic extensive cerebral edema. All patients underwent magnetic resonance imaging before radiotherapy, and 8 patients (all symptomatic at diagnosis) were analyzed. Dose ranged from 20 to 33 Gy (median, 30 Gy) in 1–5 fractions. Bevacizumab, 5 mg/kg, was administered for a median of 4 cycles (given every 2 weeks) [48]. All patients undergoing primary analysis showed an average lesion reduction of 55.8% and 63.4% in postcontrast and T2-weighted scans, respectively. Seven of eight patients had improvement in clinical symptoms, with a nearly 20-point median score improvement in performance status. At a median 5 months of follow-up after the last dose of bevacizumab, all 8 patients were alive and had achieved intracranial disease control. Radiation necrosis and recurrent brain edema were not observed [48]. This approach seems promising and safe for this group of patients presenting with a single metastatic brain lesion and extensive edema.
It is important to highlight that the bulk of reports currently available have relatively small sample sizes and short follow-up. However, the data are encouraging. Some studies do report reasonable safety and acceptable toxicity profile in combined-modality treatment. Certain series suggest better median survival duration, longer freedom from failure, and better symptom management with minimal increase in radiation toxicity.
Targeted Therapies for Breast Cancer, Lung Cancer, and Renal Cell Carcinoma
In selecting targeted therapies for brain metastases, priority is given to those with central nervous system (CNS) penetration, with sufficient ability to reach therapeutic level, and activity against a solid tumor type [26, 49]. As of this writing, no level I evidence supports the use of SRS with targeted therapy. However, several studies show promising activity of combination therapy, and multiple clinical trials are underway. The bulk of data are used in the whole-brain or RT-naïve setting. Here we present key data in SRS trials and series with targeted therapy for different histologic types.
Multiple targeted therapies have been attempted in breast cancer, most notably antiangiogenic agents, such as bevacizumab and anti-HER2 agents (including trastuzumab and lapatinib, which also targets EGFR). Trastuzumab is a standard-of-care therapy in managing patients with HER2-positive metastatic breast cancer. Although trastuzumab improves survival in patients with metastatic disease, compared with patients not receiving trastuzumab there is an apparent increase in both the incidence of brain metastasis in these patients and the CNS being the first site of relapse. The relatively large molecular size limits CNS penetration [50, 51]. Lapatanib has dual-target effect and is more likely to cross the blood-brain barrier than trastuzumab. Yomo et al. retrospectively analyzed 40 patients treated with Gamma Knife (Elekta, Stockholm, Sweden; https://www.elekta.com) SRS and disease with HER2 overexpression, 24 of whom received lapatinib. The 1-year local control of lapatinib-treated patients was 86% versus 69% for non-lapatinib-treated patients (p < .001), highlighting improved disease control of patients receiving combination therapy [38]. This is promising because a previous study reported that patients with HER2-positive breast cancer and brain metastases treated with SRS have decreased progression-free survival and overall survival [40]. Interestingly, bevacizumab has shown protective effects against radiation necrosis and CNS toxicity after SRS or hypofractionated SRS to the brain [52, 53]. In a retrospective series of breast cancer patients with brain metastases treated with WBRT with or without trastuzumab, those with HER2-positive disease receiving trastuzumab survived significantly longer than those who did not receive trastuzumab [54]. None of these patients received SRS or surgery. The bulk of patients who were HER2-positive and received trastuzumab and progressed while receiving therapy were found to die of intracranial progression. This suggests that in this instance, patients who have an actionable mutation and receive targeted therapy may further benefit from increased local therapy, such as SRS, to further improve intracranial control while extracranial disease is controlled.
Renal cell carcinoma (RCC), like melanoma, presents an interesting clinical challenge given its relative radioresistance. Dose escalation with SRS has largely overcome this to offer excellent local control of brain metastases, but further progress is possible. One Wake Forest University series reported on 61 patients with RCC metastases treated with Gamma Knife SRS with or without sunitinib, sorafenib, or temsirolimus. The median survival duration for patients receiving targeted therapy increased from 7.2 to 16.6 months, with freedom from local failure increasing from 60% to 93% [33]. Safety and excellent local control has been shown in RCC patients given simultaneous antiangiogenic sorafenib or sunitinib therapy [32]. Again, this continues to be an active area of study to further establish safety and efficacy.
The tyrosine kinase inhibitor erlotinib in EGFR-mutant lung adenocarcinoma has already shown CNS penetration and activity. Agents of this class can be used in first-line treatment of metastatic patients. Gerber et al. found equivalent survival in lung cancer patients with brain metastases treated with upfront erlotinib versus standard-of-care whole-brain radiation therapy [55]. Although RTOG 0320 found a potentially deleterious effect to combination SRS and erlotinib, Welsh et al. found no significant additional neurotoxicity in a phase II study that added erlotinib to whole brain RT. The overall response rate was 86%. Compared with historic controls, patients had longer overall survival with combination therapy and patients with EGFR-mutant disease had particularly longer survival than those with EGFR wild-type disease [47, 56]. Other agents of interest in lung cancer include the ALK mutation-targeted crizotinib, also approved for first-line treatment of ALK-positive lung cancer. The role of all these agents when combined with SRS is unclear, but the potential CNS activity of each agent warrants further study.
Melanoma and Immunotherapy
Targeted therapies have revolutionized the treatment of melanoma, particularly those with BRAF V600E mutations, allowing for improved progression-free survival and even overall survival. Medications that have activity in brain metastases include the BRAF kinase inhibitors dabrafenib and vemurafenib and the MEK inhibitor trametinib. This is critical considering that median duration of survival is 4 months in patients with melanoma brain metastases in previously reported series [57]. A multicenter phase II trial of dabrafenib in patients with BRAF-mutant melanoma with brain metastases found the drug to have significant activity in the central nervous system. Intracranial disease control (including complete response, partial response, and stable disease) for Val600Glu BRAF-mutant patients was greater than 80%; control was less in Val600Lys BRAF-mutant patients. Twenty-two percent of patients experienced a grade 3 or worse toxicity, with 30% experiencing a serious adverse event, including fever, intracranial hemorrhage, and squamous cell carcinoma. Toxicity was acceptable in this population [58]. Vemurafenib alone similarly showed activity in patients with previously treated melanoma brain metastasis. Ten of 24 patients in this open-label pilot study achieved at least a partial response, as well as a reduced need for steroids or improved KPS. Four of 24 patients experienced a grade 3 adverse event, including squamous cell carcinoma [59]. Given these studies, it is important to note that BRAF inhibitors do exhibit significant CNS penetration and activity.
Ipilimumab, an immunomodulating monoclonal antibody, alone also has shown activity in patient with both asymptomatic and symptomatic melanoma brain metastases. Activity was improved in patients with smaller asymptomatic lesions compared with those with symptomatic lesions and using steroids. This study established that ipilimumab alone has CNS activity without significant unexpected adverse effects [60]. SRS combined with an anti-cytotoxic T-lymphocyte-associated protein 4 (anti-CTLA4) agent ipilimumab was shown in one series to improve median survival duration from 4.9 to 21.3 months; the result was largely confirmed by another study [34, 61]. In a small series of BRAF V600E-mutant patients treated with SRS and vemurafenib, nearly half showed partial or complete response [41]. Recently published data by Kiess et al. show safety and efficacy of SRS combined with ipilimumab. A total of 113 lesions were treated in 46 patients. Patients treated with SRS before or concurrently with ipilimumab had superior regional recurrence rate and overall survival compared with patients who received SRS after ipilimumab. The authors considered the 20% grade 3 or 4 toxicity to be acceptable [42].
SRS combined with an anti-CTLA4 ipilimumab was shown in one series to improve median survival duration from 4.9 to 21.3 months; the result was largely confirmed by another study. In a small series of BRAF V600E-mutant patients treated with SRS and vemurafenib, nearly half showed partial or complete response.
Other reports do show some difficulty in combining RT with BRAF inhibitors. Ly et al. reported on 185 lesions in 52 patients treated with SRS, with or without BRAF inhibition. One-year local control was higher in patients with BRAF mutations who received BRAF inhibitors (85% versus 51.5%; p = .0077). However, patients treated with BRAF inhibitors had higher rates of intratumoral hemorrhage [62]. In a review of patients treated with SRS and BRAF inhibitors from 2005 to 2012, Patel et al. described 12 of 72 patients with melanoma metastases treated concurrently with BRAF inhibitors. Local control was similar between the groups, but patients who received BRAF inhibitors exhibited higher rates and more symptomatic radiation necrosis [63]. Radiation necrosis mimicked rapid intracranial progression, with symptoms in 2 patients treated with vemurafenib after completing SRS [64]. These medications generally show CNS activity and acceptable safety profiles, but BRAF inhibitor use is showing evidence of increased radiation necrosis. Data continue to emerge because this remains an exciting area of research; multiple phase II and phase III trials using concurrent targeted therapy with SRS are accruing or anticipating opening.
Evaluation of Response to Targeted Agents and SRS
SRS has outstanding rates of local control. However, patients with brain metastases in general have poor prognosis and a high systemic disease burden, which may influence both progression-free and overall survival. As data regarding CNS activity of these agents improve, the best way to analyze treatment efficacy for intracranial disease with concurrent extracranial disease must be addressed.
Overall survival effects may be confounded by other salvage treatments, including surgery, radiation, and other systemic therapy to intracranial and extracranial sites. Progression-free survival and radiographic response assessments both demand precise and repeatable measurements with agreed-upon response cutoffs. Finally, attention is increasingly given to neurologic symptoms as a response measure [65].
The Response Assessment in Neuro-Oncology (RANO) group has established guidelines for objective and reproducible response measurement in the treatment of brain metastases [66]. This includes analysis of the index lesion, nonindex lesion, corticosteroid use, and clinical status. In creating the RANO criteria for brain metastases, Lin et al. have provided excellent and thoughtful insight into optimally evaluating patients with brain metastases in two reviews with attention to response criteria and neurocognitive outcomes [67, 68].
To truly appreciate the response rate for targeted agents and SRS for intracranial disease, assessment should be limited to CNS response (optimally with radiologic evaluation) and neurologic symptom assessment. Objective local control criteria may include the RANO brain metastasis measures, with neurologic symptom progression-free survival or death from intracranial disease as measures used as alternative to survival. Interval imaging studies would be conducted at scheduled time points. CNS activity, determined by early studies including the BRAF inhibitor studies noted earlier in this article, can be used to power studies and design toxicity stopping rules to assess efficacy of combined approaches.
Future Directions
The paucity of data regarding SRS combined with targeted therapy in patients with brain metastases represents an excellent opportunity for future trials and investigation. Future trials must first address safety and tolerability of combining therapy and then show efficacy in improving symptom control, local control, and potentially overall survival in a rigorous and repeatable manner.
Although nearly 50% of cancer patients will receive radiation therapy during some time in their cancer care, radiation therapy studies are underrepresented in clinical trials. Further study will require substantial additional funding in the field because the acquisition of study medication, SRS treatment, and execution of a study can be very expensive. As documented among a total of 1,415 phase III trials for cancer, only 46 (0.9%) currently study a combination of radiation therapy (stereotactic body radiation therapy, SRS, or other modalities) and targeted therapeutics [8, 69]. Furthermore, the combination of these treatments will ultimately need to show value in health service outcomes research.
Early data do reflect that SRS and targeted therapy show an acceptable level of toxicity in certain histologic types and therapeutics. It is an active area of study regarding which combination of targeted therapy and SRS shows the best local control and overall survival benefit. It is critical to identify patients, whether by histologic features, performance status, extracranial disease control, and intracranial disease burden, who may best benefit from this emerging combined-modality approach.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Author Contributions
Conception/Design: Fabio Ynoe Moraes, Neil K. Taunk, Gustavo Nader Marta, John H. Suh, Yoshiya Yamada
Provision of study material or patients: Fabio Ynoe Moraes, Neil K. Taunk, Gustavo Nader Marta
Collection and/or assembly of data: Fabio Ynoe Moraes, Neil K. Taunk, Gustavo Nader Marta
Data analysis and interpretation: Fabio Ynoe Moraes, Neil K. Taunk, Gustavo Nader Marta
Manuscript writing: Fabio Ynoe Moraes, Neil K. Taunk, Gustavo Nader Marta, John H. Suh, Yoshiya Yamada
Final approval of manuscript: Fabio Ynoe Moraes, Neil K. Taunk, Gustavo Nader Marta, John H. Suh, Yoshiya Yamada
Disclosures
Yoshiya Yamada: Varian Medical Systems (C/A), Institute for Medical Education (H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Patchell RA. The management of brain metastases. Cancer Treat Rev. 2003;29:533–540. doi: 10.1016/s0305-7372(03)00105-1. [DOI] [PubMed] [Google Scholar]
- 2.Johnson JD, Young B. Demographics of brain metastasis. Neurosurg Clin N Am. 1996;7:337–344. [PubMed] [Google Scholar]
- 3.Sundermeyer ML, Meropol NJ, Rogatko A, et al. Changing patterns of bone and brain metastases in patients with colorectal cancer. Clin Colorectal Cancer. 2005;5:108–113. doi: 10.3816/ccc.2005.n.022. [DOI] [PubMed] [Google Scholar]
- 4.Wen PY, Loeffler JS Management of brain metastases. Oncology (Williston Park) 1999;13 941–954, 957–961; discussion 961–962, 969. [PubMed] [Google Scholar]
- 5.Alexander E, 3rd, Moriarty TM, Davis RB, et al. Stereotactic radiosurgery for the definitive, noninvasive treatment of brain metastases. J Natl Cancer Inst. 1995;87:34–40. doi: 10.1093/jnci/87.1.34. [DOI] [PubMed] [Google Scholar]
- 6.Flickinger JC, Kondziolka D, Lunsford LD, et al. A multi-institutional experience with stereotactic radiosurgery for solitary brain metastasis. Int J Radiat Oncol Biol Phys. 1994;28:797–802. doi: 10.1016/0360-3016(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 7.Pirzkall A, Debus J, Lohr F, et al. Radiosurgery alone or in combination with whole-brain radiotherapy for brain metastases. J Clin Oncol. 1998;16:3563–3569. doi: 10.1200/JCO.1998.16.11.3563. [DOI] [PubMed] [Google Scholar]
- 8.Morris ZS, Harari PM. Interaction of radiation therapy with molecular targeted agents. J Clin Oncol. 2014;32:2886–2893. doi: 10.1200/JCO.2014.55.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheehan JP, Yen CP, Lee CC, et al. Cranial stereotactic radiosurgery: Current status of the initial paradigm shifter. J Clin Oncol. 2014;32:2836–2846. doi: 10.1200/JCO.2013.53.7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown PD, Brown CA, Pollock BE, et al. Stereotactic radiosurgery for patients with “radioresistant” brain metastases. Neurosurgery. 2002;51:656–665; discussion 665–667. [PubMed] [Google Scholar]
- 11.Chang EL, Selek U, Hassenbusch SJ, 3rd, et al. Outcome variation among “radioresistant” brain metastases treated with stereotactic radiosurgery. Neurosurgery. 2005;56:936–945; discussion 936–945. [PubMed] [Google Scholar]
- 12.Gaspar LE, Scott C, Murray K, et al. Validation of the RTOG recursive partitioning analysis (RPA) classification for brain metastases. Int J Radiat Oncol Biol Phys. 2000;47:1001–1006. doi: 10.1016/s0360-3016(00)00547-2. [DOI] [PubMed] [Google Scholar]
- 13.Suh JH. Stereotactic radiosurgery for the management of brain metastases. N Engl J Med. 2010;362:1119–1127. doi: 10.1056/NEJMct0806951. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto M, Kawabe T, Sato Y, et al. A case-matched study of stereotactic radiosurgery for patients with multiple brain metastases: Comparing treatment results for 1-4 vs ≥ 5 tumors: Clinical article. J Neurosurg. 2013;118:1258–1268. doi: 10.3171/2013.3.JNS121900. [DOI] [PubMed] [Google Scholar]
- 15.Serizawa T, Yamamoto M, Sato Y, et al. Gamma Knife surgery as sole treatment for multiple brain metastases: 2-center retrospective review of 1508 cases meeting the inclusion criteria of the JLGK0901 multi-institutional prospective study. J Neurosurg. 2010;113(suppl):48–52. doi: 10.3171/2010.8.GKS10838. [DOI] [PubMed] [Google Scholar]
- 16.Shaw E, Scott C, Souhami L, et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: Final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys. 2000;47:291–298. doi: 10.1016/s0360-3016(99)00507-6. [DOI] [PubMed] [Google Scholar]
- 17.Balagamwala EH, Chao ST, Suh JH. Principles of radiobiology of stereotactic radiosurgery and clinical applications in the central nervous system. Technol Cancer Res Treat. 2012;11:3–13. doi: 10.7785/tcrt.2012.500229. [DOI] [PubMed] [Google Scholar]
- 18.Brown JM, Carlson DJ, Brenner DJ The tumor radiobiology of SRS and SBRT: Are more than the 5 Rs involved? Int J Radiat Oncol Biol Phys. 2014;88:254–262. doi: 10.1016/j.ijrobp.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuks Z, Kolesnick R. Engaging the vascular component of the tumor response. Cancer Cell. 2005;8:89–91. doi: 10.1016/j.ccr.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 20.Kolesnick R, Fuks Z. Radiation and ceramide-induced apoptosis. Oncogene. 2003;22:5897–5906. doi: 10.1038/sj.onc.1206702. [DOI] [PubMed] [Google Scholar]
- 21.Park C, Papiez L, Zhang S, et al. Universal survival curve and single fraction equivalent dose: useful tools in understanding potency of ablative radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70:847–852. doi: 10.1016/j.ijrobp.2007.10.059. [DOI] [PubMed] [Google Scholar]
- 22.Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: A paradigm shift. J Natl Cancer Inst. 2013;105:256–265. doi: 10.1093/jnci/djs629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wallner PE, Steinberg ML, McBride WH, et al. A fork in the road: Choosing the path of relevance. Int J Radiat Oncol Biol Phys. 2015;92:214–216. doi: 10.1016/j.ijrobp.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 24.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 25.Wilson GD, Bentzen SM, Harari PM. Biologic basis for combining drugs with radiation. Semin Radiat Oncol. 2006;16:2–9. doi: 10.1016/j.semradonc.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 26.Lin NU. Targeted therapies in brain metastases. Curr Treat Options Neurol. 2014;16:276. doi: 10.1007/s11940-013-0276-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeng J, Baik C, Bhatia S, et al. Combination of stereotactic ablative body radiation with targeted therapies. Lancet Oncol. 2014;15:e426–e434. doi: 10.1016/S1470-2045(14)70026-9. [DOI] [PubMed] [Google Scholar]
- 28.Shen Y, Ruan M, Luo Q, et al. Brain metastasis from follicular thyroid carcinoma: Treatment with sorafenib. Thyroid. 2012;22:856–860. doi: 10.1089/thy.2011.0419. [DOI] [PubMed] [Google Scholar]
- 29.Sanna G, Petralia G, Cossu Rocca M, et al. Long survival in a patient with brain metastases from breast cancer. Clin Med Oncol. 2008;2:103–108. doi: 10.4137/cmo.s317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruzevick J, Nicholas S, Redmond K, et al. A patient with HIV treated with ipilimumab and stereotactic radiosurgery for melanoma metastases to the brain. Case Rep Oncol Med. 2013;2013:946392. doi: 10.1155/2013/946392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carlson JA, Nooruddin Z, Rusthoven C, et al. Trastuzumab emtansine and stereotactic radiosurgery: An unexpected increase in clinically significant brain edema. Neuro-oncol. 2014;16:1006–1009. doi: 10.1093/neuonc/not329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Staehler M, Haseke N, Nuhn P, et al. Simultaneous anti-angiogenic therapy and single-fraction radiosurgery in clinically relevant metastases from renal cell carcinoma. BJU Int. 2011;108:673–678. doi: 10.1111/j.1464-410X.2010.09895.x. [DOI] [PubMed] [Google Scholar]
- 33.Cochran DC, Chan MD, Aklilu M, et al. The effect of targeted agents on outcomes in patients with brain metastases from renal cell carcinoma treated with Gamma Knife surgery. J Neurosurg. 2012;116:978–983. doi: 10.3171/2012.2.JNS111353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knisely JP, Yu JB, Flanigan J, et al. Radiosurgery for melanoma brain metastases in the ipilimumab era and the possibility of longer survival. J Neurosurg. 2012;117:227–233. doi: 10.3171/2012.5.JNS111929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silk AW, Bassetti MF, West BT, et al. Ipilimumab and radiation therapy for melanoma brain metastases. Cancer Med. 2013;2:899–906. doi: 10.1002/cam4.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vickers MM, Al-Harbi H, Choueiri TK, et al. Prognostic factors of survival for patients with metastatic renal cell carcinoma with brain metastases treated with targeted therapy: Results from the international metastatic renal cell carcinoma database consortium. Clin Genitourin Cancer. 2013;11:311–315. doi: 10.1016/j.clgc.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 37.Mathew M, Tam M, Ott PA, et al. Ipilimumab in melanoma with limited brain metastases treated with stereotactic radiosurgery. Melanoma Res. 2013;23:191–195. doi: 10.1097/CMR.0b013e32835f3d90. [DOI] [PubMed] [Google Scholar]
- 38.Yomo S, Hayashi M, Cho N. Impacts of HER2-overexpression and molecular targeting therapy on the efficacy of stereotactic radiosurgery for brain metastases from breast cancer. J Neurooncol. 2013;112:199–207. doi: 10.1007/s11060-013-1046-1. [DOI] [PubMed] [Google Scholar]
- 39.Tazi K, Hathaway A, Chiuzan C, et al. Survival of melanoma patients with brain metastases treated with ipilimumab and stereotactic radiosurgery. Cancer Med. 2015;4:1–6. doi: 10.1002/cam4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tam M, Narayana A, Raza S, et al. Role of HER2 status in the treatment for brain metastases arising from breast cancer with stereotactic radiosurgery. Med Oncol. 2014;31:832. doi: 10.1007/s12032-013-0832-0. [DOI] [PubMed] [Google Scholar]
- 41.Narayana A, Mathew M, Tam M, et al. Vemurafenib and radiation therapy in melanoma brain metastases. J Neurooncol. 2013;113:411–416. doi: 10.1007/s11060-013-1127-1. [DOI] [PubMed] [Google Scholar]
- 42.Kiess AP, Wolchok JD, Barker CA, et al. Stereotactic radiosurgery for melanoma brain metastases in patients receiving ipilimumab: Safety profile and efficacy of combined treatment. Int J Radiat Oncol Biol Phys. 2015;92:368–375. doi: 10.1016/j.ijrobp.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomas Jefferson University. Ipilimumab and whole-brain radiation therapy or stereotactic radiosurgery in treating patients with melanoma with brain metastases. ClinicalTrials.gov. 2015. Available at https://clinicaltrials.gov/ct2/show/NCT01703507. Accessed July 21, 2015.
- 44.University Health Network. Stereotactic radiosurgery with sunitinib for brain metastases. ClinicalTrials.gov. 2015. Available at https://clinicaltrials.gov/ct2/show/NCT00981890. Accessed July 21, 2015.
- 45.University of California, San Francisco. A phase 2 prospective trial of dabrafenib with stereotactic radiosurgery in BRAFV600E melanoma brain metastases. ClinicalTrials.gov. 2015. Available at https://clinicaltrials.gov/ct2/show/NCT01721603. Accessed July 21, 2015.
- 46.Vanderbilt-Ingram Cancer Center. Sorafenib tosylate and stereotactic radiosurgery in treating patients with brain metastases. ClinicalTrials.gov. 2015. Available at https://clinicaltrials.gov/ct2/show/NCT01276210. Accessed July 21, 2015.
- 47.Sperduto PW, Wang M, Robins HI, et al. A phase 3 trial of whole brain radiation therapy and stereotactic radiosurgery alone versus WBRT and SRS with temozolomide or erlotinib for non-small cell lung cancer and 1 to 3 brain metastases: Radiation Therapy Oncology Group 0320. Int J Radiat Oncol Biol Phys. 2013;85:1312–1318. doi: 10.1016/j.ijrobp.2012.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Y, Wang E, Pan L, et al. A new strategy of CyberKnife treatment system based radiosurgery followed by early use of adjuvant bevacizumab treatment for brain metastasis with extensive cerebral edema. J Neurooncol. 2014;119:369–376. doi: 10.1007/s11060-014-1488-0. [DOI] [PubMed] [Google Scholar]
- 49.Preusser M, Capper D, Ilhan-Mutlu A, et al. Brain metastases: Pathobiology and emerging targeted therapies. Acta Neuropathol. 2012;123:205–222. doi: 10.1007/s00401-011-0933-9. [DOI] [PubMed] [Google Scholar]
- 50.Yin W, Jiang Y, Shen Z, et al. Trastuzumab in the adjuvant treatment of HER2-positive early breast cancer patients: A meta-analysis of published randomized controlled trials. PloS One. 2011;6:e21030. doi: 10.1371/journal.pone.0021030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Musolino A, Ciccolallo L, Panebianco M, et al. Multifactorial central nervous system recurrence susceptibility in patients with HER2-positive breast cancer: Epidemiological and clinical data from a population-based cancer registry study. Cancer. 2011;117:1837–1846. doi: 10.1002/cncr.25771. [DOI] [PubMed] [Google Scholar]
- 52.Deibert CP, Ahluwalia MS, Sheehan JP, et al. Bevacizumab for refractory adverse radiation effects after stereotactic radiosurgery. J Neurooncol. 2013;115:217–223. doi: 10.1007/s11060-013-1214-3. [DOI] [PubMed] [Google Scholar]
- 53.Boothe D, Young R, Yamada Y, et al. Bevacizumab as a treatment for radiation necrosis of brain metastases post stereotactic radiosurgery. Neuro-oncol. 2013;15:1257–1263. doi: 10.1093/neuonc/not085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Le Scodan R, Jouanneau L, Massard C, et al. Brain metastases from breast cancer: Prognostic significance of HER-2 overexpression, effect of trastuzumab and cause of death. BMC Cancer. 2011;11:395. doi: 10.1186/1471-2407-11-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gerber NK, Yamada Y, Rimner A, et al. Erlotinib versus radiation therapy for brain metastases in patients with EGFR-mutant lung adenocarcinoma. Int J Radiat Oncol Biol Phys. 2014;89:322–329. doi: 10.1016/j.ijrobp.2014.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Welsh JW, Komaki R, Amini A, et al. Phase II trial of erlotinib plus concurrent whole-brain radiation therapy for patients with brain metastases from non-small-cell lung cancer. J Clin Oncol. 2013;31:895–902. doi: 10.1200/JCO.2011.40.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fife KM, Colman MH, Stevens GN, et al. Determinants of outcome in melanoma patients with cerebral metastases. J Clin Oncol. 2004;22:1293–1300. doi: 10.1200/JCO.2004.08.140. [DOI] [PubMed] [Google Scholar]
- 58.Long GV, Trefzer U, Davies MA, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): A multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:1087–1095. doi: 10.1016/S1470-2045(12)70431-X. [DOI] [PubMed] [Google Scholar]
- 59.Dummer R, Goldinger SM, Turtschi CP, et al. Vemurafenib in patients with BRAF(V600) mutation-positive melanoma with symptomatic brain metastases: Final results of an open-label pilot study. Eur J Cancer. 2014;50:611–621. doi: 10.1016/j.ejca.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 60.Margolin K, Ernstoff MS, Hamid O, et al. Ipilimumab in patients with melanoma and brain metastases: An open-label, phase 2 trial. Lancet Oncol. 2012;13:459–465. doi: 10.1016/S1470-2045(12)70090-6. [DOI] [PubMed] [Google Scholar]
- 61.Schartz NE, Farges C, Madelaine I, et al. Complete regression of a previously untreated melanoma brain metastasis with ipilimumab. Melanoma Res. 2010;20:247–250. doi: 10.1097/CMR.0b013e3283364a37. [DOI] [PubMed] [Google Scholar]
- 62.Ly D, Bagshaw HP, Anker CJ, et al. Local control after stereotactic radiosurgery for brain metastases in patients with melanoma with and without BRAF mutation and treatment. J Neurosurg. 2015;123:395–401. doi: 10.3171/2014.9.JNS141425. [DOI] [PubMed] [Google Scholar]
- 63.(P011) stereotactic radiosurgery and BRAF inhibitor therapy for melanoma brain metastases is associated with increased risk for radiation necrosis. Oncology (Williston Park) 2015;29(suppl 1). [PubMed]
- 64.Liebner DA, Walston SA, Cavaliere R, et al. Radiation necrosis mimicking rapid intracranial progression of melanoma metastasis in two patients treated with vemurafenib. Melanoma Res. 2014;24:172–176. doi: 10.1097/CMR.0000000000000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Quant EC, Wen PY. Response assessment in neuro-oncology. Curr Oncol Rep. 2011;13:50–56. doi: 10.1007/s11912-010-0143-y. [DOI] [PubMed] [Google Scholar]
- 66.Lin NU, Lee EQ, Aoyama H, et al. Response assessment criteria for brain metastases: Proposal from the RANO group. Lancet Oncol. 2015;16:e270–e278. doi: 10.1016/S1470-2045(15)70057-4. [DOI] [PubMed] [Google Scholar]
- 67.Lin NU, Lee EQ, Aoyama H, et al. Challenges relating to solid tumour brain metastases in clinical trials, part 1: patient population, response, and progression. A report from the RANO group. Lancet Oncol. 2013;14:e396–e406. doi: 10.1016/S1470-2045(13)70311-5. [DOI] [PubMed] [Google Scholar]
- 68.Lin NU, Wefel JS, Lee EQ, et al. Challenges relating to solid tumour brain metastases in clinical trials, part 2: neurocognitive, neurological, and quality-of-life outcomes. A report from the RANO group. Lancet Oncol. 2013;14:e407–e416. doi: 10.1016/S1470-2045(13)70308-5. [DOI] [PubMed] [Google Scholar]
- 69.Jagsi R, Wilson LD. Research funding for radiation oncology: An unfortunately small sliver of an inadequate pie. Int J Radiat Oncol Biol Phys. 2013;86:216–217. doi: 10.1016/j.ijrobp.2013.02.028. [DOI] [PubMed] [Google Scholar]