A basal Ki67 proliferation index >50% should be considered an independent predictive factor for pathological complete response reached after neoadjuvant chemotherapy, suggesting that cell proliferation is a phenomenon closely related to chemosensitivity. These findings could help to identify patients with a potentially favorable long-term prognosis.
Keywords: Ki67, Breast cancer, Neoadjuvant chemotherapy, Chemosensitivity, Predictive factor
Abstract
Background.
In the neoadjuvant setting, changes in the proliferation marker Ki67 are associated with primary endocrine treatment efficacy, but its value as a predictor of response to chemotherapy is still controversial.
Patients and Methods.
We analyzed 262 patients with centralized basal Ki67 immunohistochemical evaluation derived from 4 GEICAM (Spanish Breast Cancer Group) clinical trials of neoadjuvant chemotherapy for breast cancer. The objective was to identify the optimal threshold for Ki67 using the receiver-operating characteristic curve method to maximize its predictive value for chemotherapy benefit. We also evaluated the predictive role of the defined Ki67 cutoffs for molecular subtypes defined by estrogen receptor (ER) and human epidermal growth factor receptor 2 (HER2).
Results.
A basal Ki67 cutpoint of 50% predicted pathological complete response (pCR). Patients with Ki67 >50% achieved a pCR rate of 40% (36 of 91) versus a pCR rate of 19% in patients with Ki67 ≤50% (33 of 171) (p = .0004). Ki67 predictive value was especially relevant in ER-HER2− and ER-HER2+ patients (pCR rates of 42% and 64%, respectively, in patients with Ki67 >50% versus 15% and 45%, respectively, in patients with Ki67 ≤50%; p = .0337 and .3238, respectively). Both multivariate analyses confirmed the independent predictive value of the Ki67 cutpoint of 50%.
Conclusion.
Basal Ki67 proliferation index >50% should be considered an independent predictive factor for pCR reached after neoadjuvant chemotherapy, suggesting that cell proliferation is a phenomenon closely related to chemosensitivity. These findings could help to identify a group of patients with a potentially favorable long-term prognosis.
Implications for Practice:
The use of basal Ki67 status as a predictive factor of chemotherapy benefit could facilitate the identification of a patient subpopulation with high probability of achieving pathological complete response when treated with primary chemotherapy, and thus with a potentially favorable long-term prognosis.
Abstract
摘要
背景. 在新辅助治疗中, 增殖标记物 Ki67 的改变与新辅助内分泌治疗的有效性有关, 但其作为化疗治疗反应预测因素的价值仍然存有争议。
患者与方法. 我们分析了来自 4 项 GEICAM (西班牙乳腺癌小组) 临床试验中乳腺癌新辅助化疗的 262 例患者, 对基底细胞 Ki67 集中进行免疫组化评价。研究旨在通过采用受试者操作特征曲线方法使 Ki67 对化疗获益的预测值最大化, 确定 Ki67 的最佳阈值。我们还评价了根据雌激素受体 (ER) 和人类表皮生长因子受体2 (HER2) 确定的明确 Ki67 临界值对分子亚型的预测作用。
结果. 基底细胞 Ki67 临界值为 50%时可预测病理学完全缓解 (pCR)。Ki67 > 50%的患者 pCR 率可达到 40% (36/91例), 而 Ki67 ≤ 50%的患者 pCR 率为 19% (33/171例) (P = 0.000 4)。Ki67 的预测值与 ER- HER2-和 ER- HER2+患者尤为相关 (pCR率: Ki67 > 50%的患者中分别为 42%和 64%, P = 0.033 7; Ki67 ≤ 50%的患者中分别为 15%和 45%, P = 0.323 8)。两种多因素分析均确认独立预测值为 Ki67 临界值为 50%。
结论. 基底细胞 Ki67 增殖指数> 50%应作为新辅助化疗后达到 pCR 的独立预测因素, 提示细胞增殖是与化疗敏感性密切相关的现象。这些结果可能有助于鉴别出可能有良好远期预后的患者组。The Oncologist 2016;21:150–155
对临床实践的提示: 使用基底细胞 Ki67 状态作为化疗获益的预测因素可有助于鉴别新辅助化疗中有较高可能达到病理学完全缓解的患者亚组, 这类患者长期预后可能较好。
Introduction
In breast cancer, Ki67 immunohistochemical (IHC) determination is the most widely used biomarker of cell proliferation. Despite this, Ki67 status is not considered a robust prognostic or predictive factor because of the limited reproducibility of results, the variability in cutpoints used, and the different clinical scenarios in which it has been studied. Thus, it has not been recommended as a predictive factor in common clinical practice [1].
The role of Ki67 as a predictive factor of response to neoadjuvant hormone therapy has been well-established [2, 3]. However, its value is less obvious in the prediction of response to neoadjuvant chemotherapy [4, 5]. Several retrospective studies have associated high levels of Ki67 with higher pathological complete response (pCR) rates [6–11]. However, other studies have failed to confirm these data [12, 13]. This inconsistency could be related to the fact that in nearly all studies the choice of cutpoints to define high Ki67 levels has been based on empirical observations without any biological justification or proper statistical approach. A wide range of high Ki67 levels has been communicated, with the cutpoint of 10% to 25% being the most commonly used [14]. In addition, its predictive role regardless estrogen receptor (ER) and human epidermal growth factor receptor 2 (HER2) status has not been ascertained.
The main objective of this study was to analyze the predictive role of Ki67 for neoadjuvant chemotherapy efficacy. Our investigation was based on data from four clinical trials carried out by GEICAM (the Spanish Breast Cancer Group). First, we identified the optimal cutpoint of Ki67 to maximize its predictive value; then, we evaluated the predictive role of Ki67 in relation to ER and HER2 status.
Patients And Methods
Study Population
We analyzed the data from 262 patients with available centralized Ki67 IHC determination and pathological response data, derived from four GEICAM clinical trials of neoadjuvant chemotherapy for breast cancer: GEICAM/2002-01 (34 patients, all subtypes), GEICAM/2003-03 (32 patients, HER2 overexpressed), GEICAM 2006-03 (119 patients, luminal subtype [defined by IHC as ER+ and/or progesterone receptor (PR)+, HER2−] and triple-negative subtype [ER−, PR−, HER2−]), and GEICAM/2006-14 (77 patients, HER2 overexpressed). In all these trials, patients were treated with anthracycline and taxane-based neoadjuvant chemotherapy; anti-HER2 therapy (trastuzumab or lapatinib) was administered for most patients with HER2 overexpression (Table 1). Detailed descriptions of these trials have been published elsewhere [15–19]. The 262 patients involved represented 73% of the 361 patients participating in the four trials combined. These trials were performed in accordance with the Declaration of Helsinki, approved by the ethics committees at all participating institutions (supplemental online Table 3) and the Spanish Health Authority, and registered at http://www.clinicaltrials.gov (NCT00128856, NCT00129896, NCT00432172, NCT00841828). All patients provided written informed consent for trial participation and molecular analyses.
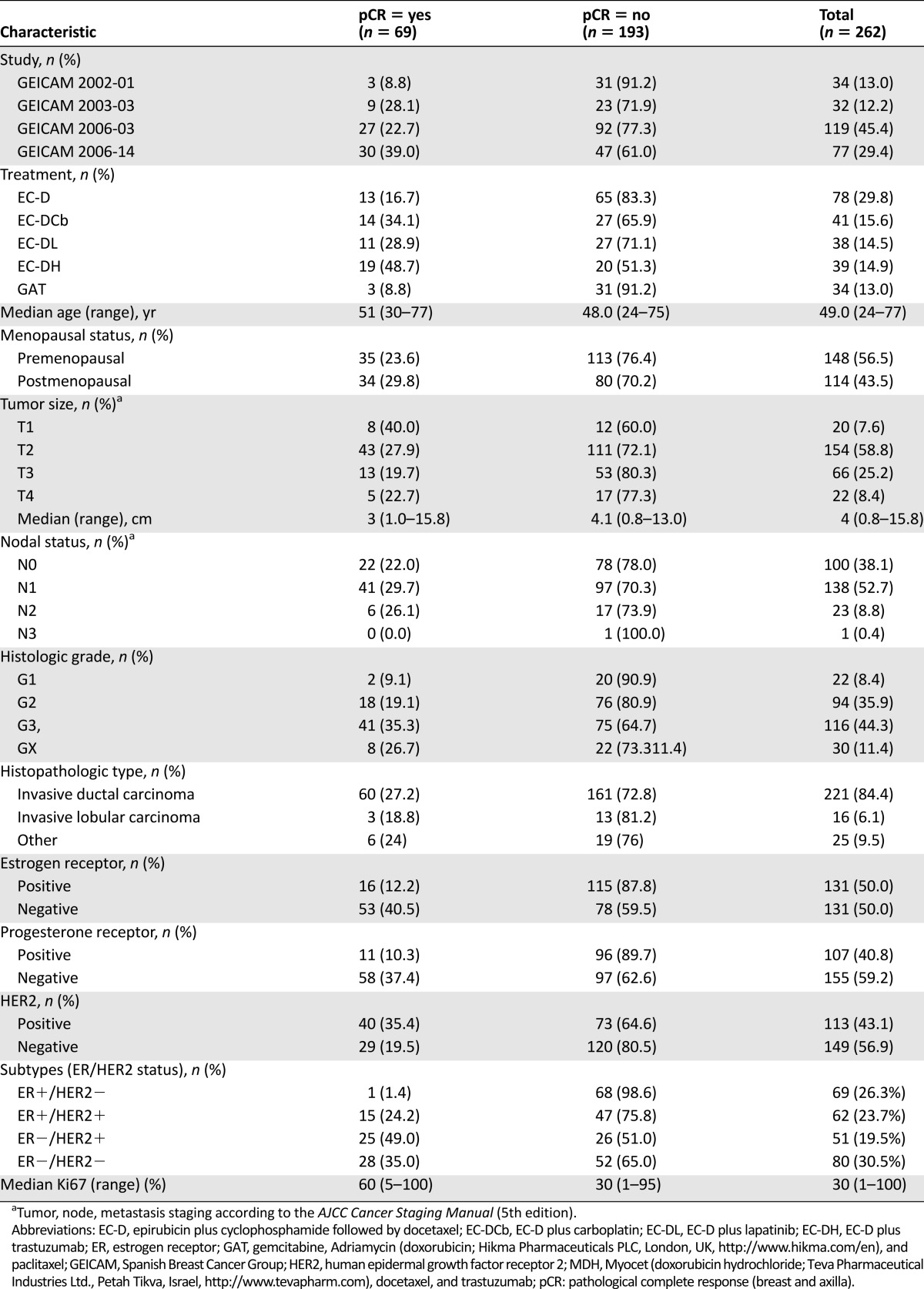
Table 1.
Patient characteristics by pathological complete response in breast and axilla
IHC and/or Fluorescence In Situ Hybridization Determination of Ki67, ER, PR, and HER2
Biomarker analysis was carried out by IHC and/or fluorescence in situ hybridization (FISH) at a central laboratory on the available pretreatment tumor samples from those patients. We performed immunostaining using formalin-fixed, paraffin-embedded tissue sections as previously described [20].
Ki-67 was assessed using the mouse monoclonal antibody (mAb) clone MIB1 (Dako, Glostrup, Denmark, http://www.dako.com). The Ki67 proliferation index was defined as the mean of tumor cells with marker expression, including the hot spots.
For ER and PR expression, sections were incubated with primary mAb to ER: clone SP1 for GEICAM/2002-01 and GEICAM/2006-03 (Dako), clone EP1 for GEICAM/2006-14 (Dako); mAb to PR clone 1A6 (Novocastra, Leica Biosystems, Nussloch, Germany, http://www.leicabiosystems.com) for GEICAM/2002-01, clone PgR636 (Dako) for 2006-14, and clone Y85 (Vitro, Madrid, Spain, http://www.vitro.bio/) for GEICAM/2006-03. IHC determination was performed by using the EnVision FLEX system (Dako). ER and PR were scored with reference to the proportion of stained tumor cells and were classified as positive according to American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) guidelines [21].
HER2 expression was determined by HercepTest (Dako) for GEICAM/2002-01, GEICAM/2003-03, and GEICAM/2006-03, and amplification was confirmed by PathVysion FISH probes (Abbott Molecular, Des Plaines, IL, https://www.abbottmolecular.com/us/home.html), following ASCO/CAP guidelines [22].
All assays were evaluated by three expert pathologists (F.R., F.P., J.A.A.) blinded to pathological response. Discrepancies were solved by consensus between them.
Definition of pCR
We defined pCR on the basis of the Miller and Payne criteria [23] as the complete disappearance of the invasive tumor in the mammary gland (grade 5) and the absence of tumor in the axillary lymph nodes examined by axillary clearance after neoadjuvant therapy or negative sentinel node before the start of therapy.
Statistical Analysis
Receiver-operating characteristic (ROC) curve analysis was used to determine the optimal cutpoint for Ki67 by calculating the sensitivity and specificity indices corresponding to Ki67 cutpoints selected for every 10 units (range, 1–100). Cutpoints divide the study population into groups of high and low expression, which we correlated with pCR. We considered the optimal cutpoint as the one having the highest combined sensitivity and specificity values [24]; we prioritized those with higher sensitivity indices under equal circumstances. We then performed univariate and multivariate logistical regression analyses to examine the association between clinical-pathological variables and pCR. Multivariate models included only variables that exhibited a univariate association with the dependent variable, pCR (p < .25). We used the area under the curve (AUC) parameter to evaluate the model, including Ki67 and other clinical-pathological variables, and to assess Ki67’s ability to discriminate between patients with and without pCR. All analyses were performed by using the Statistical Analysis System (SAS) Enterprise Guide 5.1 software (SAS Institute Inc., Cary, NC, https://www.sas.com/).
Results
Patient Characteristics
Patients’ characteristics are described in Table 1. The median age was 49 years, and 57% of patients were premenopausal. Fifty-nine percent had T2 stage disease, and 62% were clinically node-positive. Most patients (84%) had an invasive ductal carcinoma, whereas 6% had lobular and 10% other type. The histological grade was mainly 2 (36%) or 3 (44%), ER positivity was seen in 50% of tumors, and PR and HER2 negativity was seen in 60% and 57% of cases, respectively. The distribution by ER and HER2 status was as follows: ER+/HER2− tumors in 69 (26%) patients, ER+/HER2+ in 62 (24%) patients, ER−/HER2+ in 51 (20%) patients, and ER−/HER2− in 80 (30%) patients. The median proportion of cells stained for Ki67 was 30% (quartile 1, 15%; quartile 3, 65%). Supplemental online Figure 1 shows the distribution of the proportion of staining for Ki67 in 10-expression intervals.
pCR Rates and ROC Curves
Of the 262 patients, 69 (26%) achieved pCR, similar to the rate observed in the whole population (85 of the 355 [24%] patients with available pathological CR data included in the 4 trials). The pCR rate was higher in small tumors, those with histological grade 3, ER- or PR-negative tumors, and those with high Ki67 indices (Table 1).
Results based on ROC curve method showed that the Ki67 cutpoints with the highest combined sensitivity and specificity values were 60 and 50, with 50% showing a higher sensitivity index (data not shown). On the basis of this cutpoint (Ki67 >50%), high Ki67 was seen in 35% of patients (91 of 262) included in this analysis. Fifty-three percent of the pCRs (36 of 69) were achieved in patients with Ki67 >50%, and 72% of patients without pCR (138 of 193) had tumors with Ki67 ≤50%. Patients with Ki67 >50% achieved a pCR rate of 40% (36 of 91 cases) and patients with Ki67 ≤50% had a pCR rate of 19% (33 of 171 cases) (p = .0004) (Table 1).
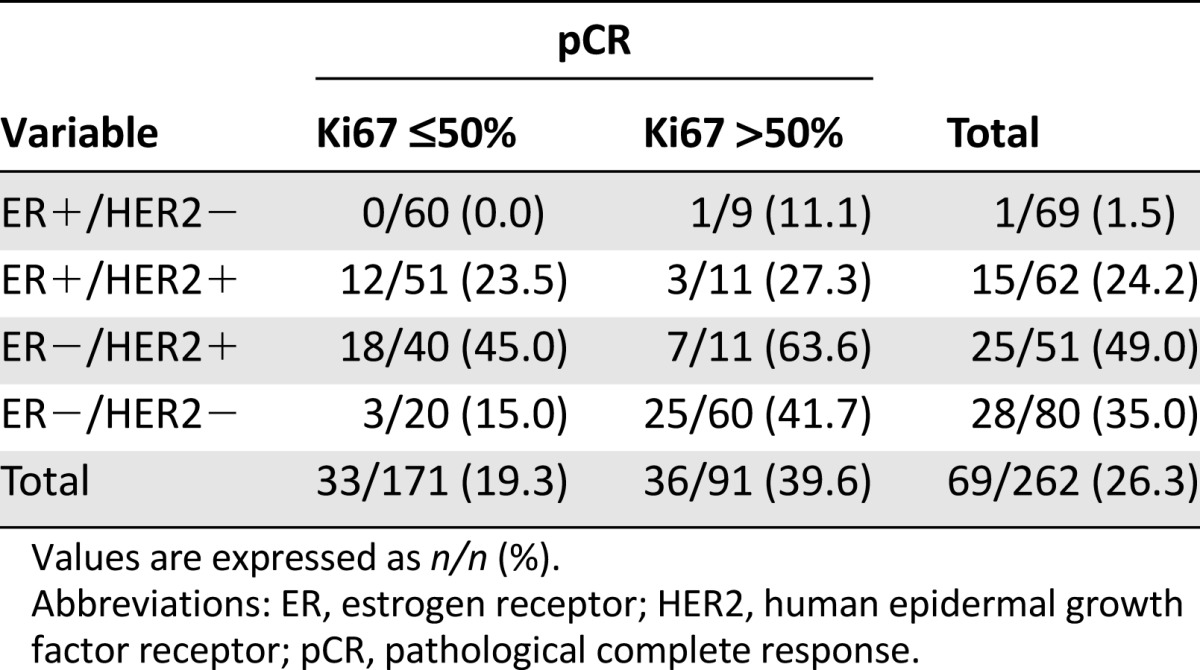
Table 2 describes pCR rates according to ER and HER2 status and Ki67 index. Only 1 of the 69 patients with ER+/HER2− tumors (1.5%) achieved pCR. In contrast, 15 of the 62 ER+/HER2+ patients (24%), 28 of the 80 ER−/HER2− patients (35%), and 25 of the 51 ER−/HER2+ patients (49%) achieved pCR. The 50% cutpoint of Ki67 predicted better pCRs, specifically in patients with ER−/HER2− (42% high versus 15% low; p = .0337) and ER−/HER2+ (64% high versus 45% low; p = .3238) tumors.
Table 2.
Rate of pathological complete response for breast and axilla by estrogen receptor status, human epidermal growth factor receptor 2 status, and Ki67 score
Univariate and Multivariate Analysis of pCR Predictive Factors
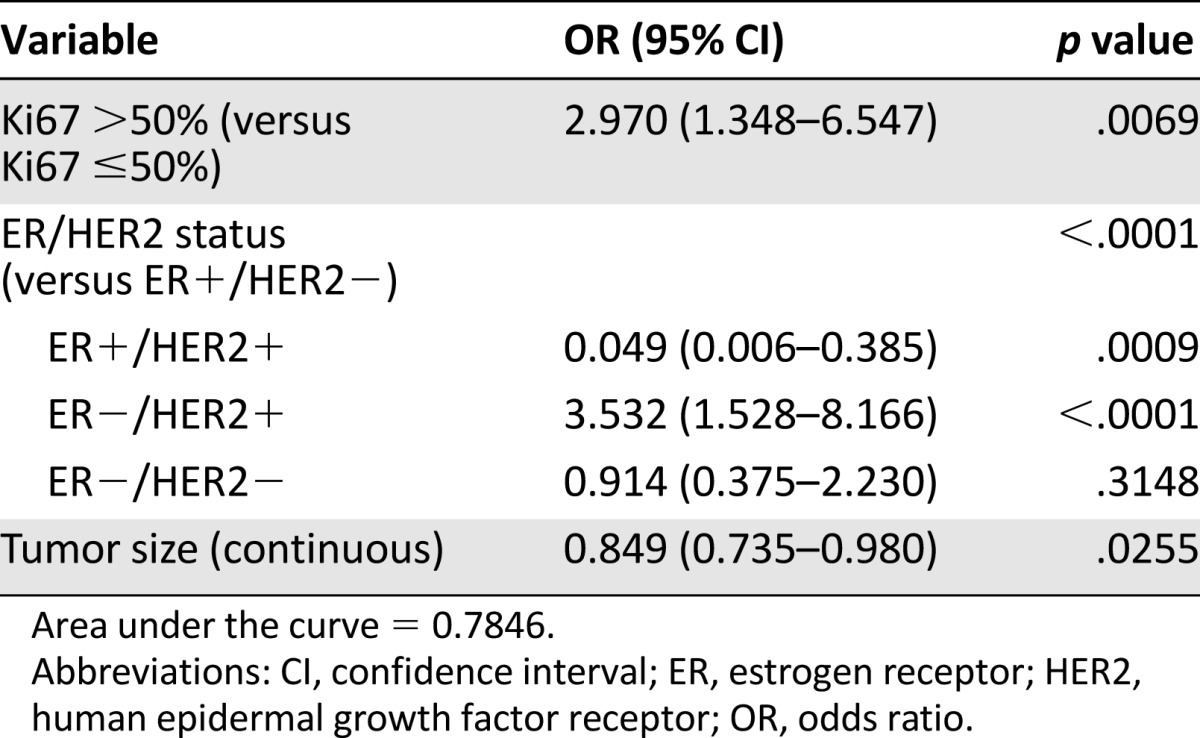
The univariate analyses (supplemental online Table 1) showed a statistically significant association between pCR and small tumor size (considered as a continuous variable) (p = .0368), high histological grade (p = .0185), ER− status (p ≤ .0001), PR− status (p < .0001), and HER2+ status (p = .0041). A high proliferation level determined by Ki67 quantitative measurement (odds ratio [OR]: 1.02; 95% confidence interval [CI]: 1.01–1.03; p < .0001) or by considering a Ki67 >50% (OR: 2.74; 95% CI: 1.55–4.82; p = .0005) exhibited a significant association with pCR.
In the multivariate analyses (Table 3), factors showing an independent and statistically significant association with pCR included a Ki67 index >50%, ER− and HER2+ status, and smaller tumor size (AUC = 0.7846).
Table 3.
Multivariate analyses for factors associated to pathological complete response in breast and axilla
Multivariate analyses including only the most chemosensitive patient subpopulation (that with ER−/HER2− and ER−/HER2+ tumors) (supplemental online Table 2) showed that Ki67 >50% and HER2+ status were independent predictive factors for pCR (AUC: 0.6178).
Discussion
According to robust retrospective analyses of clinical trial data [2, 3], the assessment of cellular proliferation based on Ki67 determination may be used as a predictive factor for the efficacy of breast cancer neoadjuvant hormone therapy. However, its role as an independent predictive factor for efficacy of neoadjuvant chemotherapy is not that well-established [4]. Thus, routine Ki67 assessment has not been recommended when patients receive primary chemotherapy [25]. The reason is that despite data indicating the correlation between high ki67 and pCR, most of these data are derived from retrospective studies other than clinical trials [6–11]. Furthermore, Ki67 cutpoints used in these studies were selected empirically (with thresholds associated with the mean of observed values in the study population) or were arbitrarily established at 10%–25% [14].
Our method—ROC curve analysis based on sensitivity and specificity indices to discriminate patients achieving pCR in a specific range of biomarker values—was aimed at identifying an optimal Ki67 cutpoint. Our results showed that 50% was the optimal cutpoint and identified two subpopulations: patients with a high Ki67 level (>50%) achieving a pCR rate of 40% (36 of 91 cases) and patients with a low Ki67 level (≤50%) with a pCR rate of 19% (33 of 171 cases). In the multivariate analyses, this effect was confirmed to be independent of other pCR-related factors, such as ER or HER2 status.
Ki67’s predictive value in breast cancer is of special relevance in the population potentially most responsive to neoadjuvant chemotherapy. Including the ER−/HER2− and ER−/HER2+ patients, we observed a pCR rate of 45% (32 of 71 cases) for those with Ki67 >50% and a pCR rate of 35% (21 of 60) in patients with Ki67 ≤50%. In this chemosensitive subgroup, basal Ki67 indices >50% were also an independent predictor for pCR. The long-term prognostic value of pCR among patients treated with neoadjuvant chemotherapy has been well-established [26, 27]. Recent findings have shed light on this prognostic relationship, which seems to apply especially to patients with HER2+, triple-negative (ER−/PR−/HER2−), or high-risk ER+ tumors [28, 29]. In fact, regarding drug approval, the Food and Drug Administration has adopted pCR in these patient subgroups as a surrogate marker of long-term treatment efficacy [30].
Thus, a Ki67 index >50% may be predictive of high pCR rates of postneoadjuvant chemotherapy (especially in the ER−/HER2− and ER−/HER2+ population). This cutpoint may potentially identify a subpopulation of breast cancer patients with a favorable long-term prognosis after achieving pCR with primary chemotherapy. Similar findings have been described for management of neuroendocrine tumors. Nadler et al. [31] established that Ki-67 is a reliable pathological grading marker in determining tumor grade, according to the European Neuroendocrine Tumor Society guidelines and the 2010 World Health Organization classification. Moreover, in patients with advanced gastrointestinal neuroendocrine carcinoma, Sorbye et al. [32] suggested that the Ki67 threshold of 55% was the best cutoff predicting rate of response to chemotherapy.
The results of this study should be interpreted in the context of its weaknesses. First, the sample size is somewhat limited. Second, the centralized Ki67 determination may invite questions about its reproducibility in daily practice. This is especially relevant given that its interobserver variability is often mentioned as one of the limitations of Ki67 being defined by IHC as a robust proliferation biomarker [4]. However, it should be said that the interpretation of different genetic signatures is also subject to variability. Nevertheless, independently of the set of genes used, all of them captured the same subpopulation with a poor prognosis [33, 34], with mainly high-proliferation tumors, especially among ER+ cases [35, 36]. In addition, in other studies IHC-detected Ki67 levels were associated with quantitatively assessed proliferation in first-generation genetic signatures [37–39]. Finally, different studies have reported a high interlaboratory variability in Ki67 scoring on breast tumors among some of the world's most experienced pathologists [40, 41]. Clinical decision-making regarding treatment options in breast cancer often relies on the application of a Ki67 cutoff to classify patients into “Ki67 high” or “Ki67 low” risk groups. Reported data suggest that even if a consensus Ki67 cutoff is agreed upon, discordant Ki67 measurements between observers for low proliferation/luminal A breast tumors have been estimated in 50% of studied cases; the discrepancies in high rates of Ki67 have been lower [41]. Thus, a definition of a Ki67 threshold >50% might improve the reproducibility of scoring results between laboratories and observers and would help to identify tumors with a high proliferation rate and worse outcome.
Conclusion
A basal Ki67 index >50% could be considered an independent predictive factor for pCR reached after neoadjuvant chemotherapy. From a biological perspective, these results suggest that cell proliferation is a phenomenon closely related to chemosensitivity. From a clinical perspective, these findings could facilitate the identification of a patient subpopulation with high probability of achieving pCR when treated with primary chemotherapy, and thus with a potentially favorable long-term prognosis.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
This work was supported by funds from Fondo Europeo de Desarrollo Regional (Red Tematica de Investigacion Cooperativa en Cancer): RD12/0036/0076 (J.A. and A.B.), RD12/0036/0051 (J.A. and J.R.), RD12/0036/0070 (A.L.), and Retics Biobank RD/09/0076/00101 (F.R.). J.A. and F.R. are recipients of intensification grant ISCIII.
Author Contributions
Conception/Design: Emilio Alba
Provision of study material or patients: Emilio Alba, Ana Lluch, Antonio Anton-Torres, Pedro Sanchez-Rovira, Joan Albanell, Lourdes Calvo, Jose Antonio Lopez García-Asenjo, Jose Palacios, Jose Ignacio Chacon, Amparo Ruiz, Juan De la Haba-Rodriguez, Miguel A. Segui-Palmer, Beatriz Cirauqui, Mireia Margeli, Arrate Plazaola, Agusti Barnadas, Federico Rojo
Collection and/or assembly of data: Emilio Alba, Ana Lluch, Antonio Anton-Torres, Pedro Sanchez-Rovira, Joan Albanell, Lourdes Calvo, Jose Ignacio Chacon, Amparo Ruiz, Juan De la Haba-Rodriguez, Miguel A. Segui-Palmer, Beatriz Cirauqui, Mireia Margeli, Arrate Plazaola, Agusti Barnadas, Rosalia Caballero, Federico Rojo
Data analysis and interpretation: Emilio Alba, Ana Lluch, Nuria Ribelles, Maribel Casas, Rosalia Caballero, Eva Carrasco, Federico Rojo
Manuscript writing: Emilio Alba, Nuria Ribelles, Maribel Casas, Rosalia Caballero, Eva Carrasco, Federico Rojo
Final approval of manuscript: Emilio Alba, Nuria Ribelles, Antonio Anton-Torres, Pedro Sanchez-Rovira, Joan Albanell, Lourdes Calvo, Jose Antonio Lopez García-Asenjo, Jose Palacios, Jose Ignacio Chacon, Amparo Ruiz, Juan De la Haba-Rodriguez, Miguel A. Segui-Palmer, Beatriz Cirauqui, Mireia Margeli, Arrate Plazaola, Agusti Barnadas, Maribel Casas, Rosalia Caballero, Eva Carrasco, Federico Rojo
Disclosures
Agusti Barnadas: Pfizer, Celgene, Roche (C/A), Roche, Novartis, Celgene (H), Roche, Celgene (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Harris L, Fritsche H, Mennel R, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25:5287–5312. doi: 10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- 2.Dowsett M, Smith IE, Ebbs SR, et al. Short-term changes in Ki-67 during neoadjuvant treatment of primary breast cancer with anastrozole or tamoxifen alone or combined correlate with recurrence-free survival. Clin Cancer Res. 2005;11:951s–958s. [PubMed] [Google Scholar]
- 3.Ellis MJ, Tao Y, Luo J, et al. Outcome prediction for estrogen receptor-positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics. J Natl Cancer Inst. 2008;100:1380–1388. doi: 10.1093/jnci/djn309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dowsett M, Nielsen TO, A’Hern R, et al. Assessment of Ki67 in breast cancer: Recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst. 2011;103:1656–1664. doi: 10.1093/jnci/djr393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andre F, Arnedos M, Goubar A, et al. Ki67—no evidence for its use in node-positive breast cancer. Nat Rev Clin Oncol. 2015;12:296–301. doi: 10.1038/nrclinonc.2015.46. [DOI] [PubMed] [Google Scholar]
- 6.Colleoni M, Viale G, Zahrieh D, et al. Expression of ER, PgR, HER1, HER2, and response: A study of preoperative chemotherapy. Ann Oncol. 2008;19:465–472. doi: 10.1093/annonc/mdm509. [DOI] [PubMed] [Google Scholar]
- 7.Denkert C, Loibl S, Müller BM, et al. Ki67 levels as predictive and prognostic parameters in pretherapeutic breast cancer core biopsies: A translational investigation in the neoadjuvant GeparTrio trial. Ann Oncol. 2013;24:2786–2793. doi: 10.1093/annonc/mdt350. [DOI] [PubMed] [Google Scholar]
- 8.Esserman LJ, Berry DA, DeMichele A, et al. Pathologic complete response predicts recurrence-free survival more effectively by cancer subset: Results from the I-SPY 1 TRIAL--CALGB 150007/150012, ACRIN 6657. J Clin Oncol. 2012;30:3242–3249. doi: 10.1200/JCO.2011.39.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fasching PA, Heusinger K, Haeberle L, et al. Ki67, chemotherapy response, and prognosis in breast cancer patients receiving neoadjuvant treatment. BMC Cancer. 2011;11:486. doi: 10.1186/1471-2407-11-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones RL, Salter J, A’Hern R, et al. Relationship between oestrogen receptor status and proliferation in predicting response and long-term outcome to neoadjuvant chemotherapy for breast cancer. Breast Cancer Res Treat. 2010;119:315–323. doi: 10.1007/s10549-009-0329-x. [DOI] [PubMed] [Google Scholar]
- 11.Keam B, Im SA, Lee KH, et al. Ki-67 can be used for further classification of triple negative breast cancer into two subtypes with different response and prognosis. Breast Cancer Res. 2011;13:R22. doi: 10.1186/bcr2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guarneri V, Piacentini F, Ficarra G, et al. A prognostic model based on nodal status and Ki-67 predicts the risk of recurrence and death in breast cancer patients with residual disease after preoperative chemotherapy. Ann Oncol. 2009;20:1193–1198. doi: 10.1093/annonc/mdn761. [DOI] [PubMed] [Google Scholar]
- 13.von Minckwitz G, Sinn HP, Raab G, et al. Clinical response after two cycles compared to HER2, Ki-67, p53, and bcl-2 in independently predicting a pathological complete response after preoperative chemotherapy in patients with operable carcinoma of the breast. Breast Cancer Res. 2008;10:R30. doi: 10.1186/bcr1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luporsi E, André F, Spyratos F, et al. Ki-67: level of evidence and methodological considerations for its role in the clinical management of breast cancer: Analytical and critical review. Breast Cancer Res Treat. 2012;132:895–915. doi: 10.1007/s10549-011-1837-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alba E, Albanell J, de la Haba J, et al. Trastuzumab or lapatinib with standard chemotherapy for HER2-positive breast cancer: results from the GEICAM/2006-14 trial. Br J Cancer. 2014;110:1139–1147. doi: 10.1038/bjc.2013.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alba E, Calvo L, Albanell J, et al. Chemotherapy (CT) and hormonotherapy (HT) as neoadjuvant treatment in luminal breast cancer patients: Results from the GEICAM/2006-03, a multicenter, randomized, phase-II study. Ann Oncol. 2012;23:3069–3074. doi: 10.1093/annonc/mds132. [DOI] [PubMed] [Google Scholar]
- 17.Alba E, Chacon JI, Lluch A, et al. A randomized phase II trial of platinum salts in basal-like breast cancer patients in the neoadjuvant setting. Results from the GEICAM/2006-03, multicenter study. Breast Cancer Res Treat. 2012;136:487–493. doi: 10.1007/s10549-012-2100-y. [DOI] [PubMed] [Google Scholar]
- 18.Antón A, Ruiz A, Plazaola A, et al. Phase II clinical trial of liposomal-encapsulated doxorubicin citrate and docetaxel, associated with trastuzumab, as neoadjuvant treatment in stages II and IIIA HER2-overexpressing breast cancer patients. GEICAM 2003-03 study. Ann Oncol. 2011;22:74–79. doi: 10.1093/annonc/mdq317. [DOI] [PubMed] [Google Scholar]
- 19.Sánchez-Rovira P, Antón A, Barnadas A, et al. Classical markers like ER and ki-67, but also survivin and pERK, could be involved in the pathological response to gemcitabine, adriamycin and paclitaxel (GAT) in locally advanced breast cancer patients: results from the GEICAM/2002-01 phase II study. Clin Transl Oncol. 2012;14:430–436. doi: 10.1007/s12094-012-0820-4. [DOI] [PubMed] [Google Scholar]
- 20.Rojo F, García-Parra J, Zazo S, et al. Nuclear PARP-1 protein overexpression is associated with poor overall survival in early breast cancer. Ann Oncol. 2012;23:1156–1164. doi: 10.1093/annonc/mdr361. [DOI] [PubMed] [Google Scholar]
- 21.Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Arch Pathol Lab Med. 2010;134:907–922. doi: 10.5858/134.6.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 23.Ogston KN, Miller ID, Payne S, et al. A new histological grading system to assess response of breast cancers to primary chemotherapy: Prognostic significance and survival. Breast. 2003;12:320–327. doi: 10.1016/s0960-9776(03)00106-1. [DOI] [PubMed] [Google Scholar]
- 24.Metz CE. Basic principles of ROC analysis. Semin Nucl Med. 1978;8:283–298. doi: 10.1016/s0001-2998(78)80014-2. [DOI] [PubMed] [Google Scholar]
- 25.Berruti A, Generali D, Kaufmann M, et al. International expert consensus on primary systemic therapy in the management of early breast cancer: Highlights of the fourth symposium on primary systemic therapy in the management of operable breast cancer, Cremona, Italy (2010) J Natl Cancer Inst Monogr. 2011;2011:147–151. doi: 10.1093/jncimonographs/lgr037. [DOI] [PubMed] [Google Scholar]
- 26.Chollet P, Amat S, Cure H, et al. Prognostic significance of a complete pathological response after induction chemotherapy in operable breast cancer. Br J Cancer. 2002;86:1041–1046. doi: 10.1038/sj.bjc.6600210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: Updates of national surgical adjuvant breast and bowel project protocols B-18 and B-27. J Clin Oncol. 2008;26:778–785. doi: 10.1200/JCO.2007.15.0235. [DOI] [PubMed] [Google Scholar]
- 28.Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 29.von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30:1796–1804. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 30.Prowell TM, Pazdur R. Pathological complete response and accelerated drug approval in early breast cancer. N Engl J Med. 2012;366:2438–2441. doi: 10.1056/NEJMp1205737. [DOI] [PubMed] [Google Scholar]
- 31.Nadler A, Cukier M, Rowsell C, et al. Ki-67 is a reliable pathological grading marker for neuroendocrine tumors. Virchows Arch. 2013;462:501–505. doi: 10.1007/s00428-013-1410-8. [DOI] [PubMed] [Google Scholar]
- 32.Sorbye H, Welin S, Langer SW, et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): The NORDIC NEC study. Ann Oncol. 2013;24:152–160. doi: 10.1093/annonc/mds276. [DOI] [PubMed] [Google Scholar]
- 33.Fan C, Oh DS, Wessels L, et al. Concordance among gene-expression-based predictors for breast cancer. N Engl J Med. 2006;355:560–569. doi: 10.1056/NEJMoa052933. [DOI] [PubMed] [Google Scholar]
- 34.Prat A, Parker JS, Fan C, et al. PAM50 assay and the three-gene model for identifying the major and clinically relevant molecular subtypes of breast cancer. Breast Cancer Res Treat. 2012;135:301–306. doi: 10.1007/s10549-012-2143-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Desmedt C, Haibe-Kains B, Wirapati P, et al. Biological processes associated with breast cancer clinical outcome depend on the molecular subtypes. Clin Cancer Res. 2008;14:5158–5165. doi: 10.1158/1078-0432.CCR-07-4756. [DOI] [PubMed] [Google Scholar]
- 36.Wirapati P, Sotiriou C, Kunkel S, et al. Meta-analysis of gene expression profiles in breast cancer: Toward a unified understanding of breast cancer subtyping and prognosis signatures. Breast Cancer Res. 2008;10:R65. doi: 10.1186/bcr2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cuzick J, Dowsett M, Pineda S, et al. Prognostic value of a combined estrogen receptor, progesterone receptor, Ki-67, and human epidermal growth factor receptor 2 immunohistochemical score and comparison with the Genomic Health recurrence score in early breast cancer. J Clin Oncol. 2011;29:4273–4278. doi: 10.1200/JCO.2010.31.2835. [DOI] [PubMed] [Google Scholar]
- 38.Niikura N, Iwamoto T, Masuda S, et al. Immunohistochemical Ki67 labeling index has similar proliferation predictive power to various gene signatures in breast cancer. Cancer Sci. 2012;103:1508–1512. doi: 10.1111/j.1349-7006.2012.02319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams DJ, Cohen C, Darrow M, et al. Proliferation (Ki-67 and phosphohistone H3) and oncotype DX recurrence score in estrogen receptor-positive breast cancer. Appl Immunohistochem Mol Morphol. 2011;19:431–436. doi: 10.1097/PAI.0b013e318206d23d. [DOI] [PubMed] [Google Scholar]
- 40.Polley MY, Leung SC, Gao D, et al. An international study to increase concordance in Ki67 scoring. Mod Pathol. 2015;28:778–786. doi: 10.1038/modpathol.2015.38. [DOI] [PubMed] [Google Scholar]
- 41.Polley MY, Leung SC, McShane LM, et al. An international Ki67 reproducibility study. J Natl Cancer Inst. 2013;105:1897–1906. doi: 10.1093/jnci/djt306. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.