Anthracycline-containing chemotherapy (Anth-C) is associated with long-term cardiovascular mortality. In the years after Anth-C, ventricular-arterial coupling was significantly attenuated during exercise, primarily owing to decreased left ventricular contractility. No differences in arterial elastance were observed. The previously reported adverse effects of Anth-C on the vasculature do not appear to persist in the years following treatment as vascular structure and function were comparable to those of controls.
Keywords: Cardiotoxicity, Exercise, Echocardiography, Vascular, Contractility
Abstract
Background.
Anthracycline-containing chemotherapy (Anth-C) is associated with long-term cardiovascular mortality. Although cardiovascular risk assessment has traditionally focused on the heart, evidence has demonstrated that vascular dysfunction also occurs during and up to 1 year following Anth-C. Whether vascular dysfunction persists long-term or negatively influences cardiac function remains unknown. Hence, the present study evaluated ventricular-arterial coupling, in concert with measures of vascular structure and function, in the years following Anth-C.
Methods.
Arterial elastance (Ea), end-systolic elastance (Ees), and ventricular-arterial coupling (Ea/Ees) were measured during rest and exercise using echocardiography. Resting vascular function (flow-mediated dilation) and structure (carotid intima-media thickness, arterial stiffness) were also measured.
Results.
Thirty breast cancer survivors (6.5 ± 3.6 years after Anth-C) with normal left ventricular ejection fraction (LVEF) (60% ± 6%) and 30 matched controls were studied. At rest, no differences were found in Ea, Ees, Ea/Ees, or LVEF between groups. The normal exercise-induced increase in Ees was attenuated in survivors at 50% and 75% of maximal workload (p < .01). Ea/Ees was also higher at all workloads in the survivors compared with the controls (p < .01). No differences in vascular structure and function were observed between the two groups (p > .05).
Conclusion.
In the years after Anth-C, ventricular-arterial coupling was significantly attenuated during exercise, primarily owing to decreased LV contractility (indicated by a reduced Ees). This subclinical dysfunction appears to be isolated to the heart, as no differences in Ea were observed. The previously reported adverse effects of Anth-C on the vasculature appear to not persist in the years after treatment, as vascular structure and function were comparable to controls.
Implications for Practice:
Anthracycline-induced cardiotoxicity results in significantly impaired ventricular-arterial coupling in the years following chemotherapy, owing specifically to decreased left ventricular contractility. This subclinical dysfunction was identified only under exercise stress. A comprehensive evaluation of vascular structure and function yielded no differences between those treated with anthracyclines and controls. Combined with a stress stimulus, ventricular-arterial coupling might hold significant value beyond characterization of integrative cardiovascular function, in particular as a part of a risk-stratification strategy after anthracycline-containing chemotherapy. Although vascular function and structure were not different in this cohort, this does not undermine the importance of identifying vascular (dys)function in this population, because increases in net arterial load during exercise might amplify the effect of reductions in contractility on cardiovascular function after anthracycline-containing chemotherapy.
Abstract
摘要
背景. 含蒽环类的化疗方案 (Anth-C) 与远期心血管死亡率相关。尽管心血管风险的评估一般关注的是心脏, 但已有证据证实 Anth-C 治疗期间和治疗后至多 1 年内也会发生血管功能障碍。血管功能障碍是否会持续存在, 或者是否对心脏功能有不利影响, 都尚属未知。因此, 本研究对 Anth-C 治疗后数年中的心室-动脉偶联进行了评价, 同时测量了血管结构及功能。
方法. 在静息和活动状态下使用超声心动图对动脉弹性 (Ea)、收缩末期动脉弹性 (Ees) 和心室-动脉偶联 (Ea/Ees) 进行测量。还测量了静息状态下的血管功能 (血流介导性舒张) 与结构 (颈动脉内膜中层厚度、动脉硬度)。
结果. 我们对 30 例左心室射血分数 (LVEF) 正常 (60%±6%) 的乳腺癌存活患者 (Anth-C 治疗后 6.5±3.6 年) 及 30 例匹配对照进行了研究。静息状态下组间 Ea、Ees、Ea/Ees 和 LVEF 均无差异。正常人活动引起 Ees 增加, 而在乳腺癌存活者的 Ees 在最大工作负荷的 50%和 75%出现下降 (P < 0.01)。存活者在所有工作负荷下的 Ea/Ees 均高于对照 (P < 0.01)。两组的血管结构和功能之间未见差异 (P > 0.05)。
结论. Anth-C 治疗后数年, 运动期间的心室-动脉偶联显著变差, 主要是由左心室收缩力下降 (Ees 降低提示) 所致。由于未观察到 Ea 的区别, 这一亚临床功能障碍看似与心脏无关。既往研究报告 Anth-C 对血管的不良作用似乎不会持续至治疗后数年, 因为数年后患者的血管结构与功能与对照相似。The Oncologist 2016;21:141–149
对临床实践的提示: 蒽环类引起的心脏毒性导致了化疗数年后患者显著的心室-血管偶联受损, 这是由于左心室收缩性下降所致。这一亚临床功能障碍仅见于活动应激下。对血管结构和功能进行综合评价未发现曾接受蒽环类治疗的受试者与对照之间存在差异。结合应激刺激, 心室-动脉偶联可能在综合心血管功能以外具有明显的价值, 尤其是作为含蒽环类方案化疗后进行风险分层策略的一部分。尽管这一队列中血管功能和结构未发生变化, 但并未削弱识别血管功能 (障碍) 对于该人群的重要性, 因为在接受含蒽环类药物化疗后, 活动期间的净动脉负荷增加可能会放大心血管功能中收缩性降低的效应。
Introduction
Early-stage breast cancer patients are at greater risk of developing cardiovascular disease relative to age-matched healthy women [1] primarily owing to the direct cardiotoxic effects of adjuvant anthracycline-containing chemotherapy (Anth-C) combined with other direct (e.g., hormone therapy) and indirect (e.g., physical inactivity) perturbations that occur across the cancer continuum [2]. To date, investigators have focused almost exclusively on the cardiac-specific effects of Anth-C (primarily through evaluation of resting assessment of left ventricular ejection fraction [LVEF]), with minimal attention to vascular damage. Increased aortic stiffness [3, 4], endothelial dysfunction [5], and arterial remodeling [6] have been demonstrated during or up to 1 year following Anth-C. However, whether these impairments in vascular structure (e.g., arterial stiffness and carotid intima-media thickness [CIMT]) and function (e.g., endothelial-dependent flow-mediated dilation [FMD]) persist in the years following Anth-C and whether they contribute to the impaired cardiac function observed in breast cancer survivors is currently unknown.
The heart and systemic vasculature are integral components of the cardiovascular network, with changes in one component affecting performance of the other. Understanding not only how the heart and systemic vasculature function independently, but also how they interact (termed ventricular-arterial coupling) is important when evaluating global cardiovascular function [7]. Ventricular-arterial coupling combines a measure of net arterial load or arterial elastance (Ea) and end-systolic elastance (Ees), a load-independent measure indicating LV contractility, specifically under stress [8]. Although ventricular-arterial coupling (Ea/Ees) is inversely related to LVEF [9], it provides information beyond systolic function by also evaluating alterations in arterial function or ventricular function, or both. Under stress, Ea/Ees is a surrogate measure of cardiovascular reserve capacity (CVRC), because it permits integrative assessment of the heart and vasculature’s ability to respond to an increased workload [7, 10]. During exercise, the cardiovascular system favors Ees to supply appropriate cardiac output, resulting in a reduction in Ea/Ees [10]. If Anth-C increases Ea and/or reduces Ees by having a direct effect on the vasculature or myocardium, respectively, the reduction in Ea/Ees commonly observed with exercise will be greatly attenuated, indicative of reduced CVRC. Pathologic impairments in CVRC are etiologic in many chronic disease conditions [11], and surrogate measures of CVRC such as Ea/Ees are prognostic of mortality and cardiovascular events [12].
Studies to date have only examined vascular function in the short-term and only examined heart and vascular function in isolation, primarily under resting conditions. Therefore, the purpose of the present study was to evaluate both the independent and the integrative effects of the heart and vasculature at rest and during exercise in breast cancer survivors with preserved LVEF (>50%) who had received Anth-C. We hypothesized that the normal reduction in Ea/Ees would be attenuated during exercise in breast cancer survivors. Furthermore, we hypothesized that CIMT, arterial stiffness, and FMD would be significantly impaired in the patients compared to the controls.
Methods
Patients and Study Design
Using a cross-sectional design, 30 estrogen receptor-positive, HER2-negative early-stage (stage I-III) breast cancer survivors treated with adjuvant Anth-C 2–15 years previously were recruited from the British Columbia Cancer Agency Cancer Registry. Also, 30 age-, body mass index (BMI)-, and activity level-matched women were recruited for comparison purposes. The research complied with the Declaration of Helsinki, and written informed consent that had received institutional ethics board approval was obtained from all participants before initiating the study. All study assessments were performed over a period of 3 days and were standardized across patient and control subjects. A detailed description of the study methods is provided in the supplemental online data.
Measurements
Cardiopulmonary Exercise Testing
Each participant performed an incremental cardiopulmonary exercise test to symptom limitation on an upright electrically braked cycle ergometer (Ergoline 800S; CareFusion Corp., San Diego, CA, http://www.carefusion.com) with expired-gas analysis (SensorMedics Vmax 29C; CareFusion Corp.) according to American Thoracic Society Guidelines as modified for cancer populations [13].
Echocardiographic Measurements of Cardiac Function and Ventricular-Arterial Coupling
Two-dimensional transthoracic echocardiographic images (IE33; Philips, Amsterdam, The Netherlands, http://www.philips.ca/healthcare/solutions/ultrasound) were performed in the apical four- and two-chamber views to determine the left ventricular end-diastolic volume (EDV), end-systolic volume (ESV), and LVEF by the modified Simpson rule [14]. Measures were obtained at rest and during steady-state exercise using a discontinuous exercise protocol at 25%, 50%, and 75% of the subject’s maximal work rate (Wmax). Diastolic filling parameters were collected at rest according to published guidelines [15]. Ea was calculated as the ratio of end-systolic pressure (ESP) to stroke volume (SV) [16]. ESP was determined by the validated equation 0.9 × systolic blood pressure (SBP) measured by manual sphygmomanometry [17]. Ees was calculated using the validated single beat technique, using the measurements of blood pressure, stroke volume, LVEF, and pre-ejection and systolic ejection time intervals from LV outflow Doppler, as previously described [18]. A trained sonographer who was unaware of the group allocation performed all echocardiographic assessments and analysis.
Measurement of Vascular Structure and Function
Central (carotid-femoral pulse wave velocity [PWV]) and peripheral (carotid-radial PWV) arterial stiffness were assessed using handheld tonometers (SPT-301; Millar Instruments, Houston, TX, http://www.millar.com), adhering to international guidelines [19]. Local arterial stiffness of the carotid artery was determined using a previously reported method [20] to calculate arterial compliance, distensibility, and β-stiffness index. Our standard error of measurement for PWV is 0.17 m/s, with a coefficient of variation of 3.5%. CIMT was measured with the subject in the supine position using an 8-MHz high-frequency linear array transducer. Images were taken of the far wall, 1 cm proximal to the carotid bulb. The CIMT at end diastole (1 frame before the R interval) of 10 successive beats was recorded and averaged. Our standard error of measurement for CIMT is 0.039 mm, with a coefficient of variation of 5.3%.
Endothelial function was evaluated using the FMD technique, which measures flow-mediated endothelial-dependent vasodilation of the brachial artery, according to international guidelines [21]. To assess endothelial-independent vasodilation of the brachial artery, a sublingual 400-μg spray dose of glyceryl trinitrate (GTN) was administered, and changes in diameter were recorded for >5 minutes. Our standard error of measurement for FMD is 0.3%, with a coefficient of variation of 3.6%.
Statistical Analysis
To examine the differences between groups, the Wilcoxon rank sum test for independent nonparametric samples was used for continuous variables and Fisher’s exact test for categorical variables. No adjustments were made for multiple comparisons. A two-sided significance level of 0.05 was used for all statistical tests. Simple regression analysis was also performed to study the associations between the time from Anth-C completion and performance of vascular and ventricular-arterial coupling measures. All statistical analyses were conducted using SAS, version 9.3 (SAS Institute, Cary, NC, http://www.sas.com), by an independent statistician. An a priori sample size calculation was performed using preliminary data from our group, which demonstrated that breast cancer survivors had an Ea/Ees of 0.11 higher than that of the age-, BMI-, and activity-matched controls at 75% Wmax. Using this difference in the primary outcome (i.e., Ea/Ees of 0.11), a sample of 30 subjects per group were required, assuming a SD of the change score of 0.15, a power of 0.8, and using a two-tailed test with an α of 0.05.
Results
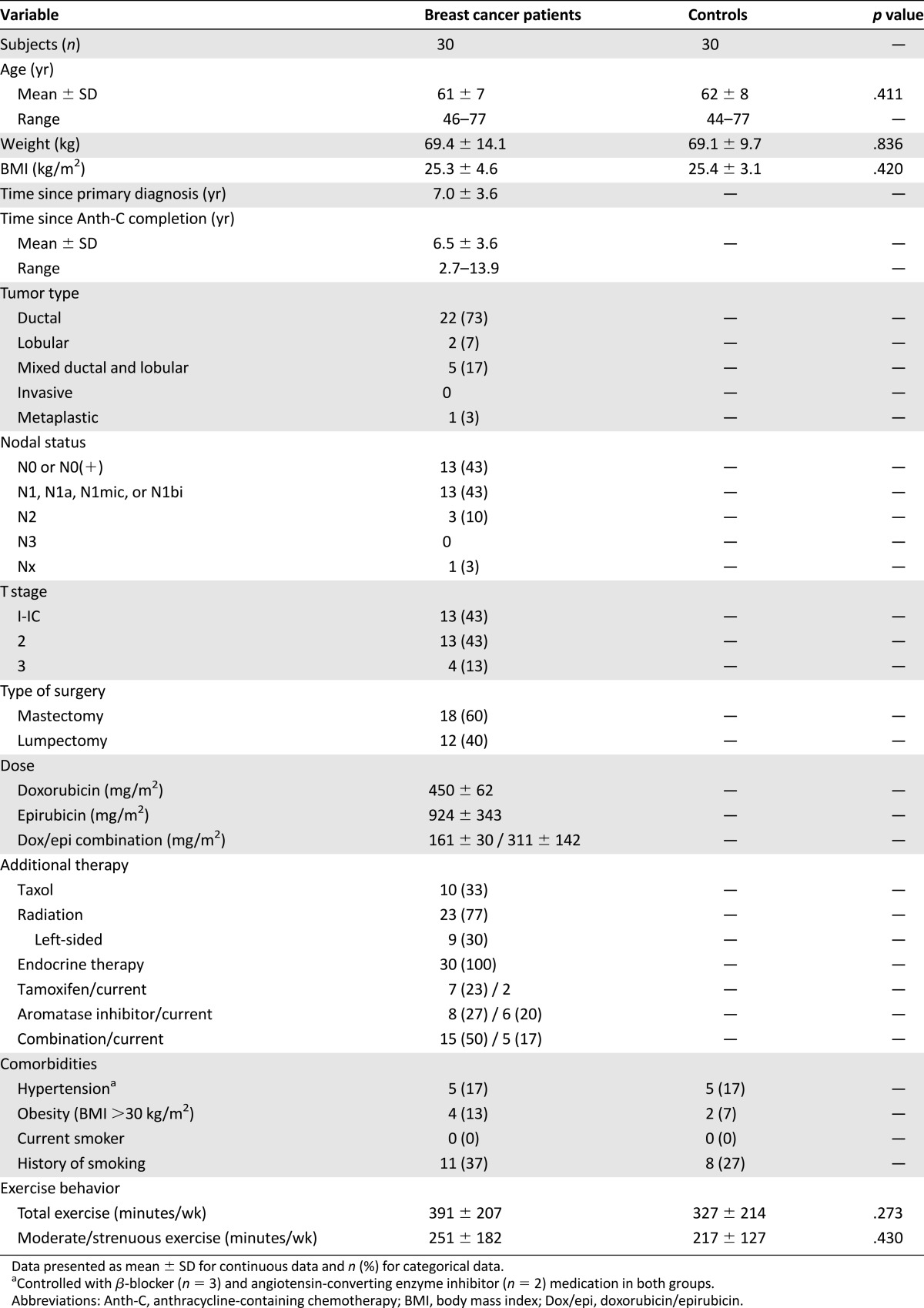
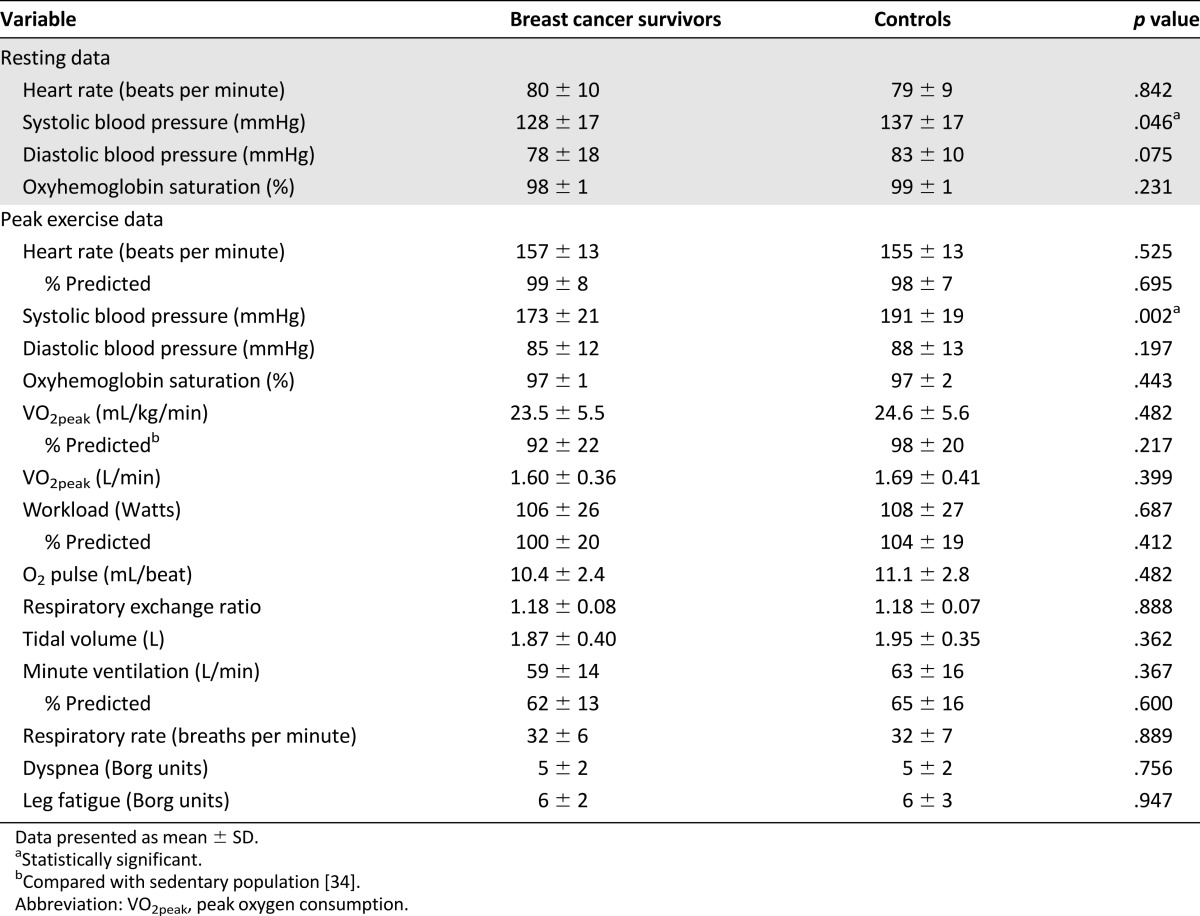
Patients were recruited from May 2011 to October 2013. A total of 145 potentially eligible patients were contacted for the study, and 33 (23%) were interested in participation. Of these, 3 patients were unable to participate, and 30 completed all study assessments. Thirty matched controls were also recruited. The study flow is presented in supplemental online Figure 1. The subject characteristics are presented in Table 1. No significant differences were found in the baseline characteristics between survivors and controls. The cardiopulmonary exercise data are presented in Table 2. The peak oxygen consumption (VO2peak) and Wmax were not different between groups.
Table 1.
Subject characteristics
Table 2.
Cardiopulmonary responses to exercise
Ventricular-Arterial Coupling
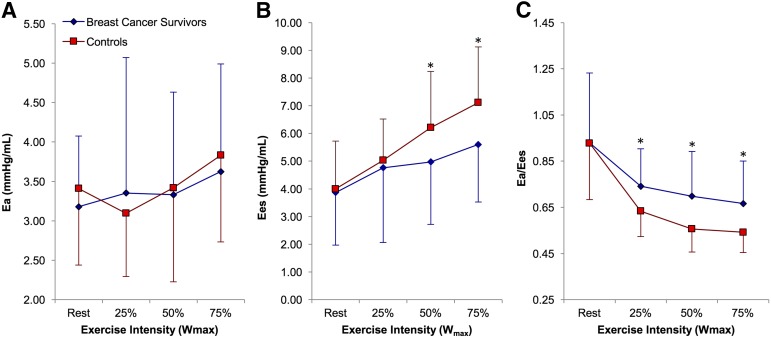
The measures of ventricular-arterial coupling are presented in Figure 1. No significant differences were observed for the resting measures of ventricular-arterial coupling. Ea was also not different at any exercise intensity between the two groups. Ees was reduced in survivors compared with control subjects at 50% and 75% Wmax (p < .01). The Ea/Ees ratio was also higher at 25%, 50%, and 75% Wmax (p < .01) in survivors compared with controls. No significant relationships were found between the time from Anth-C completion and the measures of ventricular-arterial coupling. Individual Ea/Ees responses from rest to 75% Wmax are presented in supplemental online Figure 2. The cardiovascular responses stratified by the change in Ea during exercise are presented in supplemental online Figure 3.
Figure 1.
Graphs showing the ventricular-arterial coupling measures used in the present study. Arterial elastance (A), end-systolic elastance (B), and Ea/Ees ratio (C) at different intensities of exercise. Error bars denote the SD of the mean. ∗, p ≤ .01.
Abbreviations: Ea, arterial elastance; Ees, end-systolic elastance; Ea/Ees ratio, ventricular-arterial coupling.
Cardiac Function
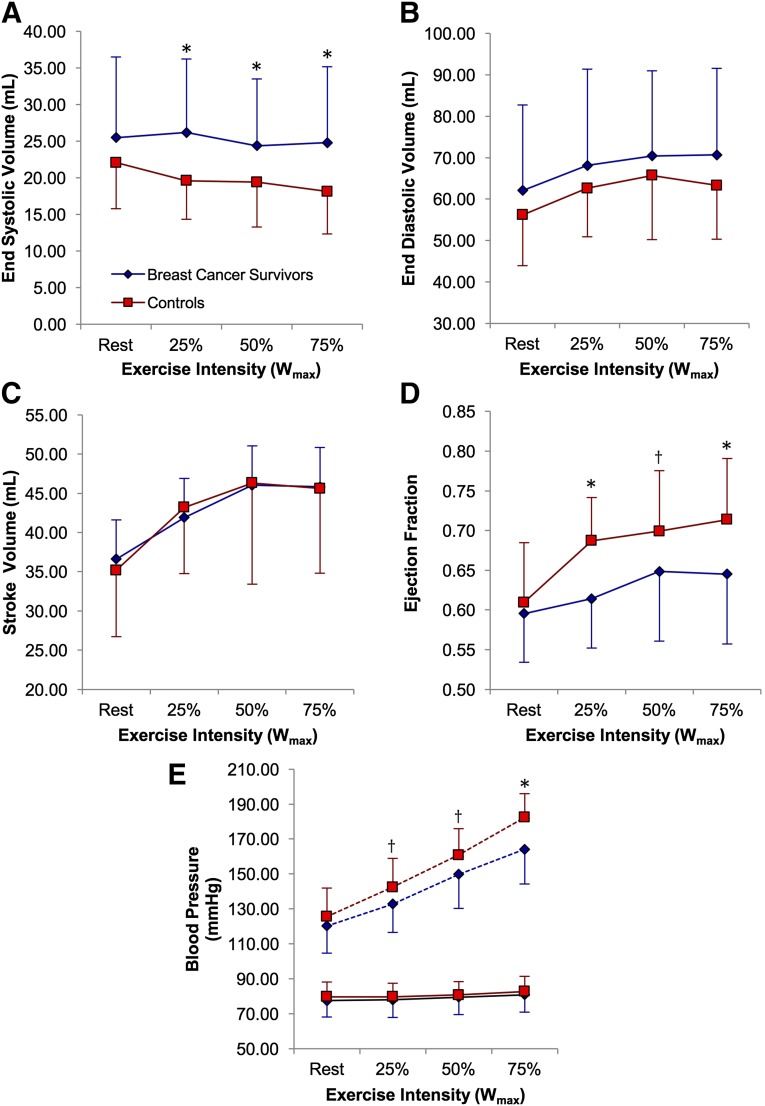
The measures of cardiac function are presented in Figure 2. At rest, the measures of LV systolic function were not different between the survivors and controls. ESV was elevated in survivors at 25% (p < .01), 50% (p < .05), and 75% Wmax (p < .01). SBP was lower in survivors at all intensities (p < .05), and heart rate was elevated at rest and 25% Wmax (p < .05). EDV and SV were not different at any exercise intensity between the two groups. LVEF was not different at rest but was reduced at 25% (p < .001), 50% (p < .05), and 75% Wmax (p < .01) in survivors. Early and late peak ventricular filling velocities and pressure gradients measured at rest were unchanged, although deceleration time and mitral deceleration index were reduced in survivors (p < .05; supplemental online Table 1).
Figure 2.
Graphs showing the measures of cardiac function. End-systolic volume (A), end-diastolic volume (B), stroke volume (C), ejection fraction (D), and systolic (dashed line) and diastolic (solid line) blood pressure (E) at rest and during different intensities of exercise. Error bars denote the SD of the mean. ∗, p ≤ .01; †, p ≤ .05.
Abbreviation: Wmax, maximal work rate.
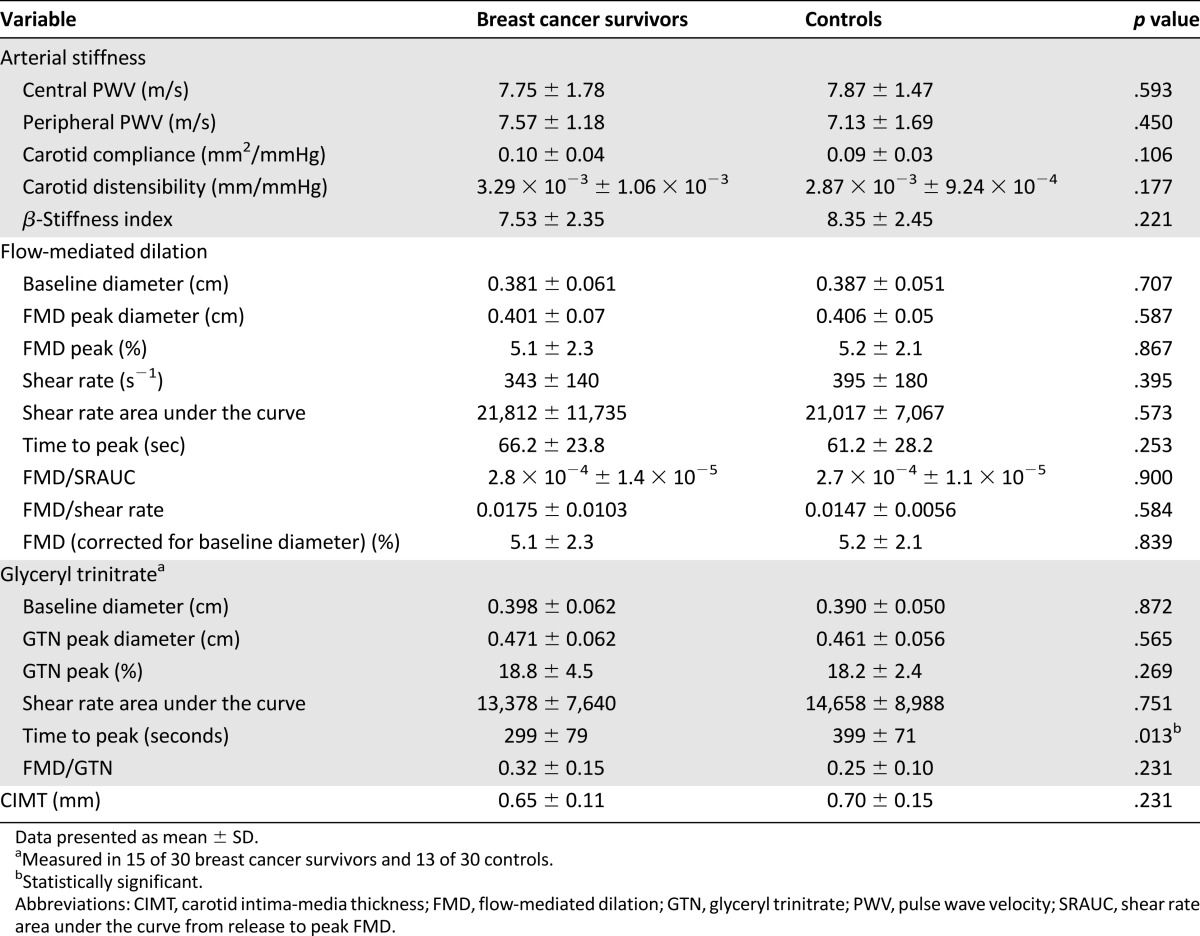
Vascular Structure and Function
The measures of vascular structure and function are presented in Table 3. The baseline and peak diameters, shear rate, shear stimulus (measured by the shear rate area under the curve from release to peak FMD [SRAUC]), time to peak dilation, FMD normalized to shear rate, SRAUC, and baseline diameters were not different between the two groups. Also, no differences were found in endothelial-independent vasodilation between groups, except for a faster time to peak dilation after GTN administration in survivors (p < .05). No differences were found in CIMT, central and peripheral arterial stiffness, carotid compliance, distensibility, or the β-stiffness index. Also, no significant relationships were found between the time from Anth-C completion and any vascular measure.
Table 3.
Vascular structure and function
Discussion
To our knowledge, this is the first study to use ventricular-arterial coupling to investigate the integrative effects of the heart and vasculature in any cancer population. In support of our primary hypothesis, the expected decrease in Ea/Ees was attenuated during exercise in breast cancer survivors compared to controls. This finding primarily resulted from a decreased Ees, as Ea was unchanged during exercise. Contrary to our secondary hypothesis, no differences in vascular structure and function were observed in breast cancer survivors in the years following treatment, suggesting that sustained Anth-C dysfunction is isolated to the heart. It is important to highlight that the differences in ventricular-arterial coupling were only unveiled with exercise, because no differences in Ea/Ees (or LVEF) were observed at rest, emphasizing the importance of stress-based assessments to expose subclinical cardiovascular dysfunction after Anth-C.
Exercise-Induced Changes in Ventricular-Arterial Coupling Are Attenuated in the Years Following Anth-C
In healthy individuals, Ees markedly increases with increasing exercise intensity, reflecting acute changes in LV contractility [10]. It is well-established that Anth-C directly injures the myocardium [22], which induces structural changes that can perturb myocardial contractile function beyond that commonly seen with aging [23]. In the present study, Ees was normal at rest; however, the typical exercise-induced increase was attenuated at moderate intensities (i.e., 50% and 75% Wmax), indicative of contractile impairment. Thus, survivors have little or no reserve to augment contractility in response to an increase in the exercise-induced metabolic demand. Evidence of reduced LV contractility was further supported by the concomitant increases in ESV and reductions in SBP at each exercise load in survivors, without significant changes in afterload (the total peripheral resistance was not different between groups [data not shown]).
In the present study, as exercise intensity increased from rest to 75% Wmax, control subjects reduced Ea/Ees by 42%, similar to the findings from previous studies of healthy individuals (aged 40–70 years) [24, 25]. However, the reduction in Ea/Ees in survivors was only 28% and was considerably attenuated compared with that observed in healthy aging [24, 25]. This inability to appropriately reduce Ea/Ees in response to exercise stress occurred in the presence of a preserved stroke volume. The maintenance of stroke volume likely resulted from the small, albeit nonsignificant, increase in EDV (Fig. 2), thus using the Frank-Starling mechanism to compensate for the attenuated LV contractility. However, the inability of survivors to reduce Ea/Ees during exercise suggests that the CVRC is reduced, which might mitigate the capacity to further adjust to future age- or disease-related perturbations in cardiac or vascular function. For example, in healthy aging, increased Ea during exercise results in reduced utilization of the Frank-Starling mechanism and greater reliance on Ees to meet functional demand [26]. Using similar Ea stratifications (supplemental online data; supplemental online Fig. 3), survivors who increased Ea during exercise not only did not increase EDV, but also had an Ees that increased to <50% of that of controls, as well as a significant reduction in SBP. This suggests a failure to meet functional demand. These exploratory findings suggest that an increase in arterial load during exercise might amplify the effect of reductions in contractility on cardiovascular function after Anth-C. Whether these specific survivors are at highest risk of the onset of symptomatic cardiovascular disease (CVD) requires investigation.
The cardiovascular response to an acute stressor after Anth-C is not well understood, as few studies to date have used a systemic stressor in conjunction with conventional imaging to detect subclinical cardiovascular disease [27, 28]. In current oncology practice, evaluation of cardiac function is almost exclusively determined via the resting assessment of LVEF. However, the resting LVEF only provides a snapshot of cardiac performance under optimal circumstances. It is not a sensitive measure of early (subclinical) myocyte damage and is not prognostic in patients with preserved LVEF (>50%) [29]. More novel techniques, such as echocardiography [27] and magnetic resonance imaging [30], to measure subtle changes in cardiac mechanics and deformation might provide more sensitive detection of cardiac injury at rest compared with LVEF, although supporting evidence is still emerging. Although the information obtained from resting assessments with these novel techniques might become influential in risk stratification before or after Anth-C by providing diagnostic and prognostic information beyond the resting LVEF [31], the utility of these measures for evaluating integrative cardiovascular function, rather than just cardiac-specific function, is limited. Because LVEF is influenced by loading conditions and heart rate (unlike Ees), we contend that the evaluation of Ea/Ees, combined with a stress stimulus, will provide a wealth of physiologically and clinically relevant information that is both additive and complementary to current (resting LVEF) and emerging techniques. For example, given that Ea/Ees is a measure of CVRC and a profound range of individual responses in Ea/Ees from rest to exercise exist (from a 60% reduction to 30% increase; supplemental online Fig. 2), it is possible that Ea/Ees could provide incremental prognostic information in this population.
Vascular Structure and Function Are Not Different in the Years Following Anth-C
Increased aortic stiffness [3, 4], endothelial dysfunction [5], and arterial remodeling [6] have been reported up to 1 year following Anth-C administration. However, in the present study and contrary to our hypothesis, Ea was not different at rest or during any stage of exercise. Also, FMD, arterial stiffness, and CIMT were not different between the survivors at an average of ∼6.5 years following Anth-C and the controls. Endothelial dysfunction has been shown to occur in both animal models [5, 32] and clinical populations [5] immediately after Anth-C administration. With removal of the oxidative stress stimulus (Anth-C), nitric oxide (NO) bioavailability and vascular function should normalize unless (a) permanent endothelial remodeling or damage is sustained, (b) NO bioavailability is chronically reduced from another perturbation, (c) the shear stress stimulus is significantly diminished, or (d) redundant pathways that upregulate to compensate for dysfunction in the aforementioned processes are inadequate. Our data have demonstrated that in this relatively small cohort, both endothelial-dependent and -independent function appear normal in the years after treatment. This suggests that none of these aforementioned causes appear to be the case. The brachial artery endothelium has been shown to possess an inherent plasticity with the removal of an acute perturbation (e.g., after arterial catheter insertion, FMD will recover completely over a period of several months [33]). It is possible that in this sample a similar temporal pathophysiology has occurred, although prospective longitudinal assessment is required. This normal response also extends to the structure of the arterial wall, because arterial stiffness and CIMT after Anth-C administration were similar to controls. As the time from treatment was the primary difference between previous studies and the present trial, our data support that arterial structure, function, and thickness might “normalize” in the years following Anth-C. Such conclusions, however, should be interpreted with caution, because the study was powered according to our primary outcome variable (ventricular-arterial coupling), not measures of vascular structure and function.
Study Limitations
The major limitation of the present study was the cross-sectional design. However, owing to the novelty of our assessments and the need to characterize cardiovascular function in the long-term after Anth-C, we believe the data presented is a valuable step in characterizing the post-Anth-C cardiovascular phenotype in breast cancer. A survival bias should also be considered when interpreting the results of the present study. Pressure and flow were not measured directly in the present study, instead, these were estimated using noninvasive surrogates. These measures, however, have been validated at rest [18] and have been used in multiple disease populations during exercise [24, 25], yielding similar results to those shown in the present study.
Conclusion
Despite preserved cardiac function indicated by resting LVEF, the use of exercise echocardiography combined with Ea/Ees revealed subclinical dysfunction, with the heart and vasculature not appropriately coupled to deliver blood to the periphery owing to a reduced Ees. These results highlight that although reductions in ventricular contractility persist in the years following Anth-C, surprisingly, in our relatively small cohort, vascular thickness, stiffness, and function do not. Although the present study can only provide a cross-sectional “snapshot” of cardiovascular function in the years after Anth-C, we contend that such a comprehensive evaluation of subclinical dysfunction across the cardiovascular network has elucidated novel areas of focus and clarification for future study. First, although vascular function and structure were not different in the present cohort, this does not undermine the importance of identifying vascular (dys)function in this population, as increases in arterial load during exercise may amplify the effect of reductions in contractility on cardiovascular function after Anth-C. Verification of our findings, especially in those with a high comorbidity burden, is warranted. Second, it is plausible that Ea/Ees, combined with a stress stimulus, holds significant value beyond the characterization of integrative cardiovascular function and CVRC, in particular as a part of a risk-stratification strategy following Anth-C. Prospective longitudinal studies are now required to elucidate how changes in Ea/Ees from diagnosis to late survivorship are related to CVD onset after Anth-C. Such information will then provide the foundation for interventional strategies that can specifically target areas of dysfunction within the cardiovascular system to optimize cardiovascular outcomes following Anth-C exposure.
See http://www.TheOncologist.com for supplemental material available online.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Supplementary Material
Acknowledgments
We greatly acknowledge the work of the Kelowna General Hospital Echocardiography Laboratory, in particular Terri Coleman, Margaret Hill, Shelley Guenther, and Lila Mah. This work was supported by the Canadian Breast Cancer Foundation, British Columbia/Yukon Division. G.K. was supported by the Canadian Institute of Health Research Master's Award, the Frederick Banting and Charles Best Canada Graduate Scholarship, and the Bill Tymchuk Cancer Research Award Endowment Fund. N.E. is supported by a Clinical Scholar Career Award from the Michael Smith Foundation for Health Research.
Author Contributions
Conception/design: Graeme J. Koelwyn, Lee W. Jones, Neil D. Eves
Provision of study material or patients: Susan L. Ellard, Neil D. Eves
Collection and/or assembly of data: Graeme J. Koelwyn, Nia C. Lewis, Susan L. Ellard, Jinelle C. Gelinas, J. Douglass Rolf, Bernie Melzer, Samantha M. Thomas, Neil D. Eves
Data analysis and interpretation: Graeme J. Koelwyn, Nia C. Lewis, Lee W. Jones, Samantha M. Thomas, Pamela S. Douglas, Michel G. Khouri, Neil D. Eves
Manuscript writing: Graeme J. Koelwyn, Lee W. Jones, Neil D. Eves
Final approval of manuscript: Graeme J. Koelwyn, Nia C. Lewis, Susan L. Ellard, Lee W. Jones, Jinelle C. Gelinas, J. Douglass Rolf, Bernie Melzer, Samantha M. Thomas, Pamela S. Douglas, Michel G. Khouri, Neil D. Eves
Disclosures
The authors indicated no financial relationships.
References
- 1.Hooning MJ, Botma A, Aleman BM, et al. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J Natl Cancer Inst. 2007;99:365–375. doi: 10.1093/jnci/djk064. [DOI] [PubMed] [Google Scholar]
- 2.Koelwyn GJ, Khouri M, Mackey JR, et al. Running on empty: Cardiovascular reserve capacity and late effects of therapy in cancer survivorship. J Clin Oncol. 2012;30:4458–4461. doi: 10.1200/JCO.2012.44.0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaosuwannakit N, D’Agostino R, Jr, Hamilton CA, et al. Aortic stiffness increases upon receipt of anthracycline chemotherapy. J Clin Oncol. 2010;28:166–172. doi: 10.1200/JCO.2009.23.8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drafts BC, Twomley KM, D’Agostino R, Jr, et al. Low to moderate dose anthracycline-based chemotherapy is associated with early noninvasive imaging evidence of subclinical cardiovascular disease. JACC Cardiovasc Imaging. 2013;6:877–885. doi: 10.1016/j.jcmg.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duquaine D, Hirsch GA, Chakrabarti A, et al. Rapid-onset endothelial dysfunction with Adriamycin: Evidence for a dysfunctional nitric oxide synthase. Vasc Med. 2003;8:101–107. doi: 10.1191/1358863x03vm476oa. [DOI] [PubMed] [Google Scholar]
- 6.Kalábová H, Melichar B, Ungermann L, et al. Intima-media thickness, myocardial perfusion and laboratory risk factors of atherosclerosis in patients with breast cancer treated with anthracycline-based chemotherapy. Med Oncol. 2011;28:1281–1287. doi: 10.1007/s12032-010-9593-1. [DOI] [PubMed] [Google Scholar]
- 7.Kass DA. Ventricular arterial stiffening: Integrating the pathophysiology. Hypertension. 2005;46:185–193. doi: 10.1161/01.HYP.0000168053.34306.d4. [DOI] [PubMed] [Google Scholar]
- 8.Sagawa K. The ventricular pressure-volume diagram revisited. Circ Res. 1978;43:677–687. doi: 10.1161/01.res.43.5.677. [DOI] [PubMed] [Google Scholar]
- 9.Cohen-Solal A, Caviezel B, Laperche T, et al. Effects of aging on left ventricular-arterial coupling in man: Assessment by means of arterial effective and left ventricular elastances. J Hum Hypertens. 1996;10:111–116. [PubMed] [Google Scholar]
- 10.Chantler PD, Lakatta EG, Najjar SS. Arterial-ventricular coupling: Mechanistic insights into cardiovascular performance at rest and during exercise. J Appl Physiol (1985) 2008;105:1342–1351. doi: 10.1152/japplphysiol.90600.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sicari R, Nihoyannopoulos P, Evangelista A, et al. Stress echocardiography expert consensus statement—Executive summary: European association of echocardiography (EAE) (a registered branch of the ESC) Eur Heart J. 2009;30:278–289. doi: 10.1093/eurheartj/ehn492. [DOI] [PubMed] [Google Scholar]
- 12.Ky B, French B, May Khan A, et al. Ventricular-arterial coupling, remodeling, and prognosis in chronic heart failure. J Am Coll Cardiol. 2013;62:1165–1172. doi: 10.1016/j.jacc.2013.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones LW, Eves ND, Haykowsky M, et al. Cardiorespiratory exercise testing in clinical oncology research: Systematic review and practice recommendations. Lancet Oncol. 2008;9:757–765. doi: 10.1016/S1470-2045(08)70195-5. [DOI] [PubMed] [Google Scholar]
- 14.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: A report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr. 2009;10:165–193. doi: 10.1093/ejechocard/jep007. [DOI] [PubMed] [Google Scholar]
- 16.Sunagawa K, Maughan WL, Burkhoff D, et al. Left ventricular interaction with arterial load studied in isolated canine ventricle. Am J Physiol. 1983;245:H773–H780. doi: 10.1152/ajpheart.1983.245.5.H773. [DOI] [PubMed] [Google Scholar]
- 17.Kelly RP, Ting CT, Yang TM, et al. Effective arterial elastance as index of arterial vascular load in humans. Circulation. 1992;86:513–521. doi: 10.1161/01.cir.86.2.513. [DOI] [PubMed] [Google Scholar]
- 18.Chen CH, Fetics B, Nevo E, et al. Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. J Am Coll Cardiol. 2001;38:2028–2034. doi: 10.1016/s0735-1097(01)01651-5. [DOI] [PubMed] [Google Scholar]
- 19.Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 20.Koelwyn GJ, Currie KD, MacDonald MJ, et al. Ultrasonography and tonometry for the assessment of human arterial stiffness. In: Ainslie P, editor. Applied Aspects of Ultrasonography in Humans. Rijeka, Croatia: InTech Europe; 2012. [Google Scholar]
- 21.Thijssen DH, Black MA, Pyke KE, et al. Assessment of flow-mediated dilation in humans: A methodological and physiological guideline. Am J Physiol Heart Circ Physiol. 2011;300:H2–H12. doi: 10.1152/ajpheart.00471.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mackay B, Ewer MS, Carrasco CH, et al. Assessment of anthracycline cardiomyopathy by endomyocardial biopsy. Ultrastruct Pathol. 1994;18:203–211. doi: 10.3109/01913129409016291. [DOI] [PubMed] [Google Scholar]
- 23.Scott JM, Khakoo A, Mackey JR, et al. Modulation of anthracycline-induced cardiotoxicity by aerobic exercise in breast cancer: Current evidence and underlying mechanisms. Circulation. 2011;124:642–650. doi: 10.1161/CIRCULATIONAHA.111.021774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borlaug BA, Olson TP, Lam CS, et al. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2010;56:845–854. doi: 10.1016/j.jacc.2010.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fournier SB, Reger BL, Donley DA, et al. Exercise reveals impairments in left ventricular systolic function in patients with metabolic syndrome. Exp Physiol. 2014;99:149–163. doi: 10.1113/expphysiol.2013.075796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chantler PD, Melenovsky V, Schulman SP, et al. Use of the Frank-Starling mechanism during exercise is linked to exercise-induced changes in arterial load. Am J Physiol Heart Circ Physiol. 2012;302:H349–H358. doi: 10.1152/ajpheart.00147.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khouri MG, Hornsby WE, Risum N, et al. Utility of 3-dimensional echocardiography, global longitudinal strain, and exercise stress echocardiography to detect cardiac dysfunction in breast cancer patients treated with doxorubicin-containing adjuvant therapy. Breast Cancer Res Treat. 2014;143:531–539. doi: 10.1007/s10549-013-2818-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKillop JH, Bristow MR, Goris ML, et al. Sensitivity and specificity of radionuclide ejection fractions in doxorubicin cardiotoxicity. Am Heart J. 1983;106:1048–1056. doi: 10.1016/0002-8703(83)90651-8. [DOI] [PubMed] [Google Scholar]
- 29.Wang TJ, Evans JC, Benjamin EJ, et al. Natural history of asymptomatic left ventricular systolic dysfunction in the community. Circulation. 2003;108:977–982. doi: 10.1161/01.CIR.0000085166.44904.79. [DOI] [PubMed] [Google Scholar]
- 30.Lightfoot JC, D’Agostino RB, Jr, Hamilton CA, et al. Novel approach to early detection of doxorubicin cardiotoxicity by gadolinium-enhanced cardiovascular magnetic resonance imaging in an experimental model. Circ Cardiovasc Imaging. 2010;3:550–558. doi: 10.1161/CIRCIMAGING.109.918540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khouri MG, Douglas PS, Mackey JR, et al. Cancer therapy-induced cardiac toxicity in early breast cancer: Addressing the unresolved issues. Circulation. 2012;126:2749–2763. doi: 10.1161/CIRCULATIONAHA.112.100560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayward R, Hydock D, Gibson N, et al. Tissue retention of doxorubicin and its effects on cardiac, smooth, and skeletal muscle function. J Physiol Biochem. 2013;69:177–187. doi: 10.1007/s13105-012-0200-0. [DOI] [PubMed] [Google Scholar]
- 33.Dawson EA, Rathore S, Cable NT, et al. Impact of catheter insertion using the radial approach on vasodilatation in humans. Clin Sci (Lond) 2010;118:633–640. doi: 10.1042/CS20090548. [DOI] [PubMed] [Google Scholar]
- 34.Fitzgerald MD, Tanaka H, Tran ZV, et al. Age-related declines in maximal aerobic capacity in regularly exercising vs. sedentary women: A meta-analysis. J Appl Physiol (1985) 1997;83:160–165. doi: 10.1152/jappl.1997.83.1.160. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.