Abstract
HIV-1 envelope glycoprotein gp120 (gp120) is a major virulence protein implicated in the pathogenesis of HIV-associated neurocognitive disorders (HAND). Although gp120 has been suggested to cause synaptic and neuronal injuries by disrupting NMDA receptor (NMDAR) function, the underlying mechanism is unclear. Here, we show that gp120Bal down-regulates the phosphorylation of the NMDAR subunit 1 NR1 (at Ser896 and Ser897), which is essential for NMDAR function. This effect of gp120Bal is blocked by specific antagonists of both NMDA and AMPA receptors, indicating a critical role of synaptic activation. Furthermore, AMD3100 and maraviroc, antagonists of CCR5 and CXCR4 chemokine receptors, respectively, inhibit the effect of gp120Bal on NR1, suggesting that CXCR4 and CCR5 activation are involved. These findings may provide mechanistic insights into the synaptopathogenesis caused by HIV-1 infection.
Introduction
According to a recent estimate, there are over 35 million people worldwide living with human immunodeficiency virus (HIV) infection (UNAIDS/WHO) (https://www.aids.gov/hiv-aids-basics/hiv-aids-101/global-statistics/). While HIV-1 is best known for its devastating damage to the immune system that leads to acquired immunodeficiency syndrome (AIDS), the virus also infects the central nervous system (CNS) and cause neurological diseases, especially HIV-associated neurocognitive disorders (HAND). Patients with HAND have cognitive and/or sensorimotor deficits, including mental slowing, memory loss, difficulties in performing complex tasks that require executive functions, and motor disorders (McArthur et al, 2005). Although highly active antiretroviral therapy (HAART) can suppress viral burden to nearly undetectable levels in cerebrospinal fluid (CSF) and serum, HAND is still observed in as many as half of HIV-1-infected patients (Cysique et al, 2004; Heaton et al, 2010; Heaton et al, 2011). Thus, it is important to develop rationale-based adjunctive therapy to combat the disorders.
Previous studies showed that HIV-1 infection leads to encephalitis and neuronal apoptosis (Alirezaei et al, 2007; Bachis et al, 2009; Hayward, 2004; Mattson et al, 2005). However, these pathological manifestations and the number of HIV-infected cells do not seem to closely correlate to the HIV-associated cognitive impairments (Glass et al, 1995; Glass et al, 1993). In contrast, synaptodendritic damage seems to better correlate with the presence and severity of the cognitive deficits (Ellis et al, 2007; Everall et al, 1999; Masliah et al, 1997; Sa et al, 2004). Synaptodendritic injuries were observed in HIV patients (Sa et al, 2004), gp120 transgenic mice (Toggas et al, 1994), HIV-1 transgenic rats (Rao et al, 2011) and in neuron cultures exposed to exogenous gp120 (Iskander et al, 2004; Kim et al, 2011). HIV-1 gp120, which can modulate various cellular process by activating its chemokine co-receptors CXCR4 or CCR5, was shown to cause synaptodendritic degeneration (Bachis et al, 2009; Kaul et al, 2007). In addition, axonal damage may also be associated with HAND, as indicated by the elevated neurofilament protein (NFL) in CSF of patients with HIV dementia (Ellis et al, 2007).
The synapses may degenerate in HIV patients, as indicated by the decrease in the number of dendritic spines (Iskander et al, 2004; Masliah et al, 1997; Sa et al, 2004). Synapse loss also seems to occur in the gp120 transgenic mice (Toggas et al, 1994). In addition to synaptic degeneration, HIV-1 proteins may also interfere with synaptic activity (Haughey and Mattson, 2002; Kasyanov et al, 2006; Krogh et al, 2014; Nath et al, 1996; Tovar et al, 2013; Xu et al, 2011). For instance, gp120 was shown to activate N-methyl D-aspartate receptor (NMDAR), and this could induce synaptic damage (Gemignani et al, 2000; Kaul and Lipton, 1999; Lipton, 1992; Pattarini et al, 1998; Toggas et al, 1996; Viviani et al, 2006; Yang et al, 2013). Interestingly, T-cell-tropic (T-tropic) gp120 IIIB promoted trafficking and surface clustering of NMDA receptors in membrane microdomains in cultured hippocampal neurons. The gp120 IIIB-regulated NMDAR trafficking was associated with the phosphorylation of the NMDAR subunit NR1 (also known as the GluN1 subunit) at its C-terminal serine 896 (Ser896) and serine 897 (Ser897) (Xu et al, 2011). It has been shown that phosphorylation at these residues are important posttranslational modifications for NMDAR to be transported from the Golgi complex and thus is a critical step in the process of NMDAR surface expression, which is required for it to become functional at synapses (Scott et al, 2003; Scott et al, 2001).
HIV-1 viruses isolated from the central nervous system (CNS) are mainly macrophage (M)-tropic (Gonzalez-Perez et al, 2012; Gorry et al, 2001; Peters et al, 2004). The M-tropic gp120Bal uses CCR5 (rather than CXCR4 for T-tropic gp120) as a co-receptor. Previous studies indicated that gp120Bal mediates HIV neurotoxicity by a process different from gp120 IIIB (Bachis et al, 2010). We were interested in the effects of HIV-1 gp120Bal on NMDA receptors. Intriguingly, we found that, when applied to primary cortical cultures, gp120Bal transiently decreased the level of pSer896 NR1 and pSer897 NR1. These findings indicate that gp120Bal may impair synaptic NMDAR activity by interfering with NR1 trafficking. In addition, our studies show that the gp120Bal-induced down-regulation of pSer896 and pSer897 NR1 is diminished by NMDAR and AMPAR antagonists, as well as the antagonists of CCR5 and CXCR4. Thus, the results suggest that gp120Bal down-regulates the phosphorylated NR1 by a process that depends on synaptic activity and HIV-1 co-receptor activation.
Materials and Methods
Animals
All experiments were performed following the protocols that were approved by the Institutional Animal Care and Use Committee at the University of Texas Medical Branch and were in accord with National Institutes of Health guidelines. Young adult C57BL/6 mice (2–3 months old; 20–25 g body weight) were used. Mice were housed in a constant temperature environment (23 ± 3°C) with standard bedding, free access to food and water, and under a 12/12 h light/dark cycle.
Primary cortical cultures
Primary cortical cultures were prepared from mouse embryos as described previously (Ru et al, 2012). Briefly, cortices were dissected from C57BL/6J mouse embryos (E18), and cells were dissociated with trypsin in Hank’s balanced salt solution (Invitrogen) with gentle trituration. Cells were plated on poly-D-lysine (30,000–70,000; Sigma) coated dishes at a density of 1.5 × 105 cells/cm2 in DMEM medium (Invitrogen) containing 10% FBS (Gibco). Three hours after plating, the medium were replaced with Neurobasal Medium (Invitrogen) supplemented with 2% B27 (Invitrogen) and 0.5mM L-glutamine (Invitrogen). One-third of the medium was replenished every 3 days until 12–14 days in vitro (DIV). Only morphologically healthy cultures were used for drug treatments.
Materials
HIV-1 coat protein gp120Bal (Cat # 4961) was obtained from the NIH AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, NIH. The protein in PBS was aliquoted and stored at −80°C until use. Gp120 protein with only a single freeze-thaw cycle was used. Maraviroc (Cat# 11580, NIH AIDS Research and Reference Reagent Program) and D-APV (cat# 165304; Calbiochem) were dissolved in ultrapure water. Bicyclam JM-2987 (AMD3100) (Cat#8128; NIH AIDS Research and Reference Reagent Program) and CNQX (Cat#C239; Sigma) were prepared in DMSO. Antibodies for immunoblotting were: anti-NR1 (1:2,000; Cat # 06-311; Millipore), anti-phospho-NR1 (Ser896) (1:1000; Cat#06-640; Millipore), anti-phospho-NR1 (Ser897) (1:1000; Cat#ABN99; Millipore), caspase-3 (1:1000; Cat#9662; Cell Signaling Technology) and anti-β-actin (1:1,000; Cat # sc-1616-R; Santa Cruz Biotechnology) antibodies. Antibodies for immunofluorescence staining are: microtubule-associated protein-2 (MAP-2) (1:500; Cat#ab5392, Abcam), Cleaved Caspase-3 (Asp175) (5A1E) (1:200, Cat# 9661, Cell Signaling Technology).
Direct transcutaneous intrathecal injection (i.t.)
For direct transcutaneous intrathecal injection of gp120Bal, a modified version (Li et al, 2013) of the original method (Hylden and Wilcox, 1980) was used. Mice were initially anaesthetized by inhaling 2.5% isoflurane, a 30.5-gauge stainless steel needle attached to a 10μl Hamilton syringe (Hamilton, Reno, NV) was used for i.t. injections. To locate the intervertebral space between L5 and L6, the experimenter used the left index finger to palpate the tip of the sixth lumbar vertebra (L6) and used the right hand to insert the needle into the intervertebral space at a 45° angle. A correct intrathecal placement of the needle tip was judged by a tail twitch. Then, 100ng (in 5μl) gp120 protein was injected. Mice injected with vehicle (PBS) were used as controls.
Immunofluorescent staining
Cultured primary cortical cells were fixed with 4% PFA for 20 min, and blocked in 5% BSA in 0.1M PBS with 0.3% Triton X-100. Fixed cells were incubated overnight (4 °C) with chicken anti-MAP2 and rabbit anti-cleaved caspase-3, followed by fluorescently conjugated secondary antibodies (FITC-conjugated goat anti-chicken lgY and cys3-conjungated goat anti-rabbit IgG) (1:200, Jackson ImmunoResearch Laboratories) at room temperature for 1 h. DAPI was added to visualize cell nuclei when mounting. Images were captured using a confocal microscope (Nikon). Quantitative analysis of captured images was performed using ImageJ. Three to five independent experiments were performed for each treatment.
Western blotting
Cortical cultures in 12-well plates (12–14 DIV) were rinsed with PBS and lysed immediately with 100 μl of 2× SDS-PAGE sample buffer (1×: 62.5 mM Tris, pH 6.8, 2% SDS, 5% 2-mercaptoethanol, 10% glycerol, and 0.0025% bromophenol blue). For in vivo analysis, mice were anesthetized and sacrificed, followed by collecting the L4–L6 lumbar spinal cord segments. The spinal cord tissues from individual animals were homogenized in 500 μl of RIPA lysis buffer (50mM Tris-HCl, pH 7.2, 150mM NaCl, 0.1% SDS, 1% Triton X-100, 0.5% sodium deoxycholate, 1mM EDTA, 1% protease inhibitor mixture (cat# P8340; Sigma-Aldrich) and 1% phosphatase inhibitor 2/3 (cat # P5726/P0044; Sigma-Aldrich). Protein lysates were prepared and the concentrations were determined using BCA protein assay kit (product 23227; Pierce) as previously described (Li et al, 2013). Equal amounts of protein (30μg/lane) were separated on 10% Tris-glycine polyacrylamide gels and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore). Immunoblots were blocked for 2 h in Tris-buffered saline Tween-20 (TBST; 20 mM Tris–HCl, 150 mM NaCl, pH 7.5, 0.05% Tween-20) containing 5% non-fat milk. Then, blocked blots were sequentially incubated with primary and secondary antibody. Protein bands were visualized using the Enhanced Chemiluminescence kit (Pierce), and the intensities of non-saturated bands on Western blots were quantified by densitometry analysis with NIH ImageJ. β-actin was included as a loading control.
Statistical analysis
Quantitative summary data were expressed as means ± SEM. Statistical analysis was performed with Prism 5 software. We used the one-way analysis of variance (ANOVA) with a Tukey post-hoc test for comparisons among multiple groups, one-way repeated measure ANOVA to analyze in vivo data, and two-way ANOVA with a Bonferroni post-hoc test to analyze two categorical explanatory variables.
Results
gp120Bal induces transient decrease of phosphorylated NR1 in vitro and in vivo
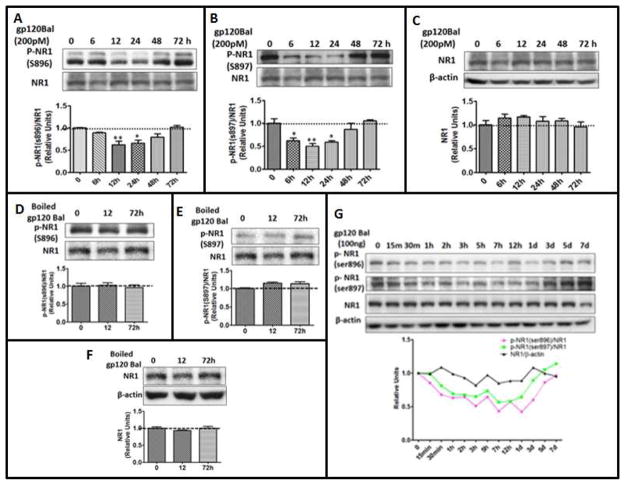
The NR1 subunit of NMDAR is an essential subunit that is ubiquitously expressed throughout the brain. Phosphorylation is a key mechanism that regulates the localization and channel function of NMDARs (Scott et al, 2003). Previous studies indicated that phosphorylation of NR1 at Ser897 and Ser896 contributes to HIV gp120 IIIB-induced NMDAR trafficking and surface expression in cultured hippocampal neurons (Xu et al, 2011). We wanted to determine in this study if gp120Bal acts similarly in the regulation of NR1 phosphorylation. Interestingly, instead of stimulating NR1 phosphorylation, we observed that exposure of primary cortical culture to 200pM gp120Bal transiently decreases pNR1 (Ser896, Ser897). A significant decrease of pSer896-NR1 was detected between 12 h and 24 h post gp120Bal application (Fig. 1A), while a decrease in pSer897-NR1 occurred between 6 h and 24 h (Fig. 1B). The levels of both pSer896-NR1 and pSer897-NR1 returned to baseline after 72 h of gp120Bal treatment (Fig. 1A and 1B). On the other hand, we did not observe significant changes of total NR1 during this period (Fig. 1C). In addition, when treated with heat-denatured gp120Bal, we did not detect decreases of the phosphorylated NR1 at Ser896, Ser897, or total NR1 (Fig. 1D–F), indicating that the ability of gp120Bal to down-regulate phosphorylated NR1 requires its bioactivity. To determine the effects of gp120Bal on pNR1 levels in vivo, we injected gp120Bal intrathecally into adult mice and measured the pSer896 NR1 and pSer897 NR1 levels in the L4–L6 spinal segments at various time points following gp120Bal administration. Our results showed that compared to total NR1, which was unchanged, both pSer896-NR1 and pSer897-NR1 gradually decreased after gp120 injection (Fig. 1G). The levels of both forms of pNR1 returned to baseline by day 5 (Fig. 1G). These results indicate that HIV-1 gp120Bal down-regulates phosphorylated NR1 (Ser896 and S897) both in vitro and in vivo.
Fig. 1. gp120Bal treatment caused time-dependent decrease of phosphorylated NR1 both in vitro and in vivo.
A–C, time course of gp120Bal-induced down-regulation of phosphorylated NR1. Cortical cultures (14 DIV) were incubated with 200pM gp120Bal for the indicated times. Cell lysates were analyzed by immunoblotting using antibodies against pNR1 (at Ser896) (A), pNR1 (Ser897) (B) and total NR1 (C). D–E, heat-inactivated gp120Bal did not decrease the level of pNR1. Protein levels of pSer896 NR1 (D), pSer897 NR1 (E) and total NR1 (F) were detected by immunoblotting in cortical cultures (14DIV) after treated with heat-inactivated gp120Bal. (G) Gp120Bal decreased phosphorylated NR1 in the spinal cord. Mice were i.t. injected with 100ng gp120Bal for the indicated times and followed by immunoblotting of the total NR1 and phosphorylated NR1 in the L4–L6 spinal cord, p-NR1(ser896)/NR1 vs. NR1/β-actin, p<0.001, p-NR1(ser897)/NR1 vs NR1/β-actin, p<0.01. Quantitative graphs are shown as mean ± SEM (*P<0.05; **P<0.01; ***P<0.001 vs. time 0, n≥ 3).
gp120Bal treatment does not cause significant neuron loss and dendrites degeneration
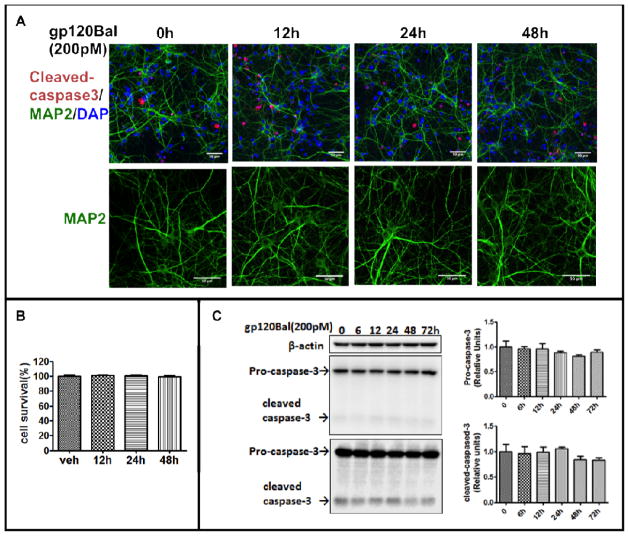
Previous studies show that gp120-induced CXCR4 activation cause neurotoxicity and lead to neuron death (Hesselgesser et al, 1998; Kaul et al, 2001; Meucci et al, 1998). gp120-induced CCR5 activation also causes neuronal apoptosis, but its natural ligands “regulated-and-normal-T-cell-expressed-and-secreted” (RANTES, CCL5) and MIP-1β prevent the gp120 neurotoxicity (Bachis and Mocchetti, 2005; Kaul and Lipton, 1999; Kaul et al, 2007; Meucci et al, 1998). To test whether gp120Bal (200pM) can cause the neural cell apoptosis or dendritic degeneration under our experimental conditions, we stained cleaved-caspase-3 to identify apoptotic cells. We found that gp120 treatment did not significantly increase cleaved caspase-3-positive cells (Fig. 2A and 2B). This finding was confirmed by Western blot analysis (Fig. 2C). MAP2 staining, showed that gp120Bal treatment did not alter dendritic morphology (lower panel in Fig. 2A). Thus, gp120Bal did not cause apparent neuron apoptosis and dendritic degeneration under our experimental conditions.
Fig. 2. gp120Bal (200pM) did not affect cell survival.
Cultured primary cortical neurons that had been exposed to recombinant gp120Bal (200pM) were stained for MAP-2 (green) and cleaved-caspase-3 (red) proteins (A). Apoptotic cells with cleaved-caspase-3 staining were counted (B). The protein levels of caspase-3 and cleaved-caspase-3 after gp120Bal treatment were also determined by Western blot analysis (C). Scale bar, 50 μm.
gp120Bal-induced decreases of pSer896-NR1 and pSer897-NR1 require the activation of NMDA and AMPA receptors
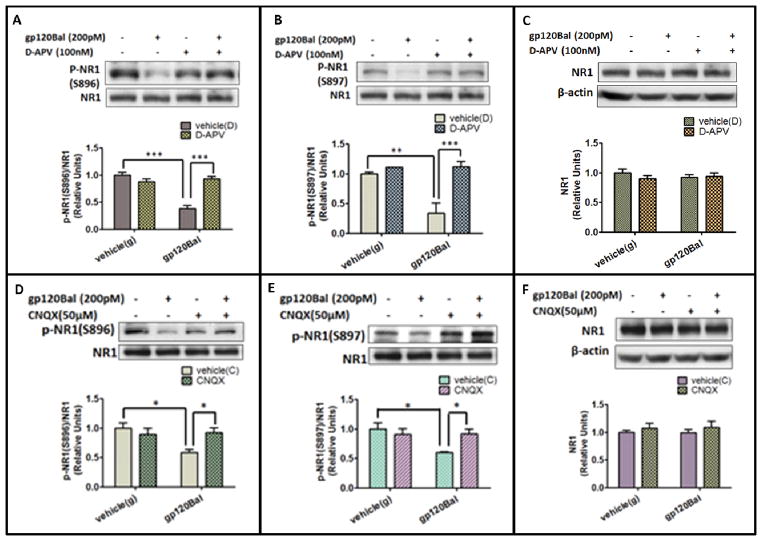
Next, we wanted to characterize the mechanism by which gp120Bal induces the decrease of phosphorylated NR1. Because previous studies show that gp120 leads to neuronal degeneration by its excitotoxicity (Haughey and Mattson, 2002; Lannuzel et al, 1995; Lipton, 1992; Potter et al, 2013; Toggas et al, 1996), we tested the role of NMDAR and AMPAR activation. We used D-APV (100 μM) to block NMDARs (Morris, 1989), and CNQX (50 μM) to block AMPARs (Honore et al, 1988). We found that although gp120 (200 pM, 12 h) significantly down-regulated the phosphorylation of NR1 at Ser896 by 41% (p<0.001) (Fig. 3A), and Ser897 by 40% (p<0.01) (Fig. 3B), pre-treating the cultures with D-APV completely blocked the gp120Bal-induced decrease of pSer896-NR1 (p<0.05 compared to only gp120Bal treatment; Fig. 3A) and pSer897-NR1 (p<0.05 compared to only gp120Bal treatment; Fig. 3B). Similarly, the AMPAR antagonist CNQX also blocked the effect of gp120Bal on phosphorylated NR1 at Ser896 (Fig. 3D) and Ser897 (Fig. 3E). However, D-APV and CNQX treatment have no effects on the total amount of NR1 (Fig. 3C and 3F). These data indicated that the activation of both NMDARs and AMPARs are critical for gp120Bal to down-regulate phosphorylated NR1.
Fig. 3. gp120Bal decreased NR1 phosphorylation via NMDARs and AMPARs.
A–C, effects of D-APV on gp120Bal-induced decrease of p-Ser896 NR1 (A), p-Ser897 NR1 (B) and total NR1 (C). Primary cortical cultures (14 DIV) were pretreated with vehicle or 100 μM D-APV for 30 min and then incubated with 200 pM gp120Bal for 12 h, followed by western blot analysis. D–F, effects of CNQX on gp120Bal-induced decrease of p-Ser896 NR1 (D), p-Ser897 NR1 (E) and total NR1 (F). Primary cortical cultures (14 DIV) were pretreated with vehicle or 50 μM CNQX for 30 min and then co-cultured with 200 pM gp120 Bal for 12 h, followed by western blot. Quantitative results in graphs are shown as mean ± SEM (*P<0.05; **P<0.01, n≥ 3, vehicle (g) is vehicle of gp120Bal; vehicle (D) is vehicle of D-APV; vehicle (C) is vehicle of CNQX).
Chemokine receptors CCR5 and CXCR4 contribute to gp120Bal-induced decrease of phosphorylated NR1
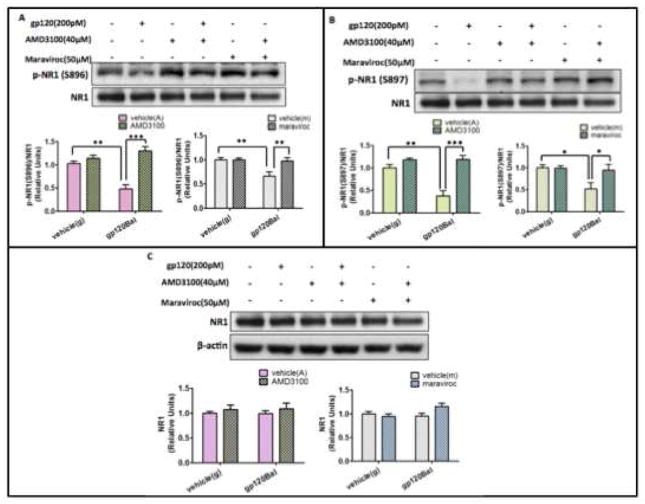
HIV productively infects macrophages and microglia but not neurons, which do not express CD4. Nonetheless, chemokine receptors CXCR4 and CCR5 serve as co-receptors for gp120 and mediate neuronal damage (Bachis et al, 2009; Catani et al, 2000; Kaul et al, 2007). While M-tropic HIV-1 strains (e.g. Bal) preferably uses CCR5 as a co-receptor (Alkhatib et al, 1996; Choe et al, 1996; Dragic et al, 1996), T-tropic strain (e.g. IIIB) use CXCR4 (Feng et al, 1996). Dual-tropic strains (e.g. SF2) can use both CCR5 and CXCR4 as co-receptors (Collman et al, 1992; Yi et al, 1999). Thus, we performed studies to determine the potential roles of CCR5 and CXCR4 in the gp120Bal-induced decreases of pSer896-NR1 and pSer897-NR1. We employed AMD3100 and maraviroc to inhibit CCR5 and CXCR4, respectively. As we had seen before, gp120Bal treatment caused significant decreases in the levels of p-Ser896-NR1 and p-Ser897-NR1 after 12 h of treatment. However, pre-treating the cells with either AMD3100 or maraviroc for 30 min blocked the gp120Bal-induced decreases of pSer896-NR1 (Fig. 4A; p<0.05, compared to only gp120Bal treatment) and pSer897-NR1 (Fig. 4B; p<0.05, compared to only gp120Bal treatment)). Neither AMD3100 nor maraviroc had any effect on total NR1 (Fig. 4C). These results suggest that gp120Bal induces the down-regulation of phosphorylated NR1 via CXCR4 and CCR5.
Fig. 4. Chemokine receptors CCR5 and CXCR4 are critical for gp120Bal-induced decrease of pSer986-NR1 and pSer897 NR1.
A–B, AMD3100 and maraviroc abolished gp120Bal-induced decrease of pSer986-NR1 (A) and pSer986-NR1 (B). C, AMD3100 and maraviroc did not change the amount of total NR1. The primary cortical cultures were pre-treated with the AMD3100 (μM) or maraviroc (50 μM) for 30 min, followed by gp120Bal (200 pM) administration for another 12 hours. Cell lysates were analyzed by immunoblotting. Data are expressed as mean ± SEM from at least three independent experiments (*P<0.05; **P<0.01, n≥ 3, vehicle (g) is vehicle of gp120Bal; vehicle (A) is vehicle of AMD3100; vehicle (M) is vehicle of maraviroc).
Discussion
NMDARs play important roles in synaptic transmission and plasticity, as well as memory formation (Bliss and Collingridge, 1993; Morris, 1989). Prior studies suggest that NMDARs are activated by gp120, which leads to neuronal damage or death (Dreyer et al, 1990; Geeraerts et al, 2006; Kaul and Lipton, 1999; Lipton et al, 1991; Medina et al, 1999). The gp120-induced and NMDAR-mediated neuronal injuries likely contribute to HAND such as HIV-1-associated dementia. Despite these significant advancements, we still know little about how gp120 may regulate NMDARs to activate them. Our data described in this paper reveal for the first time a transient effect of gp120Bal on the down-regulation of phosphorylated NR1 (Ser896 and Ser897), both in cortical primary cultures and in the mouse spinal cord. The gp120-induced decrease of NR1 phosphorylation is apparently not a consequence of neurotoxicity (Fig. 2). Since phosphorylation is a key regulatory mechanism for NMDAR trafficking, synaptic targeting, and surface expression (Chen and Roche, 2007; Scott et al, 2003), our findings suggest that gp120Bal may disturb NMDAR function, especially at synapses. The reduced phosphorylation of NR1 at Ser896 and Ser897 may affect the receptor’s clustering and decrease its sensitivity to glutamate. However, whether the decreased phosphorylation plays a role in the pathogenesis of HAND or itself is a compensatory response to the HAND is unknown. In this context, it is interesting to note that gp120 IIIB enhances the phosphorylation of the NR1 at both Ser896 and Ser897. It also promotes NMDAR trafficking and surface expression (Xu et al, 2011). We currently do not know how the different types of gp120 cause apparently opposite effects on NR1 phosphorylation.
Our data indicate that activation of NMDARs and AMPARs is essential for gp120Bal to down-regulate pSer896-NR1 and pSer897-NR1. NR1 is an essential subunit of functional NMDARs that can be phosphorylated by a variety of protein kinases. For example, NR1 is phosphorylated by protein kinase C (PKC) on two residues (Ser890 and Ser896) (Tingley et al, 1997). PKCα phosphorylates Ser896 and PKCγ phosphorylates Ser890 (Sanchez-Perez and Felipo, 2005). cAMP-dependent protein kinase A (PKA) phosphorylates NR1 on Ser897 (Tingley et al, 1997). Ser896 and Ser897 are highly phosphorylated in the endoplasmic reticulum and Golgi. The phosphorylation of Ser896 and Ser897 increases the surface expression of NMDARs, suggesting that the phosphorylation of these sites is an important regulatory mechanism for NMDAR intracellular trafficking (Scott et al, 2003; Scott et al, 2001) It is well established that Ca2+ influx following NMDA receptor activation leads to the activation of many kinases, including PKC and PKA. Normally, NMDA receptor activation is primed by AMPA receptor activation, which causes membrane depolarization and relieves of Mg2+ blockage of the NMDA receptors. Thus, the simplest interpretation of the observed CNQX effect is that it impairs NMDA receptor activation induced by gp120, and consequently the phosphorylation of NR1. Indeed, inhibition of NMDA receptors by APV also blocked the NR1 phosphorylation. Since serine/threonine protein phosphatases such as protein phosphatases 1 (PP1), 2A (PP2A), and 2B (PP2B or calcineurin) regulate the activities of NMDARs (Tong et al, 1995; Wang et al, 1994; Westphal et al, 1999), the level of the phosphorylated NR1 is presumably determined by the competing action of the kinases and phosphatases. We observed that the CCR5 antagonist maraviroc blocked the effect of gp120Bal in down-regulating NR1 phosphorylation. This finding indicates that gp120Bal binds to its co-receptor CCR5 to elicit the change. To our surprise, the CXCR4 antagonist AMD3100 was also able to block the gp120Bal-induced decrease of phosphorylated NR1 (Fig. 4A and 4B). Although CXCR4 is the preferable co-receptor for T-tropic gp120, some M-tropic HIV-1 strains can use CXCR4 for entry into monocyte-derived macrophages (Gorry et al, 2001; Gray et al, 2009). Thus, it is possible that some gp120Bal may bind to CXCR4 to induce the effect (Igarashi et al, 2003). Another possibility is that CXCR4 activation by its natural ligand such as CXCL12 induced by gp120Bal contributes to the down-regulation of NR1 phosphorylation.. CXCL12 has been shown to promote neuronal apoptosis (Bezzi et al, 2001; Hesselgesser et al, 1998; Kaul and Lipton, 1999; Zheng et al, 1999). Because blocking either CCR5 or CXCR4 was able to diminish the effect of gp120Bal, the down-regulation of phosphorylated NR1 is likely a consequence of a combined effect of both CCR5 and CXCR4 signaling. For example, following interaction with their specific ligands, activated chemokine receptors could induce higher concentrations of intracellular free calcium (Kaul et al, 2001; Lannuzel et al, 1995; Meucci et al, 1998), which potentially facilitates action potential firing and neuronal excitation. Chemokine binding can also activate the tyrosine kinase Pyk2 (Davis et al, 1997), the p38 mitogen-activated protein kinase pathway (Kaul and Lipton, 1999), or calcineurin (Shepherd et al, 2012; Shepherd et al, 2013). Therefore, CCR5- and CXCR4-mediated dysregulation of the kinases and/or phosphatases may contribute to the downregulation of phosphorylated NR1.
Acknowledgments
HIV-1 gp120, Maraviroc and AMD3100 were obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH. This work was supported by NIH grants NS079166 and DA036165 to SJT.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Alirezaei M, Watry DD, Flynn CF, Kiosses WB, Masliah E, Williams BR, Kaul M, Lipton SA, Fox HS. Human immunodeficiency virus-1/surface glycoprotein 120 induces apoptosis through RNA-activated protein kinase signaling in neurons. J Neurosci. 2007;27:11047–55. doi: 10.1523/JNEUROSCI.2733-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkhatib G, Combadiere C, Broder CC, Feng Y, Kennedy PE, Murphy PM, Berger EA. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–8. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- Bachis A, Biggio F, Major EO, Mocchetti I. M- and T-tropic HIVs promote apoptosis in rat neurons. J Neuroimmune Pharmacol. 2009;4:150–60. doi: 10.1007/s11481-008-9141-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachis A, Cruz MI, Mocchetti I. M-tropic HIV envelope protein gp120 exhibits a different neuropathological profile than T-tropic gp120 in rat striatum. Eur J Neurosci. 2010;32:570–8. doi: 10.1111/j.1460-9568.2010.07325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachis A, Mocchetti I. Brain-derived neurotrophic factor is neuroprotective against human immunodeficiency virus-1 envelope proteins. Neuroprotective Agents. 2005;1053:247–257. doi: 10.1196/annals.1344.022. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Domercq M, Brambilla L, Galli R, Schols D, De Clercq E, Vescovi A, Bagetta G, Kollias G, Meldolesi J, Volterra A. CXCR4-activated astrocyte glutamate release via TNFalpha: amplification by microglia triggers neurotoxicity. Nat Neurosci. 2001;4:702–10. doi: 10.1038/89490. [DOI] [PubMed] [Google Scholar]
- Bliss TVP, Collingridge GL. A Synaptic Model of Memory - Long-Term Potentiation in the Hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Catani MV, Corasaniti MT, Navarra M, Nistico G, Finazzi-Agro A, Melino G. gp120 induces cell death in human neuroblastoma cells through the CXCR4 and CCR5 chemokine receptors. J Neurochem. 2000;74:2373–9. doi: 10.1046/j.1471-4159.2000.0742373.x. [DOI] [PubMed] [Google Scholar]
- Chen BS, Roche KW. Regulation of NMDA receptors by phosphorylation. Neuropharmacology. 2007;53:362–368. doi: 10.1016/j.neuropharm.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath PD, Wu L, Mackay CR, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–48. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- Collman R, Balliet JW, Gregory SA, Friedman H, Kolson DL, Nathanson N, Srinivasan A. An infectious molecular clone of an unusual macrophage-tropic and highly cytopathic strain of human immunodeficiency virus type 1. J Virol. 1992;66:7517–21. doi: 10.1128/jvi.66.12.7517-7521.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cysique LA, Maruff P, Brew BJ. Prevalence and pattern of neuropsychological impairment in human immunodeficiency virus-infected/acquired immunodeficiency syndrome (HIV/AIDS) patients across pre- and post-highly active antiretroviral therapy eras: a combined study of two cohorts. Journal of Neurovirology. 2004;10:350–7. doi: 10.1080/13550280490521078. [DOI] [PubMed] [Google Scholar]
- Davis CB, Dikic I, Unutmaz D, Hill CM, Arthos J, Siani MA, Thompson DA, Schlessinger J, Littman DR. Signal transduction due to HIV-1 envelope interactions with chemokine receptors CXCR4 or CCR5. Journal of Experimental Medicine. 1997;186:1793–1798. doi: 10.1084/jem.186.10.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragic T, Litwin V, Allaway GP, Martin SR, Huang Y, Nagashima KA, Cayanan C, Maddon PJ, Koup RA, Moore JP, Paxton WA. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–73. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- Dreyer EB, Kaiser PK, Offermann JT, Lipton SA. HIV-1 coat protein neurotoxicity prevented by calcium channel antagonists. Science. 1990;248:364–7. doi: 10.1126/science.2326646. [DOI] [PubMed] [Google Scholar]
- Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci. 2007;8:33–44. doi: 10.1038/nrn2040. [DOI] [PubMed] [Google Scholar]
- Everall IP, Heaton RK, Marcotte TD, Ellis RJ, McCutchan JA, Atkinson JH, Grant I, Mallory M, Masliah E. Cortical synaptic density is reduced in mild to moderate human immunodeficiency virus neurocognitive disorder. HNRC Group. HIV Neurobehavioral Research Center. Brain Pathol. 1999;9:209–17. doi: 10.1111/j.1750-3639.1999.tb00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–7. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- Geeraerts T, Deiva K, M’Sika I, Salim H, Hery C, Tardieu M. Effects of SDF-1alpha and gp120IIIB on apoptotic pathways in SK-N-SH neuroblastoma cells. Neurosci Lett. 2006;399:115–20. doi: 10.1016/j.neulet.2006.01.033. [DOI] [PubMed] [Google Scholar]
- Gemignani A, Paudice P, Pittaluga A, Raiteri M. The HIV-1 coat protein gp120 and some of its fragments potently activate native cerebral NMDA receptors mediating neuropeptide release. Eur J Neurosci. 2000;12:2839–46. doi: 10.1046/j.1460-9568.2000.00172.x. [DOI] [PubMed] [Google Scholar]
- Glass JD, Fedor H, Wesselingh SL, McArthur JC. Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Annals of Neurology. 1995;38:755–62. doi: 10.1002/ana.410380510. [DOI] [PubMed] [Google Scholar]
- Glass JD, Wesselingh SL, Selnes OA, Mcarthur JC. Clinical-Neuropathologic Correlation in Hiv-Associated Dementia. Neurology. 1993;43:2230–2237. doi: 10.1212/wnl.43.11.2230. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Perez MP, O’Connell O, Lin R, Sullivan WM, Bell J, Simmonds P, Clapham PR. Independent evolution of macrophage-tropism and increased charge between HIV-1 R5 envelopes present in brain and immune tissue. Retrovirology. 2012;9:20. doi: 10.1186/1742-4690-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorry PR, Bristol G, Zack JA, Ritola K, Swanstrom R, Birch CJ, Bell JE, Bannert N, Crawford K, Wang H, Schols D, De Clercq E, Kunstman K, Wolinsky SM, Gabuzda D. Macrophage tropism of human immunodeficiency virus type 1 isolates from brain and lymphoid tissues predicts neurotropism independent of coreceptor specificity. J Virol. 2001;75:10073–89. doi: 10.1128/JVI.75.21.10073-10089.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray L, Roche M, Churchill MJ, Sterjovski J, Ellett A, Poumbourios P, Sherieff S, Wang B, Saksena N, Purcell DF, Wesselingh S, Cunningham AL, Brew BJ, Gabuzda D, Gorry PR. Tissue-specific sequence alterations in the human immunodeficiency virus type 1 envelope favoring CCR5 usage contribute to persistence of dual-tropic virus in the brain. J Virol. 2009;83:5430–41. doi: 10.1128/JVI.02648-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughey NJ, Mattson MP. Calcium dysregulation and neuronal apoptosis by the HIV-1 proteins Tat and gp120. J Acquir Immune Defic Syndr. 2002;31(Suppl 2):S55–61. doi: 10.1097/00126334-200210012-00005. [DOI] [PubMed] [Google Scholar]
- Hayward P. Viral proteins cause cell death in HIV-associated dementia. Lancet Neurol. 2004;3:325. doi: 10.1016/s1474-4422(04)00780-x. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I, Grp C. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy CHARTER Study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, LeBlanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I, Grp CH. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. Journal of Neurovirology. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselgesser J, Taub D, Baskar P, Greenberg M, Hoxie J, Kolson DL, Horuk R. Neuronal apoptosis induced by HIV-1 gp120 and the chemokine SDF-1 alpha is mediated by the chemokine receptor CXCR4. Curr Biol. 1998;8:595–8. doi: 10.1016/s0960-9822(98)70230-1. [DOI] [PubMed] [Google Scholar]
- Honore T, Davies SN, Drejer J, Fletcher EJ, Jacobsen P, Lodge D, Nielsen FE. Quinoxalinediones: potent competitive non-NMDA glutamate receptor antagonists. Science. 1988;241:701–3. doi: 10.1126/science.2899909. [DOI] [PubMed] [Google Scholar]
- Hylden JL, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol. 1980;67:313–6. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- Igarashi T, Donau OK, Imamichi H, Dumaurier MJ, Sadjadpour R, Plishka RJ, Buckler-White A, Buckler C, Suffredini AF, Lane HC, Moore JP, Martin MA. Macrophage-tropic simian/human immunodeficiency virus chimeras use CXCR4, not CCR5, for infections of rhesus macaque peripheral blood mononuclear cells and alveolar macrophages. J Virol. 2003;77:13042–52. doi: 10.1128/JVI.77.24.13042-13052.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iskander S, Walsh KA, Hammond RR. Human CNS cultures exposed to HIV-1 gp120 reproduce dendritic injuries of HIV-1-associated dementia. J Neuroinflammation. 2004;1:7. doi: 10.1186/1742-2094-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasyanov A, Tamamura H, Fujii N, Xiong H. HIV-1 gp120 enhances giant depolarizing potentials via chemokine receptor CXCR4 in neonatal rat hippocampus. Eur J Neurosci. 2006;23:1120–8. doi: 10.1111/j.1460-9568.2006.04646.x. [DOI] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–94. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Kaul M, Lipton SA. Chemokines and activated macrophages in HIV gp120-induced neuronal apoptosis. Proc Natl Acad Sci U S A. 1999;96:8212–6. doi: 10.1073/pnas.96.14.8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M, Ma Q, Medders KE, Desai MK, Lipton SA. HIV-1 coreceptors CCR5 and CXCR4 both mediate neuronal cell death but CCR5 paradoxically can also contribute to protection. Cell Death Differ. 2007;14:296–305. doi: 10.1038/sj.cdd.4402006. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Shin AH, Thayer SA. Activation of cannabinoid type 2 receptors inhibits HIV-1 envelope glycoprotein gp120-induced synapse loss. Mol Pharmacol. 2011;80:357–66. doi: 10.1124/mol.111.071647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogh KA, Wydeven N, Wickman K, Thayer SA. HIV-1 protein Tat produces biphasic changes in NMDA-evoked increases in intracellular Ca2+ concentration via activation of Src kinase and nitric oxide signaling pathways. J Neurochem. 2014;130:642–56. doi: 10.1111/jnc.12724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lannuzel A, Lledo PM, Lamghitnia HO, Vincent JD, Tardieu M. HIV-1 envelope proteins gp120 and gp160 potentiate NMDA-induced [Ca2+]i increase, alter [Ca2+]i homeostasis and induce neurotoxicity in human embryonic neurons. Eur J Neurosci. 1995;7:2285–93. doi: 10.1111/j.1460-9568.1995.tb00649.x. [DOI] [PubMed] [Google Scholar]
- Li B, Shi Y, Shu J, Gao J, Wu P, Tang SJ. Wingless-type mammary tumor virus integration site family, member 5A (Wnt5a) regulates human immunodeficiency virus type 1 (HIV-1) envelope glycoprotein 120 (gp120)-induced expression of pro-inflammatory cytokines via the Ca2+/calmodulin-dependent protein kinase II (CaMKII) and c-Jun N-terminal kinase (JNK) signaling pathways. J Biol Chem. 2013;288:13610–9. doi: 10.1074/jbc.M112.381046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton SA. Memantine prevents HIV coat protein-induced neuronal injury in vitro. Neurology. 1992;42:1403–5. doi: 10.1212/wnl.42.7.1403. [DOI] [PubMed] [Google Scholar]
- Lipton SA, Sucher NJ, Kaiser PK, Dreyer EB. Synergistic effects of HIV coat protein and NMDA receptor-mediated neurotoxicity. Neuron. 1991;7:111–8. doi: 10.1016/0896-6273(91)90079-f. [DOI] [PubMed] [Google Scholar]
- Masliah E, Heaton RK, Marcotte TD, Ellis RJ, Wiley CA, Mallory M, Achim CL, McCutchan JA, Nelson JA, Atkinson JH, Grant I. Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. HNRC Group. The HIV Neurobehavioral Research Center. Annals of Neurology. 1997;42:963–72. doi: 10.1002/ana.410420618. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Haughey NJ, Nath A. Cell death in HIV dementia. Cell Death Differ. 2005;12(Suppl 1):893–904. doi: 10.1038/sj.cdd.4401577. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Brew BJ, Nath A. Neurological complications of HIV infection. Lancet Neurol. 2005;4:543–55. doi: 10.1016/S1474-4422(05)70165-4. [DOI] [PubMed] [Google Scholar]
- Medina I, Ghose S, Ben-Ari Y. Mobilization of intracellular calcium stores participates in the rise of [Ca2+]i and the toxic actions of the HIV coat protein GP120. Eur J Neurosci. 1999;11:1167–78. doi: 10.1046/j.1460-9568.1999.00550.x. [DOI] [PubMed] [Google Scholar]
- Meucci O, Fatatis A, Simen AA, Bushell TJ, Gray PW, Miller RJ. Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc Natl Acad Sci U S A. 1998;95:14500–5. doi: 10.1073/pnas.95.24.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG. Synaptic plasticity and learning: selective impairment of learning rats and blockade of long-term potentiation in vivo by the N-methyl-D-aspartate receptor antagonist AP5. J Neurosci. 1989;9:3040–57. doi: 10.1523/JNEUROSCI.09-09-03040.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A, Psooy K, Martin C, Knudsen B, Magnuson DS, Haughey N, Geiger JD. Identification of a human immunodeficiency virus type 1 Tat epitope that is neuroexcitatory and neurotoxic. J Virol. 1996;70:1475–80. doi: 10.1128/jvi.70.3.1475-1480.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattarini R, Pittaluga A, Raiteri M. The human immunodeficiency virus-1 envelope protein gp120 binds through its V3 sequence to the glycine site of N-methyl-D-aspartate receptors mediating noradrenaline release in the hippocampus. Neuroscience. 1998;87:147–57. doi: 10.1016/s0306-4522(98)00125-0. [DOI] [PubMed] [Google Scholar]
- Peters PJ, Bhattacharya J, Hibbitts S, Dittmar MT, Simmons G, Bell J, Simmonds P, Clapham PR. Biological analysis of human immunodeficiency virus type 1 R5 envelopes amplified from brain and lymph node tissues of AIDS patients with neuropathology reveals two distinct tropism phenotypes and identifies envelopes in the brain that confer an enhanced tropism and fusigenicity for macrophages. J Virol. 2004;78:6915–26. doi: 10.1128/JVI.78.13.6915-6926.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter MC, Figuera-Losada M, Rojas C, Slusher BS. Targeting the glutamatergic system for the treatment of HIV-associated neurocognitive disorders. J Neuroimmune Pharmacol. 2013;8:594–607. doi: 10.1007/s11481-013-9442-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao JS, Kim HW, Kellom M, Greenstein D, Chen M, Kraft AD, Harry GJ, Rapoport SI, Basselin M. Increased neuroinflammatory and arachidonic acid cascade markers, and reduced synaptic proteins, in brain of HIV-1 transgenic rats. J Neuroinflammation. 2011;8:101. doi: 10.1186/1742-2094-8-101. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ru W, Peng Y, Zhong L, Tang SJ. A role of the mammalian target of rapamycin (mTOR) in glutamate-induced down-regulation of tuberous sclerosis complex proteins 2 (TSC2) J Mol Neurosci. 2012;47:340–5. doi: 10.1007/s12031-012-9753-1. [DOI] [PubMed] [Google Scholar]
- Sa MJ, Madeira MD, Ruela C, Volk B, Mota-Miranda A, Paula-Barbosa MM. Dendritic changes in the hippocampal formation of AIDS patients: a quantitative Golgi study. Acta Neuropathol. 2004;107:97–110. doi: 10.1007/s00401-003-0781-3. [DOI] [PubMed] [Google Scholar]
- Sanchez-Perez AM, Felipo V. Serines 890 and 896 of the NMDA receptor subunit NR1 are differentially phosphorylated by protein kinase C isoforms. Neurochemistry International. 2005;47:84–91. doi: 10.1016/j.neuint.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Scott DB, Blanpied TA, Ehlers MD. Coordinated PKA and PKC phosphorylation suppresses RXR-mediated ER retention and regulates the surface delivery of NMDA receptors. Neuropharmacology. 2003;45:755–67. doi: 10.1016/s0028-3908(03)00250-8. [DOI] [PubMed] [Google Scholar]
- Scott DB, Blanpied TA, Swanson GT, Zhang C, Ehlers MD. An NMDA receptor ER retention signal regulated by phosphorylation and alternative splicing. J Neurosci. 2001;21:3063–72. doi: 10.1523/JNEUROSCI.21-09-03063.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd AJ, Loo L, Gupte RP, Mickle AD, Mohapatra DP. Distinct modifications in Kv2.1 channel via chemokine receptor CXCR4 regulate neuronal survival-death dynamics. J Neurosci. 2012;32:17725–39. doi: 10.1523/JNEUROSCI.3029-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd AJ, Loo L, Mohapatra DP. Chemokine co-receptor CCR5/CXCR4-dependent modulation of Kv2.1 channel confers acute neuroprotection to HIV-1 glycoprotein gp120 exposure. PLoS One. 2013;8:e76698. doi: 10.1371/journal.pone.0076698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingley WG, Ehlers MD, Kameyama K, Doherty C, Ptak JB, Riley CT, Huganir RL. Characterization of protein kinase A and protein kinase C phosphorylation of the N-methyl-D-aspartate receptor NR1 subunit using phosphorylation site-specific antibodies. Journal of Biological Chemistry. 1997;272:5157–5166. doi: 10.1074/jbc.272.8.5157. [DOI] [PubMed] [Google Scholar]
- Toggas SM, Masliah E, Mucke L. Prevention of HIV-1 gp120-induced neuronal damage in the central nervous system of transgenic mice by the NMDA receptor antagonist memantine. Brain Res. 1996;706:303–7. doi: 10.1016/0006-8993(95)01197-8. [DOI] [PubMed] [Google Scholar]
- Toggas SM, Masliah E, Rockenstein EM, Rall GF, Abraham CR, Mucke L. Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature. 1994;367:188–93. doi: 10.1038/367188a0. [DOI] [PubMed] [Google Scholar]
- Tong G, Shepherd D, Jahr CE. Synaptic desensitization of NMDA receptors by calcineurin. Science. 1995;267:1510–2. doi: 10.1126/science.7878472. [DOI] [PubMed] [Google Scholar]
- Tovar YRLB, Kolson DL, Bandaru VV, Drewes JL, Graham DR, Haughey NJ. Adenosine triphosphate released from HIV-infected macrophages regulates glutamatergic tone and dendritic spine density on neurons. J Neuroimmune Pharmacol. 2013;8:998–1009. doi: 10.1007/s11481-013-9471-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viviani B, Gardoni F, Bartesaghi S, Corsini E, Facchi A, Galli CL, Di Luca M, Marinovich M. Interleukin-1 beta released by gp120 drives neural death through tyrosine phosphorylation and trafficking of NMDA receptors. J Biol Chem. 2006;281:30212–22. doi: 10.1074/jbc.M602156200. [DOI] [PubMed] [Google Scholar]
- Wang LY, Orser BA, Brautigan DL, MacDonald JF. Regulation of NMDA receptors in cultured hippocampal neurons by protein phosphatases 1 and 2A. Nature. 1994;369:230–2. doi: 10.1038/369230a0. [DOI] [PubMed] [Google Scholar]
- Westphal RS, Tavalin SJ, Lin JW, Alto NM, Fraser ID, Langeberg LK, Sheng M, Scott JD. Regulation of NMDA receptors by an associated phosphatase-kinase signaling complex. Science. 1999;285:93–6. doi: 10.1126/science.285.5424.93. [DOI] [PubMed] [Google Scholar]
- Xu H, Bae M, Tovar-y-Romo LB, Patel N, Bandaru VV, Pomerantz D, Steiner JP, Haughey NJ. The human immunodeficiency virus coat protein gp120 promotes forward trafficking and surface clustering of NMDA receptors in membrane microdomains. J Neurosci. 2011;31:17074–90. doi: 10.1523/JNEUROSCI.4072-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Hu D, Xia J, Liu J, Zhang G, Gendelman HE, Boukli NM, Xiong H. Enhancement of NMDA receptor-mediated excitatory postsynaptic currents by gp120-treated macrophages: implications for HIV-1-associated neuropathology. J Neuroimmune Pharmacol. 2013;8:921–33. doi: 10.1007/s11481-013-9468-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi Y, Isaacs SN, Williams DA, Frank I, Schols D, De Clercq E, Kolson DL, Collman RG. Role of CXCR4 in cell-cell fusion and infection of monocyte-derived macrophages by primary human immunodeficiency virus type 1 (HIV-1) strains: two distinct mechanisms of HIV-1 dual tropism. J Virol. 1999;73:7117–25. doi: 10.1128/jvi.73.9.7117-7125.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Thylin MR, Ghorpade A, Xiong H, Persidsky Y, Cotter R, Niemann D, Che M, Zeng YC, Gelbard HA, Shepard RB, Swartz JM, Gendelman HE. Intracellular CXCR4 signaling, neuronal apoptosis and neuropathogenic mechanisms of HIV-1-associated dementia. J Neuroimmunol. 1999;98:185–200. doi: 10.1016/s0165-5728(99)00049-1. [DOI] [PubMed] [Google Scholar]