Abstract
Immunotherapy directed against tau is a promising treatment strategy for Alzheimer’s Disease (AD) and tauopathies. We review initial studies on tau-directed immunotherapy, and present data from our laboratory testing antibodies using the rTg4510 mouse model, which deposits tau in forebrain neurons. Numerous antibodies have been tested for their efficacy in treating both pathology and cognitive function, in different mouse models, by different routes of administration, and at different ages or durations. We report, here, that the conformation-specific antibody MC-1 produces some degree of improvement to both cognition and pathology in rTg4510. Pathological improvements as measured by Gallyas staining for fully formed tangles and phosphorylated tau appeared four days after intracranial injection into the hippocampus. We also examined markers for microglial activation, which did not appear impacted from treatment. Behavioral effects were noted after continuous infusion of antibodies into the lateral ventricle for approximately 2 weeks. We examined basic motor skills, which were not impacted by treatment, but did note cognitive improvements with both novel object and radial arm water maze testing. Our results support earlier reports in the initial review presented here, and collectively show promise for this strategy of treatment. The general absence of extracellular tau deposits may avoid the opsonization and phagocytosis mechanisms activated by antibodies against amyloid, and make anti tau approaches a safer method of immunotherapy for Alzheimer’s disease.
Keywords: Vaccination, active immunization, passive immunization, dementia, rTg4510, Gallyas, tau, transgenic mice
Introduction
Dementias are a growing burdensome health condition, affecting an increasingly greater percentage of the world’s population. People are living longer, and are surviving conditions such as heart disease and cancer, which are becoming relatively treatable and are declining as ‘cause(s) of death’. Alzheimer’s Disease (AD) is the most common and most studied dementia associated with tau accumulation, however there are several other neurodegenerative disorders which are also classified as tauopathies. The term tauopathy suggests that there is some deposition of the protein tau metabolism and pathology occurs in association with this deposition.
AD is the leading cause of dementia, accounting for 50 to 80 percent of dementia cases, and the prevalence of the disease is projected to increase significantly as the baby-boom generation retires and longevity continues to increase. AD is characterized by severe cognitive decline with age, ultimately requiring continuous caregiving and eventually death. The pathology of AD is characterized by the presence of extracellular amyloid plaques, intracellular neurofibrillary tangles (NFT) composed of hyperphosphorylated tau protein, neuron loss, and evidence of inflammation indicated by the presence of reactive microglia and astrocytes, as previously reviewed (Lee et al., 2001, Medeiros et al., 2010). Frontotemporal Lobe Dementia (FTLD) is a rare form of dementia that is somewhat related to AD, most notably in the pathology of hyperphosphorylated tau and macroscopic brain shrinkage. It has a more rapid onset than AD, with symptoms that reflect personality changes more than memory loss (Lashley et al., 2015). Like AD, there are no known treatments or cures for FTLD. Other tauopathies, where tau becomes pathologic, include frontotemporal dementia with associated Parkinsonism linked to chromosome 17 (FTDP-17), Pick’s Disease, corticobasal degeneration, and argyrophilic grain disease. These diseases have different origins and symptoms, but all share pathologic forms of tau as a major correlative factor underlying the disease (Braak et al., 1993, Utton et al., 2005).
Tau exists as a normal protein within cells to assist in stability of the cytoarchitecture, especially in neurons. It binds to microtubules to provide structural support for axons, and it also facilitates trafficking of important intracellular compounds and organelles, as reviewed by (Morris et al., 2011) and others. It is considered to be a key protein for normal neural functioning; however there are numerous paths by which it can be rendered unstable, or pathological. Post-translational modification is one way that tau can change from beneficial to detrimental; hyperphosphorylation, nitration, acetylation and truncation are examples of post-translational modifications that can significantly alter tau function (Wang et al., 2014). In addition, while considered a natively unfolded protein, tau takes on multiple tertiary conformations, which hinder its ability to perform the intended function and ultimately render it as a toxic entity that leads to neurodegenerative disease (Yu et al., 2012). Tau can become misfolded, leading to aggregation, which can lead to ubiquitination and breakdown by the ubiquitin-proteasomal-system; larger aggregates require the autophagy system for breakdown and removal (Wang and Mandelkow, 2012, Castrillo and Oliver, 2016).
Initial efforts for treating tauopathies have focused on blocking hyperphosphorylation by using kinase inhibitors, which has been thought to be a primary initiating factor for aggregation (Sui et al., 2015). Additional treatment approaches have included: aggregation inhibition using various types of small molecules (O’Leary et al., 2010); degradation of aggregates and clearance by enhancing metabolic processes such as those initiated by heat shock proteins (HSP), the UPS and/or autophagy (Fontaine et al., 2015); direct stabilization of microtubules using paclitaxel, EpoD, or other known microtubule stabilizing agents (Brunden et al., 2010); proteolysis or use of proteases and other methods of aggregate degradation (Guerrero-Munoz et al., 2014) (Utton et al., 2005, Oddo et al., 2009, Gotz et al., 2012, Himmelstein et al., 2012, Mandelkow and Mandelkow, 2012, Wolfe, 2012, Guerrero-Munoz et al., 2014).
While these potential treatment strategies continue to be explored, an emerging approach that has shown promise in mouse models of tau deposition is immunotherapy. Vaccination is the most common form of immunotherapy, which typically involves administration of antigen, often with adjuvant, to actively increase the body’s production of antibodies. Additionally, immunotherapy can be accomplished by injecting antibodies or antisera against the unwanted substance, thereby eliminating the need for the recipient’s body to produce its own antibodies (passive immunization) (Lee et al., 2001, Wisniewski and Boutajangout, 2010).
An adjuvant is frequently given with active vaccines to boost the immune system’s response to the foreign substance (the immunogen). Adjuvants help improve immunogenicity of the administered compound by activating macrophages and other innate immune system components, such as microglia in the central nervous system (CNS). They increase the antigen-presentation of the immunogen to lymphocytes, thereby increasing endogenous production of antibodies by stimulating the immune system to produce more of a response than the antigen alone would (Wilcock and Colton, 2008, Agadjanyan et al., 2015, Halle et al., 2015). Most well-tolerated adjuvants used in humans consist of aluminum-based compounds such as alum, which stimulates a Th2 (anti-inflammatory) cytokine response (Ghochikyan et al., 2006). “Freund’s Adjuvant” (either Complete or Incomplete, CFA or IFA) is also commonly used experimentally, and stimulates a Th1 cytokine response (pro-inflammatory) (Billiau and Matthys, 2001). Another common adjuvant, Quil A, is often used in veterinary practices, and the equivalent for human clinical studies is called QS-1 (discussed below) (Ghochikyan et al., 2006, Ragupathi et al., 2010).
Tau has become a focus for immunotherapy studies, in part because of some of the issues and limitations of Aβ immunotherapy in clinical research trials, as previously reviewed (Lemere, 2013), and in part because tau pathology is more directly linked to the symptoms, progression, and severity of AD (Braak et al., 1993, Oddo et al., 2009). There is a much stronger correlation between cognitive decline and the development of tau aggregates than with Aβ deposition (Sigurdsson, 2008). The best strategy for treating AD might prove to be one that targets both Aβ and tau in concert.
ACTIVE IMMUNIZATION
Aβ immunotherapy began with investigations of active immunization using various forms of Aβ formulated as a vaccine, first in mice and then in human clinical trials. Passive immunization, which consists of injecting purified antibodies, followed preclinical research on active immunotherapy, as previously reviewed (Morgan, 2011). A similar course has evolved in tau immunotherapy. Active immunization was examined first (Table 1), using various mouse models and immunogens for tau, in addition to multiple adjuvants. The next step was to consider passive immunization with various antibodies and multiple mouse models (see following section). Early studies showed that both active and passive forms of tau-directed immunization clear some of the pathological forms of tau. Even though tau has traditionally been considered an intracellular protein, recent studies have shown it to be released extracellularly and transmit pathology between cells (Lee et al., 2001, Kfoury et al., 2012).
TABLE 1.
Active Immunization using tau antigens
| Authors | Antigen, Dose & Adjuvant | Mouse Model, incl. mutation, if present | Age at first inj | Duration | Techniques and markers examined | Details/Results |
|---|---|---|---|---|---|---|
| Asuni et al, J Neurosci 2007 | 100 μl Tau379–408 @ 1 mg/ml with Adju-Phos adjuvant, sc bi-weekly for first month, then monthly | JNPL3 (P301L) | 2 mo | 3 – 5 mo | IHC and Western Blot for MC-1 & PHF1, Rotarod, NOR | ↓ MC-1 and PHF 1 by IHC; ↑ PHF 1 in soluble fraction by Western, ↑ time on rotarod; No cognitive change in NOR; noted antibody uptake into neurons |
| Boutajangout, et al, J Neurosci 2010 | 100 μg Tau379–408 + 100 μl Alum, i.p.; 3 injections bi-weekly, then monthly | hTau/PS1 | 3–4 mo | 5 – 6 mo | IHC for PHF1 & AT8, PHF1 by Western, Rotarod, Traverse Beam RAM, NOR, CFSM | ↓ PHF1 and AT8, ↓ soluble PHF1, no change in rotarod performance, improved performance in RAM and NOR |
| Boimel et al, Exp Neurol 2010 | sc injection of 100 μg each of 3 KLH-linked phos-tau-peptides (aa’s ~ 195 – 238) with i.p. CFA + PT. Adjuvant booster @ 48 hrs, then sc antigen booster 1 wk later | E257T/P301S | 4 to 12 mo | 2 wks | Clinical evaluation of paralysis; Gallyas staining (NFT); IHC for AT8, AT180, and glial markers GFAP, MAC3, CD45R and CD4; Bielschowsky-Hematoxylin and lectin staining; double immunofluorescence for CD11b, F4/80 and MAC3; & lysosomal staining for Cathepsin D | No ↑ in paralysis; no difference in monocyte infiltration (Bielschowsky Hemotoxylin staining), CD45R & CD4 staining showed limited B or T cell infiltration, no macrophages detected by MAC3 & no axonal damage noted by these stains, so encephalogic risk is minimal; injected tau present in sera & blood, not parenchyma; ↓ NFTs, ↓ AT8, ↓ AT180, ↑ microglia by lectin staining, ↓ Cathepsin D |
| Bi et al, PLOS1 2011 | 100 μg KLH-linked peptide (aa’s 395–406) with CFA (first) then with IFA 2 & 4 wks after | pR5 (P301L) | 4, 8, or 18 mo | 1 mo | Gallyas staining for tangles, IHC for PHF1 & pSer422, GFAP staining | ↓ NFTs, PHF1 and pSer422; ↑ GFAP in old mice, trend ↑ in mid-aged mice, no difference in GFAP in young after vaccine |
| Troquier et al, Curr Alz Res 2012 | 100 μg Y14T or Y10A (pSer422 epitope) i.p. + CFA; 2nd injection 2 wks later with IFA, then monthly | THY-Tau22 (4R @ g272v & p301S with Thy 1.2 promoter) | 2 or 3.5 mos | 14 wks or 18 wks | IHC, Modified Y-maze, ELISA, Western | No changes in AT8, ↓ in AT100 & pSer422, vaccines ↑ performance to wt levels, ↑ tau in sera in correlation with duration of treatment & memory performance, ↓ AT100 & pSer422 in SDS-insoluble fraction |
| Rozenstein-Tsalkovich et al, Exp Neurol 2013 | Sc injection of 3 phos-tau-peptides @ 100 μg, in IFA and PT, given twice bi-weekly, then 50 μl peptide monthly | E257T/P301S | 6 or 12 mos | 8 – 14 wks | Experimental autoimmune encephalomyelitis test, IHC, behavioral observation | ↑ monocyte infiltration with memory improvement, no microglial change, treated mice developed paralysis |
| Selenica et al, J Neuroinflam 2014 | Sc administration of 100 μg tau (human wt or P301L tau) with 20 μg Quil-A adjuvant. 3 bi-weekly injections followed with a 10 -week resting period then boosters every 3 weeks for 3 additional times | rTg4510 | 5 mos | 19 wks, including a 10-day resting period | [3-thymidine] assay to identify T-cell proliferation; epitope mapping via microarray; IHC for tau species, and glial markers CD45, CD11b and GFAP | ↑ antibody production, ↑ cellular response: ↓ CD45, CD11b and GFAP; ↓ H150 & AT8; 5 new immunogenic epitopes identified by microarray: 2 N-terminal (aa’s 9–15 and 21–27), two in the proline-rich domain (aa’s 168–174 and 220–228) and 1 in the C-terminal (aa’s 427–438); 5 new immunogenic epitopes were identified: 2 N-terminal (aa’s9–15 and 21–27), two in the proline-rich domain (aa’s 168–174 and 220–228) and 1 in the C-terminal (aa’s 427–438) |
Notes:
sc = subcutaneous
i.p. = intraperitoneal
IHC = immunohistochemistry
NOR = Novel Object Recognition
RAM = Radial Arm Maze
CFSM = Closed Field Symmetrical Maze
KLH = keyhole limpet hemocyanin
aa’s = amino acids
CFT = complete Freund’s adjuvant
PT = pertussis toxin
IFA = incomplete Freund’s adjuvant
NFT = neurofibrillary tangles
GFAP = glial fibrillary acidic protein
wt = wild-type
First attempts at tau immunotherapy were performed by a group in Israel (Rosenmann, 2006) who injected full-length recombinant human tau protein with CFA and pertussis toxin (PT) into wild type C57BL/6 mice to see if this might cause an autoimmune response such as encephalomyelitis, which was observed in clinical trials with Aβ vaccination (Orgogozo et al., 2003). The CFA+PT adjuvant was chosen for its proinflammatory effect to identify immunotherapy safety issues. Using this approach, they detected encephalomyelitis along with NFT formation 1.5 to 5 months post-injection. This study demonstrated that by giving tau to non-transgenic animals in the context of severe innate immune activation, tau pathology could be initiated. In an attempt to target toxic forms of tau and avoid deleterious side effects, a follow-up study involved injecting a combination of 3 phospho-tau peptides, along with CFA and PT, peripherally, into two NFT mouse models (E257T; P301S), hypothesizing a reduction of pathology (Boimel et al., 2010). This peptide combination covered 5 pathogenic phospho- tau residues corresponding to the epitopes for antibodies AT 8, AT 100 and AT 180. Encephalopathic symptoms were not seen up to 8 months post-injection, however significant reductions were seen in NFTs and phospho-tau by staining for Gallyas, AT 8 and AT 180. Microglial population increased in response to the phospho-tau peptide plus adjuvant injections. These effects were still seen 8 months after a single injection followed by one boost.
This group further examined the exaggerated effects of proinflammatory adjuvants (Rozenstein-Tsalkovich et al., 2013) by administering a 3 phospho-tau peptide antigens combined with CFA and PT. This combination produces a pro-inflammatory (Th1) response, thereby creating an inflammation-based mechanism of treatment. However, given the initial results from Aβ-targeted immunotherapy in human trials, this type of Th1 biased immunization increased the risk of (apparently) autoimmune encephalitis-like responses. The CFA adjuvant, with added PT, increases blood-brain barrier permeability of antigen, but consistent with the results from anti-Aβ vaccine clinical trials this pro-inflammatory condition can lead to encephalopathic conditions. They noted monocyte infiltration, however no added microglial activation, in response to the adjuvants alone. Antibody production was observed resulting from the p-tau immunizations. Nontransgenic (NTg) mice and double mutant E57T/P301S tau mice revealed significantly more inflammation in the NTg mice compared to the tau mice.
Ittner’s lab reported, in 2011, a study using different adjuvants over the course of administration (Bi et al., 2011). This study examined active immunization using an antigen covering amino acids 395 – 406 (the PHF1 epitope) in pR5 mice overexpressing P301L mutation under the Thy1.2 promoter. Mice, aged 4, 8, and 18 mos, were given three injections of the 12-amino acid antigen conjugated to keyhole limpet hemocyanin (KLH) with CFA adjuvant, followed by injection of antigen with IFA. Four months after they received the initial immunization, the mice showed notable antibody titers. Six to nine months following the initial administration, they collected tissue for anatomical localization and quantification of tau pathology. They identified reductions in Gallyas staining, immuno-staining for PHF1, and pSer422. In addition the oldest group of mice showed a marked significant increase in astrocytosis and there was a trend for increasing levels of GFAP in the middle-aged group of mice.
Following this initial focus on pSer396, Troquier et al (Troquier et al., 2012) studied the pSer422 epitope as a potential therapeutic target. They used THY-1 promoter Tau22 mice, which demonstrate conformationally-based tau pathology at the pSer422 epitope, which is more easily accessible to immunotherapy than other phosphorylated tau epitopes, to investigate the effects of providing active immunotherapy that is specific to pSer422. These mice display pathology starting at 3 months of age. They first tested two immunogens that specifically targeted the pSer422 epitope (one included 7 amino acids, the other 11 amino acids) combined with CFA adjuvant. The mice received immunization for 14 weeks. They then chose the most immunogenic epitope to treat 3.5 mo old mice (15 weeks of age) for 18 weeks followed by analysis for performance in a 2-trial Y-maze test. Pathology was also assessed using immunohistochemistry analysis (IHC) for AT100 and pSer422, along with Western Blotting for various phospho-tau markers. The behavioral testing showed that vaccinated mice performed similar to NTg animals, based on time spent in the novel arm compared to unvaccinated transgenic controls. The Sarkosyl insoluble tau fraction demonstrated significantly reduced pathology for AT100 and pSer422 by Western analysis. IHC revealed a trend toward reduction of these pathological markers in CA1. They also noticed an apparent efflux of tau from brain parenchyma to the blood sera, suggesting an increase of clearance from brain to systemic mechanisms of clearance, referred to as a peripheral sink (Citron, 2010, Morgan, 2011).
A very nicely designed series of studies on tau immunotherapy was conducted in the laboratory of Einar Sigurdsson at NYU. Initial work by Sigurdsson’s group (Asuni et al., 2007) examined the effects of active immunization with 4E6G7, a computer-designed immunogen peptide of tau covering amino acids 379–408, which includes the PHF-1 phosphorylation sites of Ser396 and Ser404 (a late-stage phosphoepitope in NFTs). This immunogen, or the Alum adjuvant as a control, was injected subcutaneously between 2 and 5 months into JNPL3 P301L mice (Lewis et al, 2000), which express 4R/0N tau including a FTDP-17-associated tau mutation P301L (Denk and Wade-Martins, 2009) using the mouse prion promoter. These mice develop pathology primarily in the motor cortex, brainstem, and spinal cord, typically resulting in hind limb paralysis after 9 months of age. Alum was chosen as an adjuvant because it is well tolerated and leads to a Th2-type immune response. The vaccinated mice were found to have reductions in insoluble tau and increases in soluble tau, and they exhibited improved performance on rotarod and balance beam tests. Additionally, a gender difference was noted, with females having more tau pathology before treatment, followed by similar levels to males post-treatment, suggesting that more pathological tau was prevented in the females (Bayer et al., 2005, Sigurdsson, 2008). To address the question of the antibody distribution within the brain and whether or not they could enter neurons, Sigurdsson’s group collected and purified antibodies generated against the tau immunogen, then FITC-labeled them, and injected the antibodies into the carotid arteries of the JNPL3 P301L mice. Later, histochemistry identified labeled antibodies in the brain, within neurons, and co-localized with markers for pathologic tau. Interestingly, the same labeled antibodies also were injected into NTg animals and no uptake into the brain was observed, suggesting that the blood-brain barrier is likely compromised in the transgenic animals, and probably also in humans with tauopathies, which would facilitate antibody passage into the brain (Sigurdsson, 2009).The mouse model used by Sigurdsson et al in their initial studies does not necessarily represent AD pathology; it is primarily a model of motor pathology that progresses so quickly that cognitive maze testing is not practical. However, this was a pioneering study that indicated immunization against tau could be a successful therapeutic approach in tauopathies and perhaps AD.
They followed this with work using a different mouse model that mimics AD more closely, exhibiting development of forebrain tangles and cognitive deficits. This model was created by crossing htau mice with M146L-mutation PS1 mice maintained on a mouse-tau knock-out background. These mice displayed early (before 2 months of age), rapidly progressing tau pathology in cortical and hippocampal areas. The same immunogen 4E6G7 was administered intraperitoneally (i.p.) with alum adjuvant, with controls receiving adjuvant alone. Antibody titers were measured by ELISA, and were notable, throughout the experiment. Behavioral testing included the same motor tests as described above, and also the radial arm maze, the closed field symmetrical maze, and novel object recognition testing. Immunization resulted in significant performance improvement in all three cognitive tests compared to controls. IHC for PHF1 revealed significant decreases in the immunized mice vs. controls. Soluble PHF1 tau was also reduced as measured by Western blotting. Most notable is that the behavioral improvements correlated well with the levels of PHF1 pathology. (Boutajangout et al., 2010).
Most recently reported work by our research group examined active immunization using different agents, to map relevant epitopes to target with vaccination (Selenica et al., 2014). Specifically, human full-length wild-type tau or P301L tau was administered subcutaneously with Quil A adjuvant into rTg4510 mice for 6 weeks (three biweekly injections), rested for 10 weeks then administered 3 additional injections. High titer antibody production in sera was verified and the best antigenic sites on tau were estimated by epitope mapping. The immune response resulted in greatest anti-tau antibody production of isotype IgG1, followed by IgG2b, IgG2a, then IgM. Analysis of IFN-γ demonstrated a strong T-cell response in splenocytes from vaccinated mice. Two N-terminal epitopes, two in the proline-rich domain, and one C-terminal epitope were identified. A 7-amino acid epitope at amino acids 21 – 27 proved to have the most robust binding to antisera, and this epitope corresponds to a caspase-cleavage site. The two epitopes in the proline-rich domain contain phosphorylation sites, perhaps leading to tau dissociation from microtubules and tangle initiation. Microglial activation, as assessed by CD45 and CD11b IHC revealed decreased inflammation in brains of the vaccinated mice. H150 total-tau staining was reduced in response to the wild-type tau administration, GFAP was reduced in the P301L administration, and AT8 and CD45 staining were reduced in response to both vaccines.
A summary of these active immunization studies against tau pathology is reported in Table 1.
PASSIVE IMMUNIZATION
Sigurdsson et al (2011) also pioneered passive tau-directed immunotherapy efforts (Table 2), by injecting the monoclonal antibody, PHF1, i.p., into JNPL3 mice. Two to 3-month old mice were given PHF1 weekly, or pooled mouse IgG as a control. At 5 to 6 months of age, behavioral testing (traverse or balance beam, rotarod, and open field) was conducted. The dentate gyrus, motor cortex, and brainstem were evaluated for pathology using IHC for PHF1 to compare pathological forms of tau to total tau levels. They found a significant reduction of PHF1 staining in the dentate gyrus along with a strong trend for reduction in the motor cortex, but no difference in the brain stem. Western blotting for PHF1 and CP13/B19 revealed significant decreases of insoluble pathological tau, but no differences in soluble or Sarkosyl soluble tau. Behavioral analysis resulted in fewer footslips on the traverse beam in the treated group, while the other motor testing did not appear to be affected. Because this mouse model primarily exhibits motor deficits, cognitive performance was not evaluated. The results do, however, show great promise for passive immunotherapy directed against tau, although the authors noted that this passive-immunotherapy approach seemed to be less efficacious than the prior active-immunization approach directed at essentially the same region of phosphorylated tau. This conclusion was based on the absence of an effect in the brainstem, despite effectiveness in the dentate gyrus. They also noted, however, that passive immunization is likely to be safer, by avoiding a potentially adverse T cell response, such as that found in the AN1792 vaccine study against the amyloid peptide (Boutajangout et al., 2011).
TABLE 2.
Passive Immunization using tau antibodies
| Authors | Antibody, Dose & Administration | Mouse Models, incl. mutation if present | Age at first injection | Duration | Details/Results |
|---|---|---|---|---|---|
| Chai, et al, JBC 2011 | PHF1 or MC-1, 15 mg/kg, peripheral injection (not specified) | JNPL3 (P301L) and/or P301S | 2 mos | 2 – 3 mos | ↓ insol AT8, no change HT 7 by Western, ↑ Time on rotarod, ↓ AT8 & PG5 by IHC; Correlation of AT8 signals in P1 fraction with Sarkosyl-insoluble fractions to show that the preparations were valid |
| Boutajangout, et al, J. Neurochem 2011 | i.p. injection of PHF1 | Female JNPL3 |
9 – 12 wks | 2 – 3 mos | ↑ Time on balance beam at 5 – 6 mos, but no difference on rotarod performance; ↓ PHF1/B19 ratio, ↓ PHF1 (alone), ↓ CP13/B19 ratio, ↓ CP13 (alone) in Sarkosyl fraction for Western; ↓ PHF1 in right Dentate Gyrus by IHC |
| d’Abramo, et al, PLOS1 2013 | i.p. injection of PHF1, MC-1, or DA31: 250 μg/125 μl, for a total of 10 mg/kg weekly | Female JNPL3 |
3 or 7 mos | 3 – 4 mos | ↓ CP13 in CA1 with MC-1 treatment by ELISA, ↓ RZ3 in CA1 with DA31 treatment and ↓ CP13 from MC-1 in older mice by IHC; ↓ RZ3 in CA1 of older DA31 treated mice (total tau Western Blot), ↓ in total tau with MC-1, No change with DA3 (Western Blot of insoluble fraction), no changes in survival |
| Yanamandro, et al, Cell/Neuron 2013 | icv injection of their own tau antibodies: Continuous icv infusion of 7.2 μg/d, for 6 weeks of: HJ8 series (anti human tau); HJ8.5 = AA’s 25 – 30; HJ9 series (anti mouse tau); HJ9.3 = AA’s 306 – 320 (repeat domain); HJ9.4 = AA’s 7 – 13 (N-terminal); Or HJ3 series (anti Aβ, mouse) | P301S | 6 mos | 3 mos | HJ8.5 ↓ AT8 in piriform & entorhinal Cx, hippocampus & amygdala (by IHC), HJ9.3 ↓ AT8 to lesser degrees in similar regions, HJ9.4 had less ↓, & no change in piriform Cx; ThioS and PHF1 staining yielded similar results. Staining for activated microglia strongly correlated with AT8 staining; HJ8.5 & HJ9.3 ↓ activated microglia in piriform & entorhinal Cx and amygdala. HJ9.4 showed lower effect on microglia; ThioS and PHF1 had similar results. Staining for activated microglia strongly correlated with AT8 staining; HJ8.5 & HJ9.3 ↓ activated microglia in piriform & entohinal Cx and amygdala. HJ9.4 had lesser effect on microglia. Significant (>50%) ↓ of detergent-insoluble tau (Western) from HJ8.5 and HJ9.3 treatment; > human tau than mouse tau in the FA “insoluble” fractions, ↓ by treatment; “seeding” ↓ by HJ8.5 and HJ9.3, not HJ9.4, in correlation with insoluble tau. No differences in motor testing, Contextual Fear Conditioning ↑ from all treatments with HJ8.5 = most robust freezing, Cued Fear Conditioning not affected by treatments |
| Castillo-Carranza, et al, J. Neurosci 2014 | icv (1 μl @ 1 mg/ml) or iv (30 μg/animal) injection of TOMA (tau oligomeric monoclonal antibody): single injection; either icv or iv, then 4 days until tissue analysis | JNPL3 | 4 or 8 mos | single injection | icv ↑ time on rotarod, to near-wild type levels, correlating w/reductions of oligos; icv treatment ↓ immunofluorescence of Thr231 and T22 as co-localized with DAPI; Single iv injection ↑ time on rotarod and ↑ alternations in Y maze 4 days post-injection; Single icv injection ↓ oligomeric tau (T22); Single iv injection ↑ oligo and HT7 ELISA levels in serum, correlating to ↓ levels in the brain; Single iv injection had no effect on AT8 or Gallyas staining; only T22 staining for oligos was reduced; Single iv injection did not change Western blot analysis for AT8 and/or PHF-13; Western analyses showed ↓ oligo levels, while Tau 5 showed ↑ monomeric tau, by both administrations. |
| Walls, et al, Neurosci Letters 2014 | i.c. injection of either AT8, 4G8 or IgG; 2 μg/animal | 3xTg (swAPP/PS1/P301L) | 15 – 18 mos | 1 – 4 wks | HT7, AT8 and Gallyas staining ↓ 1 – 2 weeks post-injection of AT8; From 3 weeks on, levels of tau (phospho, and normal) returned to IgG-treated (control) levels; 6E10 levels reduced due to 4G8 administration, following a similar pattern; AT8 administration did not effect Aβ levels, and 4G8 did not affect tau |
| Collin, et al, Brain 2014 | i.p. injection of MAb86 a pSer422-specific antibody, 60 mg/kg with 3 day intervals | TauPS2APP (P301L tau) | acute: 16-mo; chronic: 10-mo | Acute = 48 H, chronic = 1 wk | MAb86 specifically binds to tau phosphorylated at Ser422, shown in Western (Sarkosyl fraction); Immunofluorescence in CA1 verified binding; double staining with flotillin showed binding and internalization via lipid rafts; Chronic treatment delayed tau pathology and favored lysosomal clearance as shown by ELISA. |
Notes:
IHC = immunohistochemistry
i.p. = intraperitoneal
icv = intracranial ventricular
FA = formic acid extract
iv = intravenous
i.c. = intracranial
In parallel work, Martin Citron, then at Eli Lilly was investigating passive immunotherapy directed at tau using two different tau-overexpressing mouse lines, the JNPL3 mouse and P301S mutant PS19 line. The JNPL3 mice received 15 mg/kg of antibody three times per week, while the P301S mice (which demonstrate more rapid progression; pathology becoming evident at younger ages) received 15 mg/kg twice weekly. They tested two different types of anti-tau antibodies: first giving systemic i.p. PHF1 administration, or i.p. administration of MC-1, an antibody specific to conformational alterations of tau (Chai et al., 2011). The mice were given antibody or an IgG1 control hybridoma from non-immunized mice, starting at 2 months of age, continuing until 6 months. Western blotting revealed reductions in phosphorylated tau (AT8) in the insoluble fraction, but no change in total tau levels. This was confirmed in an ELISA assay using the AT8 antibody, which revealed a significant and substantial reduction of phosphorylated tau in response to both administered antibodies. They then administered the same antibodies to P301S mice from age 2 to 5 months, tested motor performance and showed a significant improvement on the rotarod for both antibody groups compared to controls. The rate and progression of improvement in motor deficits was greater for the P301S mice, which exhibit a more rapid onset of pathology, than the JNPL3 mice. Similarly, there appeared to be a delayed onset of pathology in the P301S mice. ELISA analysis revealed 45 percent reduction in pathology with PHF1 administration, and a 33 percent reduction in pathology after MC-1 administration. IHC also revealed reduced tau pathology after administration of either antibody compared to control, and no significant difference in microglia or astrocyte activation.
Work in Peter Davies’ lab has followed on these investigations using JNPL3 mice. PHF1, MC-1, or the pan-tau antibody DA31, were administerd to either 4 or 7 month old mice for 4 months. They showed that MC-1 treatment reduced CP-13 staining in CA1 of the hippocampus as well as reducing RZ3 staining, in response to the antibodies directed against pathological tau isoforms. Their study looked for IgG in neurons, but failed to identify any, leading to questions of mechanism of action (d’Abramo, 2013)
Yanamandra et al examined the effects and actions of various extracellular tau-targeting antibodies on P301S mice (Lee et al., 2001, Yanamandra et al., 2013). Ongoing collaborations between Marc Diamond and David Holtzman have resulted in the creation of a spectrum of antibodies with varying activities for binding extracellular tau, with a general emphasis on preventing extracellular aggregation and “seeding” (propagation) of tau pathology. They have created a line of tau antibodies called the HJ8 series, which is raised against full-length human tau, as well as a HJ9 series that are directed against mouse tau (Espinoza et al., 2008). These antibodies were developed in response to observations of extracellular tau appearance was followed by seeding of pathology into nearby cells. They hypothesized that tau can aggregate extracellularly, then become absorbed by adjacent cells, thereby transmitting the pathology. They employed intracerebroventricular (ICV) administration using 3 of their antibodies: HJ3.4 directed against Aβ, HJ8.5 an anti-human tau antibody, and HJ9.3 & HJ9.4, which are anti-mouse tau. ICV provided continuous delivery of the antibodies, for 3 months, into 6-month old P301S mice. Clearance of antibody from the CNS to the periphery was identified by temporary infusion of biotinylated HJ8.5 followed by analysis of CSF and plasma, during the course of the ICV treatment. Behavioral analysis revealed no differences in motor function, but there was a significant improvement in contextual fear conditioning. The HJ8.5 treated mice exhibited far greater freezing levels than control tau mice, and to a lesser degree the HJ9.4 treated mice also exhibited significantly more freezing.
Pathological evaluation revealed that administration of HJ8.5, HJ9.3 and HJ9.4 reduced immunostaining for AT8 (pSer202/pThr205), in the piriform cortex, amygdala, and entorhinal cortex, with HJ8.5 demonstrating the most robust improvement in pathology. HJ9.4 did not show significant reductions in the piriform cortex, however. These results were consistent between male and female mice, however the males had greater overall pathology. These results correlated with reductions in staining for PHF 1 (pSer396/404) as well as reductions in the microglial marker CD68. Additionally, a semi-quantitative ranking of ThioS staining revealed a reduction of tau deposition in the HJ8.5-treated mice compared to controls.
Biochemical analysis of tau levels was determined using ELISA on 3 fractions from the anterior cortex: an aqueous fraction produced using RAB buffer, a detergent soluble fraction using RIPA buffer, and an insoluble fraction obtained by formic acid (FA) digestion of the resulting pellet. The aqueous and RIPA-soluble fractions did not reveal significant changes in total tau levels as detected using their total-tau antibody HJ8.7, however the detergent insoluble FA fractions demonstrated a significant decrease (> 50%) of total tau in the HJ8.5 and HJ9.3 treated animals. Western blot analysis, using mouse polyclonal antibodies, revealed similar results to the staining for AT8 and CD68 in response to these two treatments. The HJ9.4-treated animals did not demonstrate such reductions. These results were specific to human, not mouse, tau as assessed by ELISA using species-specific tau markers. AT8 levels followed a similar trend in the FA fractions based on treatment. They further treated HEK293 cells with the RAB lysates from the treated mice; FRET analysis identified significantly lower seeding levels in response to the H8.5 and HJ9.3 (but not HJ9.4) treatments that correlated with the FA-fraction ELISA tau levels. This study emphasized monomeric, extracellular tau in interstitial fluid (ISF), which was previously thought to be a very unlikely location for tau protein until recently. Tau may aggregate extracellularly as well as intracellularly, thereby passing pathology on to nearby cells (Yamada et al., 2011).
Alternatively, Rakez Kayed’s laboratory has been examining antibodies that are specific to oligomeric tau. Oligomers of tau, as well as other proteins, have been identified as possibly being the most pathogenic form of these prion-like proteins (Mandelkow et al., 1996, Hoover et al., 2010, Medeiros et al., 2010, Lasagna-Reeves et al., 2011b) To this end, Kayed’s laboratory developed and characterized, novel antibodies, T22 and TOMA, that specifically target tau oligomers, and not monomers or stable NFTs (Lasagna-Reeves et al., 2012a, Lasagna-Reeves et al., 2012b). T22 is a rabbit antiserum that targets oligomers for analysis, whereas TOMA is a mouse monoclonal antibody that has been tested as an immunotherapy agent. Initially, they administered recombinant tau aggregates to 2-month old BALB/c mice along with CFA. The monoclonal antibodies derived from hybridomas from these mice were screened for specificity of tau oligomer targeting. In vitro testing, by ELISA, Western and dot blot demonstrated that T22 selectively marked oligomeric tau, but not monomeric tau, when compared to Tau 5 which recognizes all forms of tau; T22 did not bind to monomeric tau, while Tau 5 recognized all tau forms, thus demonstrating that T22 is a specific marker for tau oligomers. This original oligomer-specific polyclonal antiserum for tau enabled more specific identification of tau oligomers, so that characterization of tau pathology could be assessed in a new manner, then treatment approaches could be evaluated with a focus on oligomer-based pathology and improvements due to treatments.
Kayed’s lab further created and validated a mouse monoclonal oligomer-specific antibody, TOMA (tau oligomer monoclonal antibody), which they administered to tau transgenic mice to examine its potential as a new immunotherapeutic approach for treating tauopathies (Castillo-Carranza et al., 2014a) TOMA was administered by ICV or IV injection to 8 month-old male JNPL3 mice to examine the effects of oligomer-targeted treatment of tau pathology in vivo. Their ICV protocol was to inject 1 μg TOMA into each lateral ventricle one time. IV injection involved dilating the blood vessels in the tail, then injecting 30 μg of antibody. An additional group of 8 month-old mice received a tail-vein injection of 30 μg of biotinylated TOMA. Blood was collected before, and at several intervals after, injection to measure antibody concentration; brain tissue was collected to identify delivery of the antibody across the blood-brain barrier and into brain parenchyma. In vivo imaging of these animals revealed that the biotinylated TOMA did cross into both the brain and spinal cord. TOMA administration restored behavioral performance to that of wild-type mice in the tests conducted. The single ICV injection normalized rotarod performance to NTg levels. Additionally, the single IV administration yielded improvements in Y maze performance, which persisted through re-testing 2 months later. Pathology was evaluated using Western Blot, and probing for oligomeric-only tau using the polyclonal T22, in comparison to total monomeric tau, using Tau 5. ICV injection of TOMA led to a strong reduction in T22, but not Tau 5 staining by immunoblot; results were confirmed by immunofluorescent IHC staining, and were further correlated to reductions in pThr231 detection of tau aggregates, which has been shown to be associated with oligomers in human tissue (Lasagna-Reeves et al., 2011a). HT7 results showed increased levels while various other phospho-tau markers were decreased, suggesting a possible transfer of oligomeric tau to monomers and early aggregates. The single IV dose of TOMA, while showing improvements in Y-maze and rotarod performance, also revealed reductions in tau oligomers (assessed by Western blot, probed with rabbit-anti tau antibody), as well as reductions in ELISA levels of tau oligomers. Further anatomical evaluation using immunofluorescence showed reduced levels of oligomers in cell bodies and axons of CA1 neurons compared to controls, and there were no reductions in AT8 or Gallyas staining, suggesting that changes in monomeric and NFT forms of tau were not involved in the improvements demonstrated here. ELISA and Western blot analysis using numerous other phospho-forms of tau did not show differences in either of the treatment protocols. They concluded that oligomeric tau clearance leads to behavioral improvement, whereas earlier monomers, phospho-forms, and even fully formed NFTS did not need to be cleared for these functional improvements.
The next step in AD-related immunotherapy involved passive immunotherapy to 3xTG animals to identify benefits to both tau and Aβ pathologies. Frank LaFerla’s laboratory (Walls et al., 2014) has created a transgenic mouse line with the swAPP/PS1 and tau P301L mutations (3xTg mouse line), with the intent to examine correlated effects and treatments for both Aβ and tau pathology (Walls et al., 2014). Mice aged 15 – 18 months old were administered the AT8 anti-tau antibody, which recognizes the pSer202 and pThr205 epitopes (Walls et al., 2014). The mice were given AT8, 4G8 (an anti-Aβ antibody) or IgG control, intracranially, into the CA1 region of the hippocampus. A single injection was followed by tissue collection at weekly intervals (1 to 4 weeks post-injection), then IHC to identify changes in pathology. The AT8-injected animals showed a dramatic reduction in tau pathology one-week post-injection (HT7 measurements). This reduction continued through week 2, but starting at week 3 the levels of tau pathology started returning to levels seen in IgG-treated controls. AT8 staining was also reduced 1-week post-injection, as was Gallyas staining for fully-formed NFTs. No changes in Aβ pathology were noted by staining for (6E10 measurements) in response to AT8 injection. However injection of 4G8 resulted in Aβ reductions for 1–2 weeks followed by recovery back to baseline levels, analogous to the transient reductions in tau found with AT8.
A summary of these studies is reported in table 2.
MECHANISMS OF ACTION
The results summarized above have demonstrated that varied methods of applying anti-tau antibodies are capable of clearing tau pathology, in parallel with improving behavior. The question that obviously arises is: what is the mechanism by which this clearance is occurring?
The earlier work conducted by Sigurdsson’s group has suggested that the tau-antibodies are taken up by neuronal cells. Then autophagy and induction of lysosomal activity degrades and clears the protein. This is the preferred pathway for degradation of misfolded proteins, oligomers and aggregates. These ‘bad’ proteins become sequestered into autophagosomes and then are enzymatically degraded (Ding and Yin, 2008). The autophagy pathway can be overwhelmed in pathologic conditions, leading to accumulation of aggregates in the cytosol, which leads to formation of fibrils, paired helical filaments, and other β-pleated sheet formations such as those seen in AD (Sigurdsson, 2009, Morris et al., 2011).
Yanamandra’s group suggested an extracellular method of tau clearance. Their antibodies were able to impede uptake of extracellular tau aggregates and inhibit prion-like propagation of tau between cells. They demonstrated that, by binding extracellular tau aggregates, intracellular tau pathology could be delayed or prevented from passing on to a neighboring cell (Yanamandra et al., 2013).
Kayed’s group (Castillo-Carranza et al., 2014b) demonstrated that TOMA did not need to be internalized to reduce tau burden. Their results argued for a distinct clearance of tau pathology extracellularly. This extracellular clearance could also result in a peripheral sink type of mechanism by drawing tau oligomers from the brain and into the circulation, not unlike what has been observed for some anti-Aβ antibodies (Morgan, 2011).
Another potential mechanism of clearance that has largely been considered for both Aβ and tau immunotherapy involves microglial phagocytosis and removal, quite possibly in conjunction with inflammatory mechanisms. Sigurdsson’s group addressed the role of inflammation and the immune system in tau pathology’s clearance by microglial phagocytosis. They concluded indications did not favor this route of elimination (Sigurdsson, 2008).
Kayed’s group (Castillo-Carranza et al., 2014b) analyzed brain sections from treated and control groups using ELISA and immunofluorescence, for IL-6, IL-1β and Iba1, and found no differences between any of the antibody treatment groups, thereby suggesting that microglial activation and inflammation were not major factors in their model. Their analysis of serum and CSF suggest that oligomeric tau clearance occurs extracellularly and leads to elevated breakdown products in systems designed for full-body elimination, vs simply eliminating these polymers from neuronal cells.
Roche Pharmaceuticals has also examined tau immunotherapy using a triple transgenic model, and has followed on the earlier lines of targeting pSer422 with passive immunotherapy (Collin et al., 2014). Their 3x mouse-line is TauPS2APP; swAPP mice crossed with PS2, then P301L tau mutant mice. These mice received either an acute treatment, or a chronic treatment of their cultured MAb86 antibody (phosphor tau 416 – 430, produced in rabbit) that was either mouse IgG1 (for chronic administration) or human IgG1 (for shorter administrations) Acute treatment consisted of two i.p. doses, 3 days apart, of the “human” MAb86, in 16 month-old mice, with tissue collection 2 days after the last injection. Chronic administration began with 10-month old mice, which received weekly i.p. doses of the “mouse”-antibody for 16 weeks, followed by tissue collection a week later. Their pSer422 monoclonal antibody, MAb86, specifically bound to tau phosphorylated at Ser422, but not unphosphorylated tau at that location. MAb86 specific binding was located to the CA1 region of the hippocampus of TauPS2APP mice, as shown by immunofluorescence, and it was further shown to be intracellular (somatic or dendritic) binding, suggesting that the antibody is taken into neuronal cells. Double staining with MAb86 and flotillin 1 (marker for lipid rafts) indicated localization in lipid rafts within the plasma membrane. Further localization with lysosomes corroborated the prior studies that suggested anti-tau antibodies are internalized, leading pathologic forms of tau to lysosomes for degradation and removal. They further demonstrated that chronic administration of MAb86 delayed tau pathology, while favoring lysosomal clearance using ELISA and immunofluorescence.
In summary, there are multiple mechanisms anti-tau antibodies might engage when used for the clearance of pathological forms of tau. These include stimulation of intracellular degradation, possibly following internalization of complexes, neutralization of intracellular aggregates, or neutralization of extracellular aggregates, preventing internalization and/or spreading of pathologic tau. Moreover, the peripheral sink mechanism, which reveals increased blood levels of tau aggregates, has been reported and might aid in tau clearance from the brain. At this stage, there is not much evidence favoring an opsonization and enhanced phagocytosis as a mechanism for tau clearance, as supported by work with anti-Aβ antibodies, but this cannot yet be ruled out. Just as it is likely that different anti-Aβ antibodies employ different or multiple methods of clearing amyloid (Morgan, 2011), it is equally plausible that different anti-tau antibodies employ different or multiple mechanisms to stem the propagation of, or clearance of tau. Hence, there is not a right or a wrong mechanism of immunotherapy in opposing tau action in tau depositing mice. The apparent mechanistic differences observed by different research teams need not be viewed as conflicts. In fact they might be collectively integrated in attempts to identify the most efficacious monoclonal antibodies (possessing more than one mechanism of action).
RECENT WORK IN OUR LABORATORY
Previous work in our laboratory has shown that peripheral administration of anti-Aβ antibody was successful in reducing diffuse amyloid and compact plaques in the brain, and this was accompanied by improved performance in the radial arm water maze (Wilcock et al., 2004). Multiple microglial markers were modified by immunotherapy suggesting that one likely mechanism of clearance involved opsonization and Fcγ receptor-mediated phagocytosis. Our work in tau immunotherapy has been less conclusive, to date. So far, our results have suggested some positive impacts from tau passive immunotherapy, however the most effective tau antibody remains elusive.
We’ve treated several ages of rTg4510 mice, which demonstrate rapid tau deposition resembling that of advanced AD or FTLD patients (Santacruz et al., 2005). This mouse line expresses the 4R0N tau isoform with a P301L mutation under control of a tetracycline (tet) promoter. High-level forebrain neuron-expression of tau is driven by crossing with a CamKII-driven Tet-transactivator-protein transgenic mouse. These mice develop robust, progressive, age-dependent, pre-tangle and NFT pathology starting at 3 months of age. In addition to robust tau pathology, this model develops readily observable brain shrinkage visible as early as 6 months of age. Concurrent with the tau pathology is neuronal loss and atrophy. Behaviorally these mice exhibit extreme hyperactivity and impairment in a large number of cognitive function tests (Brownlow et al., 2014)
Our initial studies examined rTg4510 mice at an age when robust tau pathology is well established (between 11 and 13 months of age). Our studies used intracranial injection of several tau antibodies, into the frontal cortex and hippocampus of rTg4510 mice. Injections were performed unilaterally using convection-enhanced delivery (CED), as described in (Carty et al., 2010), at a rate of 2.5 μl/min, resulting in a total of 2 μg IgG per injection site. The contralateral hemisphere was untreated and used to normalize for differences in starting amounts of deposition. Four days post-injection, tissue was collected for IHC and histological staining to compare tau-pathology markers between the groups. NFTs were identified using Gallyas silver staining (Brownlow et al 2014). We also examined total tau using the rabbit polyclonal antibody H150 (Santa Cruz), rabbit-anti pSer199/202 (AnaSpec), and rabbit-anti pSer396 (AnaSpec). CD45 and Iba-1, markers for microglial activation, were also examined, as well as total neuron population via Nissl staining. Sections were imaged with a digital scanning microscope (Mirax) and fractional area stained measured by image analysis of entire regions from 4–6 sections per region. Results are expressed as a fraction of the uninjected contralateral hemisphere.
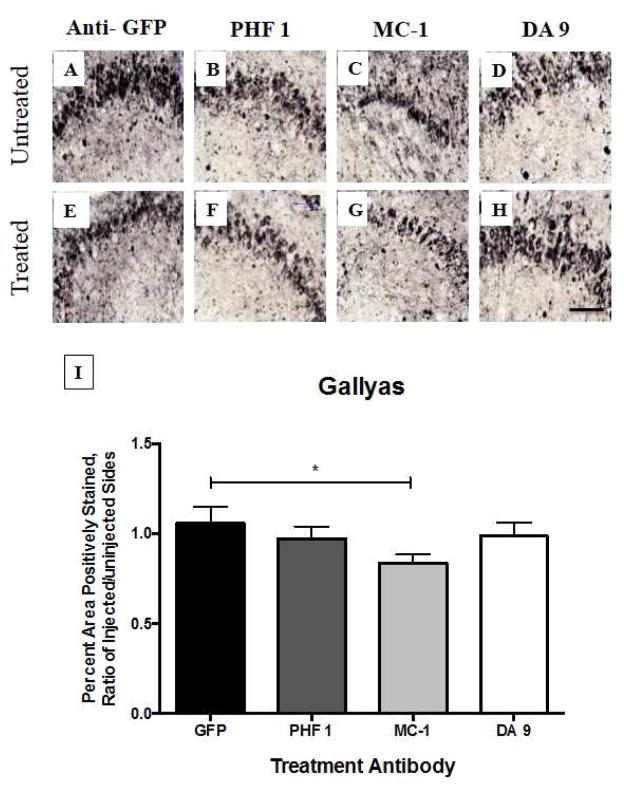
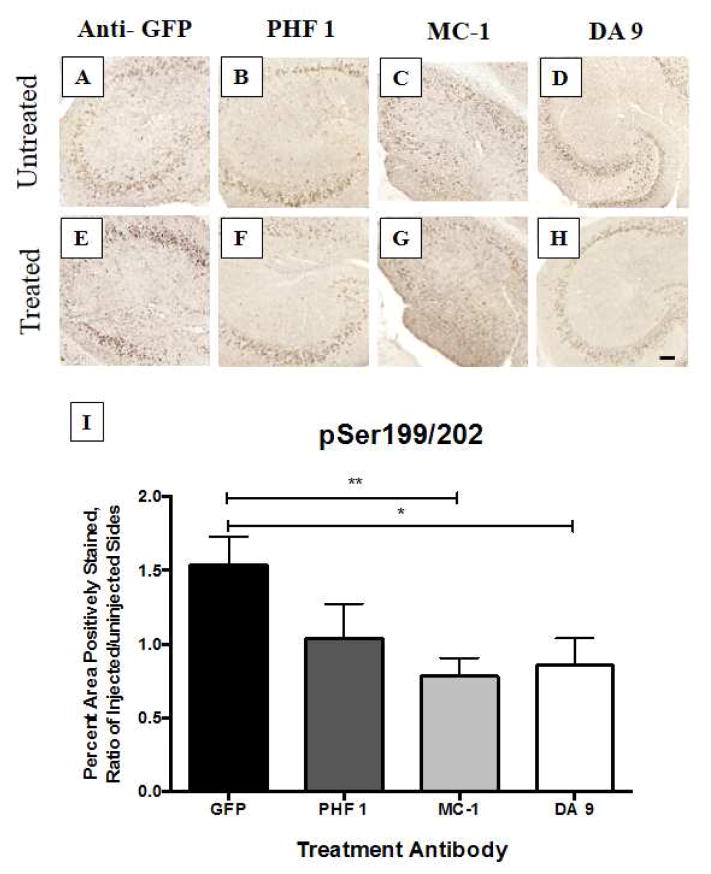
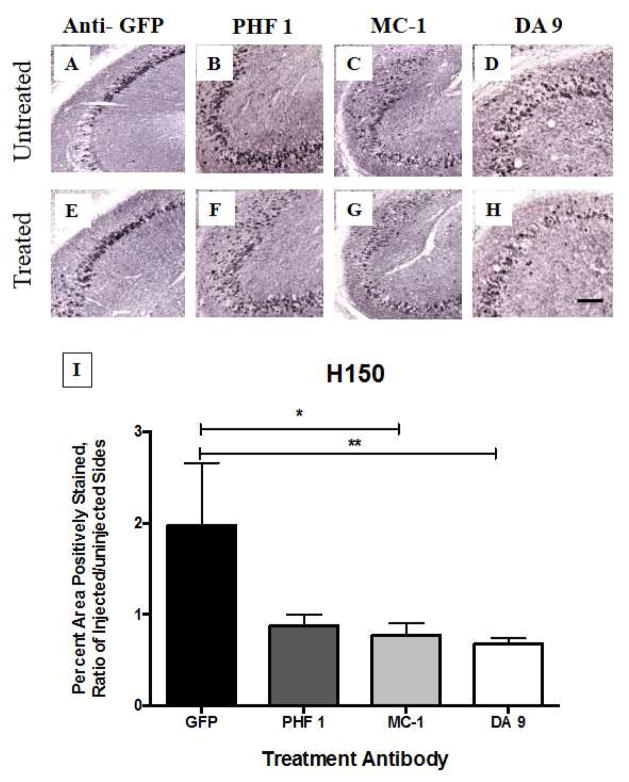
We identified significant reductions in Gallyas staining (Figure 1) as well as Ser199/202 (Figure 2) and H150 (Figure 3) staining in the hippocampus of mice treated with the antibody MC-1 when compared to mice treated with the antibody directed against the non-mammalian protein green fluorescent protein (GFP), which was not expected to remove tau deposits. We also examined staining for pSer396 and relative microglial activation, because microglial activation is one of the common pathological features of AD (Table 3). No notable increase in microglial activation, as examined by CD45, was identified which is an encouraging finding because over-activation of the innate immune system has proven to be a challenge in clinical studies of Aβ immunotherapy. Nonetheless, all tau-depositing mice have much more CD45 than their non-transgenic littermates. Similarly, we did not observe notable difference in neuronal population between the different treatments when using Nissl staining.
Fig. 1. Gallyas silver staining of hippocampus, four days after intracranial injection of anti-tau antibodies into rTg4510 mice.
One-year old rTg4510 mice (n = 6 – 8 animals per group) received intracranial injection (by convection enhanced delivery, CED) of potential tau-treatment antibodies into the right hemisphere. The left hemisphere remained untreated. Three different types of antibodies were tested, in comparison to an anti-GFP control injection. PHF-1 is a phosphorylated form of tau, MC-1 is a conformation-dependent antibody, and DA-9 is a pan-tau antibody. MC-1 treatment demonstrated a reduction in NFTs (p < 0.05 by ANOVA), while the other antibodies did not. Scale bar = 100 μm
Fig. 2. Analysis of hyperphosphorylated tau (pSer199/202) in hippocampus, four days after intracranial injection of antibodies into one-year old rTg4510 mice.
The right hemisphere pathology, normalized by the uninjected left hemisphere, showed a marked response to two of the three different antibodies, compared to the anti-GFP control injection. DA 9 treatment yielded a moderate reduction in pSer199/202 staining (p < 0.05), while MC-1 treatment led to an even more dramatic reduction of pSer199/202 (p< 0.01; n = 6 – 8 animals per group)). Statistics were computed using StatView software and ANOVA analysis. Scale bar = 100 μm
Fig. 3. H150 immunostaining following antibody treatment to rTg4510 mice.
One year old rTg4510 mice (n = 6 – 7 per group) received intracranial injections of treatment antibodies into the right hippocampus. Four days later, tissue was collected and the treated (right) hippocampus was compared to the untreated (left) hippocampus to identify differences in pathology by IHC. Results were compared between antibody treatments and anti-GFP control injections. Both MC-1 and DA 9 treatments led to significant reductions in H150 pathology (p < 0.05) compared to the anti-GFP control treatments. Results were analyzed using StatView software, for the non-parametric Mann Whitney U Test. Scale bar = 100 μm
Table 3.
Other staining results following intracranial injection of antibodies
| STAIN | Anti-GFP Treatment Mean +/− SEM | MC-1 Treatment Mean +/− SEM | PHF1 Treatment Mean +/− SEM | DA9 Treatment Mean +/− SEM |
|---|---|---|---|---|
| pSer396 | 1.03 +/− 0.21 | 0.90 +/− 0.156 | 0.75 +/− 0.14 | 1.36 +/− 0.22 |
| CD45 | 1.06 +/− 0.13 | 0.96 +/− 0.12 | 1.13 +/− 0.11 | 1.06 +/− 0.10 |
Sub-populations of mice were stained with other phospho-epitopes of tau; here we show an example using pSer396, which did not demonstrate significant reduction in pathology, similar to others tested. Additionally, we examined the microglial marker CD45 to note if antibody treatment had any impact on pro-inflammatory response, which was not reflected here.
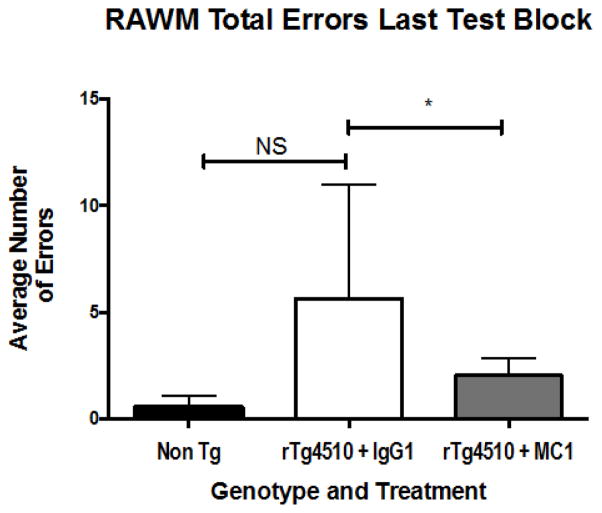
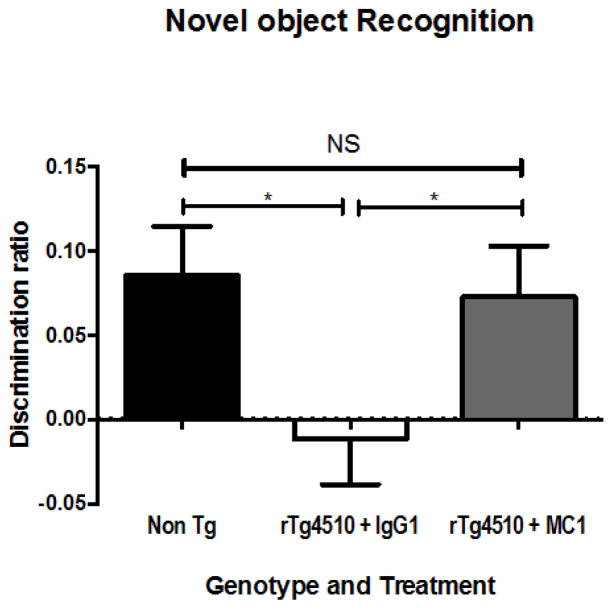
We conducted a subsequent study using 10 – 12 month-old mice, infused with either MC-1, Tau 5 or TOMA, or IgG1 (as a control) antibodies . The mice where implanted with mini-osmotic pumps (Alzet) filled with 20 μg of either MC-1, Tau 5, or TOMA, or IgG control, (all of which were 1 mg/ml) into the right lateral ventricle for continuous delivery of 0.5 μg a day over 28 days, using mini-osmotic pumps (Alzet) as described previously (Selenica et al., 2013, Brownlow et al., 2014). This route of administration allowed for behavioral testing as well as pathology evaluation. Activity testing for open field, revealed a genotype effect but no treatment effect; no differences were noted between the treated mice and controls when comparing tau-depositing transgenic mice to non-transgenic littermates (Table 4). Cognitive assessment (as in Brownlow et al, 2014) did reveal performance improvements in both novel object recognition testing (Figure 4) and radial arm water maze (RAWM – Figure 5) in response to MC-1 treatment when compared to IgG1 treated mice.
Table 4.
Activity testing results
| Activity Test | Non Transgenic mean +/− SEM | rTg4510 + IgG1 mean +/− SEM | rTg4510 + MC-1 Mean +/− SEM | rTg4510 + Tau 5 Mean +/− SEM |
|---|---|---|---|---|
| Open Field Distance Traveled | 50.6 +/− 5.9 | 126 +/− 28 | 149 +/− 26 | 143 +/− 26 |
| Rotarod – Total time | 40.5 +/− 5.1 | 57.3 +/− 6.7 | 44.4 +/− 6.7 | 48.2 +/− 6.0 |
| Y-Maze – Number of Entries | 38.9 +/− 3.6 | 51.8 +/− 10.1 | 44.4 +/− 8.9 | 46.4 +/− 4.8 |
| Y-Maze - Alternations | 55.2 +/− 3.7 | 58.3 +/− 6.4 | 64.0 +/− 5.8 | 61.4 +/− 4.2 |
Our laboratory has observed that rTg4510 mice typically display much greater activity levels than NonTg mice, as mentioned above. Such activity is demonstrated here, by comparing distance traveled in the Open Field test, the time on the Rotarod and the number of entries in the Y-Maze. Slight increases are also shown in the number of alternations in the Y-Maze. Treatment of the rTg4510 mice with either MC-1 or Tau 5 did not significantly impact performance compared to IgG1 treatment.
Fig. 4. Cognitive-behavioral results from rTg4510 mice after receiving 2 weeks of continuous infusion of anti-tau antibodies into the right ventricle.
Mice that had received two weeks of antibody infusion were subjected to a battery of behavioral testing, with emphasis on cognitive performance. Novel object recognition testing revealed that the IgG1 control antibody (n = 9) did not lead to performance improvement compared to Non Tg mice (n = 8). In contrast, MC-1 treated mice (n = 8) showed cognitive performance levels that were nearly indistinguishable from the Non Tg mice. ANOVA statistical analysis was performed using StatView software
Fig. 5. Spatial memory testing, using the radial arm water maze (RAWM), to identify cognitive differences between treated and untreated rTg4510 mice.
Mice received anti-tau treatments of MC-1 (n = 8), or Tau 5 (n=9), by continuous infusion into the right ventricle. Two weeks later, they were tested for cognitive performance in the RAWM. Results were compared to an IgG1 control treatment (n=9). A significant reduction in the number of errors in MC-1 treated mice compared to the IgG1 treated control group was identified, as highlighted by the average number of errors during the last block of testing. MC-1 treated mice, at this time point, had not achieved Non Tg levels (n=8), however the mice performed significantly better than the IgG1 controls as calculated by StatView ANOVA (p < 0.05)
Overall these data suggest that MC-1, a conformation specific antibody developed by Peter Davies, has greater efficacy than several other antibodies when administered ICV. These data encourage consideration of a humanized form of this antibody or related human antibodies for testing in early stage cases of AD.
FUTURE DIRECTIONS
Tau provides many different strategies for treatment given its different isoforms, post-translational modifications, conformations, and more. Presently, it appears that targeting oligomers of tau is likely to be the focus for many of these studies. Multiple groups have been investigating oligomeric forms of amyloid and tau and consider these to be the most toxic forms of the proteins. As the groundwork continues to be laid in tau immunotherapy studies, it is likely that the next generation of immunotherapies will be directed at oligomers, as Kayed’s lab has been doing. Additionally, many have suggested a future immunotherapeutic approach targeting Aβ and tau in concert, as noted by recent work in LaFerla’s laboratory (Medeiros et al., 2011). Interestingly, the general success obtained with anti-tau approaches might favor tau immunotherapy over anti-amyloid immunotherapy. The low levels of extracellular tau coupled with the absence of general microglial activation may avoid the edema and hemorrhage observed with anti-amyloid immunotherapy in mice and humans (Wilcock et al., 2007, Sperling et al., 2012).
On the clinical front, a tau active vaccine entered into a Phase 1 human clinical trial; an agent named AADvac1. Axon Neuroscience SE began a clinical study in mid 2013, recruiting subjects for a Phase I clinical study on the safety and tolerance, of this active immunotherapy agent: a synthetic tau peptide conjugated to KLH, also using aluminum hydroxide as an adjuvant to further stimulate the immune response. The reported protocol consists of treating patients with mild to moderate AD three times, over a course of three months, then assessing response by measuring neuropsychiatric measures, cognitive testing, MRI, and blood biomarkers. Additional measures of immune response to the treatment will be obtained to verify the responses as immune-mediated.
Acknowledgments
The authors thank Dr. Peter Davies for the generous gifts of monoclonal antibodies MC-1, PHF1 and DA9, for the treatments reported above. We also thank the vivarium staff at the USF Health Byrd Alzheimer’s Disease Institute for their help in maintaining the health and care of our mouse colony, including maintaining ethical standards and compliance as described in the “Guide for the Care and Use of Laboratory Animals” in an AAALAC-accredited facility. All procedures using mice were approved by the Institutional Animal Care and Use Committee of the University of South Florida. All applicable international, and/or institutional guidelines for the care and use of animals were followed. This work was supported by NS076308 and by the USF Health Byrd Alzheimer’s Institute.
References
- Alzheimer’s Association. Alzheimer’s Disease Facts and Figures. Alzheimer’s Association; Chicago, IL: 2011. ClinicalTrials.gov identifier: NCT01850238 , Axon Neuroscience SE (2013) [DOI] [PubMed] [Google Scholar]
- Agadjanyan MG, Petrovsky N, Ghochikyan A. A fresh perspective from immunologists and vaccine researchers: Active vaccination strategies to prevent and reverse Alzheimer’s disease. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2015 doi: 10.1016/j.jalz.2015.06.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asuni AA, Boutajangout A, Quartermain D, Sigurdsson EM. Immunotherapy targeting pathological tau conformers in a tangle mouse model reduces brain pathology with associated functional improvements. J Neurosci. 2007;27:9115–9129. doi: 10.1523/JNEUROSCI.2361-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer AJ, Bullock R, Jones RW, Wilkinson D, Paterson KR, Jenkins L, Millais SB, Donoghue S. Evaluation of the safety and immunogenicity of synthetic Abeta42 (AN1792) in patients with AD. Neurology. 2005;64:94–101. doi: 10.1212/01.WNL.0000148604.77591.67. [DOI] [PubMed] [Google Scholar]
- Bi M, Ittner A, Ke YD, Gotz J, Ittner LM. Tau-targeted immunization impedes progression of neurofibrillary histopathology in aged P301L tau transgenic mice. PLoS One. 2011;6:e26860. doi: 10.1371/journal.pone.0026860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billiau A, Matthys P. Modes of action of Freund’s adjuvants in experimental models of autoimmune diseases. Journal of leukocyte biology. 2001;70:849–860. [PubMed] [Google Scholar]
- Boimel M, Grigoriadis N, Lourbopoulos A, Haber E, Abramsky O, Rosenmann H. Efficacy and safety of immunization with phosphorylated tau against neurofibrillary tangles in mice. Exp Neurol. 2010;224:472–485. doi: 10.1016/j.expneurol.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Boutajangout A, Ingadottir J, Davies P, Sigurdsson EM. Passive immunization targeting pathological phospho-tau protein in a mouse model reduces functional decline and clears tau aggregates from the brain. J Neurochem. 2011 doi: 10.1111/j.1471-4159.2011.07337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutajangout A, Quartermain D, Sigurdsson EM. Immunotherapy targeting pathological tau prevents cognitive decline in a new tangle mouse model. J Neurosci. 2010;30:16559–16566. doi: 10.1523/JNEUROSCI.4363-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E, Bohl J. Staging of Alzheimer-related cortical destruction. Eur Neurol. 1993;33:403–408. doi: 10.1159/000116984. [DOI] [PubMed] [Google Scholar]
- Brownlow ML, Joly-Amado A, Azam S, Elza M, Selenica ML, Pappas C, Small B, Engelman R, Gordon MN, Morgan D. Partial rescue of memory deficits induced by calorie restriction in a mouse model of tau deposition. Behavioural brain research. 2014;271:79–88. doi: 10.1016/j.bbr.2014.06.001. [DOI] [PubMed] [Google Scholar]
- Brunden KR, Zhang B, Carroll J, Yao Y, Potuzak JS, Hogan AM, Iba M, James MJ, Xie SX, Ballatore C, Smith AB, 3rd, Lee VM, Trojanowski JQ. Epothilone D improves microtubule density, axonal integrity, and cognition in a transgenic mouse model of tauopathy. J Neurosci. 2010;30:13861–13866. doi: 10.1523/JNEUROSCI.3059-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carty N, Lee D, Dickey C, Ceballos-Diaz C, Jansen-West K, Golde TE, Gordon MN, Morgan D, Nash K. Convection-enhanced delivery and systemic mannitol increase gene product distribution of AAV vectors 5, 8, and 9 and increase gene product in the adult mouse brain. Journal of neuroscience methods. 2010;194:144–153. doi: 10.1016/j.jneumeth.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo-Carranza DL, Gerson JE, Sengupta U, Guerrero-Munoz MJ, Lasagna-Reeves CA, Kayed R. Specific targeting of tau oligomers in Htau mice prevents cognitive impairment and tau toxicity following injection with brain-derived tau oligomeric seeds. J Alzheimers Dis. 2014a;40(Suppl 1):S97–S111. doi: 10.3233/JAD-132477. [DOI] [PubMed] [Google Scholar]
- Castillo-Carranza DL, Sengupta U, Guerrero-Munoz MJ, Lasagna-Reeves CA, Gerson JE, Singh G, Estes DM, Barrett AD, Dineley KT, Jackson GR, Kayed R. Passive immunization with Tau oligomer monoclonal antibody reverses tauopathy phenotypes without affecting hyperphosphorylated neurofibrillary tangles. J Neurosci. 2014b;34:4260–4272. doi: 10.1523/JNEUROSCI.3192-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrillo JI, Oliver SG. Alzheimer’s as a Systems-Level Disease Involving the Interplay of Multiple Cellular Networks. Methods in molecular biology (Clifton, NJ) 2016;1303:3–48. doi: 10.1007/978-1-4939-2627-5_1. [DOI] [PubMed] [Google Scholar]
- Chai X, Wu S, Murray TK, Kinley R, Cella CV, Sims H, Buckner N, Hanmer J, Davies P, O’Neill MJ, Hutton ML, Citron M. Passive immunization with anti-Tau antibodies in two transgenic models: reduction of Tau pathology and delay of disease progression. The Journal of biological chemistry. 2011;286:34457–34467. doi: 10.1074/jbc.M111.229633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citron M. Alzheimer’s disease: strategies for disease modification. Nat Rev Drug Discov. 2010;9:387–398. doi: 10.1038/nrd2896. [DOI] [PubMed] [Google Scholar]
- Collin L, Bohrmann B, Gopfert U, Oroszlan-Szovik K, Ozmen L, Gruninger F. Neuronal uptake of tau/pS422 antibody and reduced progression of tau pathology in a mouse model of Alzheimer’s disease. Brain : a journal of neurology. 2014 doi: 10.1093/brain/awu213. [DOI] [PubMed] [Google Scholar]
- d’Abramo C, Acker C, Jimenez H, Davies P. Tau Passive Immunotherapy in Mutant P301L Mice: Antibody Affinity versus Specificity. PLOS ONE Volume. 2013;8:10. doi: 10.1371/journal.pone.0062402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denk F, Wade-Martins R. Knock-out and transgenic mouse models of tauopathies. Neurobiol Aging. 2009;30:1–13. doi: 10.1016/j.neurobiolaging.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding WX, Yin XM. Sorting, recognition and activation of the misfolded protein degradation pathways through macroautophagy and the proteasome. Autophagy. 2008;4:141–150. doi: 10.4161/auto.5190. [DOI] [PubMed] [Google Scholar]
- Espinoza M, de Silva R, Dickson DW, Davies P. Differential incorporation of tau isoforms in Alzheimer’s disease. J Alzheimers Dis. 2008;14:1–16. doi: 10.3233/jad-2008-14101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine SN, Rauch JN, Nordhues BA, Assimon VA, Stothert AR, Jinwal UK, Sabbagh JJ, Chang L, Stevens SM, Jr, Zuiderweg ER, Gestwicki JE, Dickey CA. Isoform-selective Genetic Inhibition of Constitutive Cytosolic Hsp70 Activity Promotes Client Tau Degradation Using an Altered Co-chaperone Complement. The Journal of biological chemistry. 2015;290:13115–13127. doi: 10.1074/jbc.M115.637595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghochikyan A, Mkrtichyan M, Petrushina I, Movsesyan N, Karapetyan A, Cribbs DH, Agadjanyan MG. Prototype Alzheimer’s disease epitope vaccine induced strong Th2-type anti-Abeta antibody response with Alum to Quil A adjuvant switch. Vaccine. 2006;24:2275–2282. doi: 10.1016/j.vaccine.2005.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotz J, Ittner A, Ittner LM. Tau-targeted treatment strategies in Alzheimer’s disease. British journal of pharmacology. 2012;165:1246–1259. doi: 10.1111/j.1476-5381.2011.01713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Munoz MJ, Castillo-Carranza DL, Kayed R. Therapeutic approaches against common structural features of toxic oligomers shared by multiple amyloidogenic proteins. Biochemical pharmacology. 2014;88:468–478. doi: 10.1016/j.bcp.2013.12.023. [DOI] [PubMed] [Google Scholar]
- Halle M, Tribout-Jover P, Lanteigne AM, Boulais J, St-Jean JR, Jodoin R, Girouard MP, Constantin F, Migneault A, Renaud F, Didierlaurent AM, Mallett CP, Burkhart D, Pilorget A, Palmantier R, Larocque D. Methods to monitor monocytes-mediated amyloid-beta uptake and phagocytosis in the context of adjuvanted immunotherapies. Journal of immunological methods. 2015 doi: 10.1016/j.jim.2015.05.002. [DOI] [PubMed] [Google Scholar]
- Himmelstein DS, Ward SM, Lancia JK, Patterson KR, Binder LI. Tau as a therapeutic target in neurodegenerative disease. Pharmacology & therapeutics. 2012;136:8–22. doi: 10.1016/j.pharmthera.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover BR, Reed MN, Su J, Penrod RD, Kotilinek LA, Grant MK, Pitstick R, Carlson GA, Lanier LM, Yuan LL, Ashe KH, Liao D. Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron. 2010;68:1067–1081. doi: 10.1016/j.neuron.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kfoury N, Holmes BB, Jiang H, Holtzman DM, Diamond MI. Trans-cellular propagation of Tau aggregation by fibrillar species. The Journal of biological chemistry. 2012;287:19440–19451. doi: 10.1074/jbc.M112.346072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasagna-Reeves CA, Castillo-Carranza DL, Jackson GR, Kayed R. Tau Oligomers as Potential Target for Immunotherapy for Alzheimer Disease and Tauopathies. Curr Alzheimer Res. 2011a doi: 10.2174/156720511796717177. [DOI] [PubMed] [Google Scholar]
- Lasagna-Reeves CA, Castillo-Carranza DL, Sengupta U, Clos AL, Jackson GR, Kayed R. Tau oligomers impair memory and induce synaptic and mitochondrial dysfunction in wild-type mice. Molecular neurodegeneration. 2011b;6:39. doi: 10.1186/1750-1326-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasagna-Reeves CA, Castillo-Carranza DL, Sengupta U, Guerrero-Munoz MJ, Kiritoshi T, Neugebauer V, Jackson GR, Kayed R. Alzheimer brain-derived tau oligomers propagate pathology from endogenous tau. Scientific reports. 2012a;2:700. doi: 10.1038/srep00700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasagna-Reeves CA, Castillo-Carranza DL, Sengupta U, Sarmiento J, Troncoso J, Jackson GR, Kayed R. Identification of oligomers at early stages of tau aggregation in Alzheimer’s disease. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2012b;26:1946–1959. doi: 10.1096/fj.11-199851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashley T, Rohrer JD, Mead S, Revesz T. Review: An update on clinical, genetic and pathological aspects of frontotemporal lobar degenerations. Neuropathology and applied neurobiology. 2015 doi: 10.1111/nan.12250. [DOI] [PubMed] [Google Scholar]
- Lee VM, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu Rev Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- Lemere CA. Immunotherapy for Alzheimer’s disease: hoops and hurdles. Molecular neurodegeneration. 2013;8:36. doi: 10.1186/1750-1326-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelkow EM, Mandelkow E. Biochemistry and cell biology of tau protein in neurofibrillary degeneration. Cold Spring Harbor perspectives in medicine. 2012;2:a006247. doi: 10.1101/cshperspect.a006247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelkow EM, Schweers O, Drewes G, Biernat J, Gustke N, Trinczek B, Mandelkow E. Structure, microtubule interactions, and phosphorylation of tau protein. Ann N Y Acad Sci. 1996;777:96–106. doi: 10.1111/j.1749-6632.1996.tb34407.x. [DOI] [PubMed] [Google Scholar]
- Medeiros R, Baglietto-Vargas D, Laferla FM. The Role of Tau in Alzheimer’s Disease and Related Disorders. CNS Neurosci Ther. 2010 doi: 10.1111/j.1755-5949.2010.00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros R, Baglietto-Vargas D, LaFerla FM. The role of tau in Alzheimer’s disease and related disorders. CNS Neurosci Ther. 2011;17:514–524. doi: 10.1111/j.1755-5949.2010.00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D. Immunotherapy for Alzheimer’s disease. J Intern Med. 2011;269:54–63. doi: 10.1111/j.1365-2796.2010.02315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris M, Maeda S, Vossel K, Mucke L. The many faces of tau. Neuron. 2011;70:410–426. doi: 10.1016/j.neuron.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary JC, 3rd, Li Q, Marinec P, Blair LJ, Congdon EE, Johnson AG, Jinwal UK, Koren J, 3rd, Jones JR, Kraft C, Peters M, Abisambra JF, Duff KE, Weeber EJ, Gestwicki JE, Dickey CA. Phenothiazine-mediated rescue of cognition in tau transgenic mice requires neuroprotection and reduced soluble tau burden. Molecular neurodegeneration. 2010;5:45. doi: 10.1186/1750-1326-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Cheng D, LaFerla FM. Genetically altering Abeta distribution from the brain to the vasculature ameliorates tau pathology. Brain Pathol. 2009;19:421–430. doi: 10.1111/j.1750-3639.2008.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orgogozo JM, Gilman S, Dartigues JF, Laurent B, Puel M, Kirby LC, Jouanny P, Dubois B, Eisner L, Flitman S, Michel BF, Boada M, Frank A, Hock C. Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology. 2003;61:46–54. doi: 10.1212/01.wnl.0000073623.84147.a8. [DOI] [PubMed] [Google Scholar]
- Ragupathi G, Damani P, Deng K, Adams MM, Hang J, George C, Livingston PO, Gin DY. Preclinical evaluation of the synthetic adjuvant SQS-21 and its constituent isomeric saponins. Vaccine. 2010;28:4260–4267. doi: 10.1016/j.vaccine.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmann H, Grigoriadis N, Karussis D, Boimel M, Touloumi O, Ovadia H, Abramsky O. Tauopathy-like Abnormalities and Neurologic Deficits in Mice Immunized With Neuronal Tau Protein. Arch Neurol. 2006;63:1459–1467. doi: 10.1001/archneur.63.10.1459. [DOI] [PubMed] [Google Scholar]
- Rozenstein-Tsalkovich L, Grigoriadis N, Lourbopoulos A, Nousiopoulou E, Kassis I, Abramsky O, Karussis D, Rosenmann H. Repeated immunization of mice with phosphorylated-tau peptides causes neuroinflammation. Exp Neurol. 2013;248:451–456. doi: 10.1016/j.expneurol.2013.07.006. [DOI] [PubMed] [Google Scholar]
- Santacruz K, Lewis J, Spires T, Paulson J, Kotilinek L, Ingelsson M, Guimaraes A, DeTure M, Ramsden M, McGowan E, Forster C, Yue M, Orne J, Janus C, Mariash A, Kuskowski M, Hyman B, Hutton M, Ashe KH. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309:476–481. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selenica ML, Brownlow M, Jimenez JP, Lee DC, Pena G, Dickey CA, Gordon MN, Morgan D. Amyloid oligomers exacerbate tau pathology in a mouse model of tauopathy. Neuro-degenerative diseases. 2013;11:165–181. doi: 10.1159/000337230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selenica ML, Davtyan H, Housley SB, Blair LJ, Gillies A, Nordhues BA, Zhang B, Liu J, Gestwicki JE, Lee DC, Gordon MN, Morgan D, Dickey CA. Epitope analysis following active immunization with tau proteins reveals immunogens implicated in tau pathogenesis. Journal of neuroinflammation. 2014;11:152. doi: 10.1186/s12974-014-0152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson EM. Immunotherapy targeting pathological tau protein in Alzheimer’s disease and related tauopathies. J Alzheimers Dis. 2008;15:157–168. doi: 10.3233/jad-2008-15202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson EM. Tau-focused immunotherapy for Alzheimer’s disease and related tauopathies. Curr Alzheimer Res. 2009;6:446–450. doi: 10.2174/156720509789207930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling R, Salloway S, Brooks DJ, Tampieri D, Barakos J, Fox NC, Raskind M, Sabbagh M, Honig LS, Porsteinsson AP, Lieberburg I, Arrighi HM, Morris KA, Lu Y, Liu E, Gregg KM, Brashear HR, Kinney GG, Black R, Grundman M. Amyloid-related imaging abnormalities in patients with Alzheimer’s disease treated with bapineuzumab: a retrospective analysis. The Lancet Neurology. 2012;11:241–249. doi: 10.1016/S1474-4422(12)70015-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui D, Liu M, Kuo MH. In vitro aggregation assays using hyperphosphorylated tau protein. Journal of visualized experiments : JoVE. 2015:e51537. doi: 10.3791/51537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troquier L, Caillierez R, Burnouf S, Fernandez-Gomez FJ, Grosjean ME, Zommer N, Sergeant N, Schraen-Maschke S, Blum D, Buee L. Targeting phospho-Ser422 by active Tau Immunotherapy in the THYTau22 mouse model: a suitable therapeutic approach. Curr Alzheimer Res. 2012;9:397–405. doi: 10.2174/156720512800492503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utton MA, Noble WJ, Hill JE, Anderton BH, Hanger DP. Molecular motors implicated in the axonal transport of tau and alpha-synuclein. J Cell Sci. 2005;118:4645–4654. doi: 10.1242/jcs.02558. [DOI] [PubMed] [Google Scholar]
- Walls KC, Ager RR, Vasilevko V, Cheng D, Medeiros R, LaFerla FM. p-Tau immunotherapy reduces soluble and insoluble tau in aged 3xTg-AD mice. Neuroscience letters. 2014;575:96–100. doi: 10.1016/j.neulet.2014.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JZ, Gao X, Wang ZH. The physiology and pathology of microtubule-associated protein tau. Essays in biochemistry. 2014;56:111–123. doi: 10.1042/bse0560111. [DOI] [PubMed] [Google Scholar]
- Wang Y, Mandelkow E. Degradation of tau protein by autophagy and proteasomal pathways. Biochem Soc Trans. 2012;40:644–652. doi: 10.1042/BST20120071. [DOI] [PubMed] [Google Scholar]
- Wilcock DM, Colton CA. Anti-amyloid-beta immunotherapy in Alzheimer’s disease: relevance of transgenic mouse studies to clinical trials. J Alzheimers Dis. 2008;15:555–569. doi: 10.3233/jad-2008-15404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcock DM, Jantzen PT, Li Q, Morgan D, Gordon MN. Amyloid-beta vaccination, but not nitro-nonsteroidal anti-inflammatory drug treatment, increases vascular amyloid and microhemorrhage while both reduce parenchymal amyloid. Neuroscience. 2007;144:950–960. doi: 10.1016/j.neuroscience.2006.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcock DM, Rojiani A, Rosenthal A, Levkowitz G, Subbarao S, Alamed J, Wilson D, Wilson N, Freeman MJ, Gordon MN, Morgan D. Passive amyloid immunotherapy clears amyloid and transiently activates microglia in a transgenic mouse model of amyloid deposition. J Neurosci. 2004;24:6144–6151. doi: 10.1523/JNEUROSCI.1090-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski T, Boutajangout A. Immunotherapeutic approaches for Alzheimer’s disease in transgenic mouse models. Brain Struct Funct. 2010;214:201–218. doi: 10.1007/s00429-009-0236-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe MS. The role of tau in neurodegenerative diseases and its potential as a therapeutic target. Scientifica. 2012;2012:796024. doi: 10.6064/2012/796024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Cirrito JR, Stewart FR, Jiang H, Finn MB, Holmes BB, Binder LI, Mandelkow EM, Diamond MI, Lee VM, Holtzman DM. In vivo microdialysis reveals age-dependent decrease of brain interstitial fluid tau levels in P301S human tau transgenic mice. J Neurosci. 2011;31:13110–13117. doi: 10.1523/JNEUROSCI.2569-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanamandra K, Kfoury N, Jiang H, Mahan TE, Ma S, Maloney SE, Wozniak DF, Diamond MI, Holtzman DM. Anti-tau antibodies that block tau aggregate seeding in vitro markedly decrease pathology and improve cognition in vivo. Neuron. 2013;80:402–414. doi: 10.1016/j.neuron.2013.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Luo Y, Dinkel P, Zheng J, Wei G, Margittai M, Nussinov R, Ma B. Cross-seeding and conformational selection between three- and four-repeat human Tau proteins. The Journal of biological chemistry. 2012;287:14950–14959. doi: 10.1074/jbc.M112.340794. [DOI] [PMC free article] [PubMed] [Google Scholar]