Abstract
Despite being empirically designed based on a simple understanding of TCR signaling, T cells engineered with chimeric antigen receptors (CARs) have been remarkably successful in treating patients with advanced refractory B cell malignancies. However, many challenges remain in improving the safety and efficacy of this therapy and extending it toward the treatment of epithelial cancers. Other aspects TCR signaling beyond those directly provided by CD3ζ and CD28 phosphorylation strongly influence a T cell’s ability to differentiate and acquire full effector functions. Here, we discuss how the principles of TCR recognition, including spatial constraints, Kon/Koff rates, and synapse formation, along with in-depth analysis of CAR signaling might be applied to develop safer and more effective synthetic tumor targeting receptors.
Introduction
Advances in genetic engineering combined with an improved understanding of T cell recognition have led to the design of synthetic tumor targeting receptors, termed chimeric antigen receptors (CARs) that can be introduced into human T cells to redirect antigen specificity and enhance function in adoptive immunotherapy. The basic concept underlying the design of CARs is to link an extracellular ligand recognition domain, typically a single-chain variable fragment (scFv), to an intracellular signaling module that includes CD3ζ to induce T cell activation upon antigen binding. The modular structure has been extended from first-generation CARs with only a CD3ζ signaling domain to second and third generation CARs that link the signaling endodomains of CD28, 4-1BB, or OX40 to CD3ζ in an attempt to mimic costimulation (signal 2) that is provided during TCR recognition by antigen presenting cells, and required for full physiologic T cell activation (Figure 1) [1,2]. The approach of providing one or more co-stimulatory signals in “cis” in second and third generation CARs augments cytokine production and proliferation of CAR-T cells in vitro, and second generation CD19-specific CARs carrying CD28 or 4-1BB signaling moieties have demonstrated potent in vivo antitumor activity in pre-clinical models and clinical trials for B cell malignancies [3–14]
Figure 1. Signaling of conventional and CAR T cells.
Left: Delivery of signals 1 and 2 to conventional T cells is initiated by the TCR interacting with pMHC on an APC. The spatial distance between the T cell and the APC is ~15nm, which physically excludes from the synapse the inhibitory receptor CD45 because of its large ectodomain. Interaction of CD4/CD8 co-receptors with MHC recruits Lck to the TCR complex, where it phosphorylates and activates Zap70, which provides signal 1. Ligation of the co-stimulatory receptor CD28 by CD80/CD86 results in PI3K activation and delivers signal 2 for full T cell activation. Right: Single receptor design showing a second-generation CAR containing CD3z and CD28 endodomains in cis. Activation of these CARs by a single tumor antigen is sufficient to deliver both signals 1 and 2 in cis, resulting in T cell activation. The spatial distance between CAR T cells and target tumor cells is not known, nor is it known whether this distance is small enough to physically exclude the phosphatase CD45 from the synapse. It is also unknown whether CARs interact with endogenous TCR/CD3z or CD4/CD8 co-receptors.
Despite being empirically designed based on a simple understanding of T cell receptor (TCR) signaling, T cells engineered with CARs specific for the lineage restricted CD19 molecule have been remarkably successful in treating patients with advanced refractory B cell malignancies. Complete tumor regression is achieved in a substantial fraction of patients in multiple studies (Table 1); however, serious cytokine release syndromes and organ toxicities related to activation of T cell effector functions through the CAR are observed in patients with a high tumor burden [10,12–14]. Because CARs confer HLA independent recognition, all patients with tumors that express the target antigen are potentially eligible for therapy, and numerous labs in academia and the biotechnology industry are now working on extending this novel therapy to common epithelial cancers. Success in this endeavor may require improving tumor-specificity, sensitivity, and safety of CARs, and understanding how synthetically constructed receptors direct T cell effector function and fate.
Table 1.
Clinical Trials of CD19 CAR-T cell therapy
| Study | CAR Design | Trial Design | Malignancy | Outcome | Refs |
|---|---|---|---|---|---|
| Kochenderfer et al. 2010 | Murine CD19scFv-CD28/CD3ζ | Case Report | Follicular Lymphoma | PR | 3 |
| Brentjens et al. 2011 | Murine CD19 scFv -CD28/CD3ζ | Pilot (9 patients) | Chronic Lymphocytic Leukemia (n=8), Acute Lymphoblastic Leukemia (n=1) | 1 PR | 4 |
|
Kalos et al. 2011 Porter et al. 2011 |
Murine CD19 scFv–4-1BB/CD3ζ | Pilot (3 patients) | Chronic Lymphocytic Leukemia (n=3) | 2CR, 1PR | 5, 6 |
| Savoldo et al. 2011 | Murine CD19 scFv-CD3ζ and CD28/CD3ζ | Pilot (6 patients) | Non Hodgkin Lymphoma (n=6) | 2 SD | 7 |
| Kochenderfer et al 2012 | Murine CD19scFv-CD28/CD3ζ | Pilot (8 patients) | Non Hodgkin Lymphoma (n=4) Chronic Lymphocytic Leukemia (n=4) | 1 CR, 5 PR | 8 |
|
Brentjens et al. 2013 Davila et al. 2014 |
Murine CD19 scFv-CD28/CD3ζ | Phase 1 (16 patients) | Acute Lymphoblastic Leukemia | CR – 88% | 9, 10 |
| Grupp et al. 2013 | Murine CD19 scFv–4-1BB/CD3ζ | Pilot (2 patients) | Acute Lymphoblastic Leukemia | 2CR | 11 |
| Maude et al. 2014 | Murine CD19 scFv–4-1BB/CD3ζ | Phase 1/2 (30 patients) | Acute Lymphoblastic Leukemia | CR - 90% | 12 |
| Lee et al. 2015 | Murine CD19scFv-CD28/CD3ζ | Phase 1 (21 patients) | Acute Lymphoblastic Leukemia | CR - 70% | 13 |
| Kochenderfer et al. 2015 | Murine CD19scFv-CD28/CD3ζ | Phase 1 (15 patients) | Non Hodgkin Lymphoma (n=11) Chronic Lymphocytic Leukemia (n=4) | CR – 53% PR – 26% |
14 |
CR – complete remission; PR – partial remission; SD – stable disease
The design and function of synthetic CARs might be improved by applying insights from recent studies of TCR recognition. Downstream signaling, T cell function and cell fate determination are significantly impacted by TCR affinity for MHC/peptide complexes determined by Kon/Koff rates, evolutionarily determined spatial constraints between the T cell and APC, immunological synapse formation, TCR clustering, and interaction of CD4 and CD8 co-receptors with MHC [15–17]. Below, we discuss how specificity, structural constraints imposed by T cell:target cell interactions, and receptor affinity impact CAR design and function, within a framework of the current understanding of how these features of TCR signaling impact T cell recognition.
Specificity: discriminating tumor cells from normal cells
A major challenge in CAR design is ensuring elimination of tumor cells while sparing healthy tissue and minimizing toxicity. Conventional T cells have an extraordinary ability to distinguish foreign peptide-MHC (pMHC) from self pMHC through their TCR. As few as 1–10 agonist pMHC in a sea of thousands of self pMHC can trigger T cell activation [18–20]. This specificity is achieved in part by selection in the thymus of a TCR repertoire enriched for low avidity to self pMHC but containing sufficient diversity to include TCRs with high avidity for foreign pMHC. Specificity is further dictated by the requirement for two simultaneous signals delivered through TCR/CD3ζ (signal 1) and co-stimulatory receptors (signal 2) on T cells, both of which are provided only by activated APCs.
In the case of CARs, specificity and safety are determined by the choice of target molecule, and most targets identified thus far are not entirely tumor restricted in their expression. For example, CD19 and CD20 are expressed on both malignant and healthy B cells and as such, T cells expressing second-generation CARs specific for these antigens eradicate tumor cells but also destroy healthy B cells, resulting in B cell depletion that is sustained as long as functional CAR-T cells persist in vivo [10,12–14]. The co-expression of a conditional suicide gene in CAR-T cells that could be activated to eliminate them or regulating expression of the CAR could overcome this side effect since the B cell pool is constantly repopulated from hematopoietic stem cells. CAR-T cells can be transduced with an inducible form of caspase-9 (iCasp9), which dimerizes and is activated upon administration of the drug AP1903, leading to rapid apoptosis of the T cell. The iCasp9 gene has been incorporated into vectors for preclinical studies and demonstrates effective inducible suicide activity in phase 1 clinical trials [21]. Co-expressed cell surface markers such as a truncated epidermal growth factor receptor and CD20 have also been incorporated into CAR vectors and could serve as targets for antibody mediated elimination of CAR-T cells [22,23]. Another approach is to regulate expression of the CAR itself by placing it under the control of regulatory elements that can be turned on or off by delivery of small molecule, such as the Tet-on or doxycycline-inducible systems that have been used in vitro [24]. In other cancers, complete tumor-specificity might be achieved by targeting neo-antigens resulting from oncogenic mutations such as the EGFRvIII, which is deleted in exons 2–7 and exhibits constitutive signaling in the absence of ligand binding, or molecules expressed in development and on tumor cells but not normally expressed in adult tissue such as ROR1 and GD2 [25–27]. Other candidate molecules for CAR-T cell therapy such as Her2/Neu, mesothelin, and MUC16 are overexpressed in tumors relative to normal tissue [28–30], but it remains to be determined whether CARs can be designed with avidity thresholds that are sufficiently tuned to distinguish target cells based on high and low levels of antigen expression. It seems likely that with such targets, toxicity will be a potentially serious issue, as will the escape of tumor variants that express low levels of antigen.
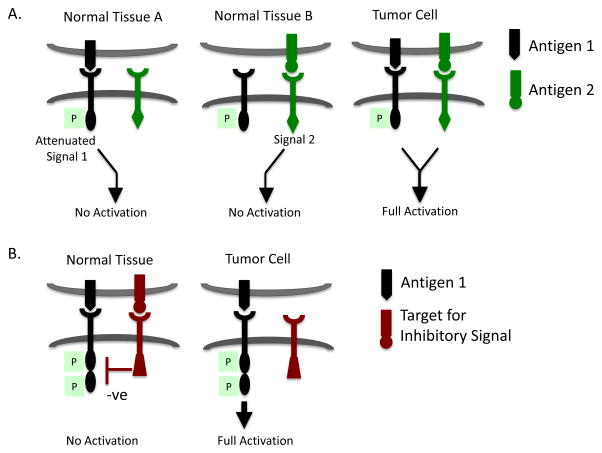
The requirement for co-stimulation (signal 2) provided by activated APCs ensures that TCR signaling is only effective when antigen is encountered in the appropriate context. Several groups are attempting to apply this two-signal concept to CAR design to improve tumor-specificity and limit off-tumor recognition of normal cells by co-expressing two CARs with different binding domains (Figure 2A). This dual receptor strategy has proved to be challenging and its success may depend on the choice of CARs to use and antigens to target. For example, Wilkie et al. used a split receptor system to target human epidermal growth factor 2 (Her2) and mucin 1 (Muc1), both of which are commonly overexpressed in many tumors. The Her2 binding CAR was designed with only a CD3ζ signaling domain, while a second CAR specific for Muc1 provided the co-stimulatory signaling domain of CD28, essentially reproducing the signal 1 and 2 checkpoints of T cell activation [31]. Such dual-specific CAR-T cells only proliferated when they received both signals 1 and 2 in response to Her2/Muc1 double-positive target cells, but not in response to Her2 or Muc1 single-positive cells. However, these dual-specific CAR-T cells were capable of lysing Her2+Muc1- and Her2+Muc1+ cells alike in vitro, demonstrating that the delivery of signal 1 only was sufficient for cytolysis. Grada et al. encountered a similar problem in their split receptor system targeting CD19 and Her2, where dual-specific CAR-T cells lysed both single CD19+ or Her2+ target cells [32]. The inability to discriminate between single and double-positive target cells seriously limits the ability of these CARs to improve targeting specificity over that of single input CARs. Kloss et al. demonstrated that it may be possible to titer down the signaling strength of the CAR delivering “signal 1”, such that full T cell activation depends entirely on signaling through co-stimulatory CAR. They screened scFvs specific for the prostate stem cell antigen (PSCA) in a CAR format and selected a suboptimal CAR that when expressed in T cells alone was insufficient to induce killing of PSCA+ tumor cells in vitro or in vivo. When this suboptimal CAR was co-expressed with a second co-stimulatory CAR that delivered CD28 and 4-1BB signals upon recognition of prostate specific membrane antigen (PSMA), the T cells were capable of recognizing dual PSCA+ PSMA+ tumor cells in vivo [33]. This strategy thus provides one way in which CAR specificity can be increased to minimize the toxicity of off-tumor effects.
Figure 2. Split receptor designs to enhance tumor selective recognition.
A. Enhancing tumor selectivity using chimeric costimulatory receptors. To reduce the potential for CAR-T cells to recognize normal tissues that may express the target antigen, dual CARs have been designed such that two molecules both of which are expressed on tumor cells must be engaged to deliver signals 1 and 2 and fully activate CAR-T cells. Normal cells that express only one of these antigens do not signal T cells sufficiently for full activation. The CAR that delivers signal 1 may need to be attenuated in signaling, such as by mutating one or more ITAMs in CD3ζ or by reducing the affinity of the scFv.
B. Enhancing tumor selectivity using inhibitory chimeric receptors. In this scenario, a CAR that delivers a dominant inhibitory signal such as might be mediated via a PD1 endodomain for example, is co-expressed with a CAR capable of full T cell activation. Engagement of both target antigens on normal cells would suppress T cell activation, whereas recognition of only the activating ligand on tumor cells would result in T cell activation and effector function.
Another way to increase the tumor-specificity of CARs is to use a split receptor system to provide negative signaling in the presence of normal, but not tumor, tissue. Such dominant inhibition of TCR signaling is yet another principle of conventional T cell biology. Upon activation, T cells up-regulate inhibitory receptors like PD-1 and CTLA-4 which dampen TCR signaling, either through competition for co-stimulatory ligands (e.g. CTLA-4 for the CD28-ligands CD80 and CD86), or via engagement of ligands up-regulated on APCs by pro-inflammatory cytokines (e.g. PD-1 for PD-L1 and PD-L2). These negative feedback mechanisms prevent excess T cell activity and minimize pro-inflammatory damage to the host. The importance of this regulation is demonstrated by the autoimmunity and lymphoproliferative disease that develop in PD-1 and CTLA-4 knockout mice [34,35]. Moreover, PD-L1 is constitutively expressed at sites of immune privilege, such as the neurons of the eye and trophoblasts in the placenta, thereby protecting these tissues from immune attack [36]. Such inhibitory receptors are also commonly up-regulated on “exhausted” T cells during chronic infection and cancer after prolonged exposure to antigen, and immunotherapies aimed at blocking the PD-1 and CTLA-4 pathways have met with significant clinical success in boosting T cell activity toward viral and tumor antigens [37,38].
A similar logic can be applied to regulating CAR recognition, where co-expression of a second inhibitory CAR specific for an antigen expressed on normal, but not tumor, tissue would protect normal tissue from CAR-T cell-mediated attack (Figure 2B). Fedorov et al. demonstrated the feasibility of this approach by co-expressing a CD19-specific activating CAR carrying the CD3ζ and CD28 endodomains and a PSMA-specific inhibitory CAR carrying the endodomain of PD-1 or CTLA-4 [39]. CAR-T cells were activated when exposed to target cells expressing CD19 in the absence of PSMA, but their ability to proliferate, lyse target cells, and secrete pro-inflammatory cytokines were inhibited by target cells co-expressing PSMA. This principle is particularly attractive for enhancing the specificity of activating CARs that recognize ligands expressed on tumor cells and some normal cells. However, in addition to the issue of which ligands restricted to normal cells to select to deliver the negative signal, a significant challenge in designing inhibitory CARs is that the mechanisms by which these inhibitory receptors exert their function in TCR signaling are not fully understood. For example, PD-1 inhibits TCR signaling by co-localizing with TCR microclusters in the synapse, and this co-localization is required for its ability to recruit SHP2 and dephosphorylate proximal TCR signaling proteins like CD3ζ, Zap70, and PKCθ [40]. Thus, the ability of the inhibitory CAR to co-localize with the activating CAR may be particularly important when using the PD-1 endodomain. By contrast, CTLA-4 is thought to inhibit TCR signaling by competing with CD28 for binding to CD80/CD86 and physically excluding CD28 from the synapse, thereby dampening co-stimulatory signaling [41]. Recent work even suggests that the primary purpose of the CTLA-4 endodomain is to regulate the surface expression of CTLA-4 rather than to induce downstream inhibitory signaling [42]. This mechanism of inhibition would be predicted to be less effective against second- or third-generation activating CARs, which already carry co-stimulatory endodomains. In fact, there is some evidence that the PD-1 endodomain is more effective than the CTLA-4 endodomain at inhibiting second-generation CAR activity [39].
Ultimately, we will need better assays to compare how the use of split receptors differ from conventional TCR signaling and might be engineered to better mimic the regulation of TCR signaling. Past studies on TCR signaling have taken a reductionist approach, using planar lipid bilayers as artificial APCs to monitor the spatial-temporal dynamics of specific proteins during formation of the immunological synapse [43]. Such a method could be particularly informative for estimating the density of ligands needed to deliver activating or inhibitory signals via co-expressed CARs, and might provide a platform for testing whether specific antigens would make good targets for co-stimulatory or co-inhibitory CARs based on their expression levels. In addition, several studies have demonstrated that the ability of co-stimulatory or co-inhibitory receptors to co-localize with TCRs at the synapse is critical for their stimulatory or inhibitory function, respectively [40,41]. Thus, live cell imaging techniques like confocal microscopy should prove useful in providing detailed insight into the spatial and temporal dynamics of CAR signaling and identifying how CAR split receptor systems deviate from conventional T cell signaling.
Structural constraints in T cell:target cell interactions
Structural and spatial elements of TCR recognition have evolved for precise regulation of the interaction between a T cell and its target cell, and can be challenging to recapitulate with synthetic receptors. During formation of the immunological synapse, the ~15nm distance between a T cell and APC is dictated by the structures of the TCR and pMHC. This close proximity is important to exclude the phosphatases CD45 and CD148, which have large ectodomains longer than 15nm, from the synapse to allow TCR induced tyrosine phosphorylation to be initiated in the absence of these negative regulators. In support of the importance of this molecular segregation at the site of TCR engagement, a mutated form of CD45 with a shortened ectodomain attenuates T cell signaling [44,45].
The spatial distance between CARs and their target antigens may be equally important for effective initiation of T cell signaling, but depends on an entirely different set of structural elements related to the location of the epitope on the target molecule and the spacer domain between the scFv and the T cell membrane. Several studies have demonstrated that the same epitope can activate CAR-T cells with greater efficiency when expressed in a more membrane-proximal position than a membrane-distal position [46–50]. For example, Hombach et al. demonstrated that CAR T cells recognizing the membrane-distal “N” epitope of the carcinoembryonic antigen (CEA) were only modestly activated; however, when they engineered recombinant CEA protein to express the N epitope in a membrane-proximal position, the same CAR T cells were activated more efficiently [46]. This suggests that targeting some membrane distal epitopes on tumor cells may permit large phosphatases like CD45 and CD148 to enter the synapse and inhibit phosphorylation events initiated by CAR engagement. Tailoring the extracellular spacer sequence between the T cell membrane and the ligand-binding scFv to promote synapse formation can assist in overcoming steric constraints imposed by the location of the target epitope. Work in our lab has demonstrated that, when targeting a membrane-distal epitope on the receptor tyrosine kinase ROR1, which is expressed on many solid tumors, CARs with a shortened extracellular spacer conferred superior recognition of ROR1+ tumor cells and induction of T cell effector function in vitro compared to the same scFv with a longer spacer [51]. The shortened spacer may decrease the distance between T cell and target cells and maintain exclusion of larger inhibitory molecules from the synapse. By contrast, when targeting a membrane-proximal epitope on ROR1, a longer extracellular spacer resulted in greater effector T cell function, indicating that the optimal spacer length of CARs will likely vary based on the position of the target epitope [52]. Thus, the design of CARs for novel targets should factor in the location of the target epitope and customize the spacer length to optimize CAR-T cell signaling.
Live-imaging technologies could prove particularly informative for studying the spatial and temporal dynamics of synapse formation between CAR-T cells and tumor cells. Using retroviral or lentiviral gene-transfer methods, signaling proteins fused to GFP can be imaged in primary T cells [53], and T cell:APC conjugates can even be imaged in vivo [54]. These methods can be used to determine whether altering the CAR spacer length affects synapse formation and/or the ability of CD45 or CD148 to localize to the synapse. Interestingly, a recent study demonstrated that co-culturing HEK cells expressing a CD19 CAR with CD19+ Raji cells resulted in clustering of receptors at the APC:T cell interface, recruitment of Zap70, and exclusion CD45 from the synapse [55]. A caveat of this study is the use of HEK cells rather than T cells; nevertheless, the results suggest that at least in some contexts, CD45 is excluded from the synapse established between CAR-T cells and their targets. The CD19 CAR-T cell interface, however, was highly convoluted and irregular, distinct from the more even and ordered TCR-pMHC interface, suggesting that some aspects of synapse formation may be distinct in CAR-T cells. A more comprehensive analysis of primary CAR-T cells and target tumor cells, both in vitro and in vivo, will be needed to understand how the formation, composition, and organization of the synapse compares to conventional T cells.
Affinity and sensitivity considerations
Tumor antigens are often expressed at low levels on the cell surface, and this can present an obstacle to eliciting effective CAR-T cell signaling. In tumors in which CAR-T cells are effective in the clinic such as chronic lymphocytic leukemia (CLL) and ALL for example, the number of CD19 molecules per tumor cell has been estimated to be ~10,000, which 2–5 fold higher than alternative targets such as ROR1. The lower level of target antigen density that is required to elicit effective T cell activation will likely vary for different target antigens and CAR constructs depending on CAR expression, scFv affinity, spatial constraints, and on tumor cell expression of adhesion and co-stimulatory ligands that provide signals distinct from the CAR. A recent study demonstrated that for a CD20-specific CAR, ~200 CD20 molecules per target cell were required for lytic activity, while the requirement for cytokine production was ~10-fold higher. Consequently, CD20 specific CAR-T cells could efficiently kill even CD20-downregulated lymphoma and leukemia targets in vitro [56]. If further refined, such studies may be useful in determining the antigen density threshold required for CAR activity and predicting whether tumor cells or healthy tissues will elicit CAR activation.
Some direction for designing CARs that elicit T cell activation towards very low-density antigens may be provided from the study of conventional TCRs, which display an exquisite sensitivity to low-density antigen. Imaging studies suggest that even a single agonist pMHC can trigger cytokine production from some naïve T cells [18]. The mechanisms underlying TCR sensitivity and concomitant specificity are not fully understood and continue to be an important area of investigation. One way sensitivity is achieved is through the activation of co-stimulatory receptors, which can lower the activation threshold for TCRs. In the absence of CD28 ligation, T cells require very high numbers of TCR-pMHC interactions and prolonged stimulation, whereas CD28 co-stimulation allows T cells to respond to lower numbers of TCR-pMHC interactions [57,58]. Likewise, other receptors like NKG2D have been shown to function as co-stimulatory receptors by enhancing IL-2 production and T cell proliferation upon TCR triggering [59]. Thus, signaling domains from such co-stimulatory receptors may be incorporated into the CAR itself or into split-receptor systems to enhance T cell sensitivity to target antigens.
Another explanation for the exquisite sensitivity of TCRs is provided by the “serial triggering” model, which suggests that for efficient T cell activation, multiple TCRs should sample the pMHC, a process which requires sufficiently low binding affinity and short Koff rates of the receptor-ligand interaction [60]. By contrast, the function of some CARs, which typically have much higher affinity measured by surface plasmon resonance than those measured for TCR/pMHC interactions, has been shown to improve with increasing scFv affinity. For example, Hudecek et al. demonstrated that when testing a panel of ROR1-specific CARs, CARs derived from a higher affinity scFv conferred greater effector function against ROR1+ mantle cell lymphoma and epithelial cancer cell lines in vitro and in vivo [51]. However, other studies indicate the existence of an avidity threshold for both CARs and TCRs, above which T cell function is not improved or is actually reduced [61–63]. In a study using CARs specific for pMHC, increased affinity and surface expression of the CAR paradoxically resulted in reduced cytolytic activity and sensitivity to low peptide concentrations relative to an αβTCR specific for the same epitope [63]. These studies suggest that a specific range in binding affinity is required for optimal CAR functionality.
The rational design of CARs will benefit from the development of tools for precise study of the Kon/Koff rates, preferably using live cells that express the CAR, to determine the target range for receptor affinity and optimize CAR sensitivity, especially to low-density antigens. One such tool was recently developed by Busch and colleagues, who used a real-time microscopy assay to study the Koff rates of TCRs on living T cells [64]. In this approach, monomeric pMHC conjugated to fluorescent dyes are multimerized on a Streptactic backbone via the Streptag sequences and are used to label viable epitope-specific T cells. Addition of D-biotin displaces the pMHC by binding to Streptactin at Streptag sequences with higher affinity, leaving the pMHC bound to TCRs. Using fluorescence microscopy, the dissociation rate of pMHC from TCRs can be quantified as the decay in fluorescence intensity over time. Such technology may similarly be used to rapidly measure Koff rates of CARs expressed in T cells that might correlate with enhanced CAR-T cell functionality in in vitro and in vivo assays against tumors that express both low or high densities of target antigen.
Another strategy is to directly screen “CAR bodies” using scFv libraries formatted as CARs and expressed through lentiviral transduction in T cells, which are then stimulated with the tumor cells of interest [65]. Current methods for screening potential scFvs involve testing against cell surface-expressed antigens. Although scFvs in solution may recognize the antigen in this context, they may not be as effective when expressed as a CAR on the T cell surface, where adhesion and other cell surface molecules affect T cell:tumor cell interactions. By expressing a library of scFv as CARs on T cells and screening the T cells for activation following antigen exposure, this strategy allows for the selection of scFvs based on their ability to induce T cell activation and proliferation, rather than just selecting for the highest affinity binders. Because current data suggests the optimal CAR affinity cannot be determined a priori for individual target molecules, such an unbiased approach that relies on CAR expression in a T cell may be particularly important to identify the ideal affinity range for a given scFv and target antigen.
Missing signal transduction modules
Another mechanism of signal amplification in TCR recognition that is lacking with CARs involves the interaction of CD4 and CD8 co-receptors with pMHC. The co-receptors CD4 and CD8 play a critical role in the initiation of TCR signaling by bringing Lck, which is bound to their cytoplasmic tails, into proximity of the TCR/CD3ζ complex, where it phosphorylates ITAMs in the CD3ζ chain, facilitating the binding and phosphorylation of Zap70 to promote its catalytic activity. However, once Lck is activated, co-receptors can associate with other TCRs weakly bound to self pMHC and mediate activation of those TCRs [66]. As a result of positive selection, TCRs are weakly self-reactive and these interactions with self pMHC are important for amplification of signals from foreign pMHC/TCR interactions [67,68]. Although CARs are clearly able to phosphorylate Zap70 and initiate downstream TCR signaling [69], whether they interact structurally with CD8 co-receptors or endogenous TCR/CD3ζ complexes is unclear. One study demonstrated that use of the CD3 transmembrane domain in construction of a CAR enabled it to associate with endogenous CD3ζ and be incorporated into TCR clusters [70], as CD3ζ is known to associate with αβ TCRs via its transmembrane region [71,72]. This study, however, was performed in Jurkat cells which have varied expression of proximal TCR signaling components relative to primary T cells, and other studies found that use of the CD3 transmembrane domain actually resulted in reduced protein stability and surface expression of the CAR compared to the CD28 transmembrane domain [7]. Thus, whether the CAR forms a synapse and clusters in a manner similar to TCRs or interacts with endogenous TCR/CD3 complexes and co-receptors remains unresolved. Strategies in CAR design that could enhance interaction with co-receptors may allow activation of a single CAR to be amplified by serial triggering of endogenous TCRs.
Alternative ligand binding domains
ScFv-based extracellular recognition domains have largely dominated the synthetic antigen receptor field. As such, development of new CARs relies heavily on constructing scFvs from murine monoclonal antibody VH and VL sequences specific for tumor associated molecules from already available hybridomas. In addition to being limited by the small repertoire of tumor-selective monoclonal antibodies, scFvs differ significantly from TCRs in at least two respects that can limit their utility -- 1) their affinity for antigen is significantly higher, which can interfere with optimal signaling as discussed above; and 2) their specificity is limited to antigens expressed on the tumor cell surface, not intracellular antigens. Developing CAR recognition domains that more closely mimic TCRs is an intriguing strategy for enhancing T cell functionality. Several groups have employed phage display and hybridoma strategies to isolate “TCR-like” antibodies, which bind tumor-derived peptides in an HLA-restricted manner [63,73]. These antibodies imitate the specificity of TCRs in their ability to recognize intracellular tumor antigens expressed on MHC; however, they still exhibit the kinetics and high-affinity binding properties of antibodies rather than TCRs [63].
Efforts to design recognition domains de novo that mimic TCR binding from proteins other than scFvs are being developed. One platform uses designed ankyrin repeat proteins (DARPins), which are derived from normal ankyrin proteins that mediate high affinity protein-protein interactions and can be extensively tuned for affinity, on and off rates, and multiplexed specificities [74]. DARPins have been selected from phage or ribosomal display libraries to bind to Her2 and EGFR, and our group is currently investigating the functionality of CARs constructed using a DARPin binding domain. Computational methodologies have also been demonstrated to be effective at designing synthetic proteins de novo to target various antigens with highly defined properties [75–77]. This approach may be particularly powerful in designing more “TCR-like” CARs that are selected not just for their specificity for a particular tumor antigen, but also for many other physical criteria such as Kon/Koff rates, binding distance from its target epitope, and the ability to interact with endogenous TCR/CD3 and/or co-receptors.
A concern when considering these strategies, however, is the potential for enhanced immunogenicity of synthetic polypeptide binding domains and/or novel sequences at fusion sites between components of CARs. Immunogenicity may be overcome in part by selecting scFvs from human libraries. In addition, fusion sites can be designed with the help of bioinformatics tools to limit the binding of peptide sequences to MHC, although antibody recognition of these sequences may still be a limitation. Efforts to “humanize” such proteins and limit their immunogenicity will be critical for their effectiveness in the clinic.
Concluding Remarks
Despite the initial and remarkable successes of CAR-T cells in the treatment of leukemia and lymphoma, the application of CAR-T cells to the treatment of common epithelial tumors, which have few truly tumor-specific target molecules and an immunosuppressive microenvironment hostile to T cells, is likely to prove challenging. Deeper analysis of CAR signaling using new tools is necessary to provide insights into how CARs differ from TCRs and might be engineered to perform more effectively in clinical settings. Future areas for research will need to incorporate more sophisticated, global approaches to define how CAR signaling pathways differ from TCRs and identify what missing elements of TCR signaling might best be incorporated into CAR design (Table 2). Mass spectrometry-based techniques enable the quantification of changes in intracellular phosphorylation events that are initiated and propagated by signals through TCRs and CARs. This technology has been employed by several groups to gain systemwide insights into the role of phosphorylation during TCR signaling in human Jurkat and murine transgenic T cells [78,79]. Unbiased analysis of the phosphoproteome in CAR-T cells versus conventional T cells, both before and after receptor triggering, would help identify which signaling pathways are engaged and how these may differ between the two cell types. Mass-spectrometry can even be extended to quantify other post-translational modifications, such as ubiquitylation and acetylation that would provide a more comprehensive overview of the protein modifications that result from CAR or TCR triggering. Likewise, genome-wide transcriptional analysis would provide another global, unbiased insight into how CAR-T cell signaling and activation dictates effector function and cell fate. Single-cell RNA sequencing may be even more informative, revealing differences in gene transcription that may be obscured by heterogeneity at the cell population level.
Table 2.
Unresolved issues in CAR T cell biology
| Unresolved Issues in CAR T Cell Biology | Experimental Approaches to Answering These Questions |
|---|---|
| Intracellular signaling pathways |
|
| Synapse formation/organization |
|
Receptor characteristics
|
|
Cell fate decisions:
|
|
An aspect of CAR-T cell biology that has not been well studied is how signaling through CARs affects the cells ability to develop heritable “memory” to tumor antigens, which may be essential for complete eradication of malignant cells. Epigenetic modifications, in the form of changes to DNA and/or histones, are known to play an important role in CD4+ helper T cell differentiation and CD8+ memory formation [80,81]. Using chromatin immunoprecipitation combined with next-generation sequencing (ChIP-seq), genome-wide changes in transcriptionally “permissive” and “repressive” histone modifications can be quantified and mapped in various cell subsets. For example, O’Shea’s group used this technology to study the epigenetic modifications that occur during the differentiation of naïve T cells into fully polarized Th1, Th2, and Th17 T helper cells and to provide insights into the plasticity of differentiated subsets [82]. Next generation sequencing can also be used to generate comprehensive genome-wide mapping of DNA methylation sites, which have been shown to play a role in the lineage-specific differentiation of hematopoietic progenitors [83]. Global analysis of the “epigenome” in CAR-T cells may provide a deeper understanding of how signaling through these synthetic receptors affects cytokine profiles, differentiation and memory potential of CAR-T cells.
The principle that T cells that are genetically modified to express novel synthetic CARs can effectively treat advanced refractory cancers has been established but many questions remain to realize the full potential of this new therapeutic modality (Box 1). Engineering safer, more effective CARs for cancer therapy will require moving beyond the traditional empiric approach to receptor design and cell engineering, ideally guided by our knowledge of TCR signaling, T cell biology, and manipulation of the tumor microenvironment. It is now apparent that binding affinity, Kon/Koff rates, and spatial constraints between CARs and target cells can influence the ability of CARs to optimally activate T cells for tumor recognition. The precise study of these parameters should provide insights for the design of new receptors that may improve anti-tumor function, particularly against solid tumors. Other genetic modifications to make T cells resistant to tumor induced immunosuppression and combining CAR-T cells with other modalities, such as checkpoint inhibitors, can be envisioned as future approaches in this rapidly evolving field.
Box 1. Outstanding Questions.
Can common epithelial cancers be successfully treated with chimeric antigen receptor modified T cells?
What are the optimal signaling and co-stimulatory domains for chimeric antigen receptors and will these differ depending on the tumor being targeted?
Can measurements of the affinity of scFvs and target ligand density on tumor cells be used to inform more effective chimeric antigen receptor design?
What additional modifications to chimeric antigen receptor modified T cells will be necessary to overcome immunosuppressive tumor microenvironments?
Can split receptor systems using “and” and “not” logic gates be effective in the clinic for specifying selective recognition of tumor and not normal cells?
How does chimeric antigen receptor signaling differ from TCR signaling in directing cell fate decisions including formation of central and effector memory subsets?
What are the alternatives to scFvs as ligand binding domains in CAR design?
How great a barrier will immunogenicity of chimeric antigen receptor modified T cells be to their utility as cancer therapeutics?
Highlights.
T cells engineered with synthetic chimeric receptors can treat established tumors
Chimeric receptors imperfectly mimic critical elements of TCR signaling
Dual receptor systems can improve specificity for tumor cells
CAR signaling of effector functions and cell fate requires further investigation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maher J, et al. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta/CD28 receptor. Nat Biotechnol. 2002;20:70–75. doi: 10.1038/nbt0102-70. [DOI] [PubMed] [Google Scholar]
- 2.Imai C, et al. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia. 2004;18:676–684. doi: 10.1038/sj.leu.2403302. [DOI] [PubMed] [Google Scholar]
- 3.Kochenderfer JN, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116:4099–4102. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brentjens RJ, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817–4828. doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalos M, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3:95ra73–95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porter DL, et al. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Savoldo B, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest. 2011;121:1822–1826. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kochenderfer JN, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–2720. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brentjens RJ, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5:177ra38–177ra38. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davila ML, et al. Efficacy and toxicity management of 19–28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6:224ra25–224ra25. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grupp SA, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maude SL, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee DW, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kochenderfer JN, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33:540–549. doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chakraborty AK, Weiss A. Insights into the initiation of TCR signaling. Nat Immunol. 2014;15:798–807. doi: 10.1038/ni.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hogquist KA, Jameson SC. The self-obsession of T cells: how TCR signaling thresholds affect fate “decisions” and effector function. Nat Immunol. 2014;15:815–823. doi: 10.1038/ni.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malissen B, et al. Integrative biology of T cell activation. Nat Immunol. 2014;15:790–797. doi: 10.1038/ni.2959. [DOI] [PubMed] [Google Scholar]
- 18.Huang J, et al. A single peptide-major histocompatibility complex ligand triggers digital cytokine secretion in CD4(+) T cells. Immunity. 2013;39:846–857. doi: 10.1016/j.immuni.2013.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sykulev Y, et al. Evidence that a single peptide-MHC complex on a target cell can elicit a cytolytic T cell response. Immunity. 1996;4:565–571. doi: 10.1016/s1074-7613(00)80483-5. [DOI] [PubMed] [Google Scholar]
- 20.Irvine DJ, et al. Direct observation of ligand recognition by T cells. Nature. 2002;419:845–849. doi: 10.1038/nature01076. [DOI] [PubMed] [Google Scholar]
- 21.Gargett T, Brown MP. The inducible caspase-9 suicide gene system as a “safety switch” to limit on-target, off-tumor toxicities of chimeric antigen receptor T cells. Front Pharmacol. 2014;5:235. doi: 10.3389/fphar.2014.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X, et al. A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells. Blood. 2011;118:1255–1263. doi: 10.1182/blood-2011-02-337360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Philip B, et al. A highly compact epitope-based marker/suicide gene for easier and safer T-cell therapy. Blood. 2014;124:1277–1287. doi: 10.1182/blood-2014-01-545020. [DOI] [PubMed] [Google Scholar]
- 24.Pluta K, et al. Tight control of transgene expression by lentivirus vectors containing second-generation tetracycline-responsive promoters. J Gene Med. 2005;7:803–817. doi: 10.1002/jgm.712. [DOI] [PubMed] [Google Scholar]
- 25.Bax DA, et al. EGFRvIII deletion mutations in pediatric high-grade glioma and response to targeted therapy in pediatric glioma cell lines. Clin Cancer Res. 2009;15:5753–5761. doi: 10.1158/1078-0432.CCR-08-3210. [DOI] [PubMed] [Google Scholar]
- 26.Hudecek M, et al. The B-cell tumor-associated antigen ROR1 can be targeted with T cells modified to express a ROR1-specific chimeric antigen receptor. Blood. 2010;116:4532–4541. doi: 10.1182/blood-2010-05-283309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu AL, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363:1324–1334. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morgan RA, et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lanitis E, et al. Redirected antitumor activity of primary human lymphocytes transduced with a fully human anti-mesothelin chimeric receptor. Mol Ther. 2012;20:633–643. doi: 10.1038/mt.2011.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chekmasova AA, et al. Successful eradication of established peritoneal ovarian tumors in SCID-Beige mice following adoptive transfer of T cells genetically targeted to the MUC16 antigen. Clin Cancer Res. 2010;16:3594–3606. doi: 10.1158/1078-0432.CCR-10-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilkie S, et al. Dual targeting of ErbB2 and MUC1 in breast cancer using chimeric antigen receptors engineered to provide complementary signaling. J Clin Immunol. 2012;32:1059–1070. doi: 10.1007/s10875-012-9689-9. [DOI] [PubMed] [Google Scholar]
- 32.Grada Z, et al. TanCAR: A Novel Bispecific Chimeric Antigen Receptor for Cancer Immunotherapy. Mol Ther Nucleic Acids. 2013;2:e105. doi: 10.1038/mtna.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kloss CC, et al. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nat Biotechnol. 2013;31:71–75. doi: 10.1038/nbt.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishimura H, et al. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 35.Khattri R, et al. Lymphoproliferative disorder in CTLA-4 knockout mice is characterized by CD28-regulated activation of Th2 responses. J Immunol. 1999;162:5784–5791. [PubMed] [Google Scholar]
- 36.Francisco LM, et al. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pauken KE, Wherry EJ. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 2015;36:265–276. doi: 10.1016/j.it.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fedorov VD, et al. PD-1- and CTLA-4-based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses. Sci Transl Med. 2013;5:215ra172–215ra172. doi: 10.1126/scitranslmed.3006597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yokosuka T, et al. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J Exp Med. 2012;209:1201–1217. doi: 10.1084/jem.20112741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yokosuka T, et al. Spatiotemporal basis of CTLA-4 costimulatory molecule-mediated negative regulation of T cell activation. Immunity. 2010;33:326–339. doi: 10.1016/j.immuni.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 42.Walker LSK, Sansom DM. Confusing signals: recent progress in CTLA-4 biology. Trends Immunol. 2015;36:63–70. doi: 10.1016/j.it.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grakoui A, et al. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 44.Cordoba SP, et al. The large ectodomains of CD45 and CD148 regulate their segregation from and inhibition of ligated T-cell receptor. Blood. 2013;121:4295–4302. doi: 10.1182/blood-2012-07-442251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Irles C, et al. CD45 ectodomain controls interaction with GEMs and Lck activity for optimal TCR signaling. Nat Immunol. 2003;4:189–197. doi: 10.1038/ni877. [DOI] [PubMed] [Google Scholar]
- 46.Hombach AA, et al. T cell activation by antibody-like immunoreceptors: the position of the binding epitope within the target molecule determines the efficiency of activation of redirected T cells. J Immunol. 2007;178:4650–4657. doi: 10.4049/jimmunol.178.7.4650. [DOI] [PubMed] [Google Scholar]
- 47.Guest RD, et al. The role of extracellular spacer regions in the optimal design of chimeric immune receptors: evaluation of four different scFvs and antigens. J Immunother. 2005;28:203–211. doi: 10.1097/01.cji.0000161397.96582.59. [DOI] [PubMed] [Google Scholar]
- 48.James SE, et al. Antigen sensitivity of CD22-specific chimeric TCR is modulated by target epitope distance from the cell membrane. J Immunol. 2008;180:7028–7038. doi: 10.4049/jimmunol.180.10.7028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Till BG, et al. CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4-1BB domains: pilot clinical trial results. Blood. 2012;119:3940–3950. doi: 10.1182/blood-2011-10-387969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haso W, et al. Anti-CD22-chimeric antigen receptors targeting B-cell precursor acute lymphoblastic leukemia. Blood. 2013;121:1165–1174. doi: 10.1182/blood-2012-06-438002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hudecek M, et al. Receptor affinity and extracellular domain modifications affect tumor recognition by ROR1-specific chimeric antigen receptor T cells. Clin Cancer Res. 2013;19:3153–3164. doi: 10.1158/1078-0432.CCR-13-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hudecek M, et al. The nonsignaling extracellular spacer domain of chimeric antigen receptors is decisive for in vivo antitumor activity. Cancer Immunol Res. 2015;3:125–135. doi: 10.1158/2326-6066.CIR-14-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ehrlich LIR, et al. Dynamics of p56lck translocation to the T cell immunological synapse following agonist and antagonist stimulation. Immunity. 2002;17:809–822. doi: 10.1016/s1074-7613(02)00481-8. [DOI] [PubMed] [Google Scholar]
- 54.Stoll S, et al. Dynamic imaging of T cell-dendritic cell interactions in lymph nodes. Science. 2002;296:1873–1876. doi: 10.1126/science.1071065. [DOI] [PubMed] [Google Scholar]
- 55.James JR, Vale RD. Biophysical mechanism of T-cell receptor triggering in a reconstituted system. Nature. 2012;487:64–69. doi: 10.1038/nature11220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Watanabe K, et al. Target antigen density governs the efficacy of anti-CD20-CD28-CD3 ζ chimeric antigen receptor-modified effector CD8+ T cells. J Immunol. 2015;194:911–920. doi: 10.4049/jimmunol.1402346. [DOI] [PubMed] [Google Scholar]
- 57.Oliveira-dos-Santos AJ, et al. CD28 costimulation is crucial for the dev elopment of spontaneous autoimmune encephalomyelitis. J Immunol. 1999;162:4490–4495. [PubMed] [Google Scholar]
- 58.Viola A, Lanzavecchia A. T cell activation determined by T cell receptor number and tunable thresholds. Science. 1996;273:104–106. doi: 10.1126/science.273.5271.104. [DOI] [PubMed] [Google Scholar]
- 59.Groh V, et al. Costimulation of CD8alphabeta T cells by NKG2D via engagement by MIC induced on virus-infected cells. Nat Immunol. 2001;2:255–260. doi: 10.1038/85321. [DOI] [PubMed] [Google Scholar]
- 60.Stone JD, et al. T-cell receptor binding affinities and kinetics: impact on T-cell activity and specificity. Immunology. 2009;126:165–176. doi: 10.1111/j.1365-2567.2008.03015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chmielewski M, et al. T cell activation by antibody-like immunoreceptors: increase in affinity of the single-chain fragment domain above threshold does not increase T cell activation against antigen-positive target cells but decreases selectivity. J Immunol. 2004;173:7647–7653. doi: 10.4049/jimmunol.173.12.7647. [DOI] [PubMed] [Google Scholar]
- 62.Thomas S, et al. Human T cells expressing affinity-matured TCR display accelerated responses but fail to recognize low density of MHC-peptide antigen. Blood. 2011;118:319–329. doi: 10.1182/blood-2010-12-326736. [DOI] [PubMed] [Google Scholar]
- 63.Oren R, et al. Functional comparison of engineered T cells carrying a native TCR versus TCR-like antibody-based chimeric antigen receptors indicates affinity/avidity thresholds. J Immunol. 2014;193:5733–5743. doi: 10.4049/jimmunol.1301769. [DOI] [PubMed] [Google Scholar]
- 64.Nauerth M, et al. TCR-ligand koff rate correlates with the protective capacity of antigen-specific CD8+ T cells for adoptive transfer. Sci Transl Med. 2013;5:192ra87–192ra87. doi: 10.1126/scitranslmed.3005958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alonso-Camino V, et al. CARbodies: Human Antibodies Against Cell Surface Tumor Antigens Selected From Repertoires Displayed on T Cell Chimeric Antigen Receptors. Mol Ther Nucleic Acids. 2013;2:e93. doi: 10.1038/mtna.2013.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hoerter JAH, et al. Coreceptor affinity for MHC defines peptide specificity requirements for TCR interaction with coagonist peptide-MHC. J Exp Med. 2013;210:1807–1821. doi: 10.1084/jem.20122528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Krogsgaard M, et al. Agonist/endogenous peptide-MHC heterodimers drive T cell activation and sensitivity. Nature. 2005;434:238–243. doi: 10.1038/nature03391. [DOI] [PubMed] [Google Scholar]
- 68.Krogsgaard M, et al. A role for “self” in T-cell activation. Semin Immunol. 2007;19:236–244. doi: 10.1016/j.smim.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Terakura S, et al. Generation of CD19-chimeric antigen receptor modified CD8+ T cells derived from virus-specific central memory T cells. Blood. 2012;119:72–82. doi: 10.1182/blood-2011-07-366419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bridgeman JS, et al. The optimal antigen response of chimeric antigen receptors harboring the CD3zeta transmembrane domain is dependent upon incorporation of the receptor into the endogenous TCR/CD3 complex. J Immunol. 2010;184:6938–6949. doi: 10.4049/jimmunol.0901766. [DOI] [PubMed] [Google Scholar]
- 71.Call ME, et al. The organizing principle in the formation of the T cell receptor-CD3 complex. Cell. 2002;111:967–979. doi: 10.1016/s0092-8674(02)01194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Blumberg RS, et al. Structure of the T-cell antigen receptor: evidence for two CD3 epsilon subunits in the T-cell receptor-CD3 complex. Proc Natl Acad Sci USA. 1990;87:7220–7224. doi: 10.1073/pnas.87.18.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang G, et al. Anti-melanoma activity of T cells redirected with a TCR-like chimeric antigen receptor. Sci Rep. 2014;4:3571. doi: 10.1038/srep03571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tamaskovic R, et al. Designed ankyrin repeat proteins (DARPins) from research to therapy. Meth Enzymol. 2012;503:101–134. doi: 10.1016/B978-0-12-396962-0.00005-7. [DOI] [PubMed] [Google Scholar]
- 75.Procko E, et al. A computationally designed inhibitor of an Epstein-Barr viral Bcl-2 protein induces apoptosis in infected cells. Cell. 2014;157:1644–1656. doi: 10.1016/j.cell.2014.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Whitehead TA, et al. Computational design of novel protein binders and experimental affinity maturation. Meth Enzymol. 2013;523:1–19. doi: 10.1016/B978-0-12-394292-0.00001-1. [DOI] [PubMed] [Google Scholar]
- 77.Koga N, et al. Principles for designing ideal protein structures. Nature. 2012;491:222–227. doi: 10.1038/nature11600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mayya V, et al. Quantitative phosphoproteomic analysis of T cell receptor signaling reveals system-wide modulation of protein-protein interactions. Sci Signal. 2009;2:ra46–ra46. doi: 10.1126/scisignal.2000007. [DOI] [PubMed] [Google Scholar]
- 79.Navarro MN, et al. Phosphoproteomic analysis reveals an intrinsic pathway for the regulation of histone deacetylase 7 that controls the function of cytotoxic T lymphocytes. Nat Immunol. 2011;12:352–361. doi: 10.1038/ni.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kanno Y, et al. Transcriptional and epigenetic control of T helper cell specification: molecular mechanisms underlying commitment and plasticity. Annu Rev Immunol. 2012;30:707–731. doi: 10.1146/annurev-immunol-020711-075058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weng NP, et al. The molecular basis of the memory T cell response: differential gene expression and its epigenetic regulation. Nat Rev Immunol. 2012;12:306–315. doi: 10.1038/nri3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wei G, et al. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity. 2009;30:155–167. doi: 10.1016/j.immuni.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ji H, et al. Comprehensive methylome map of lineage commitment from haematopoietic progenitors. Nature. 2010;467:338–342. doi: 10.1038/nature09367. [DOI] [PMC free article] [PubMed] [Google Scholar]