Abstract
Fungal rhinosinusitis (FRS) comprises a spectrum of disease processes that vary in clinical presentation, histologic appearances, and biological significance. FRS can be acute or chronic and is most commonly classified as non-invasive or invasive based on whether fungi have invaded into tissue. This manuscript will review the pathologic classification of FRS.
Keywords: Rhinosinusitis, Fungal ball, Allergic fungal rhinosinusitis, Acute invasive fungal rhinosinusitis, Aspergillus, Allergic mucin
Introduction
Fungal rhinosinusitis (FRS) comprises a spectrum of disease processes, which vary in clinical presentation, histologic appearances, and biological significance. FRS can be acute (aggressive; symptoms <30 days), subacute (symptoms 30–90 days), and chronic (indolent; symptoms >90 days) [1–7]. FRS is most commonly classified as non-invasive or invasive based on whether fungi have invaded into tissue. While FRS has been medically known for decades, it has been more recently that FRS terminology and pathologic classification has been further defined and reviewed [1, 2].
Fungal Rhinosinusitis: Classification
As noted above, FRS is classified as either non-invasive or invasive. Non-invasive sinonasal fungal diseases include: saprophytic fungal infestation, fungal ball, and allergic fungal rhinosinusitis. The invasive forms of FRS include: acute, chronic, and chronic granulomatous. While non-invasive FRS is a serious condition requiring surgical and medical intervention, invasive forms of disease more often result in significant morbidity and mortality, particularly if left untreated.
Non-invasive Fungal Rhinosinusitis
Saprophytic Fungal Infestation
The recently proposed FRS guidelines added an additional category for non-invasive FRS referred to as saprophytic fungal infestation [1, 2]. This category was proposed to classify fungal colonization of the sinonasal tract usually following a surgical procedure or traumatic event that results in inflamed and ulcerated/crusted sinonasal mucosa with the presence of surface fungal infection without tissue invasion. While this form of FRS has been least described in the literature, it is felt that this form of FRS may precede the development of a “fungus” ball.
Fungal Ball
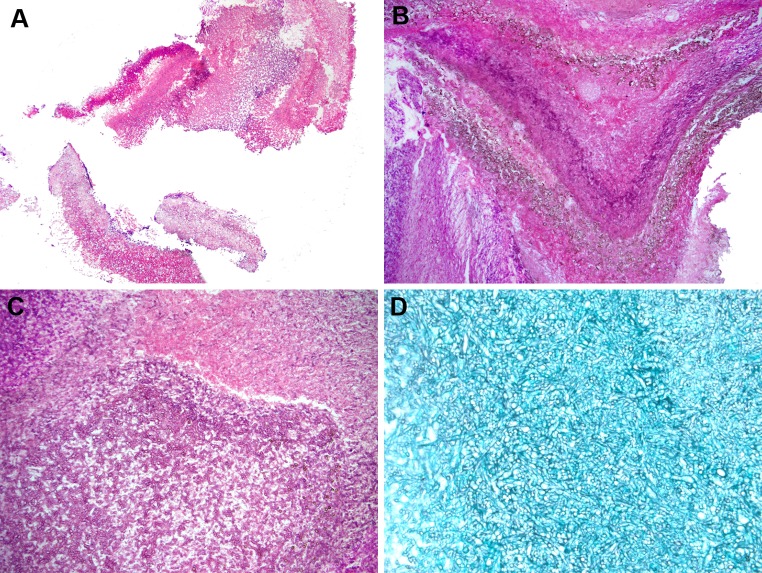
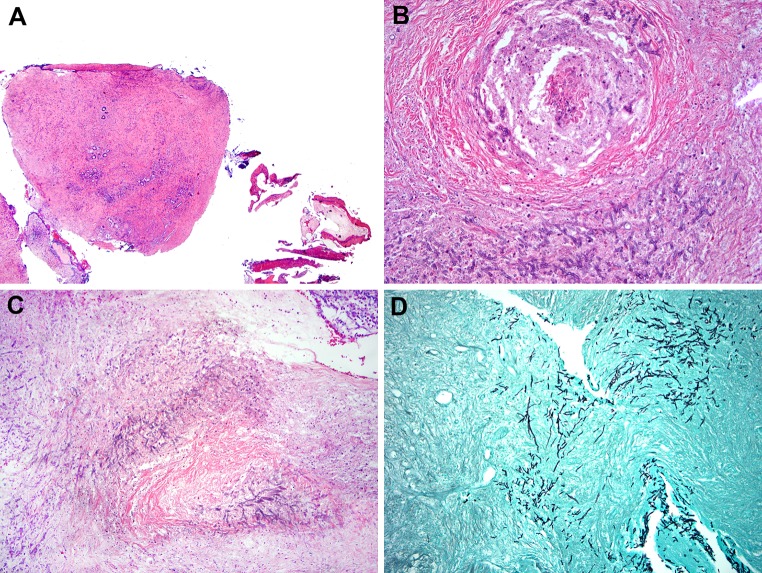
A second form of non-invasive FRS is the fungal ball, an extramucosal, entangled mass of fungi usually associated with minimal mucosal inflammation [1–7]. The most recent FRS guidelines consider fungal ball to be the most appropriate term for this entity as opposed to previously used terms, such as “mycetoma” (a term used to describe a chronic, soft tissue granulomatous fungal infection) and “aspergilloma” (fungal organisms other than Aspergillus sp. can cause a fungal ball) [1, 2]. For unknown reasons, fungal balls are more commonly identified unilaterally in the maxillary sinus in middle aged to elderly females [6, 8, 9]. Histologically, fungal balls are characterized by entangled masses of fungal organisms or masses of fungi embedded in fibrinous, necrotic exudate, with minimal mucosal inflammatory reaction (Fig. 1). By definition, no tissue invasion or granulomatous reaction is present in the surrounding tissue [1, 2]. On low power, microscopic examination of a fungal ball may be confused with the eosinophilic (allergic) mucin seen in allergic fungal rhinosinusitis, particularly since both have a “layered” appearance. However, this confusion is lost on high power which reveals abundant fungal organisms. While prior surgery has been considered a risk factor for fungal ball development, in a recent large study, only one patient had sinonasal surgery prior to the fungal ball development; however an association with prior dental procedures has been proposed [8]. In addition, fungal balls may develop when there are blockages of the sinonasal passages such as the presence of an obstructing neoplasm. Fungal cultures are often negative; however the most commonly isolated pathogen is Aspergillus sp. [3–6, 8–12]. Treatment consists of surgical removal of the fungal material without the need for antifungal therapy.
Fig. 1.
Fungal ball a low power appearance of a maxillary sinus fungal ball from a 60 yo female. Fungal ball is composed of an entangled mass of fungal organisms (Hematoxylin and eosin; original magnification ×10). b Low power of a fungal ball showing a lamellated appearance, which can be confused with eosinophilic (allergic) mucin (EM). This fungal ball shows a layered appearance and pigmented fungal forms. Cultures grew Aspergillus niger (Hematoxylin and eosin; original magnification ×25). c Higher power of fungal ball showing an entangled mass of fungal organisms with very little inflammation (Hematoxylin and eosin; original magnification ×50). d Silver stain highlighting fungal organisms in a fungal ball (Grocott stain; original magnification ×100)
Allergic Fungal Rhinosinusitis
Allergic FRS is not considered to represent a true fungal infection but is rather a result of an inflammatory reaction toward fungi in the sinonasal tract [13–18]. Interestingly, fungi begin to inhabit the sinonasal tract during the first few months of life [19]; however only a fraction of individuals develop allergic FRS. Allergic FRS occurs in immunocompetent, atopic patients who present with symptoms of chronic rhinosinusitis (CRS) not responsive to standard conservative medical therapy. Allergic FRS is seen more commonly in warm humid climates such as in the southern and southeastern United States, India, and the Middle East, although a high incidence has also been reported in large urban areas in the Midwest and northeastern United States [3–6, 10–12].
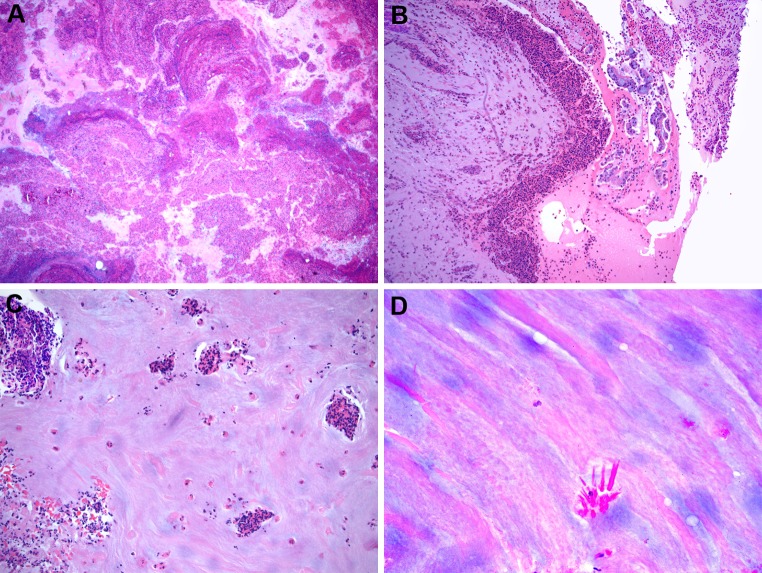
Grossly, the sinus contents from allergic FRS patients are described as inspissated, clay-like, mucin which is green, brown, or grayish in color. Microscopic examination shows eosinophilic (“allergic”) mucin that contains mucin admixed with sloughed epithelial cells, eosinophils, Charcot–Leyden crystals, eosinophilic debris, and other inflammatory cells arranged in a laminar pattern and associated with rare, scattered fungal hyphae (Figs. 2, 3). Eosinophilic mucin is now the preferred term instead of allergic mucin since there is debate regarding whether the etiology of this mucinous material, as well as allergic FRS, is allergic at all [1, 2, 17, 18, 20–29]. Fungal hyphae are occasionally seen on H and E stain but are best highlighted by histochemical stains such as silver or periodic acid Schiff (PAS) (Fig. 3). All of the material received from sinus contents of suspected allergic FRS patients should be histologically examined since fungal hyphae are often scarce. When positive, cultures from allergic FRS patients most commonly grow Dematiaceous fungi such as Alternaria sp., Bipolaris sp., Curvularia sp., etc. or Aspergillus sp. depending on geography [3, 4, 6, 10–12]. In India as well as Saudi Arabia, Aspergillus flavus appears to be the most common fungal organism cultured in allergic FRS; however, in the US, particularly in the South and Southeast, the majority of allergic FRS cases (70–90 %) are associated with dematiaceous fungi [10–16, 30]. Alternatively, in the Midwest and Northeastern US, equal incidences of dematiaceous fungi as well as Aspergillus sp. have been identified in allergic FRS patients [3–7]. Multiple fungi cultured in the same patient are not uncommon. A limited study using in situ hybridization found that about 50 % of allergic FRS patients contained Aspergillus or Penicillium rRNA in the eosinophilic mucin [31] (Fig. 3). Recent in situ hybridization (ISH) assays have also been developed to detect dematiaceous fungi in allergic FRS [32].
Fig. 2.
Allergic fungal rhinosinusitis a low power of eosinophilic (allergic) mucin showing a layered appearance of mucin admixed with inflammatory cells and debris (Hematoxylin and eosin; original magnification ×10). b Eosinophilic mucin showing collections of eosinophils and sloughed epithelial cells (Hematoxylin and eosin; original magnification ×50). c Eosinophilic mucin showing eosinophils singly and in cluster (Hematoxylin and eosin; original magnification ×50). d Eosinophilic mucin showing Charcot–Leyden crystals (Hematoxylin and eosin; original magnification ×200)
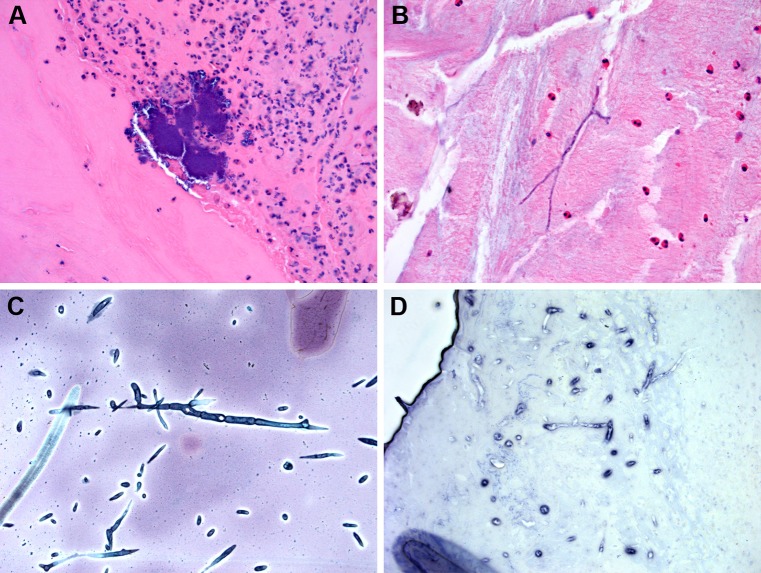
Fig. 3.
a Eosinophilic mucin often contains bacterial colonies (Hematoxylin and eosin; original magnification ×100). b Rarely, fungal organisms may be seen in eosinophilic mucin without use of specials stains. Cultures grew Curvularia sp. (Hematoxylin and eosin; original magnification ×100). c Silver stain of fungal organisms in eosinophilic mucin. Cultures grew Alternaria alternata (Grocott stain; original magnification ×200). d In situ hybridization for Aspergillus ribosomal RNA in eosinophilic mucin. Cultures confirmed A. fumigatus. (Nitroblue tetrazolium violet; original magnification ×100)
Eosinophilic mucin has also been described in patients without identifiable evidence of fungal infection by histology or conventional culture, particularly those with aspirin sensitivity syndrome. Ferguson coined the term eosinophilic mucinous rhinosinusitis to describe patients with eosinophilic mucin but without the presence of fungal organisms [33]. In Ferguson’s experience, patients with eosinophilic mucinous rhinosinusitis were clinically different from allergic FRS patients including presentation at an older age, an association with asthma and a significantly higher risk of aspirin sensitivity syndrome. However, eosinophilic mucinous rhinosinusitis as a distinct entity has been called into questioned by those that feel that sensitive methods to evaluate for fungus have not been applied fully to these patients [20, 21, 26, 29]. Guo et al. [34] using conventional Gomori methenamine silver stain (GMS) on eosinophilic mucin observed fungal organisms in only 27 % of specimens; however with trypsin pre-digestion followed by GMS, the yield was increased to 91 % of specimens. In addition, using antibodies targeting chitinase and Alternaria sp. antigens, fungi have been reported in 90–100 % of eosinophilic mucin samples, even those negative for histochemical stains [34].
The pathogenesis of allergic FRS is not completely understood. It is believed that allergic FRS is a host reaction to fungal proteins and not an actual fungal infection. Initially allergic FRS was considered a type I hypersensitivity reaction to fungi. In fact, using the diagnostic criteria of Bent and Kuhn [35], hypersensitivity to fungi is essential to the diagnosis. However, not all patients with the pathologic diagnosis of allergic FRS have systemic (or even local) hypersensitivity to fungi. More recent studies indicate that fungi induce production of eosinophil attracting Th2 cytokines and the subsequent inflammatory reaction produced results in the formation of allergic mucin [7, 17, 18, 20, 29]. Fungal proteases can induce the production of cytokines attract an influx of inflammatory cells including eosinophils which migrate through the barrier epithelial cells in response to fungi present. Interestingly, a recent review of FRS observed that a marked eosinophilic sinonasal mucosal infiltrate preceded the observed presence of fungi (either by histology or by culture) in over 30 % of allergic FRS patients [6]. Treatment of allergic FRS remains controversial but mostly the disease is treated with surgical removal of the eosinophilic mucin and steroids, although some advocate utilization of antifungal therapy.
Invasive Fungal Rhinosinusitis
Acute Invasive Fungal Rhinosinusitis
Acute invasive FRS is a devastating form of sinonasal fungal disease, which is characterized by rapid onset (<4 weeks’ duration) and an aggressive clinical course, particularly if untreated [1–7, 36–38]. Acute invasive FRS is seen in immunocompromised patients, particularly those with hematologic malignancies with low absolute neutrophil counts. Grossly, the mucosa appears pale and necrotic due to vascular thrombosis from fungal invasion. Histologically, the mucosa shows infarction vascular thrombosis and usually scant inflammatory cells. Close review shows angioinvasion of fungal forms resulting in lumenal thrombosis. While the fungi are usually seen on routine H and E stains, silver and PAS stains are often useful at highlighting the organisms particularly in vessel walls and vascular space lumens where they are often mixed with fibrin (Fig. 4). Rapid diagnosis is critical since fungal forms may grow into vital structures including the orbit and cranial cavity and patients with involvement of these structures have a significantly high morbidity and mortality rate.
Fig. 4.
Acute invasive fungal rhinosinusitis a low power view of infarcted sinonasal without significant inflammatory reaction in immunosuppressed patient with acute leukemia with acute invasive fungal rhinosinusitis Culture grew Rhizopus sp. (Hematoxylin and eosin; original magnification ×12.5). b Acute invasive FRS showing fungal organisms invading blood vessels and soft tissue. Cultures grew Aspergillus fumigatus (Hematoxylin and eosin; original magnification ×100). c Silver staining highlighting fungal hyphae in soft tissue in acute invasive FRS. Cultures grew A. fumigatus (Hematoxylin and eosin; original magnification ×100). d In situ hybridization (ISH) for Aspergillus ribosomal RNA. Note the extensive necrosis and only rare positive organisms. rRNA ISH is not always reliable on necrotic tissues (Fast red tetrazolium violet; original magnification ×100)
The diagnosis of acute invasive FRS includes histopathologic identification of tissue-invasive fungal forms, which is often performed during intraoperative consultation (frozen section) [39, 40]. Frozen section is almost always required since this technique can be performed in a rapid fashion and provide the surgeon with immediate results for treatment planning, which is usually debridement surgery followed by IV antifungals. Most cases grow either Aspergillus sp. or Rhizopus sp. Other rare pathogens such as Fusarium sp. and dematiaceous species may also cause acute invasive FRS; however, in approximately 30 % of acute invasive FRS patients, fungal cultures are negative [6, 41, 42]. Rapid ISH for rRNA targets has become a useful means for identifying fungal species in patients with acute invasive FRS (Fig. 3). In one study, ISH confirmed the fungal pathogen in culture positive cases and also identified the pathogens in culture negative cases [43]. A drawback of the procedure was the limited utility of ISH in extensively necrotic tissues such as can be seen in acute invasive FRS (Fig. 4).
Chronic Invasive and Chronic Granulomatous Fungal Rhinosinusitis
Chronic forms of invasive FRS are rare in the United States but more common in India and the Middle East [10–12]. In fact, the recently published guidelines considered whether to separate these two entities; however, it was considered that these two forms of invasive FRS had enough clinical and pathological differences to keep them as separate entities [1, 2]. By definition, patients presenting with CRS have had symptoms for greater than 12 weeks duration. Despite the fact that they are chronic in nature and slower to progress than AIFRS, they do require aggressive therapy for adequate cure.
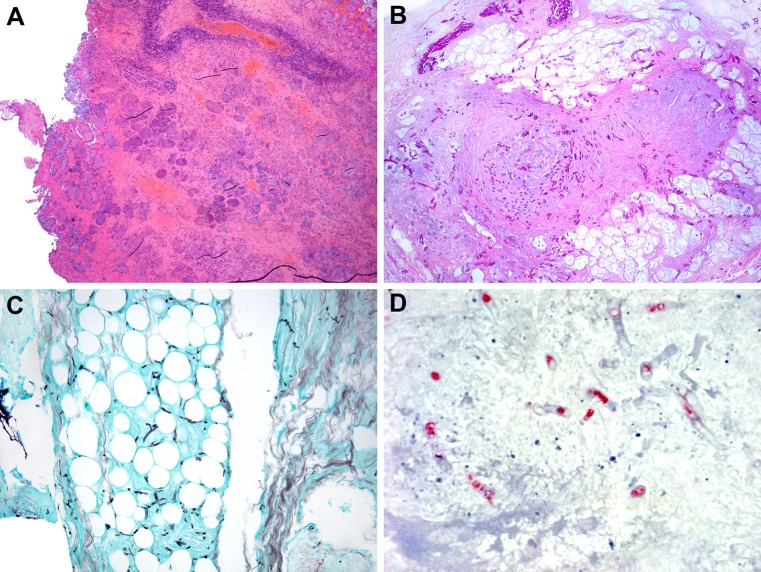
Chronic granulomatous invasive FRS is seen in immunocompetent patients, in contrast to the immunosuppressed patients in acute invasive FRS, and is endemic to India, Sudan, and Africa [1, 2]. The infection is characterized the presence of submucosal granulomatous inflammation, rare fungal hyphae, and extensive fibrosis [10–12, 44] (Fig. 5). The most common associated fungal organism is A. flavus.
Fig. 5.
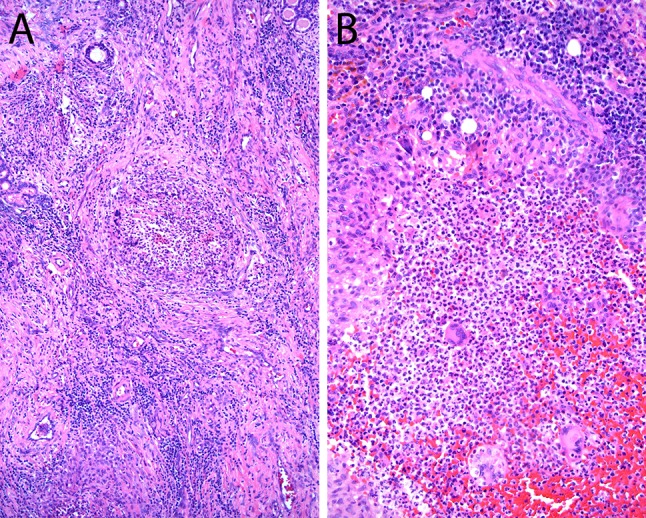
Chronic invasive granulomatous fungal rhinosinusitis a, b Granulomatous reaction toward Aspergillus flavus in patients with chronic invasive granulomatous fungal rhinosinusitis (Hematoxylin and eosin; original magnification ×25 for a and ×100 for b)
Chronic invasive FRS is a slowly growing invasive fungal infection characterized by invasion of numerous fungal organisms into the sinonasal mucosa with rare angioinvasion. This entity occurs in the background of acquired immunodeficiency syndrome, solid organ transplantation, diabetes, and in patients undergoing treatment with corticosteroids. A.fumigatus is the most common fungus isolated in these patients [1, 2, 44]. In contrast to granulomatous fungal sinusitis, fungal organisms are more numerous, there is a sparse inflammatory infiltrate, and occasionally angioinvasion (Fig. 6). Both of these forms of fungal sinusitis should be treated by surgical debridement and systemic antifungal therapy.
Fig. 6.
Chronic invasive fungal rhinosinusitis a low power of inflamed and fibrotic sinonasal mucosa in liver transplant patient with chronic invasive fungal rhinosinusitis (Hematoxylin and eosin; original magnification ×12.5). b, c Chronic invasive FRS with extensive sinonasal mucosal necrosis/infarction with visible fungal organisms in liver transplant patient with symptoms greater than 3 months duration. b Fungi within blood vessels (Hematoxylin and eosin; original magnification ×100). d Silver stain shows fungal hyphae infiltrating sinonasal mucosa in chronic invasive FRS (Hematoxylin and eosin; original magnification ×50)
References
- 1.Chakrabarti A, Das A, Panda NK. Controversies surrounding the categorization of fungal sinusitis. Med Mycol. 2009;47(suppl 1):S299–S308. doi: 10.1080/13693780802213357. [DOI] [PubMed] [Google Scholar]
- 2.Chakrabarti A, Denning DW, Ferguson BJ, et al. Fungal rhinosinusitis: a categorization and definitional scheme addressing current controversies. Laryngoscope. 2009;119:1809–1818. doi: 10.1002/lary.20520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Granville L, Chirala M, Cernoch P, Ostrowski M, Truong LD. Fungal sinusitis: histologic spectrum and correlation with culture. Hum Pathol. 2004;35:474–481. doi: 10.1016/j.humpath.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 4.Taxy J. Paranasal fungal sinusitis: contributions of histopathology to diagnosis: a report of 60 cases and literature review. Am J Surg Pathol. 2006;30(6):713–720. doi: 10.1097/00000478-200606000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Das A, Bal A, Chakrabarti A, Panda N, Joshi K. Spectrum of fungal rhinosinusitis; histopathologist’s perspective. Histopathology. 2009;54:854–859. doi: 10.1111/j.1365-2559.2009.03309.x. [DOI] [PubMed] [Google Scholar]
- 6.Montone KT, Livolsi VA, Feldman MD, Lanza DC, Palmer J, Chiu AG, Kennedy DW, Nachamkin I. Fungal rhinosinusitis: a report of 400 patients at a single university medical center. Int J Otolaryngol. 2012;2012:684835. doi: 10.1155/2012/684835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Montone K. Recent considerations in the classification and pathogenesis of fungal rhinosinusitis in pathobiology of human disease: a dynamic encyclopedia of disease mechanisms. 2014;1432–44.
- 8.Dufour X, Kauffman-Lacroix C, Ferrie JC, Goujon JM, Rodier MH, Klossek JM. Paranasal sinus fungal ball epidemiology, clinical features, and diagnosis. A retrospective analysis of 173 cases from a single center in France 1989–2002. Med Mycol. 2006;44:61–67. doi: 10.1080/13693780500235728. [DOI] [PubMed] [Google Scholar]
- 9.Nicolai P, Lombardi D, Tomenzoli D, Villaret AB, Piccioni M, Mensi M, Maroldi R. Fungus ball of the paranasal sinuses: experience in 160 patients treated with endoscopic surgery. Laryngoscope. 2009;119(11):2275–2279. doi: 10.1002/lary.20578. [DOI] [PubMed] [Google Scholar]
- 10.Michael RC, Michael JS, Ashbee RH, Mathews MS. Mycological profile of fungal sinusitis: an audit of specimens over a 7-year period in a tertiary care hospital in Tamil Nadu. Indian J Pathol Microbiol. 2008;51:493–496. doi: 10.4103/0377-4929.43738. [DOI] [PubMed] [Google Scholar]
- 11.Panda NK, Sharma SC, Chakrabarti A, Mann SBS. Paranasal sinus mycoses in north India. Mycoses. 1998;41:281–286. doi: 10.1111/j.1439-0507.1998.tb00339.x. [DOI] [PubMed] [Google Scholar]
- 12.Challa S, Uppin SG, Hanumanthu S, Panigrahi MK, Purohit AK, Sattaluri S, Borgohain R, Chava A, Vemu L, Jagarlapudi MM. Fungal rhinosinusitis: a clinicopathological study from South India. Eur Arch Otorhinolaryngol. 2010;267(8):1239–1245. doi: 10.1007/s00405-010-1202-6. [DOI] [PubMed] [Google Scholar]
- 13.Schubert MS. Allergic fungal sinusitis. Otolaryngol Clin N Am. 2004;37:301–326. doi: 10.1016/S0030-6665(03)00152-X. [DOI] [PubMed] [Google Scholar]
- 14.Schubert MS. Allergic fungal sinusitis: pathophysiology, diagnosis and management. Med Mycol. 2009;47(suppl 1):S324–S330. doi: 10.1080/13693780802314809. [DOI] [PubMed] [Google Scholar]
- 15.Schubert MS. Allergic fungal sinusitis. Clin Rev Allergy Immunol. 2006;30(3):205–216. doi: 10.1385/CRIAI:30:3:205. [DOI] [PubMed] [Google Scholar]
- 16.Laury AM, Wise SK. Allergic fungal rhinosinusitis. Am J Rhinol. 2013;27:S26–S27. doi: 10.2500/ajra.2013.27.3891. [DOI] [PubMed] [Google Scholar]
- 17.Montone K. Role of fungi in the pathophysiology of chronic rhinosinusitis: an update. Curr Allergy Asthma Rep. 2013;13(2):224–228. doi: 10.1007/s11882-012-0332-x. [DOI] [PubMed] [Google Scholar]
- 18.Montone KT. The molecular genetics of inflammatory, autoimmune, and infectious diseases of the sinonasal tract: a review. Arch Pathol Lab Med. 2014;138(6):745–753. doi: 10.5858/arpa.2013-0038-RA. [DOI] [PubMed] [Google Scholar]
- 19.Lackner A, Stammberger H, Buzina W, et al. Fungi: a normal content of human nasal mucus. Am J Rhinol. 2005;19:125–129. [PubMed] [Google Scholar]
- 20.Ponikau JU, Sherris DA, Kern EB, et al. The diagnosis and incidence of allergic fungal sinusitis. Mayo Clin Proc. 1999;74:877–884. doi: 10.4065/74.9.877. [DOI] [PubMed] [Google Scholar]
- 21.Ponikau JU, Sherris DA, Weaver A, Kita H. Treatment of chronic rhinosinusitis with intranasal amphotericin B: a randomized, placebo-controlled, double-blind pilot trial. J Allergy Clin Immunol. 2005;115:125–131. doi: 10.1016/j.jaci.2004.09.037. [DOI] [PubMed] [Google Scholar]
- 22.Ebbens FA, Fokkens WJ. The mold conundrum in chronic rhinosinusitis: where do we stand today? Curr Allergy Asthma Rep. 2008;8(2):93–101. doi: 10.1007/s11882-008-0018-6. [DOI] [PubMed] [Google Scholar]
- 23.Soler ZM, Schlosser RJ. The role of fungi in diseases of the nose and paranasal sinuses. Am J Rhinol Allergy. 2012;26:351–358. doi: 10.2500/ajra.2012.26.3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang C, Gershwin ME, Thompson GR. Fungal diseases of the nose and sinuses: an updated overview. Curr Allergy Asthma Rep. 2013;13:152–161. doi: 10.1007/s11882-012-0320-1. [DOI] [PubMed] [Google Scholar]
- 25.Callejas CA, Doglas G. Fungal rhinosinusitis: what every allergist should know. Clin Exp Allergy. 2013;43:835–849. doi: 10.1111/cea.12118. [DOI] [PubMed] [Google Scholar]
- 26.Murr AH, Goldberg AN, Pletcher SD, Dillehay K, Wymer LJ, Vesper SJ. Some chronic rhinosinusitis patients have elevated populations of fungi in their sinuses. Laryngoscope. 2012;122:1438–1445. doi: 10.1002/lary.23295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orlandi RR, Marple BF. The role of fungus in chronic rhinosinusitis. Otolaryngol Clin N Am. 2010;43:531–537. doi: 10.1016/j.otc.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 28.Orlandi RR, Marple BF, Georgelas A, et al. Immunologic response to fungus is not universally associated with rhinosinusitis. Otolaryngol Head Neck Surg. 2009;141(6):750–756. doi: 10.1016/j.otohns.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 29.Shin SH, Poinikau JU, Sherris DA, et al. Chronic rhinosinusitis: an enhanced immune response to ubiquitous airborne fungi. J Allergy Clin Immunol. 2004;114:1369–1375. doi: 10.1016/j.jaci.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 30.Al-Dousary SH. Allergic fungal sinusitis: radiological and microbiological features of 59 cases. Ann Saudi Med. 2008;28(1):17–21. doi: 10.4103/0256-4947.51772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perez LA, Lanza DC, Loevner LA, Kennedy DW, Montone KT. In situ hybridization for Aspergillus and Penicillium in allergic fungal sinusitis: a rapid means of speciating fungal pathogens in tissues. Laryngoscope. 1997;107(2):233. doi: 10.1097/00005537-199702000-00017. [DOI] [PubMed] [Google Scholar]
- 32.Montone KT, Livolsi VA, Lanza DC, Feldman MD, Kennedy DW, Palmer J, Chiu AG, Nachamkin I. Rapid in situ hybridization for dematiaceous fungi using a broad-spectrum oligonucleotide DNA probe. Diagn Mol Pathol. 2011;20(3):180–183. doi: 10.1097/PDM.0b013e31820e9c82. [DOI] [PubMed] [Google Scholar]
- 33.Ferguson BJ. Eosinophilic mucin rhinosinusitis: a distinct clinicopathologic entity. Laryngoscope. 2000;110(5):799–813. doi: 10.1097/00005537-200005000-00010. [DOI] [PubMed] [Google Scholar]
- 34.Guo C, Ghaderoshi S, Kephart GM, et al. Improving the detection of fungi in eosinophilic mucin: seeing what we could not see before. Otolaryngol Head Neck Surg. 2012;147(5):943–949. doi: 10.1177/0194599812451010. [DOI] [PubMed] [Google Scholar]
- 35.Bent JP, Kuhn F. Diagnosis of allergic fungal sinusitis. Otolaryngol Head Neck Surg. 1994;111:580–588. doi: 10.1016/S0194-5998(94)70525-9. [DOI] [PubMed] [Google Scholar]
- 36.Duggal P, Wise SK. Invasive fungal rhinosinusitis. Am J Rhinol Allergy. 2013;27:S28–S30. doi: 10.2500/ajra.2013.27.3892. [DOI] [PubMed] [Google Scholar]
- 37.Süslü AE, Oğretmenoğlu O, Süslü N, Yücel OT, Onerci TM. Acute invasive fungal rhinosinusitis: our experience with 19 patients. Eur Arch Otorhinolaryngol. 2009;266(1):77–82. doi: 10.1007/s00405-008-0694-9. [DOI] [PubMed] [Google Scholar]
- 38.Monroe MM, McLean M, Sautter N, et al. Invasive fungal rhinosinusitis: a 15 year experience with 29 patients. Laryngoscope. 2013;123:1583–1587. doi: 10.1002/lary.23978. [DOI] [PubMed] [Google Scholar]
- 39.Taxy JB, El-Zayaty S, Langerman A. Acute fungal sinusitis: natural history and the role of frozen section. Am J Clin Pathol. 2009;132(1):86–93. doi: 10.1309/AJCP9HTH9NRPMYCT. [DOI] [PubMed] [Google Scholar]
- 40.Ghadiali MT, Deckard NA, Farooq U, Astor F, Robinson P, Casiano RR. Frozen-section biopsy analysis for acute invasive fungal rhinosinusitis. Otolaryngol Head Neck Surg. 2007;136(5):714–719. doi: 10.1016/j.otohns.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 41. Derber C, Elam K, Bearman G. Invasive sinonasal disease due to dematiaceous fungi in immunocompromised individuals: case report and review of the literature. Int J Infect Dis. 2010:e329–32. [DOI] [PubMed]
- 42.Campo M, Lewis RE, Kontoyiannis DP. Invasive fusariosis in patients with hematologic malignancies at a cancer center: 1998–2009. J Infect. 2010;60(5):331–337. doi: 10.1016/j.jinf.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 43.Montone KT, LiVolsi VA, Lanza DC, Kennedy DW, Palmer J, Chiu AG, Feldman MD, Loevner LA, Nachamkin I. In situ hybridization for specific fungal organisms in acute invasive fungal rhinosinusitis. Am J Clin Pathol. 2011;135(2):190–199. doi: 10.1309/AJCPQLYZBDF30HTM. [DOI] [PubMed] [Google Scholar]
- 44.Montone KT. Differential diagnosis of necrotizing sinonasal diseases. Arch Pathol Lab Med. 2016;139(12):1508–1514. doi: 10.5858/arpa.2015-0165-RA. [DOI] [PubMed] [Google Scholar]