Abstract
The sinonasal tract is one of the least frequent sites for squamous cell carcinoma in the head and neck. However, it is still a complex tumor type for pathologists because there are numerous histologic variants with unusual morphologic features, several non-squamous carcinomas in the differential diagnosis that can have similar morphology and even squamous differentiation, and because of the increasing recognition of human papillomavirus (HPV) in a subset of the tumors. In addition, the unique and complex anatomy of the sinonasal tract can make proper staging and management of patients’ tumors quite challenging. This article reviews sinonasal tract squamous cell carcinoma in depth and provides the latest data on Schneiderian papillomas and HPV in their pathogenesis.
Keywords: Sinonasal, Squamous cell carcinoma, Human papillomavirus, Nonkeratinizing
Introduction
While squamous cell carcinoma (SCC) is the most common malignancy of the head and neck, its relative incidence across the various anatomic subsites varies. The sinonasal tract (specifically the nasal cavity and paranasal sinuses), compared to other sites, has the lowest fraction of SCC relative to other carcinoma types (~65 to 70 %). Since the sinonasal tract is the primary site for only ~3 to 5 % of all head and neck cancers [1], and since most of these tumors appear to be decreasing in incidence over time [2], this means that pathologists do not see very many of them in routine practice. It has also made it challenging to gather sufficient numbers of patients with these tumors for studies to define their morphology, molecular aspects, and proper treatment.
The incidence may be low, but sinonasal tract carcinomas have some of the most significant histologic variation of all head and neck sites, with many unusual and distinct subtypes, a relatively sizeable fraction of defined SCC variants, and also with several interesting etiologic and precursor lesion issues, such as tumors arising in Schneiderian papillomas and tumors related to human papillomavirus (HPV). This makes sinonasal tract SCC a somewhat complicated area for clinical practice and a rich subject for discussion and research. This article will review current knowledge on sinonasal SCC and its variants, differential diagnosis with other sinonasal tumors that may have similar features, the relationship to papillomas and HPV, and how all of these relate to the diagnosis and reporting of the tumors in clinical practice.
Epidemiology
Rates of sinonasal SCC have been decreasing in recent years [2]. Tumors occur predominantly in men (twice as commonly as in women) in their 50’s and 60’s. Professionals who work with wood have up to a 20 fold increased risk of developing sinonasal SCC, and leather dust and some other chemical substances used in industry have also been associated with its development [3]. While chronic inflammation can be invoked as a risk factor for tumor development in many different parts of the body, data for sinonasal SCC are limited. Indirect biological studies support chronic inflammation in the pathophysiology of industrial compound exposure and SCC development. By extension, since chronic rhinosinusitis (“allergic sinusitis”) is so common, one might speculate that it could also be a risk factor for sinonasal SCC. However, studies have not associated non-“irritant” chronic inflammation or sinonasal inflammatory polyposis with SCC development. Smoking, a very strong risk factor for SCC across head and neck subsites, only increases the risk of sinonasal SCC development by two to three fold [3].
Clinical Features
Patients with sinonasal SCC present with typical, but relatively non-specific, features of any cancer of this region. Chief complaints are primarily nasal obstruction, facial pain, rhinorrhea, and epistaxis (bleeding). Approximately 50 % arise in the nasal cavity, and 50 % in the paranasal sinuses [2], predominantly the maxillary sinus. Tumor stage is not defined by size, but rather by number and types of local structures involved for early tumors and, for late ones, by invasion of adjacent structures including orbital soft tissue, skull base, brain, and/or facial/nasal skin, all of which constitute classification of tumors as T4. Nodal metastases are relatively uncommon (~10 to 20 %) at presentation [1], and in follow up, ~10 % develop distant metastases, almost always in the presence of locoregional recurrence.
Precursor Lesions
Schneiderian papillomas are benign epithelial neoplasms of the sinonasal tract. There are three types (exophytic, inverted, and oncocytic) as defined by the World Health Organization (WHO). SCC can arise in all three, but is very rare in exophytic papillomas. It is most common in inverted papillomas, with rates as low as 2 % and as high as 27 % reported. Most of the literature, including a large collective literature review by Barnes in 2002 of 1390 patients [4], suggests that it is ~10 %. Given that a recent, large series showed a rate of only 1.9 % [5], the actual rate is probably between these two figures. Approximately 60 % of the SCC arising from inverted papillomas present synchronously. Data on rates of SCC in oncocytic papillomas are much less reliable because of their rarity, but range between 4 and 17 % [4]. The histologic features of the SCCs, and the role of HPV are discussed below.
Head and neck SCC, in general, arises from precursor squamous intraepithelial neoplasia. In the sinonasal tract, where all but the nasal vestibule is lined by respiratory epithelium, squamous metaplasia must develop prior to the squamous neoplasia. Interestingly, however, outside of SCC developing in the setting of Schneiderian papillomas, dysplastic squamous epithelium/squamous carcinoma in situ is only uncommonly found histologically in the adjacent mucosa.
Histologic Types
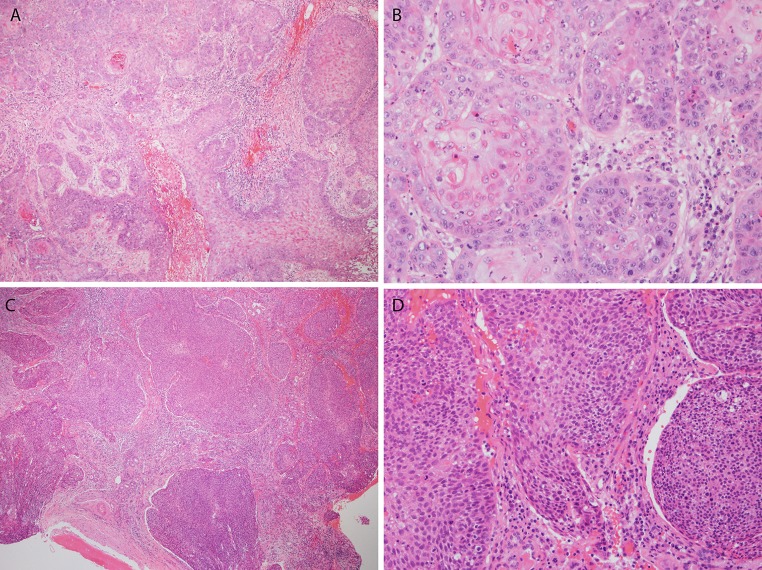
While the subtyping of SCC is pretty standard across the head and neck, there are certainly aspects unique to the tumors at specific subsites. In the sinonasal tract, the WHO classification specifies keratinizing (K or conventional) SCC, nonkeratinizing (NK) SCC, and then the other more typical variants such as basaloid, verrucous, papillary, etc. The relative fraction of KSCC versus NKSCC in the literature has varied somewhat, probably depending on subtle variations in definitions of the two. Summarizing results of the three largest studies to define them (Table 1), there were 99 KSCC (60 %) and 66 NKSCC (40 %) [6–10]. KSCC is morphologically identical to such tumors arising elsewhere (Fig. 1), with stellate, irregular nests of tumor cells in a desmoplastic stroma. Tumor cells have abundant, eosinophilic cytoplasm filled with keratin filaments. Intercellular bridges are typically prominent, and keratin production is frequent. NKSCC is similar to this same tumor type in the oropharynx. It has a “blue cell tumor” appearance with tumor cells with high nuclear to cytoplasmic ratios typically arranged in large, rounded nests or ribbons with smooth, often well demarcated, borders and little stromal desmoplasia. There is minimal maturing squamous differentiation. Central necrosis is common in the nests, and there is prominent mitotic activity and apoptosis. A very interesting feature of these tumors is that they often coat the mucosal surface with an undulating, irregular contour and, where the tumors are invading downward, have an inverted appearance with rounded nests, mimicking a Schneiderian papilloma with associated carcinoma in situ. This feature, combined with the lack of stromal desmoplasia around the deeper nests, can make the tumors look non-invasive. Some tumors can actually form mass lesions consisting only of exophytic projections of apparent carcinoma in situ but there is no clear data to say that, at least in the sinonasal tract, this type of tumor is clinically and prognostically different than clearly stromally-invasive tumors. Lesions consisting entirely of these features are still mass forming and, until better data is available, should be descriptively diagnosed and probably be clinically managed similarly to more obviously invasive tumors.
Table 1.
Transcriptionally-active high risk HPV rates for the various SCC types in the sinonasal tract
| Histologic type | Cases | HPV positivea |
|---|---|---|
| Keratinizing SCC | 99 (49.5 %) | 4/99 (4.0 %) |
| Nonkeratinizing SCC | 66 (33.3 %) | 27/66 (40.9 %) |
| Basaloid SCC | 13 (6.5 %) | 6/13 (46.2 %) |
| Papillary SCC | 10 (5.0 %) | 8/10 (80.0 %) |
| Adenosquamous carcinoma | 9 (4.5 %) | 6/9 (66.6 %) |
| Spindle cell carcinoma | 3 (1.5 %) | 0/3 (0 %) |
HPV human papillomavirus, SCC squamous cell carcinoma
aDefined as either detectable high risk HPV E6/E7 mRNA or as combined diffuse p16 expression with detectable high risk HPV DNA
Fig. 1.
Keratinizing and nonkeratinizing squamous cell carcinoma of the sinonasal tract. Keratinizing (or conventional) squamous cell carcinoma on low power (a-×4 magnification) showing large, irregular, and stellate nests of tumor cells in a lightly basophilic (desmoplastic) stroma and on high power (b-×20 magnification) showing cells with abundant, eosinophilic cytoplasmic and foci of keratinization. Nonkeratinizing squamous cell carcinoma on low power (c-×4 magnification) with large, rounded nests of blue cells with little stromal reaction, and on higher power (d-×40 magnification), oval, hyperchromatic nuclei with minimal cytoplasm and minimal areas of maturing squamous differentiation
Probably due to these somewhat unique morphologic features in sinonasal carcinomas, terminology used for them has an interesting history. Many older terms for tumors with these patterns of growth were proposed, such as “cylindrical cell” [6], “transitional cell”, and “Schneiderian” carcinoma [11]. Among the descriptors used for these tumors were palisaded basal cell layers in some of them and how they could mimic simple carcinoma in situ and/or papillomatous lesions with dysplastic epithelium, such as in the urinary bladder. Under the most recent WHO classification [11], such tumors are considered to fall under the umbrella of NKSCC, without any suggestion for grading or differentiation because of lack of established prognostic significance for it. Given that the tumors can rarely have mixed patterns with areas of mucinous differentiation in the epithelium, the more broad term “Schneiderian carcinoma” may come back into favor.
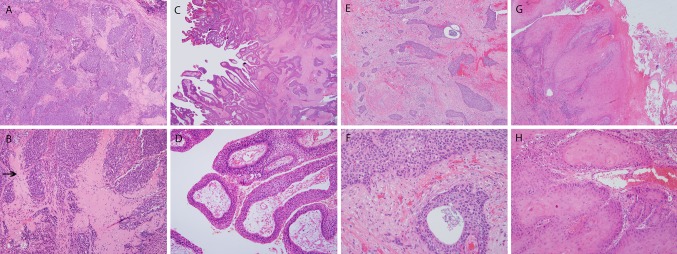
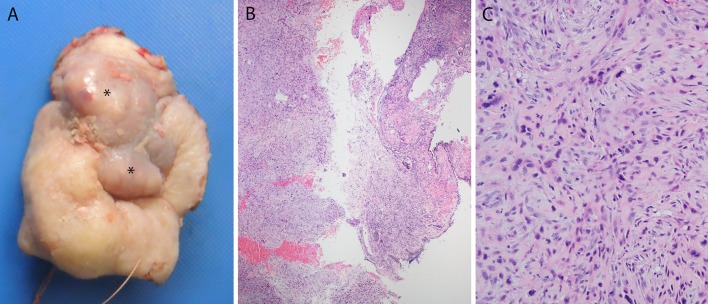
All of the major histologic variants of SCC have been described in the sinonasal region (Fig. 2) . Rates of SCC variants have varied considerably in the literature, however. In Bishop et al. [7], of a large sinonasal tumor tissue microarray cohort with 161 patients and 91 total SCC, 22 (or 24.2 %) of the tumors were specific SCC variants. However, in a large SEER-based study, other specific SCC variants accounted for only 7 % of the 4,382 SCC cases [12]. Basaloid SCC, spindle cell carcinoma, and papillary SCC are generally similar in incidence, each constituting a few percent of tumors. Papillary SCC, as mentioned above, consists of a tumor with diffuse, full thickness, malignant squamous cells but with the majority (by WHO definition) of cells lining true papillae and projecting outward into the luminal space [13] (Table 1). Despite the similarity in growth patterns, papillary SCC only rarely represents progression of a papilloma to carcinoma. Of the Schneiderian papillomas complicated by SCC in the literature, almost none went on to develop papillary SCC. Basaloid SCC should be strictly defined as a tumor of basaloid, hyperchromatic cells with round nuclei arranged in lobules and rounded nests that push against each other, divided by thin lines of hyalinized basement membrane material, with comedonecrosis, high mitosis and apoptosis, and associated with at least focal definable squamous differentiation or immunohistochemistry showing a squamous phenotype [14]. Spindle cell carcinoma consists of tumor cells with spindled or pleomorphic morphology which lose the nested, carcinomatous appearance and thus appear similar to true sarcomas. The tumors can have almost any histologic pattern with fascicular, collagenous, storiform-pleomorphic, and granulation tissue-like being some of the most common (Fig. 3). Most are biphasic tumors with a component of recognizable squamous neoplasia, either invasive SCC or squamous dysplasia. Purely sarcomatoid tumors require positive immunohistochemistry for epithelial markers such as p63, p40, and/or cytokeratin in order to make the diagnosis [15].
Fig. 2.
Histologic variants of squamous cell carcinoma. Basaloid squamous cell carcinoma consists of nested tumor with rounded profiles that “mold” to one another in a jigsaw puzzle type pattern (a-×4 magnification) with necrosis and areas of hyalinized, collagenous stroma. On higher power, the nuclei are round with abundant surrounded hyaline material forming thin lines between some nests (arrow) (b-×20 magnification). Papillary squamous cell carcinoma consists predominantly of full-thickness dysplastic squamous cells lining papillary structures (c-×1.25 magnification), with cells above the basement membrane. On higher power (d-×10 magnification), the papillae can be seen with lining cells with eosinophilic cytoplasm, angulated, hyperchromatic nuclei and no maturation. Adenosquamous carcinoma (e-×4 magnification) consists of squamous cell carcinoma, here the angulated nests in a fibrous stroma, but with “punched out” gland spaces with smooth linings. On higher power (f-×20 magnification), flocculent mucin material can be seen in the large gland space but there are also several tumor cells with basophilic, intracytoplasmic mucin globules. Verrucous carcinoma is a “hyper-differentiated” tumor consisting of very thick squamous lining with hyperkeratosis and “glassy”, brightly eosinophilic cytoplasm (g-×4 magnification). On higher power (h-×20 magnification), the tumor cells have abundant cytoplasm and pushing borders without irregular infiltration or stromal reaction
Fig. 3.
Spindle cell (sarcomatoid) carcinoma. A gross photograph of a total rhinectomy specimen for spindle cell carcinoma a shows violaceous nodules of tumor (asterisks) bulging underneath the skin of the nasal bridge. Histologically, the tumor was biphasic, consisting predominantly of keratinizing type squamous cell carcinoma but with sheet-like areas of spindle cells (b-×4 magnification) in a vaguely fascicular pattern with hyperchromatic, pleomorphic nuclei, collagenous to myxoid stroma (c-×20 magnification) and abundant mitoses, including atypical ones
Another variant that is rare in the sinonasal tract is adenosquamous carcinoma. It was the least common SCC variant in the large SEER-based study, constituting only 0.7 % of sinonasal SCC [12]. It is defined as an invasive carcinoma with obvious squamous differentiation, but associated with punched out, “gland-like” spaces, frequently with intracellular and intraluminal mucin (the latter not requisite) of any amount, even if very focal [9]. The final type is verrucous carcinoma which is distinctly uncommon in the sinonasal tract [16] with only ~20 to 25 cases reported in the literature [16]. It has the same diagnostic features in the sinonasal tract as in other head and neck sites. It must consist of very mature, thick, ribbons of exophytic and rounded epithelium with abundant, glassy cytoplasm and minimal cytologic atypia, mitotic activity limited to the basal layers, and no stromally-invasive tumor. It invades by pushing expansion of nests into tissues. It doesn’t metastasize, unless a typical invasive SCC component develops within it.
Differential Diagnosis
For KSCC, there is no significant differential diagnosis. It is typical and quite characteristic unless it is particularly poorly differentiated. However, for NKSCC, the differential diagnosis is quite broad since it is in the family of “round blue cell tumors.” There are too many tumors in this differential diagnosis to discuss in depth here. However, in brief, a helpful acronym for such tumors is “NOSE ALARM” where N stands for NUT-midline carcinoma, O for olfactory neuroblastoma, S for SCC, small cell (high grade neuroendocrine) carcinoma, and sinonasal undifferentiated carcinoma, E for Ewing’s sarcoma, A for adenoid cystic carcinoma (solid type) and HPV-related adenoid cystic-like carcinoma [7, 17], L for lymphoma, A plus R for alveolar rhabdomyosarcoma, and M for melanoma. Interestingly (and challengingly) several sinonasal “blue cell” malignancies that are not actually considered to be SCC have focal squamous neoplasia including NUT midline carcinoma, adamantinoma-like Ewing’s sarcoma, and HPV related adenoid cystic like carcinoma [7, 17]. The former two have SCC differentiation in the invasive tumor, and the latter has severe dysplasia/squamous carcinoma in situ (at least in the few reported cases).
Subtle morphologic features are very important for telling all of these tumors apart and for narrowing the differential diagnosis, and immunohistochemistry is critical. SCC, including keratinizing, NK, and basaloid types, stains consistently and diffusely for p63 and its more squamous-specific isoform p40 [18], for pancytokeratin (AE1/AE3), and for the high molecular weight cytokeratins such as 5/6 and 34βE12. Other tumors in the differential diagnosis can express p63 and p40, albeit usually focally, so caution is warranted [18]. There is no expression of neuroendocrine, hematopoietic, myoepithelial, melanocytic, or skeletal muscle differentiation markers. Solid adenoid cystic carcinoma and the newly described HPV-related adenoid cystic-like carcinoma both lack diffuse p63/p40 expression. Solid adenoid cystic carcinoma classically shows patchy p63 expression that is usually limited to the focal myoepithelial differentiation at the periphery of the nests [19]. HPV-related adenoid cystic-like carcinoma has focal ductal differentiation, expresses p63 in its abluminal cells only, and expresses myoepithelial markers such as smooth muscle actin, S-100, and calponin [7], features not seen in basaloid or NKSCC.
NKSCC, with its pushing borders, lack of stromal reaction, and ribbony nesting pattern, can resemble non-invasive lesions such as inverted papillomas. The same is true of papillary SCC with its often extensive papillary surface with tumor cells (partially or wholly) located above the basement membrane. Due to these growth patterns, while the diagnosing pathologist could consider if NKSCC or papillary SCC represent malignant transformation of an inverted papilloma, both of these SCC types consist of diffuse, full thickness, overtly carcinomatous tumor, rather than being mixed with lower grade areas or with foci of actual inverted papilloma. Further, the SCCs that arise from inverted papillomas are almost always keratinizing-type SCC and not NK or papillary SCC [20].
For spindle cell carcinoma, the differential diagnosis includes true sarcomas. Fortunately, the majority (up to 80–85 %) of spindle cell carcinomas are biphasic tumors with some component of squamous neoplasia, either invasive or in situ carcinoma. While spindle cell carcinomas resemble true sarcomas histologically, most lack any definable sarcoma pattern such as leiomyosarcoma, rhabdomyosarcoma, or osteosarcoma, this representing another histologic clue to the diagnosis. For those tumors that are not biphasic, immunohistochemical expression of p63/p40 and/or cytokeratins in the malignant cells is required for the diagnosis.
Human Papillomavirus
Transcriptionally-active HPV, as defined by the detection of both >70 % nuclear and cytoplasmic p16 expression and high risk HPV DNA by PCR or in situ hybridization (or RNA by RTPCR or in situ hybridization), has been examined in four studies over the past decade or so (Fig. 4). These have been reviewed elsewhere [21], but summarizing their results (Table 1), for NKSCC the rate was 40.9 %, which is much lower than for oropharyngeal SCC [22]. From the data, one can see that, while there is a very close (almost implicit) relationship between NKSCC (as strictly defined) and HPV in oropharyngeal SCC [23, 24], this is not the case for sinonasal NKSCC. For KSCC, only 4.0 % of tumors are related to transcriptionally-active HPV. For the other specific variants, far fewer cases have been tested, but a substantial fraction are related to transcriptionally-active HPV, particularly papillary SCC, basaloid SCC, and adenosquamous carcinoma. The rare cases of sinonasal spindle cell carcinoma have been negative. For verrucous carcinoma, studies are only available looking for HPV DNA (not combined with p16 testing) and these have shown the tumors to be negative [25]. A large study on verrucous carcinoma across the other major head and neck anatomic subsites showed that tumors are not related to transcriptionally-active high risk HPV [26] so it seems to be a similar case in the sinonasal tract. The potential prognostic significance of HPV in sinonasal SCCs is discussed below.
Fig. 4.
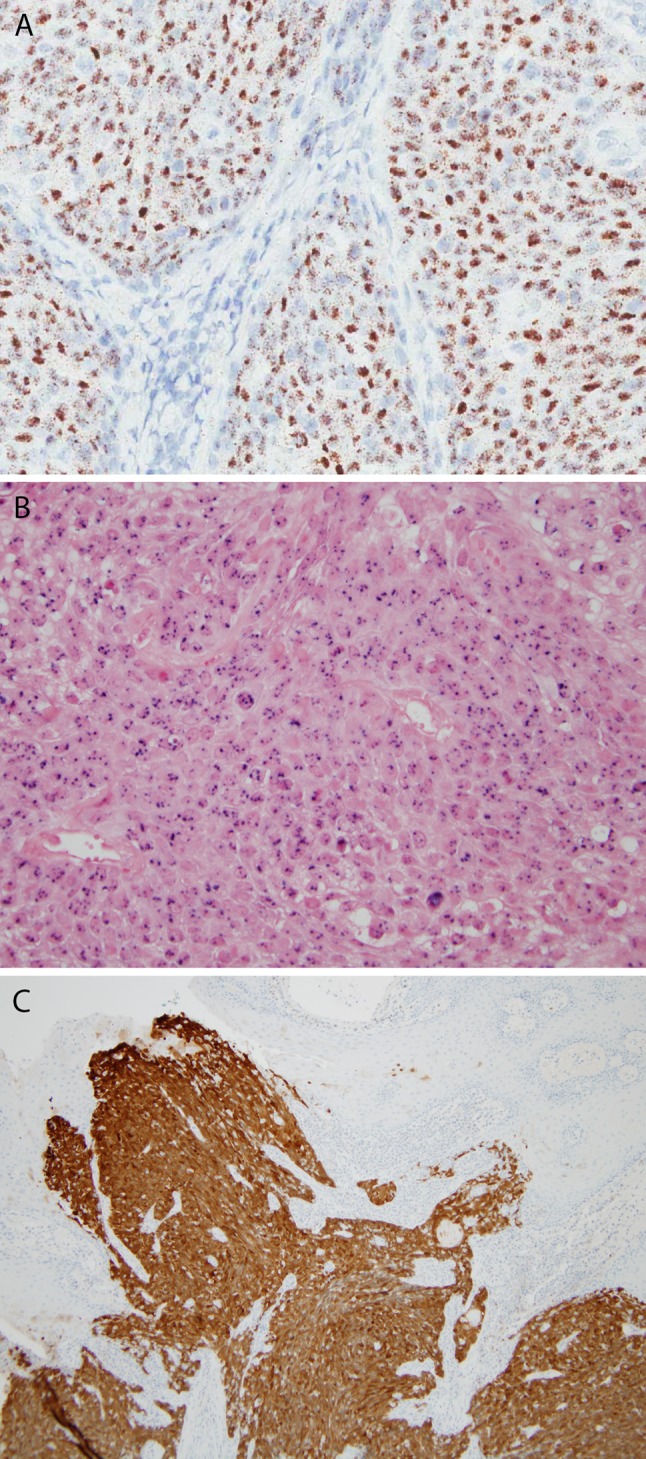
Human papillomavirus (HPV) in situ hybridization and p16 immunohistochemistry. HPV RNA can be detected by emerging in situ hybridization for E6/E7 mRNA in tissue slides, here (a-×20 magnification) with granular, brown staining in all of the tumor cells. In situ hybridization for high risk HPV DNA is common (b-×20 magnification), here showing multifocal, punctate, blue signals in the nuclei of tumor cells. p16 immunohistochemistry is a well established surrogate marker of transcriptionally-active high risk HPV in tumors with a stain pictured here where there is strong, nuclear and cytoplasmic staining (c-×4 magnification) in almost all tumor cells
Since SCC not infrequently arises from a preexisting Schneiderian papilloma, either inverted or oncocytic, and these papillomas are sometimes associated with high risk HPV, it seems possible that HPV drives the progression through these precursor lesions. In a large review of the literature in 2008, Lawson et al. [27] found that HPV DNA (low or high risk) was found in approximately 20–25 % of inverted papillomas. Further, HPV was more common in recurrent papillomas and ones with dysplasia or frank invasive carcinoma. For inverted papillomas without dysplasia or carcinoma, 22.3 % harbored HPV DNA. For those with severe dysplasia, it was 55.8 % and for those with invasive SCC, 55.1 %. Further, the ratio of high risk to low risk HPV types moved progressively toward high risk as the lesions became dysplastic or carcinomatous. This strongly suggests a role for high risk HPV in SCC development. However, the story is more complicated. Stoddard et al. [28] recently studied inverted papillomas using RNA in situ hybridization for high risk HPV and found that all 19 papillomas were positive, but most at very low signal levels. Further, as it turns out, when closely examining the studies on transcriptionally-active high risk HPV in sinonasal SCC, the subset of 27 patients with inverted papilloma-related SCC had active HPV in only 2 cases (7.4 %) [7, 20]. This suggests that the high risk HPV may be important for the growth of inverted papillomas and perhaps for carcinogenesis within them, but that it typically does not drive the growth of established tumors as it does in SCC of the oropharynx.
Treatment and Prognosis
Treatment for sinonasal SCC varies somewhat depending on the stage, patient performance status, comorbidities, and somewhat based on the tumor type. However, surgical resection with postoperative radiotherapy appears to be the optimal approach [3]. Endoscopic approaches are being used increasingly because they are less invasive, less morbid, and have less surgical complications [3]. Interestingly, while positive margin status has been shown to be adverse in sinonasal cancer resection, surgery with widely negative margins isn’t clearly better than more conservative ones, as long as clinically negative margins are achieved. Further, although no large, or randomized, studies have been performed for comparison, piecemeal resection via endoscopic approaches appears to be as effective as en bloc resection [3, 29]. Precision radiotherapy, such as intensity modulated radiation therapy and gamma knife have improved treatment and lessened morbidity, particularly for those patients with tumors adjacent to the orbits, skull base, and brain. For advanced stage tumors, particularly those that are inoperable, targeted treatments have not been defined—these are very much needed. Chemotherapy clinical trials are few and small because of the rarity of sinonasal SCC, but induction approaches are promising. Palliative chemotherapy can be used effectively for incurable tumors.
The prognosis for sinonasal SCC remains poor, averaging ~50 % at 5 years, with only an insignificant trend towards improvement over the past many decades [30]. Patients with paranasal sinus primaries have worse prognosis than those with nasal cavity tumors. Regarding histology, it is not clear that any pathologic characterization of the SCC (differentiation or SCC variant) predicts prognosis, although clearly the number of patients with specific SCC variants is really too small for informative data on their prognosis relative to each other and to conventional keratinizing type SCC. Although there is not strong data in the literature, carcinomas arising from Schneiderian papillomas may be prognostically somewhat better than de novo carcinomas [5], but this does not translate into any meaningful difference in patient management or counseling, at least currently.
The significance of HPV in these tumors has garnered a lot of attention in recent years. In the two major study cohorts that have looked for transcriptionally-active high risk HPV (i.e. p16 overexpression plus detection of HPV DNA or RNA) [7, 8] and examined survival outcomes, HPV presence was associated with statistically significantly improved prognosis in multivariate analysis in one and a trend (but not statistically significant) towards such in the other. These studies comprised only 70 (14 HPV positive [8]) and 91 (28 HPV positive [7]) patients, respectively. Thus, the results are promising for the favorable effect of HPV in sinonasal SCC, but not conclusive. Larger studies are needed. In the meantime, testing of tumors in patients with sinonasal SCC (including variants) for HPV (or its surrogate marker p16) in routine practice is not indicated. It provides no clearly defined prognostic information nor should it be used for treatment decisions.
Summary
Sinonasal SCC is the most common form of sinonasal carcinoma, but it is still relatively uncommon overall. Some tumors are associated with occupational exposures, such as to wood dust or other carcinogens. Most tumors are keratinizing (or conventional) type SCC, but there is a WHO defined NKSCC type and all of the major SCC variants also occur in the sinonasal tract. Approximately 25 % of all sinonasal SCC are associated with transcriptionally-active high risk HPV (particularly when NK morphology), and this seems to be, at least in the few early studies, associated with improved patient outcomes. The overall prognosis remains poor, with little improvement over the past several decades despite a significant decrease in treatment-related morbidity. Future studies are needed to define targeted treatments and to better define the role of high risk HPV.
References
- 1.Ansa B, Goodman M, Ward K, et al. Paranasal sinus squamous cell carcinoma incidence and survival based on surveillance, epidemiology, and end results data, 1973–2009. Cancer. 2013;119(14):2602–2610. doi: 10.1002/cncr.28108. [DOI] [PubMed] [Google Scholar]
- 2.Sanghvi S, Khan MN, Patel NR, et al. Epidemiology of sinonasal squamous cell carcinoma: a comprehensive analysis of 4994 patients. Laryngoscope. 2014;124(1):76–83. doi: 10.1002/lary.24264. [DOI] [PubMed] [Google Scholar]
- 3.Llorente JL, Lopez F, Suarez C, et al. Sinonasal carcinoma: clinical, pathological, genetic and therapeutic advances. Nat Rev Clin Oncol. 2014;11(8):460–472. doi: 10.1038/nrclinonc.2014.97. [DOI] [PubMed] [Google Scholar]
- 4.Barnes L. Schneiderian papillomas and nonsalivary glandular neoplasms of the head and neck. Mod Pathol. 2002;15(3):279–297. doi: 10.1038/modpathol.3880524. [DOI] [PubMed] [Google Scholar]
- 5.Nudell J, Chiosea S, Thompson LD. Carcinoma ex-Schneiderian papilloma (malignant transformation): a clinicopathologic and immunophenotypic study of 20 cases combined with a comprehensive review of the literature. Head Neck Pathol. 2014;8(3):269–286. doi: 10.1007/s12105-014-0527-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Mofty SK, Lu DW. Prevalence of high-risk human papillomavirus DNA in nonkeratinizing (cylindrical cell) carcinoma of the sinonasal tract: a distinct clinicopathologic and molecular disease entity. Am J Surg Pathol. 2005;29(10):1367–1372. doi: 10.1097/01.pas.0000173240.63073.fe. [DOI] [PubMed] [Google Scholar]
- 7.Bishop JA, Guo TW, Smith DF, et al. Human papillomavirus-related carcinomas of the sinonasal tract. Am J Surg Pathol. 2013;37(2):185–192. doi: 10.1097/PAS.0b013e3182698673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larque AB, Hakim S, Ordi J, et al. High-risk human papillomavirus is transcriptionally active in a subset of sinonasal squamous cell carcinomas. Mod Pathol. 2014;27(3):343–351. doi: 10.1038/modpathol.2013.155. [DOI] [PubMed] [Google Scholar]
- 9.Masand RP, El-Mofty SK, Ma XJ, et al. Adenosquamous carcinoma of the head and neck: relationship to human papillomavirus and review of the literature. Head Neck Pathol. 2011;5(2):108–116. doi: 10.1007/s12105-011-0245-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jo VY, Mills SE, Stoler MH, et al. Papillary squamous cell carcinoma of the head and neck: frequent association with human papillomavirus infection and invasive carcinoma. Am J Surg Pathol. 2009;33(11):1720–1724. doi: 10.1097/PAS.0b013e3181b6d8e6. [DOI] [PubMed] [Google Scholar]
- 11.Pilch BZ, Bouquot JE, Thompson LDR. Squamous cell carcinoma. In: Barnes EL, Eveson JW, Reichart P, Sidranksy D, editors. World health organization pathology and genetics of head and neck tumours. Lyon: IARC Press; 2005. pp. 15–17. [Google Scholar]
- 12.Vazquez A, Khan MN, Blake DM, et al. Sinonasal squamous cell carcinoma and the prognostic implications of its histologic variants: a population-based study. Int Forum Allergy Rhinol. 2015;5(1):85–91. doi: 10.1002/alr.21418. [DOI] [PubMed] [Google Scholar]
- 13.Cardesa A, Zidar N, Nadal A, et al. Papillary squamous cell carcinoma. In: Barnes L, Eveson JW, Reichart P, Sidranksy D, et al., editors. World health organization classification of tumours—pathology and genetics head and neck tumours. Lyon: IARC Press; 2005. p. 126. [Google Scholar]
- 14.Cardesa A, Zidar N, Ereno C. Basaloid squamous cell carcinoma. In: Barnes EL, Eveson JW, Reichart P, Sidranksy D, editors. World health organization classification of tumours—pathology and genetics of head and neck tumours. Lyon: IARC Press; 2005. pp. 125–126. [Google Scholar]
- 15.Cardesa A, Zidar N. Spindle cell carcinoma. In: Barnes EL, Eveson JW, Reichart P, Sidranksy D, editors. World health organization pathology and genetics—head and neck tumours. Lyon: IARC Press; 2005. pp. 127–128. [Google Scholar]
- 16.Durden FL, Jr, Moore CE, Muller S. Verrucous carcinoma of the paranasal sinuses: a case report. Ear Nose Throat J. 2010;89(7):E21–E23. doi: 10.1177/014556131008900704. [DOI] [PubMed] [Google Scholar]
- 17.Bishop JA, Ogawa T, Stelow EB, et al. Human papillomavirus-related carcinoma with adenoid cystic-like features: a peculiar variant of head and neck cancer restricted to the sinonasal tract. Am J Surg Pathol. 2013;37(6):836–844. doi: 10.1097/PAS.0b013e31827b1cd6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tilson MP, Bishop JA. Utility of p40 in the differential diagnosis of small round blue cell tumors of the sinonasal tract. Head Neck Pathol. 2014;8(2):141–145. doi: 10.1007/s12105-013-0496-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Emanuel P, Wang B, Wu M, et al. p63 Immunohistochemistry in the distinction of adenoid cystic carcinoma from basaloid squamous cell carcinoma. Mod Pathol. 2005;18(5):645–650. doi: 10.1038/modpathol.3800329. [DOI] [PubMed] [Google Scholar]
- 20.Alos L, Moyano S, Nadal A, et al. Human papillomaviruses are identified in a subgroup of sinonasal squamous cell carcinomas with favorable outcome. Cancer. 2009;115(12):2701–2709. doi: 10.1002/cncr.24309. [DOI] [PubMed] [Google Scholar]
- 21.Lewis JS, Jr, Westra WH, Thompson LD, et al. The sinonasal tract: another potential “hot spot” for carcinomas with transcriptionally-active human papillomavirus. Head Neck Pathol. 2014;8(3):241–249. doi: 10.1007/s12105-013-0514-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis JS, Jr, Khan RA, Masand RP, et al. Recognition of nonkeratinizing morphology in oropharyngeal squamous cell carcinoma—a prospective cohort and interobserver variability study. Histopathology. 2012;60(3):427–436. doi: 10.1111/j.1365-2559.2011.04092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chernock RD, El-Mofty SK, Thorstad WL, Parvin CA, Lewis JS., Jr HPV-related nonkeratinizing squamous cell carcinoma of the oropharynx: utility of microscopic features in predicting patient outcome. Head Neck Pathol. 2009;3(3):186–194. doi: 10.1007/s12105-009-0126-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orvidas LJ, Lewis JE, Olsen KD, et al. Intranasal verrucous carcinoma: relationship to inverting papilloma and human papillomavirus. Laryngoscope. 1999;109(3):371–375. doi: 10.1097/00005537-199903000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Patel KR, Chernock RD, Zhang TR, et al. Verrucous carcinomas of the head and neck, including those with associated squamous cell carcinoma, lack transcriptionally active high-risk human papillomavirus. Hum Pathol. 2013;44(11):2385–2392. doi: 10.1016/j.humpath.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 27.Lawson W, Schlecht NF, Brandwein-Gensler M. The role of the human papillomavirus in the pathogenesis of Schneiderian inverted papillomas: an analytic overview of the evidence. Head Neck Pathol. 2008;2(2):49–59. doi: 10.1007/s12105-008-0048-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stoddard DG, Jr, Keeney MG, Gao G, et al. Transcriptional Activity of HPV in Inverted Papilloma Demonstrated by In Situ Hybridization for E6/E7 mRNA. Otolaryngol Head Neck Surg. 2015;152(4):752–758. doi: 10.1177/0194599815571285. [DOI] [PubMed] [Google Scholar]
- 29.Hanna E, DeMonte F, Ibrahim S, et al. Endoscopic resection of sinonasal cancers with and without craniotomy: oncologic results. Arch Otolaryngol Head Neck Surg. 2009;135(12):1219–1224. doi: 10.1001/archoto.2009.173. [DOI] [PubMed] [Google Scholar]
- 30.Turner JH, Reh DD. Incidence and survival in patients with sinonasal cancer: a historical analysis of population-based data. Head Neck. 2012;34(6):877–885. doi: 10.1002/hed.21830. [DOI] [PubMed] [Google Scholar]