Abstract
Background
Massive bone allografts have been used for limb salvage of bone tumor resections as an alternative to endoprosthesis, although they have different outcomes and risks. The use of massive bone allografts has been thought to be associated with a high risk for infection, and there is no general consensus on the management of this complication and final outcome. Because infection is such a devastating complication of limb salvage, at times leading to loss of a limb, recognizing the risk factors for infection and the results of treatment is important.
Questions/purposes
The purposes of this study were (1) to analyze the frequency of infection in a group of patients treated with massive bone allografts; (2) to analyze risk factors such as age, sex, affected bone, type of reconstruction, operative room used, primary or revision procedure, length of postoperative antibiotic administration, and use of chemotherapy; and (3) to determine the likelihood that treatment of an infected allograft will result in a successful reconstruction.
Methods
We retrospectively analyzed the records of patients treated with massive bone allografts for a benign or malignant bone tumor or as a revision for a previous limb salvage procedure between 1985 and 2011. During this period, 673 patients were reconstructed with massive bone allografts in long bones, which included 272 osteoarticular, 246 intercalary, and 155 allograft-prosthetic composite reconstructions. Using a chart review, we ascertained the frequency of infection and reoperations after the treatment of infected allografts. Minimum followup was 2 years unless death occurred earlier (mean, 106 months; range, 6–360 months), and no patient was lost to followup. The selected variables were analyzed using multivariate logistic regression to identify risk factors for infection. We analyzed survivorship free of infection as the endpoint.
Results
During followup, 60 patients (9%) had a bacterial infection of the allograft with a survivorship free from infection of 92% at 5 years (95% confidence interval [CI], 90%–94%) and 91% at 10 years (95% CI, 89%–93%). We found that tibia allografts (p < 0.001; odds ratio [OR], 3.17; 95% CI, 1.80–5.60), male patients (p < 0.029; OR, 1.92; 95% CI, 1.08–3.49), procedures performed in a conventional operating room (p < 0.002; OR, 3.15; 95% CI, 1.58–6.62), and the use of longer periods of postoperative antibiotics (p < 0.041; OR, 2.25; 95% CI, 1.02–4.88) were patient factors associated with a greater risk of infection. In 11 patients (18%, 11 of 60 infections) the infection was controlled with antibiotics and surgical débridement; however, in 49 patients (82%, 49 of 60 infections), this approach failed, so the allograft was removed and a temporary cement spacer with antibiotic was implanted to control the infection. Forty-one patients subsequently had the spacer removed and were reconstructed after infection control with another bone allograft in 24 and an endoprostheses in 17. Four patients underwent an amputation for infection and four died of disease with the spacer in place. When we analyzed the 41 patients with a second reconstruction, 14 failed with a new infection (34%, 14 of 41 secondary reconstructed) of whom 12 had been reconstructed with bone allograft (29%) and two had endoprostheses (5%).
Conclusions
Management of infections of massive bone allografts with antibiotics and surgical débridement usually resulted in failure. Infections could be treated with resection of the allograft, antibiotics, a temporary cement spacer with antibiotics, and a repeat reconstruction; however, this approach is unlikely to be successful if a second bone allograft is used. Infections are difficult to treat, and more studies are needed, but we propose that it might be preferable to use endoprosthesis reconstruction for salvage of an infected allograft.
Level of Evidence
Level III, therapeutic study.
Introduction
Massive bone allografts are used as one option to restore bone continuity after tumor resections or massive bone losses. Although prosthetic reconstructions have improved in recent years, biological reconstruction is a functional alternative option for large extremity osseous defects [1, 3, 8, 9]. Fresh-frozen allografts have been used for many years as a reconstruction option for large bony defects of the pelvis and extremity [1].
Infection is a major cause of failure in this type of reconstruction; however, there is no general consensus regarding the management of this complication and few studies provide the results of infection treatment in massive bone allografts [2, 4, 6, 7, 10]. Although many papers that analyzed allograft reconstructions described the frequency of infection [1, 3, 8, 9, 13], only a few papers analyzed the management of this complication [2, 6, 7]. Those reports that have analyzed the frequency and treatment of bone allograft infections [2, 6, 7] did not provide the likelihood of reinfection. For that reason, we sought to analyze the frequency infection and the likelihood of its eradication using a variety of approaches at one institution in a group of patients with bone tumors who underwent massive bone allografts.
The purposes of this study were to (1) analyze the frequency of infection in a group of patients treated with massive bone allografts; (2) analyze risk factors such as age, sex, affected bone, type of reconstruction, operative room used, primary or revision procedure, length of postoperative antibiotic administration, and use of chemotherapy; and (3) determine the likelihood that treatment of an infected allograft will result in a successful reconstruction.
Patients and Methods
We retrospectively reviewed patients treated with massive bone allografts between 1985 and 2011. Minimum followup was 2 years unless death occurred earlier (mean, 106 months; range, 6–360 months), and no patient was lost to followup. We analyzed the infection percentage of 673 patients reconstructed with massive bone allografts in long bones that included 272 osteoarticular, 246 intercalary, and 155 allograft-prosthetic composite (APC) reconstructions. Mean patient age at the time of diagnosis was 30 years (range, 1–80 years). There were 359 males and 314 females. Seventy-nine reconstructions were performed in the upper limb (12%) and 594 in the lower limb (88%). There were 408 in the femur and 186 in the tibia. The original diagnoses included osteosarcoma (n = 218), giant cell tumors (n = 126), chondrosarcoma (n = 95), Ewing’s sarcoma (n = 48), bone metastasis (n = 20), fibrosarcoma (n = 15), malignant fibrohistiocytoma (n = 12), chondroblastoma (n = 12), aneurysmal bone cyst (n = 10), fibrous dysplasia (n = 7), chondromyxoid fibroma (n = 8), leiomyosarcoma (n = 5), osteoblastoma (n = 6), epithelioid hemangioendothelioma (n = 4), adamantinoma (n = 2), liposarcoma (n = 1), osteofibrous dysplasia (n = 1), and revision of another failed but not infected reconstruction (n = 83). Two hundred eighty patients received chemotherapy around the time of the resection and allograft procedure, whereas 393 did not.
During the period in question, our general indications for using allografts included patients with benign or low-grade sarcomas and those patients with high-grade sarcomas of bone or eroding into bone with clinical and imaging response to neoadjuvant chemotherapy. Another indication was for patients with failure of another reconstruction to augment bone stock. In patients receiving radiotherapy, patients with high-grade sarcomas without clinical and imaging response to neoadjuvant chemotherapy or with neurovascular tumor involvement, other approaches such as endoprostheses were used.
The surgical procedure began with resection of the lesion, including biopsy scars with appropriate bone and soft tissue margins. After being thawed in a warm solution, a fresh deep-frozen nonirradiated allograft segment, sized to fit the bone defect, was cut to the proper length. The surgical procedure was performed in a conventional operating room between 1985 and 1996, whereas from 1997 to 2011, a clean-air enclosure with vertical airflow was used. Intravenous first-generation cephalosporin (1 g) was administered immediately before surgery and postoperatively; between 1985 and 2001, they were administered for a period of 3 months (first month 4 g per day, second and third months 2 g per day), whereas since 2002, they were administered for a minimum of 24 hours or until the deep drains were discontinued (3 g per day). Patients were restricted from weightbearing for 3 to 6 months after reconstruction based on radiographic evidence of allograft healing. Followup was performed 2 weeks, 6 weeks, and 3 months after surgery, then every 3 months until 2 years, and then every 6 months. Plain radiographs and physical examination were performed at each followup.
Using a chart review, we ascertained the frequency of infection, complications, and reoperations after the treatment of infected massive bone allografts. After approval by our institution’s institutional review board, preoperative data (demographic information, including patient age, sex, affected bone, type of reconstruction, type of operating room, use of chemotherapy) and postoperative data were recorded.
Survival free of infection was estimated using the Kaplan–Meier method [5]. The statistical analysis was performed using the R programming language [11]. The variables were analyzed as associated with infection using multivariate logistic regression and included: age, sex, affected bone, type of reconstruction, operative room used, primary or revision procedure, length of postoperative antibiotic administration, and use of chemotherapy. Those variables with p < 0.05 were entered in a logistic regression analysis to assess their association with infection. Because most patients who developed an infection had it occur early in the postoperative period, we did not perform a competing risk analysis.
Results
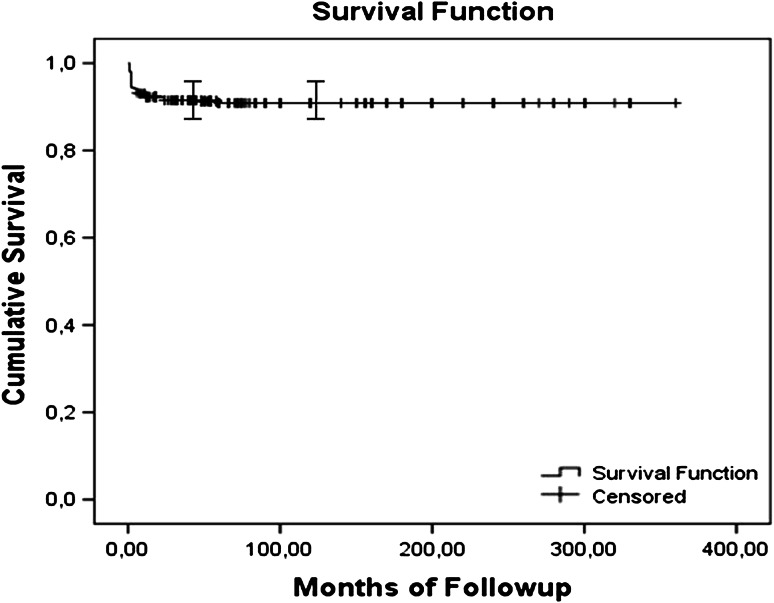
At latest followup, survivorship free from infection was 92% at 5 years (95% confidence interval [CI], 90%–94%) and 91% at 10 years (95% CI, 89%–93%) (Fig. 1). We identified 60 patients (9%) with bacterial infection of the bone allograft. There was no viral infection in this series related to the allograft used such as hepatitis virus or HIV.
Fig. 1.
A graph shows a Kaplan-Meier curve for survival free of infection. The overall survival rate for patients free of infection was 92% at 5 years (95% CI, 90%–94%) and 91% at 10 years (95% CI, 89%–93%).
We found that tibia allografts (31 infections in 186 tibias [16.6%], p < 0.001, odds ratio [OR], 3.17; 95% CI, 1.80–5.60), male patients (38 infections in 359 males [10.5%], p < 0.029; OR, 1.92; 95% CI, 1.08–3.49), procedures performed in a conventional operating room (31 infections in 147 procedures [21%], p < 0.002; OR, 3.15; 95% CI, 1.58–6.62), and the use of longer periods of postoperative antibiotics (44 infections in 303 procedures [14.5%], p < 0.041; OR, 2.25; 95% CI, 1.02–4.88]) were patient factors associated with a greater risk of infection (Table 1).
Table 1.
Multivariable analysis for predicting risk factors for infection (n = 673)
| Variable | Odds ratio | p value | 95% CI |
|---|---|---|---|
| Male | 1.92 | 0.029 | 1.08–3.49 |
| COR | 3.15 | 0.002 | 1.58–6.62 |
| LPA | 2.25 | 0.041 | 1.02–4.88 |
| Tibia | 3.17 | < 0.001 | 1.80–5.60 |
CI = confidence interval; COR = conventional operative room; LPA = longer period of antibiotics.
After sustaining the infection, in all patients, surgical débridement and antibiotics were performed as a first approach without removal of the allograft and fixation and cultures were obtained. In 46 patients a single organism was recovered that included coagulase-negative Staphylococci (17 cases), Staphylococcus aureus (13 cases), alpha-hemolytic Streptococcus (seven), Morganella morganii (three cases), Serratia marcescens (two cases), Escherichia coli (two cases), and Enterococcus faecalis (two cases); whereas 14 patients had an infection with multiple organisms. In 11 patients (18%, 11 of 60 infections) the infection was controlled with this approach; however, in 49 patients (82%, 49 of 60 infections), it failed, so the allograft was removed and a temporary cement spacer with antibiotic was implanted to control the infection. In all, cement spacer vancomycin (2 g for 40 g of cement) and aminoglycosides (1 g for 40 g of cement) (gentamicin or tobramycin) were mixed empirically to have a broad antibacterial spectrum against Gram-positive and Gram-negative bacteria. Antibiotics were administered postoperatively for 6 weeks according to the organism obtained. In four patients the infection was not controlled and an amputation was performed, and four patients died of primary disease with the cement spacer without a second reconstruction. Forty-one patients were secondarily reconstructed after infection control with at least 6 weeks without antibiotics before proceeding to the next stage, 24 with a secondary allograft reconstruction (11 APC, 10 intercalary, and three osteoarticular allografts), and 17 with an endoprostheses (Fig. 2).
Fig. 2A–D.
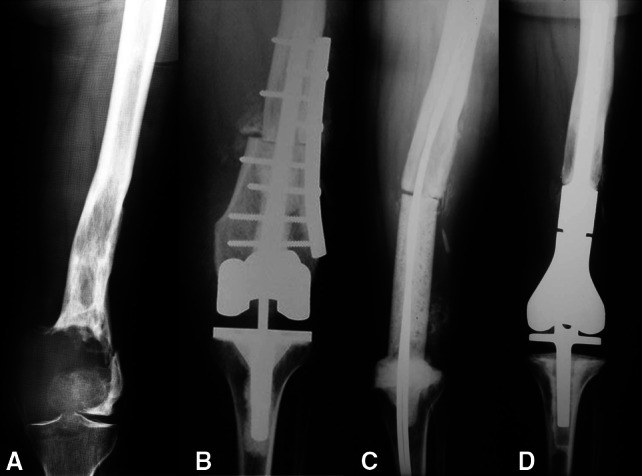
A 42-year-old female patient had a giant cell tumor of the left distal femur. She underwent reconstruction with an APC after resection of the tumor. (A) An AP radiograph of the left distal femur shows the epiphyseal tumor with fracture of the distal femur. (B) An AP radiograph obtained after resection of the tumor and reconstruction with an APC shows fixation of the allograft to the host bone with a lateral plate. (C) An AP radiograph of the left distal femur 6 months after reconstruction shows a temporary antibiotic-impregnated cement spacer that replaced the original APC owing to a deep infection. (D) Three years postoperatively, the patient’s AP radiograph of the left distal femur is shown. The patient underwent reconstruction with a modular cemented endoprosthesis.
When we analyzed the 41 patients with a second reconstruction, 14 failed (two endoprostheses and 12 allografts) with a new infection (34%) and all were treated with resection of the reconstruction and a second temporary spacer with antibiotic was implanted. These patients were reconstructed successfully with a mean followup of 60 months in six cases with an endoprosthesis, in seven cases with a new allograft (six intercalary and one APC), and in the remaining case, an amputation was performed as a result of persistent infection unable to be controlled with the temporary spacer.
Discussion
Massive bone allograft is an option used to restore function for the osseous defects created after resecting large extremity tumors and has been used for a long time for this problem. Infection is a major cause of failure in this type of reconstruction; however, there is no general consensus on how to manage this complication nor is there much data on the results of treatment once an infection occurs. We therefore studied a group of massive bone allografts of the long bones, analyzing the infection rate, identified risk factors of this specific complication, and infection control after this complication.
Our study has certain limitations. First, we analyzed infection in all types of allografts in different long bones, including intercalary, osteoarticular, and APCs, and the numbers are small enough in this retrospective series that we cannot determine reliably if site or type of graft is a risk factor. However, this allows us to have adequate numbers to analyze this specific complication. Second, the group has some inherent heterogeneity in terms of diagnosis, chemotherapy, the amount of soft tissue resection, extent of internal fixation, type of prostheses, amount of resection, and anatomic location, which could affect the incidence of infection and complications. Third, other approaches such as endoprostheses were indicated if the patient received radiotherapy, in patients with high-grade sarcomas without clinical and imaging response to neoadjuvant chemotherapy, or with neurovascular tumor involvement. These patients could be at greater risk of infection and were not reconstructed with this method. Finally, we did not exclude allografts that failed for other reasons (such as local recurrence and fracture); this suggests that our estimate of the frequency of infection of allografts may be a low estimate.
The infection frequency in this series was 9% (60 infections in 673 patients) and is similar to previous reports [2, 4, 6, 7, 10, 12, 13]. Lord et al. [6] reported a 11.7% infection rate (33 infections in 283 patients); their series did not differ with regard to age, sex, type of allograft, or site of the allograft. Dick and Strauch [2] reported a 13.3% infection rate (10 infections in 75 patients) and chemotherapy was not found to be a predisposing factor to infection when compared with the overall group. Loty et al. [7] reported 14 infections (8.5%) in 164 patients reconstructed with massive bone irradiated allografts and they did not find chemotherapy to be a risk factor.
We analyzed the risk factors for infection and found that tibia allografts, male patients, procedures performed in a conventional operating room, and the use of longer periods of postoperative antibiotics were associated with a higher risk of infection. Hernigou et al. [4] reported on 115 patients (11 infections [10%]) receiving massive allografts that were sterilized by irradiation and implanted after tumor resection and found that the risk of infection was higher when adjuvant chemotherapy and/or radiation treatments were required. Tan and Mankin [12] found that patients who lost more blood, had blood transfusions, or had longer procedures were more likely to develop an infection in 264 patients who had proximal humeral, proximal, or distal femoral resections but who did not have adjuvant chemotherapy or radiation. However, it must be noted that tibia allografts were excluded in their study, whereas in our study, the tibial reconstructions had the highest infection rate. In the largest series published, Mankin et al. [10] described 121 infections (12.8%) in 945 patients. Of that group, however, 46 of the patients had infections develop in relation to additional surgery for fracture or nonunion; therefore, the actual number of infections directly related to the allograft and not confounded by another complication is 75 (7.9%). Their series did not differ from ours with regard to age, sex, type of allograft, or site of the allograft. The infection rate for the 343 patients who received chemotherapy did not vary greatly from the mean figures for the series (14% for all patients with infections, but the infection rate for 34 patients who had radiation alone was very high when compared with patients who had no radiation [38%]). They also found a higher infection rate in intercalary arthrodesis (71 patients, 19 infections), but they did not find differences among segmental intercalary, osteoarticular, or APC.
In the present series, 18% of the infected patients (11 of 60 patients) were successfully treated with surgical débridement and antibiotics without removal of the allograft, and the percent is similar to other reports (Lord et al. 14% [6] and Loty et al. 14% [7]). Of the 49 patients (82%, 49 of 60 infections), in which the allograft was removed and a temporary cement spacer with antibiotic was implanted to control the infection, only 41 were secondary reconstructed (68%, 41 of 60 infected patients) with another bone allograft in 24 and an endoprosthesis in 17. The secondary reconstructed frequency was higher than other series (Lord et al. 33% [6], Dick and Strauch 40% [2], and Loty et al. 29% [7]). In the present series, of the 41 patients who underwent a second reconstruction, 14 failed with a new infection (34%) of whom 12 had been reconstructed with bone allograft (29%) and two had endoprostheses (5%).
In conclusion, we found that our infection frequency of 9% overall was similar to previously reported series and that infections of massive bone allografts managed with antibiotics and surgical débridement failed in most cases. The major risk factors for infection in our experience are tibia allografts, male patients, procedures performed in a conventional operating room, and the use of longer periods of postoperative antibiotics. Infections can sometimes be treated with resection of the allograft, antibiotics, a temporary cement spacer with antibiotics, and repeat reconstruction; however, these salvage reconstructions are associated with an even higher reinfection frequency if a second bone allograft is used. Based on these findings, it might be preferable to proceed to endoprosthesis reconstruction after failed infected allograft, but confirmation of this observation will need further investigation by larger studies.
Footnotes
One of the authors certifies that he (LAA-T) or she, or a member of his or her immediate family, has or may receive payments or benefits, during the study period, an amount of USD 10,000 to USD 100,000 from Stryker Americas (Miramar, FL, USA).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his institution has approved the reporting of this case report and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Aponte-Tinao LA, Ritacco LE, Albergo JI, Ayerza MA, Muscolo DL, Farfalli GL. The principles and applications of fresh frozen allografts to bone and joint reconstruction. Orthop Clin North Am. 2014;45:257–269. doi: 10.1016/j.ocl.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Dick HM, Strauch RJ. Infection of massive bone allografts. Clin Orthop Relat Res. 1994;306:46–53. [PubMed] [Google Scholar]
- 3.Gebhardt MC, Flugstad DI, Springfield DS, Mankin HJ. The use of bone allografts for limb salvage in high-grade extremity osteosarcoma. Clin Orthop Relat Res. 1991;270:181–196. [PubMed] [Google Scholar]
- 4.Hernigou P, Delepine G, Goutallier D. Infections after massive bone allografts in surgery of bone tumors of the limbs. Incidence, contributing factors, therapeutic problems. Rev Chir Orthop Reparatrice Appar Mot. 1991;77:6–13. [PubMed] [Google Scholar]
- 5.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. doi: 10.1080/01621459.1958.10501452. [DOI] [Google Scholar]
- 6.Lord CF, Gebhardt MC, Tomford WW, Mankin HJ. Infection in bone allografts. Incidence, nature, and treatment. J Bone Joint Surg Am. 1988;70:369–376. [PubMed] [Google Scholar]
- 7.Loty B, Tomeno B, Evrad J, Postel M. Infection in massive bone allografts sterilized by radiation. Int Orthop. 1994;18:164–171. doi: 10.1007/BF00192473. [DOI] [PubMed] [Google Scholar]
- 8.Mankin HJ, Gebhardt MC, Jennings LC, Springfield DS, Tomford WW. Long-term results of allograft replacement in the management of bone tumors. Clin Orthop Relat Res. 1996;324:86–97. doi: 10.1097/00003086-199603000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Mankin HJ, Gebhardt MC, Tomford WW. The use of frozen cadaveric allografts in the management of patients with bone tumors of the extremities. Orthop Clin North Am. 1987;18:275–289. [PubMed] [Google Scholar]
- 10.Mankin HJ, Hornicek FJ, Raskin KA. Infection in massive bone allografts. Clin Orthop Relat Res. 2005;432:210–216. doi: 10.1097/01.blo.0000150371.77314.52. [DOI] [PubMed] [Google Scholar]
- 11.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria; 2014. Available at: http://www.R-project.org. Accessed January 14, 2015.
- 12.Tan MH, Mankin HJ. Blood transfusion and bone allografts. Effect on infection and outcome. Clin Orthop Relat Res. 1997;340:207–214. doi: 10.1097/00003086-199707000-00027. [DOI] [PubMed] [Google Scholar]
- 13.Tomford WW, Thongphasuk J, Mankin HJ, Ferraro MJ. Frozen musculoskeletal allografts: a study of the clinical incidence and causes of infection associated with their use. J Bone Joint Surg Am. 1990;72:1137–1143. [PubMed] [Google Scholar]