Abstract
Studies on postoperative complications and survival in patients with pancreatic neuroendocrine tumors (pNET) are sparse and randomized controlled trials are not available. We reviewed all studies on postoperative complications and survival after resection of pNET. A systematic search was performed in the Cochrane Central Register of Controlled Trials, MEDLINE and EMBASE from 2000–2013. Inclusion criteria were studies of resected pNET, which described postoperative complications separately for each surgical procedure and/or 5-year survival after resection. Prospective and retrospective studies were pooled separately and overall pooled if heterogeneity was below 75 %. The random-effect model was used. Overall, 2643 studies were identified and after full-text analysis 62 studies were included. Pancreatic fistula (PF) rate of the prospective studies after tumor enucleation was 45 %; PF-rates after distal pancreatectomy, pancreatoduodenectomy, or central pancreatectomy were, respectively, 14–14–58 %. Delayed gastric emptying rates were, respectively, 5–5–18–16 %. Postoperative hemorrhage rates were, respectively, 6–1–7–4 %. In-hospital mortality rates were, respectively, 3–4–6–4 %. The 5-year overall survival (OS) and disease-specific survival (DSS) of resected pNET without synchronous resected liver metastases were, respectively, 85–93 %. Heterogeneity between included studies on 5-year OS in patients with synchronous resected liver metastases was too high to pool all studies. The 5-year DSS in patients with liver metastases was 80 %. Morbidity after pancreatic resection for pNET was mainly caused by PF. Liver resection in patients with liver metastases seems to have a positive effect on DSS. To reduce heterogeneity, ISGPS criteria and uniform patient groups should be used in the analysis of postoperative outcome and survival.
Introduction
Given the rarity of pancreatic neuroendocrine tumors (pNET), well-designed randomized controlled trials on surgical treatment for pNET are not available [1–3]. Most studies are cohort studies or case reports and therefore the level of evidence in studies on surgical treatment of pNET is limited to level III.
Studies on postoperative complications and in-hospital mortality often describe pNET as part of a larger study population. These studies include patients with pancreatic ductal adenocarcinoma, intraductal papillary mucinous neoplasm (IPMN), chronic pancreatitis, pancreatic adenomas as well as pNET [4–6]. These diagnoses may influence the postoperative complication rate and operative mortality. It is well known that patients with pancreatitis have a lower postoperative pancreatic fistula rate compared to non-pancreatitis patients [7]. Furthermore, postoperative complications after pancreatic surgery for pNET are influenced by the type of surgery, such as pancreatoduodenectomy, distal pancreatectomy, central pancreatectomy, or enucleation [8–11]. Studies analyzing postoperative complications caused by the different surgical procedures in patients with pNET are limited.
Survival of pNET patients is mainly affected by metastasis found at the time of diagnosis. The overall 5-year survival of non-functional pNET in patients with distant metastases (M1) is 43 % with a median survival of 23 months In contrast, patients with resected functional pNET without metastases (M0) have a survival rate of 90–100 % [2, 3]. Survival is often presented by tumor stages but different staging systems are used, e.g., American Joint Committee on Cancer (AJCC) staging or European Neuro Endocrine Tumor Society (ENETS) staging system [12, 13]. Another difficulty in analyzing survival of patients with pNET after resection is the inclusion of non-hereditary and hereditary patients in the same cohort. Survival outcome of patients Multiple Endocrine Neoplasia type 1 (MEN-1) or Von Hippel–Lindau (VHL) disease may be influenced since these tumors are often early diagnosed and indication for the surgical treatment can be different [3].
Considering the limitations of most studies as summarized, the aim of this study was to systematic review all studies on postoperative complications and 5-year survival in patients with resected pNET.
Methods
Search methods and identification of studies
All types of study, including cohort, case-control or case series and languages, were included. Inclusion period ranged from January 2000 till December 2013. Studies before 2000 were not included. In 2000, the WHO classification was introduced and clearly defined the phenotypes of NETs and their clinicopathological conditions. In order to reduce ambiguities and heterogeneity on pathological origin from the included studies, the time for inclusion was from 2000 to 2013 [14]. The Cochrane Central Register of Controlled trials (CENTRAL) in the Cochrane Library, MEDLINE and EMBASE were searched for studies. Also the references of the identified studies were searched to identify suitable studies.
The search strategy was supervised by the local librarian and the query terms “neuroendocrine tumor”, “carcinoid”, “pancreas”, “foregut”, “pNET”, “GEP-NET”, “pancreatoduodenectomy”, “enucleation”, “pancreatectomy”, “complications”, “fistula”, “bleeding”, “delayed gastric emptying”, “survival” or every possible variants of these terms were used. Two authors (APJJ, EJMND) independently reviewed all included studies on title and abstract and later on full text.
Inclusion criteria were all studies on resected pNET in which the postoperative complications, in-hospital mortality or survival after surgical resection was described. Postoperative complications were defined as pancreatic fistula, delayed gastric emptying, bleeding, and mortality as in-hospital mortality after resection. Finally, at least 10 patients with a pNET had to be included in the study to reduce bias and heterogeneity and to enhance scientific relevance. Studies were scored as invalid if the patients were analyzed as a part of a larger cohort of none-pNET and the data of the patients with a pNET could not be extracted from full-text analysis. Also, if not all described patients had undergone surgery and/or the resected patients have not been described separately or if studies described the postoperative complications or in-hospital mortality of the entire group and not specific after one surgical procedure, studies were scored as invalid. Finally, in order to improve homogeneity, studies were excluded from the 5-year survival analysis if all the patients of the study were affected with the MEN-1-syndrome/VHL disease or if all the included patients in the study had liver metastasis at time of surgery.
Data collection and statistical analysis
After screening on title and abstract, a full-text screening was performed to determine if the studies fulfilled the inclusion criteria. Data of postoperative complications, in-hospital mortality and survival were extracted. If possible, the complications were scored according the ISGPF/S criteria [15–17]. An overall (grade A/B/C) pancreatic fistula rate and if possible a grade B/C pancreatic fistula rate was calculated. If the grade B/C pancreatic fistula rate was not described in detail, then that study was only included in the overall pancreatic fistula proportion analysis. The same yields for delayed gastric emptying and postoperative hemorrhage. The variables of the postoperative complications and in-hospital mortality were analyzed for each surgical procedure. Studies on survival were only included if the overall 5-year survival and/or the 5-year disease-specific survival after curative resection could be extracted in patients with and/or without curative resected liver metastases. No strict definitions of a curative resection were enforced. If the survival was analyzed based on resection margins, the R0 resection margin was used.
Postoperative complications, in-hospital mortality and 5-year survival were given in proportions with a 95 % confidence interval (CI) and a meta-analysis of these proportions was performed with R [18]. The random effects model was used for expected heterogeneity. The I2 statistics was used to measure the consistency between the studies in the meta-analysis. If the I2 statistics was above 75 %, the heterogeneity was considerable and the results of proportion analysis were not suitable for a meta-analysis [19–21]. In order to make a distinction in the quality of the studies, prospective and retrospective studies were analyzed separately. From all the prospective and retrospective studies an estimated pooled proportion was calculated and if the I2 statistics were both below 75 %, all studies were pooled in an overall proportion.
Assessment of risk of bias
For the assessment of the risk of bias, the methodological index for non-randomized studies (MINORS) was used [22]. The MINORS contains 8 items: clear stated aim, inclusion consecutive patients, prospective data collection, endpoints appropriate to aim, unbiased assessment of the endpoint, appropriate follow-up period, loss to follow-up <5 % and prospective calculation of study size. Based on these eight items, the included studies will be scored to a 3-point scale from 0 to 2. An item scored 0 if the item was not reported. An item scored 1, if it was reported but inadequate and an item scored 2 if it was reported and adequate. The ideal total score would be 16. An appropriate follow-up for the studies included in the survival analysis was at least 40 months. If it was not exactly described whether all the patients were included in the follow-up, the study scored 1 point in “lost to follow-up”.
Results
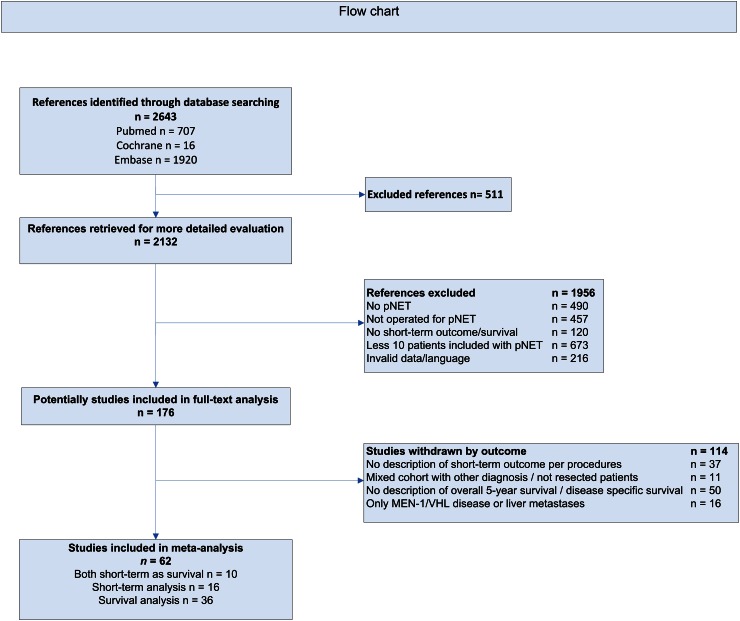
A total of 2643 studies were identified through searching the different databases, including Cochrane Central Register of Controlled trials (CENTRAL) in the Cochrane Library, MEDLINE and EMBASE. A total of 511 duplicate studies were excluded, as depicted in Fig. 1, therefore 2132 references were suitable for further assessment. Of all these references, 1956 were excluded because they did not meet the inclusion criteria or the studies were invalid. Initially 176 studies were included in the full-text search and after these articles looked through, 114 studies were withdrawn by their outcome. Finally, 62 studies were included in this meta-analysis, 10 studies for postoperative complications, in-hospital mortality and survival analysis, 16 studies for only postoperative outcome analysis and 36 for only survival analysis, as depicted in Fig. 1.
Fig. 1.
Flow Chart of the search strategy
Postoperative complications
Pancreatic fistula
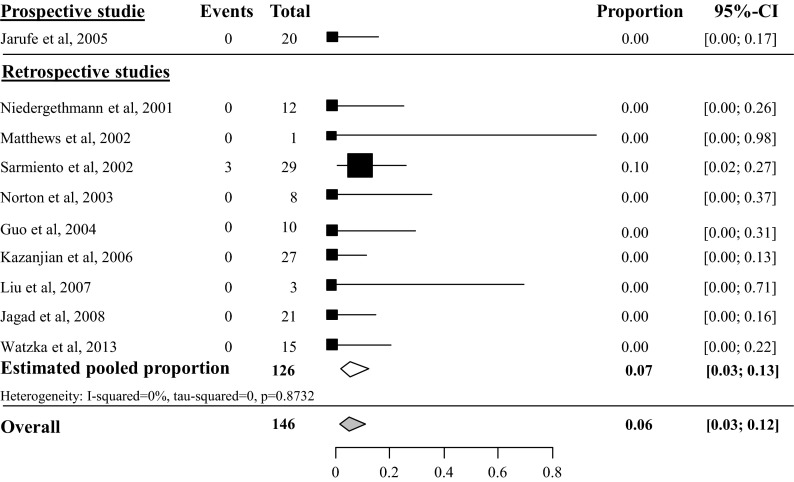
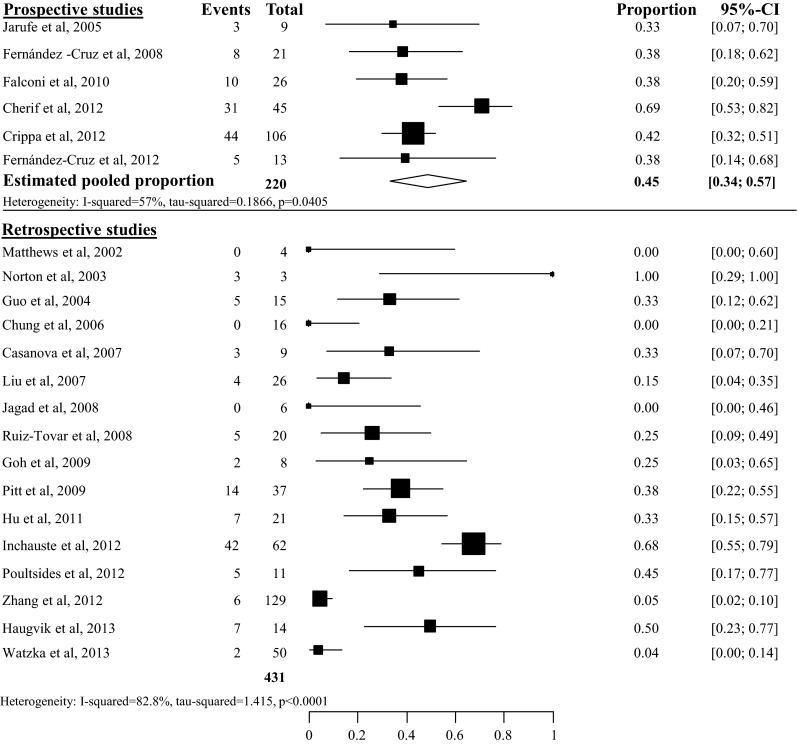
Estimated pooled pancreatic fistula (PF) rate after tumor enucleation was 45 % (95 % CI 34–57 %, I2 57 %), based on 6 prospective studies with 220 included patients [23–28]. Heterogeneity of the 16 retrospective studies was too high to pool all 22 studies, as depicted in Fig. 2 [29–44]. Overall PF rate grade B/C after tumor enucleation was 27 % (95 % CI 19–37 %), based on 8 studies with a total of 324 included patients [24, 25, 27, 28, 38, 40, 43] (see appendix Fig. 13). Overall PF rate after distal pancreatectomy was 14 % (95 % CI 10–19 %), based on 18 studies with a total of 383 included patients, as depicted in Fig. 3 [23, 24, 29–37, 39, 41–44, 45, 46]. The overall grade B/C PF rate after distal pancreatectomy was 8 % (95 % CI 2–35 %), based on 2 studies with a total of 74 included patients [24, 43] (see appendix Fig. 14). Overall PF rate after pancreatoduodenectomy was 14 % (95 % CI 9–21), based on 11 studies with a total amount of 171 included patients as depicted in Fig. 4 [23, 29–31, 34, 35, 41, 44, 46–48]. None of these studies described grade B/C PF rate in detail. Overall PF rate after central pancreatectomy was 58 % (95 % CI 41–73 %), based on four studies with a total of 56 included patients (see appendix Fig. 15) [25, 28, 34, 41]. Two studies described grade B/C PF rate ranging from 12 to 41 % (see appendix Fig. 16). Heterogeneity was too high to perform a pooled meta-analysis (I2 77 %) [25, 28].
Fig. 2.
Overall pancreatic fistula rate after tumor enucleation
Fig. 13.
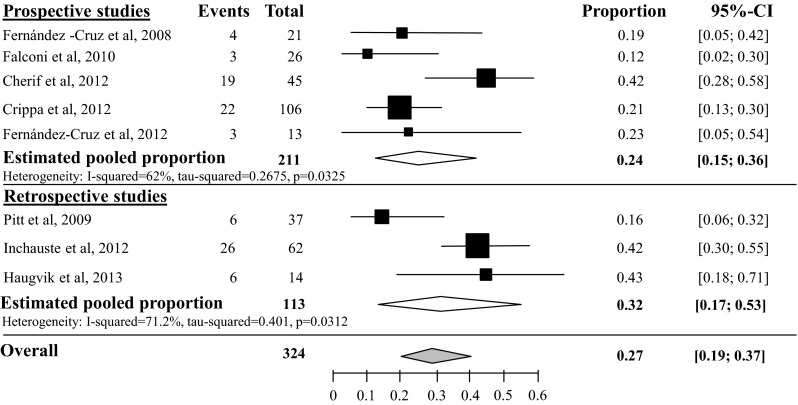
Pancreatic fistula rate grade B/C after tumor enucleation
Fig. 3.
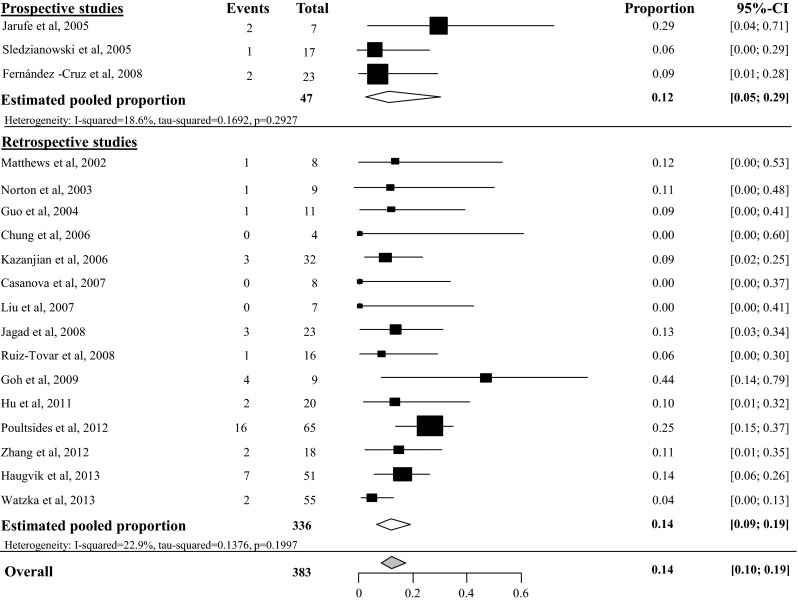
Overall pancreatic fistula rate after distal pancreatectomy
Fig. 14.
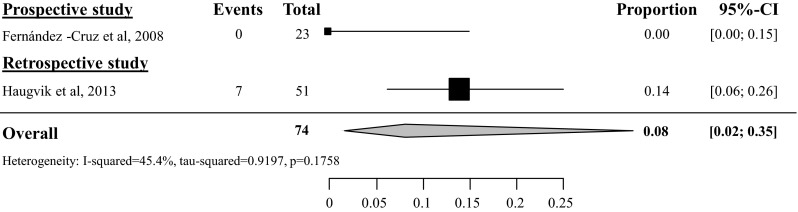
Pancreatic fistula rate grade B/C after distal pancreatectomy
Fig. 4.
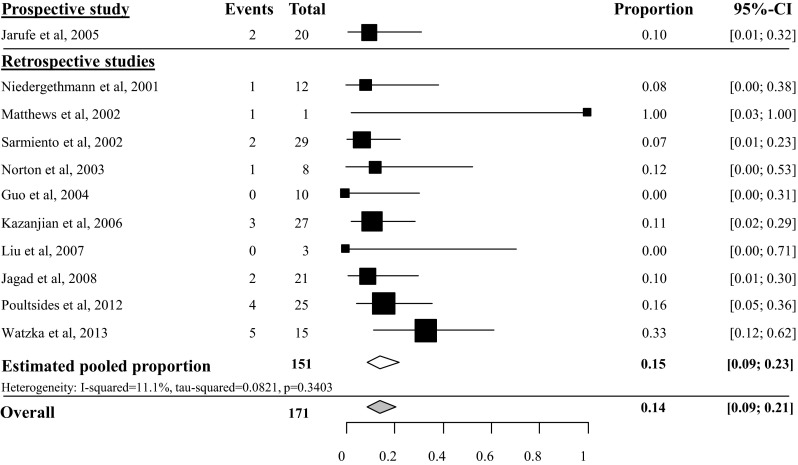
Overall pancreatic fistula rate after pancreatoduodenectomy
Fig. 15.
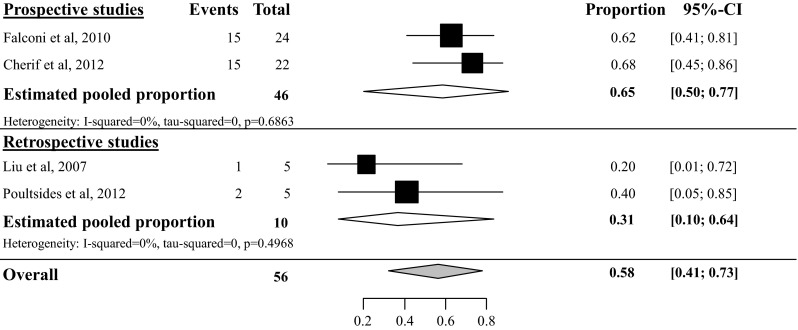
Overall pancreatic fistula rate after central pancreatectomy
Fig. 16.
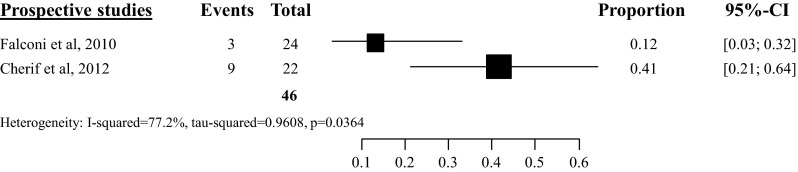
Pancreatic fistula rate grade B/C after central pancreatectomy
Delayed gastric emptying
Delayed gastric emptying (DGE) was rarely reported and only the overall DGE rate was analyzed since none of the included studies made a distinction based on the ISGPS criteria. Overall DGE rate after tumor enucleation was 5 % (95 % CI 2–10 %) based on six studies with a total amount of 231 included patients (see Fig. 5) [26, 28, 34, 35, 38, 46]. Overall DGE rate after distal pancreatectomy was 5 % (95 % CI 1–19 %, I2 12 %) [34, 35, 46], based on three studies with a total of 62 included patients (see Fig. 6), after pancreatoduodenectomy 18 % (95 % CI 10–31 %, I2 0 %) [34, 35, 46] based on three studies with a total of 51 included patients (see Fig. 7) and after central pancreatectomy, 16 % (95 % CI 1–71 %, I2 73 %) [28, 34] (see appendix Fig. 17).
Fig. 5.
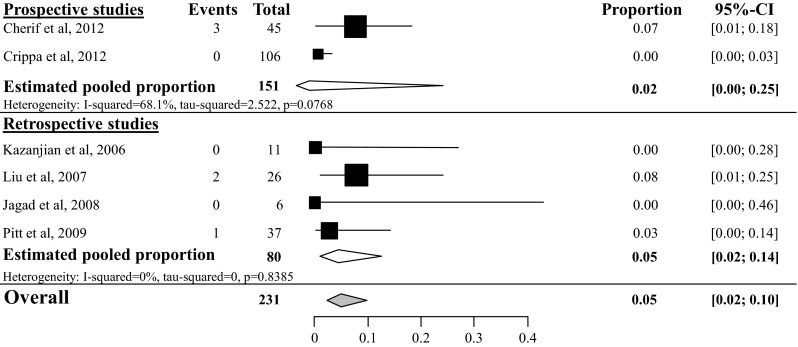
Overall delayed gastric emptying rate after tumor enucleation
Fig. 6.
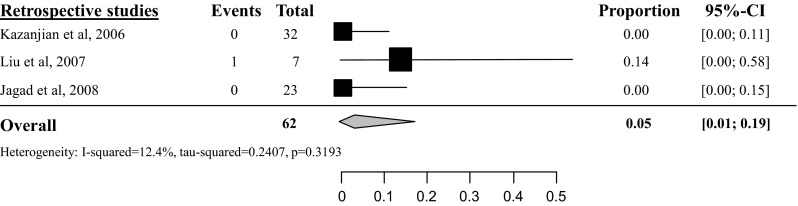
Overall delayed gastric emptying rate after distal pancreatectomy
Fig. 7.
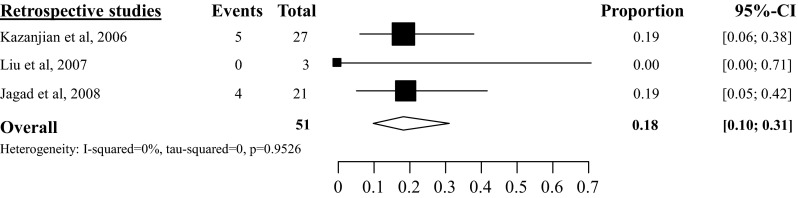
Overall delayed gastric emptying rate after pancreatoduodenectomy
Fig. 17.
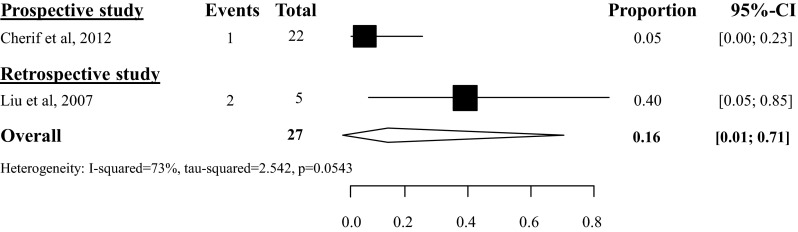
Overall delayed gastric emptying rate after central pancreatectomy
Postoperative hemorrhage
Postoperative hemorrhage was often not exactly defined according the ISGPS criteria in most studies. Therefore, a distinction between grade A and B/C hemorrhage could not be made. Six studies described the overall postoperative hemorrhage rate after tumor enucleation with a total amount of 254 included patients (see Fig. 8). In these studies, the overall postoperative hemorrhage rate was 6 % (95 % CI 3–12 %) [25, 26, 28, 35, 39, 44]. Two studies with a total amount of 62 included patients described an overall postoperative hemorrhage rate of 1 % after distal pancreatectomy (95 % CI 0–9 %, I2 0 %) [35, 44] as depicted in Fig. 9. Overall postoperative hemorrhage rate after pancreatoduodenectomy was 7 % (95 % CI 3–15 %, I2 0 %), based on four studies with a total of 77 included patients [35, 44, 47, 48] (see Fig. 10) and after central pancreatectomy 4 % (95 % CI 1–16 %, I2 0 %), based on 2 studies (see appendix Fig. 18) [25, 28].
Fig. 8.
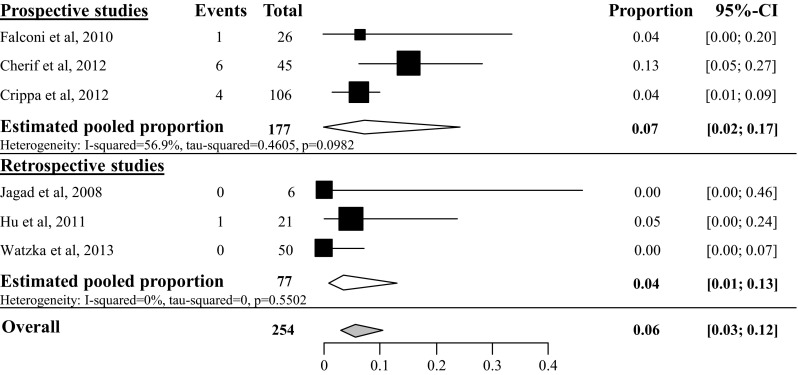
Overall postoperative hemorrhage rate after tumor enucleation
Fig. 9.
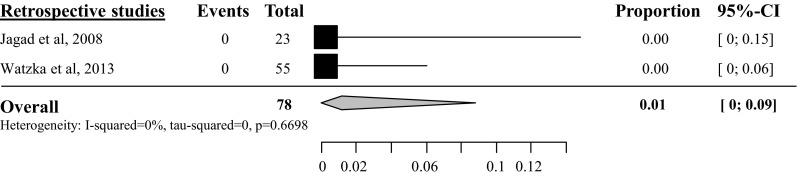
Overall postoperative hemorrhage rate after distal pancreatectomy
Fig. 10.
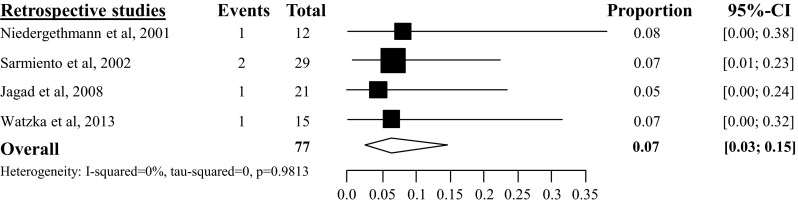
Overall postoperative hemorrhage rate after pancreatoduodenectomy
Fig. 18.
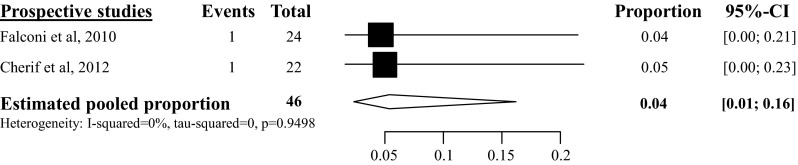
Overall postoperative hemorrhage rate after central pancreatectomy
In-hospital mortality
Overall pooled in-hospital mortality rate after tumor enucleation was 3 % (95 % CI 2–5 %), based on 20 studies with a total amount of 624 patients [23–25, 28–40, 42, 44, 46] (see appendix Fig. 19). The overall pooled in-hospital mortality after distal pancreatectomy was 4 % (95 % CI 2–7 %) [23, 24, 29–37, 39, 42, 44, 45, 46], based on 16 studies with a total of 267 included patients (see appendix Fig. 20) and 6 % after pancreatoduodenectomy (95 % CI 3–12 %), based on 10 studies with a total of 146 included patients [23, 29–31, 34, 35, 44, 46–48] (see appendix Fig. 21). The overall pooled in-hospital mortality after central pancreatectomy was 4 % (95 % CI 1–16 %), based on 3 studies with a total of 51 included patients (see appendix Fig. 22) [25, 28, 34].
Fig. 19.
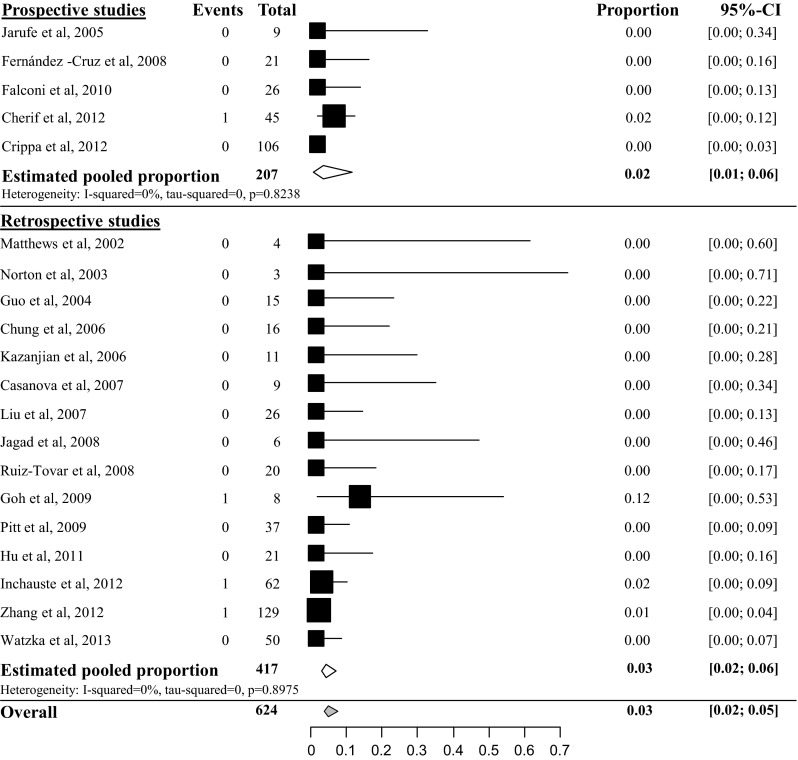
In-hospital mortality rate after tumor enucleation
Fig. 20.
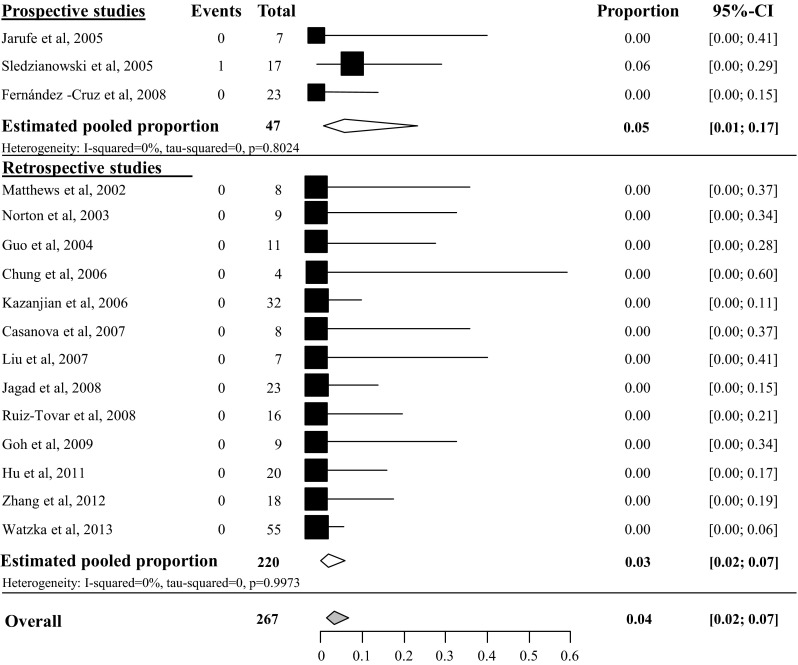
In-hospital mortality rate after distal pancreatectomy
Fig. 21.
In-hospital mortality rate after pancreatoduodenectomy
Fig. 22.
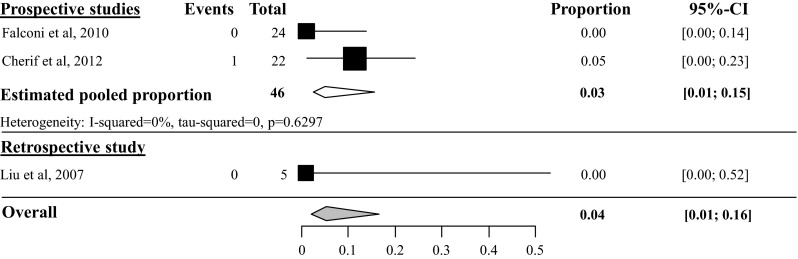
In-hospital mortality rate after central pancreatectomy
Survival analysis
The 5-year overall and disease-specific survival in patients without liver metastases
In the survival analysis, a distinction is made between studies including patients with or without resected liver metastases. In the overall 5-year survival analysis of the resected patients without liver metastases, 15 studies were analyzed with a total of 3089 included patients [28, 36, 38, 41, 49–59]. The heterogeneity between the prospective studies was too high to perform a pooled meta-analysis (I2 95 %), mainly caused by the study of Bilimoria et al. [59]. The estimated pooled proportion of the overall 5-year survival of the retrospective studies was 85 % (95 % CI 78–90 %, I2 73.5 %), see Fig. 11. In the 5-year disease-specific survival (DSS) analysis, 6 studies were included with a total amount of 420 patients [43, 50, 52, 60–62]. The overall pooled 5-year DSS after pancreatic resection was 93 % (95 % CI 88–96 %), see appendix Fig. 23.
Fig. 11.
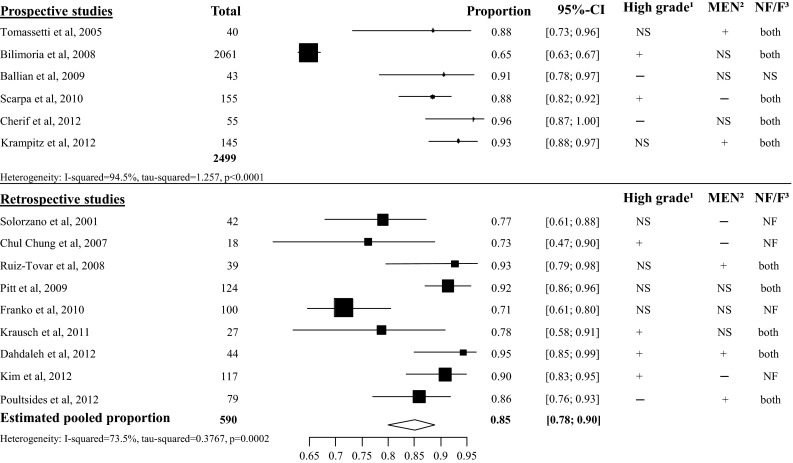
Overall 5-year survival in patients without liver metastases. 1 High grade: patients with grade 3 or poorly differentiated pNET may be included. 2 MEN: patients with a hereditary syndrome such as MEN1 syndrome or von Hippel Lindau may be included. 3 NF/F. Patients with non-functional pNET or functional pNET may be included. + Some patients are affected with the condition. − None of the patients are affected with the condition. NS not specified. The study did not specified the number of patients with the condition
Fig. 23.
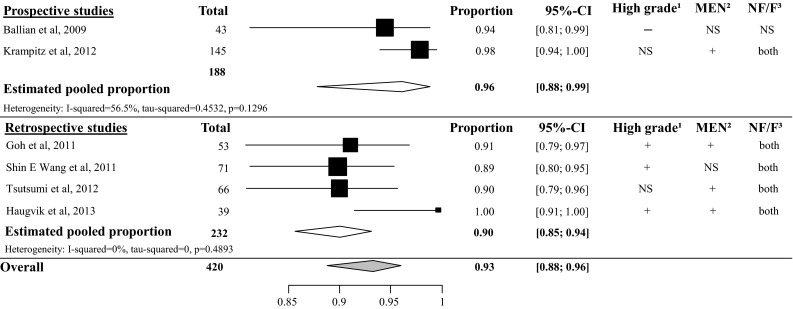
5-year disease-specific survival in patients without liver metastases. 1 High grade: patients with grade 3 or poorly differentiated pNET may be included. 2 MEN: patients with a hereditary syndrome such as MEN1 syndrome or von Hippel Lindau may be included. 3 NF/F. Patients with non-functional pNET or functional pNET may be included. + Some patients are affected with the condition. − None of the patients are affected with the condition. NS not specified. The study did not specified the number of patients with the condition
The 5-year overall and disease-specific survival in patients with liver metastases
In all the included studies, at least one patient per study had resected liver metastases. In the 5-year overall survival analysis, 23 studies were included with a total amount of 1540 patients [23, 35, 44, 46, 48, 63–80]. The heterogeneity was too high to perform an overall pooled proportion analysis, most studies included a proportion of high grade pNET (see Fig. 12). Four retrospective studies with a total of 207 included patients described the 5-year disease-specific survival in patients with liver involvement. The overall pooled 5-year DSS was 80 % (95 CI 66–90 %, I2 70 %), see appendix Fig. 24 [81–84].
Fig. 12.
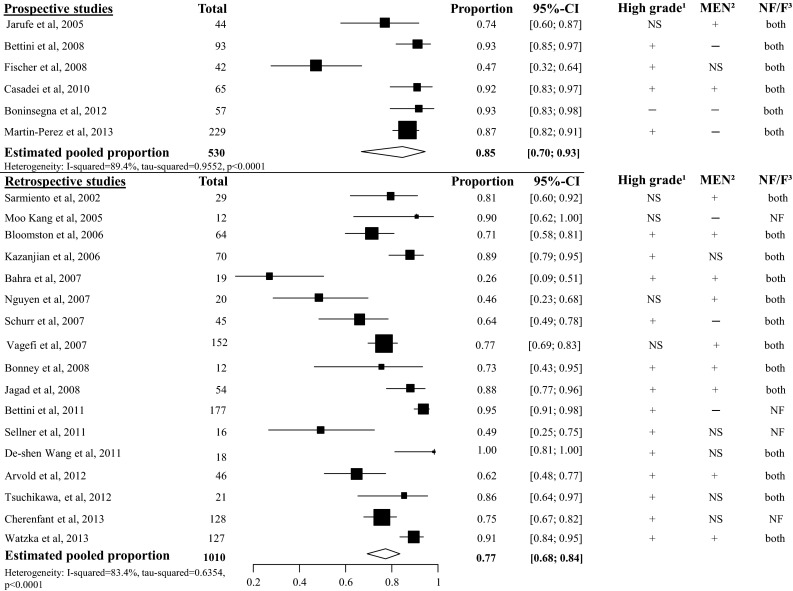
Overall 5-year survival in patients with liver metastases. 1 High grade: patients with grade 3 or poorly differentiated pNET may be included. 2 MEN: patients with a hereditary syndrome such as MEN1 syndrome or von Hippel Lindau may be included. 3 NF/F. Patients with non-functional pNET or functional pNET may be included. + Some patients are affected with the condition. − None of the patients are affected with the condition. NS not specified. The study did not specified the number of patients with the condition
Fig. 24.
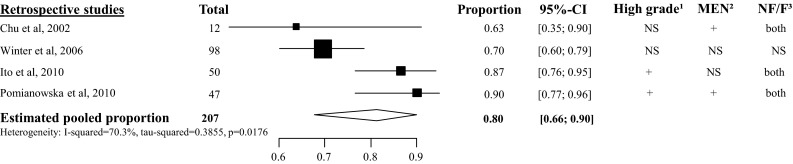
5-year disease-specific survival in patients with liver metastases. 1 High grade: patients with grade 3 or poorly differentiated pNET may be included. 2 MEN: patients with a hereditary syndrome such as MEN1 syndrome or von Hippel Lindau may be included. 3 NF/F. Patients with non-functional pNET or functional pNET may be included. + Some patients are affected with the condition. − None of the patients are affected with the condition. NS not specified. The study did not specified the number of patients with the condition
Assessment of risk of bias
On overview of the risk of bias of all the included studies is listed in Table 1. The variety of the total points ranged from 5 to 12 points. None of the studies scored on unbiased assessment of the study endpoint or prospective calculation of the study size. Overall, 33/62 studies (53 %) had a high MINOR score of ≥10 and only 8 studies (13 %) had a low MINOR score ≤7.
Table 1.
Risk of Bias according MINOR
| Study | Inclusion C/S/Ba | Clear stated aim | Inclusion consecutive patients | Prospective data collection | Endpoints appropriate to aim | Unbiased assessment of endpoint | Appropriate follow-up period | Loss to follow-up <5 % | Prospective calculation of study size | Total points |
|---|---|---|---|---|---|---|---|---|---|---|
| Niedergethmann et al. [47] | C | 1 | 2 | 0 | 1 | 0 | 2 | 2 | 0 | 8 |
| Solorzano et al. [53] | S | 1 | 2 | 0 | 1 | 0 | 1 | 2 | 0 | 7 |
| Chu et al. [81] | S | 2 | 2 | 0 | 2 | 0 | 1 | 2 | 0 | 9 |
| Matthews et al. [29] | C | 2 | 2 | 0 | 2 | 0 | 2 | 2 | 0 | 10 |
| Sarmiento et al. [48] | B | 1 | 2 | 0 | 1 | 0 | 2 | 1 | 0 | 7 |
| Guo et al. [31] | C | 1 | 2 | 0 | 1 | 0 | 1 | 1 | 0 | 6 |
| Norton et al. [30] | C | 2 | 2 | 0 | 2 | 0 | 1 | 2 | 0 | 9 |
| Jarufe et al. [23] | B | 2 | 2 | 1 | 2 | 0 | 1 | 2 | 0 | 10 |
| Kang et al. [68] | S | 2 | 2 | 0 | 2 | 0 | 1 | 2 | 0 | 9 |
| Sledzianowski et al. [45] | C | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 12 |
| Tomassetti et al. [49] | S | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 12 |
| Vagefi et al. [69] | S | 2 | 2 | 0 | 2 | 0 | 2 | 1 | 0 | 9 |
| Bloomston et al. [70] | S | 2 | 2 | 0 | 2 | 0 | 2 | 2 | 0 | 10 |
| Chung et al. [32] | C | 1 | 2 | 0 | 1 | 0 | 2 | 2 | 0 | 8 |
| Kazanjian et al. [46] | B | 2 | 2 | 0 | 2 | 0 | 2 | 2 | 0 | 10 |
| Winter et al. [82] | S | 1 | 2 | 0 | 1 | 0 | 2 | 2 | 0 | 8 |
| Bahra et al. [71] | S | 2 | 2 | 1 | 2 | 0 | 2 | 2 | 0 | 11 |
| Casanova et al. [33] | C | 0 | 2 | 0 | 0 | 0 | 2 | 2 | 0 | 6 |
| Chul Chung et al. [54] | S | 2 | 2 | 0 | 2 | 0 | 1 | 2 | 0 | 9 |
| Liu et al. [34] | C | 2 | 2 | 0 | 2 | 0 | 2 | 2 | 0 | 10 |
| Nguyen et al. [72] | S | 2 | 2 | 0 | 2 | 0 | 2 | 2 | 0 | 10 |
| Schurr et al. [73] | S | 2 | 2 | 0 | 2 | 0 | 2 | 2 | 0 | 10 |
| Bettini et al. [63] | S | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 12 |
| Bilimoria et al. [59] | S | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 12 |
| Bonney et al. [74] | S | 1 | 2 | 0 | 1 | 0 | 1 | 1 | 0 | 6 |
| Fernández-Cruz et al. [24] | C | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 12 |
| Fischer et al. [64] | S | 2 | 2 | 2 | 2 | 0 | 1 | 2 | 0 | 11 |
| Jagad et al. [35] | B | 1 | 2 | 0 | 1 | 0 | 2 | 2 | 0 | 8 |
| Ruiz-Tovar et al. [36] | B | 0 | 2 | 0 | 0 | 0 | 2 | 1 | 0 | 5 |
| Ballian et al. [50] | S | 2 | 2 | 2 | 2 | 0 | 2 | 1 | 0 | 11 |
| Goh et al. [37] | C | 2 | 2 | 0 | 2 | 0 | 2 | 1 | 0 | 9 |
| Pitt et al. [38] | B | 2 | 2 | 0 | 2 | 0 | 1 | 2 | 0 | 9 |
| Casadei et al. [65] | S | 2 | 2 | 1 | 2 | 0 | 2 | 2 | 0 | 11 |
| Falconi et al. [25] | C | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 12 |
| Franko et al. [55] | S | 2 | 2 | 0 | 2 | 0 | 1 | 2 | 0 | 9 |
| Goh et al. [60] | S | 2 | 2 | 0 | 2 | 0 | 1 | 2 | 0 | 9 |
| Ito et al. [83] | S | 2 | 2 | 0 | 2 | 0 | 2 | 2 | 0 | 10 |
| Pomianowska et al. [84] | S | 2 | 2 | 0 | 2 | 0 | 2 | 2 | 0 | 10 |
| Scarpa et al. [51] | S | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 12 |
| Arvold et al. [78] | S | 2 | 2 | 0 | 2 | 0 | 1 | 2 | 0 | 9 |
| Bettini et al. [75] | S | 2 | 2 | 0 | 2 | 0 | 2 | 2 | 0 | 10 |
| Fernández-Cruz et al. [27] | C | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 12 |
| Hu et al. [39] | C | 2 | 2 | 0 | 2 | 0 | 2 | 2 | 0 | 10 |
| Krausch et al. [56] | S | 2 | 1 | 0 | 2 | 0 | 1 | 1 | 0 | 7 |
| Sellner et al. [76] | S | 2 | 2 | 0 | 2 | 0 | 2 | 2 | 0 | 10 |
| Wang et al. [77] | S | 2 | 2 | 0 | 2 | 0 | 2 | 1 | 0 | 9 |
| Wang et al. [61] | S | 2 | 2 | 0 | 2 | 0 | 2 | 2 | 0 | 10 |
| Boninsegna et al. [66] | S | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 12 |
| Cherif et al. [28] | B | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 12 |
| Crippa et al. [26] | C | 2 | 2 | 1 | 2 | 0 | 2 | 2 | 0 | 11 |
| Dahdaleh et al. [57] | S | 2 | 2 | 0 | 2 | 0 | 2 | 1 | 0 | 9 |
| Inchauste et al. [40] | C | 2 | 2 | 0 | 2 | 0 | 2 | 2 | 0 | 10 |
| Kim et al. [58] | S | 2 | 2 | 0 | 2 | 0 | 1 | 1 | 0 | 8 |
| Krampitz et al. [52] | S | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 12 |
| Poultsides et al. [41] | B | 2 | 2 | 0 | 2 | 0 | 1 | 1 | 0 | 8 |
| Tsuchikawa et al. [79] | S | 2 | 2 | 0 | 2 | 0 | 1 | 1 | 0 | 8 |
| Tsutsumi et al. [62] | S | 2 | 2 | 0 | 2 | 0 | 2 | 2 | 0 | 10 |
| Zhang et al. [42] | C | 2 | 2 | 0 | 2 | 0 | 2 | 1 | 0 | 9 |
| Cherenfant et al. [80] | S | 1 | 2 | 0 | 2 | 0 | 1 | 1 | 0 | 7 |
| Haugvik et al. [43] | B | 2 | 2 | 0 | 2 | 0 | 2 | 2 | 0 | 10 |
| Martin-Perez et al. [67] | S | 2 | 2 | 2 | 2 | 0 | 1 | 1 | 0 | 10 |
| Watzka et al. [44] | B | 2 | 2 | 0 | 2 | 0 | 0 | 1 | 1 | 8 |
aStudy included in c complication analysis, s survival analysis or b both complication and survival analysis
Discussion
This is the first systematic review including a proportion analysis on postoperative complications, in-hospital mortality and 5-year survival in patients with a pancreatic neuroendocrine tumor. Pooled PF rate after tumor enucleation of the prospective studies was high (45 %) compared to overall pooled PF rate after distal pancreatectomy (14 %) and pancreatoduodenectomy (14 %). In patients with other diagnosis including pancreatic adenocarcinoma, the overall incidence of PF after pancreatoduodenectomy ranges from 2 % up to more than 20 % [85–88] and after distal pancreatectomy from 12–32 % [89–93] and the overall PF rate in non-pNET diagnosis is between 11 and 17 % compared to 6–34 % in patients with pNET [40, 45, 94–96]. This is coherent with the incidence of PF in patients with pNET in our review. Since the presence of PF accounts in the majority of cases for a prolonged hospital stay, the high incidence of these complications after tumor enucleation is alarming. Also the incidence of delayed gastric emptying (18 %) in patients with pNET after pancreatoduodenectomy in our review is comparable with the overall incidence from 14 to 45 % after pancreatoduodenectomy in patients with non-pNET [97–99].
The overall mortality in patients with pNET in our review is between 3 and 6 %. In the literature the recent overall mortality in non-pNET is between 0 and 4 % [91, 96, 100–102]. The overall in-hospital mortality rate in our review is slightly high. This is probably due to the fact that we included studies already from the year 2000 and centralization of pancreatic surgery takes place only since the last few years. The in-hospital mortality rate after pancreatoduodenectomy has been decreased from 15 % to even 1 or 2 % in high volume centers [103–105]. Therefore, the effect of centralization on in-hospital mortality is not shown in our review. Furthermore, in some studies on patients with pNET, pancreatic resection was not specified in pancreatoduodenectomy of distal pancreatectomy. In the analysis of the in-hospital mortality, these studies were excluded [10, 28, 40]. A second important point could be the texture of the pancreatic remnant after resection. In patients with pNET, especially small pNETs, there is no pancreatic duct dilation, no fibrosis and the pancreatic remnant is soft and viable. This is in contrast with most patients with non-pNET tumors with have a double duct sign and subsequent fibrosis of the pancreatic remnant. Since the texture of the pancreatic remnant is well known to be associated with PF and mortality this could a reasonable explanation [5, 106, 107]. Unfortunately, in this review, we could not find data on this important detail to draw conclusions concerning this point.
The analysis of postoperative complications in pancreatic surgery is more uniform since the clear definitions of these complications by the International Study Group of Pancreatic Surgery (ISGPS) [15–17]. The number of studies suitable for inclusion in the proportion analysis for pancreatic fistula grade B/C was limited. Most studies on grade B/C fistula (or delayed gastric emptying and postoperative hemorrhage) included patients with different underlying diseases. Patients with pNET were part of the studied cohort. These studies were not included in this review. Tumor enucleation is mainly indicated for pNET and therefore the number of studies for proportion analysis on grade B/C pancreatic fistula was relatively high compared to the other procedures (appendix Fig. 13). In future studies, we encourage the use of the ISGPS criteria in the analysis of postoperative complications and to describe the patients with pNET separately.
Recently, Hüttner et al. described a high incidence of pancreatic fistula after tumor enucleation in patients with all types of pancreatic neoplasm [108]. Although the authors conclude that a tumor enucleation can be performed safely and is considerable instead of a standard resection, this conclusion should be interpreted with caution. Even in high volume centers, the incidence of pancreatic fistula was comparable after both tumor enucleation and standard resection (both 23 %). Although overall length of stay and mortality after tumor enucleation is lower compared to standard resection, patients with severe pancreatic fistula will have comparable length of stay and mortality. Since specialized care for patients with PF is important for overall outcome, enucleations should also be carried out in specialized centers.
A considerable amount of studies described the 5-year survival after pancreatic resection with or without liver metastases. The 5-year disease-specific survival in patients with and without liver metastases was fairly comparable with, respectively, 93 and 80 %. Although there will be differences in tumor differentiation, functionality, or hereditary tumors, the survival rate after surgical resection in patients with liver metastases is high. An aggressive treatment in patients with liver metastases may be justified. However, both patients and tumor characteristics, such as total tumor load in the liver, are important in this treatment. In our review, the heterogeneity between the included studies in the 5-year overall survival analysis was high (Figs. 9 and 10). These differences can be explained by the patients’ characteristics of the included studies. For example, in the study of Bahra et al., patients were enrolled with at least two malignant factors such as invasion in adjacent organs, metastases, tumor invasion, tumor size ≥2 cm, and tumor grade 2 or 3 pNET [71]. Bilimoria et al. [59] also enrolled patients with distant metastases (20 %), positive lymph nodes (52.8 %), and poorly differentiated pNET (22.1 %). Most likely, a high grade/poorly differentiated tumor has more influence on survival than the presence of resected liver metastases. This hypothesis has not been analyzed in this review. In addition, in most studies no differentiation was made between functional and non-functional pNET.
Since no randomized controlled trials were available, heterogeneity was notable. During full-text analysis, some studies were not clear or incomplete on the description of the outcome. For example, studies described the postoperative after “standard pancreatic resection” but different definitions for a standard resection were used. Some studies described patients after pancreatoduodenectomy and distal pancreatectomy [10, 28, 108] while other studies described patients with all types of pancreatic resection including central pancreatectomy and total pancreatectomy [26, 38, 40]. Furthermore, some large studies, especially studies that extracted the data from the SEER database, described the survival outcome per tumor stage and most of these studies have not described an overall 5-year survival. Moreover, it was not always clear if all the included patients with stage IV disease were operated. All these studies were excluded from this review. There is no agreement of the exact cut-off value of heterogeneity in which it is accepted to perform a meta-analysis. According to the Cochrane handbook, with an I2 above 75, heterogeneity is considerable [21]. By the strict inclusion criteria, effort has been made to include homogeneous data and studies with good quality but the diversity of the studies on pNET is considerable and this review shows the best available data up till now.
Conclusion
Based on this review, we would like to recommend using uniform definitions for “pancreatic resection” or well-described “atypical resections” for a careful comparison of clinical outcome. Furthermore, the ISGPS criteria and Clavien–Dindo grading system should be used in the analysis of postoperative complications. In survival analysis, distinguishes should be made between tumor grade/tumor differentiation, patients with a hereditarily syndrome and patients with a functional or non-functional pNET. Although pNET is a rare disease, studies on postoperative outcome and survival must be uniform and clear to be able to interpret the results in the right way and to use the results in daily practice.
Acknowledgments
Sources of financial support: A.P.J. Jilesen funded by Ipsen.
Appendix
Compliance with ethical standards
Conflict of interest
No conflict of interest, no disclaimers.
References
- 1.Kulke MH, Anthony LB, Bushnell ÞDL, et al. NANETS treatment guidelines well-differentiated neuroendocrine tumors of the stomach and pancreas. Pancreas. 2010;39:735–752. doi: 10.1097/MPA.0b013e3181ebb168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jensen RT, Cadiot G, Brandi ML, et al. ENETS consensus guidelines for the management of patients with digestive neuroendocrine neoplasms: functional pancreatic endocrine tumor syndromes. Neuroendocrinology. 2012;95:98–119. doi: 10.1159/000335591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Falconi M, Bartsch DK, Eriksson B, et al. ENETS consensus guidelines for the management of patients with digestive neuroendocrine neoplasms of the digestive system: well-differentiated pancreatic non-functioning tumors. Neuroendocrinology. 2012;95:120–134. doi: 10.1159/000335587. [DOI] [PubMed] [Google Scholar]
- 4.Falconi M, Mantovani W, Crippa S, et al. Pancreatic insufficiency after different resections for benign tumours. Br J Surg. 2008;95:85–91. doi: 10.1002/bjs.5652. [DOI] [PubMed] [Google Scholar]
- 5.Fendrich V, Merz MK, Waldmann J, et al. Neuroendocrine pancreatic tumors are risk factors for pancreatic fistula after pancreatic surgery. Dig Surg. 2011;28:263–269. doi: 10.1159/000328667. [DOI] [PubMed] [Google Scholar]
- 6.Gaujoux S, Cortes A, Couvelard A, et al. Fatty pancreas and increased body mass index are risk factors of pancreatic fistula after pancreaticoduodenectomy. Surgery. 2010;148:15–23. doi: 10.1016/j.surg.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Pratt WB, Callery MP, Vollmer CM. Risk prediction for development of pancreatic fistula using the ISGPF classification scheme. World J Surg. 2008;32:419–428. doi: 10.1007/s00268-007-9388-5. [DOI] [PubMed] [Google Scholar]
- 8.Crippa S, Bassi C, Warshaw AL, et al. Middle pancreatectomy: indications, short- and long-term operative outcomes. Ann Surg. 2007;246:69–76. doi: 10.1097/01.sla.0000262790.51512.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warshaw AL. Distal pancreatectomy with preservation of the spleen. J Hepatobiliary Pancreat Sci. 2010;17:808–812. doi: 10.1007/s00534-009-0226-z. [DOI] [PubMed] [Google Scholar]
- 10.Hackert T, Hinz U, Fritz S, et al. Enucleation in pancreatic surgery: indications, technique, and outcome compared to standard pancreatic resections. Langenbeck’s Arch Surg. 2011;396:1197–1203. doi: 10.1007/s00423-011-0801-z. [DOI] [PubMed] [Google Scholar]
- 11.Gouma DJ, Van Dijkum EJMN, Obertop H. Pancreatic cancer: matters for debate. The standard diagnostic work-up and surgical treatment of pancreatic head tumours. Eur J Surg Oncol. 2000;25:113–123. doi: 10.1053/ejso.1998.0612. [DOI] [PubMed] [Google Scholar]
- 12.American Joint Committee on Cancer . AJCC Cancer staging manual. 7. New York: Springer; 2010. [Google Scholar]
- 13.Rindi G, Klöppel G, Alhman H, et al. TNM staging of foregut (neuro) endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 2006;449:395–401. doi: 10.1007/s00428-006-0250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamilton SR, Aaltonen LA (2000) World Health Organization classification of tumours, pathology and genetics of tumours of the digestive system, pp 1–134
- 15.Wente MN, Bassi C, Dervenis C, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS) Surgery. 2007;142:761–768. doi: 10.1016/j.surg.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Welsch T, Eisele H, Zschäbitz S, et al. Critical appraisal of the International Study Group of Pancreatic Surgery (ISGPS) consensus definition of postoperative hemorrhage after pancreatoduodenectomy. Langenbecks Arch Surg. 2011;396:783–791. doi: 10.1007/s00423-011-0811-x. [DOI] [PubMed] [Google Scholar]
- 17.Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 18.The R Core Team (2013) R: a language and environment for statisticalcomputing
- 19.Higgins J, Thompson S. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 20.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins J, Green S (2011) Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0, The Cochrane Collaboration
- 22.Slim K, Nini E, Forestier D, et al. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712–716. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 23.Jarufe NP, Coldham C, Orug T, et al. Neuroendocrine tumours of the pancreas: predictors of survival after surgical treatment. Dig Surg. 2005;22:157–162. doi: 10.1159/000087148. [DOI] [PubMed] [Google Scholar]
- 24.Fernández-Cruz L, Blanco L, Cosa R, Rendón H. Is laparoscopic resection adequate in patients with neuroendocrine pancreatic tumors? World J Surg. 2008;32:904–917. doi: 10.1007/s00268-008-9467-2. [DOI] [PubMed] [Google Scholar]
- 25.Falconi M, Zerbi A, Crippa S, et al. Parenchyma-preserving resections for small nonfunctioning pancreatic endocrine tumors. Ann Surg Oncol. 2010;17:1621–1627. doi: 10.1245/s10434-010-0949-8. [DOI] [PubMed] [Google Scholar]
- 26.Crippa S, Zerbi A, Boninsegna L, et al. Surgical management of insulinomas. Arch Surg. 2012;147:261–266. doi: 10.1001/archsurg.2011.1843. [DOI] [PubMed] [Google Scholar]
- 27.Fernández-Cruz L, Molina V, Vallejos R, et al. Outcome after laparoscopic enucleation for non-functional neuroendocrine pancreatic tumours. HPB (Oxford) 2012;14:171–176. doi: 10.1111/j.1477-2574.2011.00422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cherif R, Gaujoux S, Couvelard A, et al. Parenchyma-sparing resections for pancreatic neuroendocrine tumors. J Gastrointest Surg. 2012;16:2045–2055. doi: 10.1007/s11605-012-2002-7. [DOI] [PubMed] [Google Scholar]
- 29.Matthews B, Smith T, Kercher K, et al. Surgical experience with functioning pancreatic neuroendocrine tumors. Am Surg. 2002;68:660–665. [PubMed] [Google Scholar]
- 30.Norton JA, Kivlen M, Li M, et al. Morbidity and mortality of aggressive resection in patients with advanced neuroendocrine tumors. Arch Surg. 2003;138:859–866. doi: 10.1001/archsurg.138.8.859. [DOI] [PubMed] [Google Scholar]
- 31.Guo K, Liao H, Tian Y, et al. Surgical treatment of nonfunctioning islet cell tumor: report of 41 cases. Hepatobiliary Pancreat Dis Int. 2004;3:469–472. [PubMed] [Google Scholar]
- 32.Chung JC, Choi SH, Jo SH, et al. Localization and surgical treatment of the pancreatic insulinomas. ANZ J Surg. 2006;76:1051–1055. doi: 10.1111/j.1445-2197.2006.03947.x. [DOI] [PubMed] [Google Scholar]
- 33.Casanova D, Polavieja MG, Naranjo A, et al. Surgical treatment of persistent hyperinsulinemic hypoglycemia (PHH) (insulinoma and nesidioblastosis) Langenbecks Arch Surg. 2007;392:663–670. doi: 10.1007/s00423-007-0158-5. [DOI] [PubMed] [Google Scholar]
- 34.Liu H, Peng C, Zhang S, et al. Strategy for the surgical management of insulinomas: analysis of 52 cases. Dig Surg. 2007;24:463–470. doi: 10.1159/000111822. [DOI] [PubMed] [Google Scholar]
- 35.Jagad R, Koshariya M, Kawamoto J, et al. Pancreatic neuroendocrine tumors: our approach. Hepatogastroenterology. 2008;55:275–281. [PubMed] [Google Scholar]
- 36.Ruiz-Tovar J, Priego P, Martínez-Molina E, et al. Pancreatic neuroendocrine tumours. Clin Transl Oncol. 2008;10:493–497. doi: 10.1007/s12094-008-0238-1. [DOI] [PubMed] [Google Scholar]
- 37.Goh BKP, Ooi LLPJ, Cheow P-C, et al. Accurate preoperative localization of insulinomas avoids the need for blind resection and reoperation: analysis of a single institution experience with 17 surgically treated tumors over 19 years. J Gastrointest Surg. 2009;13:1071–1077. doi: 10.1007/s11605-009-0858-y. [DOI] [PubMed] [Google Scholar]
- 38.Pitt SC, Pitt HA, Baker MS, et al. Small pancreatic and periampullary neuroendocrine tumors: resect or enucleate? J Gastrointest Surg. 2009;13:1692–1698. doi: 10.1007/s11605-009-0946-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu M, Zhao G, Luo Y, Liu R. Laparoscopic versus open treatment for benign pancreatic insulinomas: an analysis of 89 cases. Surg Endosc. 2011;25:3831–3837. doi: 10.1007/s00464-011-1800-4. [DOI] [PubMed] [Google Scholar]
- 40.Inchauste SM, Lanier BJ, Libutti SK, et al. Rate of clinically significant postoperative pancreatic fistula in pancreatic neuroendocrine tumors. World J Surg. 2012;36:1517–1526. doi: 10.1007/s00268-012-1598-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poultsides GA, Huang LC, Chen Y, et al. Pancreatic neuroendocrine tumors: radiographic calcifications correlate with grade and metastasis. Ann Surg Oncol. 2012;19:2295–2303. doi: 10.1245/s10434-012-2305-7. [DOI] [PubMed] [Google Scholar]
- 42.Zhang T, Mu Y, Qu L, et al. Accurate combined preoperative localization of insulinomas aid the choice for enucleation: a single institution experience over 25 years. Hepatogastroenterology. 2012;59:1282–1285. doi: 10.5754/hge11300. [DOI] [PubMed] [Google Scholar]
- 43.Haugvik S-P, Marangos IP, Røsok BI, et al. Long-term outcome of laparoscopic surgery for pancreatic neuroendocrine tumors. World J Surg. 2013;37:582–590. doi: 10.1007/s00268-012-1893-5. [DOI] [PubMed] [Google Scholar]
- 44.Watzka FM, Laumen C, Fottner C, et al. Resection strategies for neuroendocrine pancreatic neoplasms. Langenbecks Arch Surg. 2013;398:431–440. doi: 10.1007/s00423-012-1024-7. [DOI] [PubMed] [Google Scholar]
- 45.Sledzianowski JF, Duffas JP, Muscari F, et al. Risk factors for mortality and intra-abdominal morbidity after distal pancreatectomy. Surgery. 2005;137:180–185. doi: 10.1016/j.surg.2004.06.063. [DOI] [PubMed] [Google Scholar]
- 46.Kazanjian KK, Reber HA, Hines OJ. Resection of Pancreatic Neuroendocrine Tumors. 2014;141:6–11. doi: 10.1001/archsurg.141.8.765. [DOI] [PubMed] [Google Scholar]
- 47.Niedergethmann M, Richter A, Wendl K, et al. Rare indications for a Kausch–Whipple procedure. Eur J Surg. 2001;167:115–119. doi: 10.1080/110241501750070565. [DOI] [PubMed] [Google Scholar]
- 48.Sarmiento JM, Farnell MB, Que FG, Nagorney DM. Pancreaticoduodenectomy for islet cell tumors of the head of the pancreas: long-term survival analysis. World J Surg. 2002;26:1267–1271. doi: 10.1007/s00268-002-6714-9. [DOI] [PubMed] [Google Scholar]
- 49.Tomassetti P, Campana D, Piscitelli L, et al. Endocrine pancreatic tumors: factors correlated with survival. Ann Oncol. 2005;16:1806–1810. doi: 10.1093/annonc/mdi358. [DOI] [PubMed] [Google Scholar]
- 50.Ballian N, Loeffler AG, Rajamanickam V, et al. A simplified prognostic system for resected pancreatic neuroendocrine neoplasms. HPB (Oxford) 2009;11:422–428. doi: 10.1111/j.1477-2574.2009.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scarpa A, Mantovani W, Capelli P, et al. Pancreatic endocrine tumors: improved TNM staging and histopathological grading permit a clinically efficient prognostic stratification of patients. Mod Pathol. 2010;23:824–833. doi: 10.1038/modpathol.2010.58. [DOI] [PubMed] [Google Scholar]
- 52.Krampitz G, Norton J, Poultsides G, et al. Lymph nodes and survival in pancreatic neuroendocrine tumors. Arch Surg. 2012;147:820–827. doi: 10.1001/archsurg.2012.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Solorzano CC, Lee JE, Pisters PWT, et al. Nonfunctioning islet cell carcinoma of the pancreas: survival results in a contemporary series of 163 patients. Surgery. 2001;130:1078–1085. doi: 10.1067/msy.2001.118367. [DOI] [PubMed] [Google Scholar]
- 54.Chung JC, Choi DW, Jo SH, et al. Malignant nonfunctioning endocrine tumors of the pancreas: predictive factors for survival after surgical treatment. World J Surg. 2007;31:579–585. doi: 10.1007/s00268-006-0585-4. [DOI] [PubMed] [Google Scholar]
- 55.Franko J, Feng W, Yip L, et al. Non-functional neuroendocrine carcinoma of the pancreas: incidence, tumor biology, and outcomes in 2,158 patients. J Gastrointest Surg. 2010;14:541–548. doi: 10.1007/s11605-009-1115-0. [DOI] [PubMed] [Google Scholar]
- 56.Krausch M, Anlauf M, Schott M, et al. Loss of PTEN Expression in Neuroendocrine Pancreatic Tumors. Horm meteb res. 2011;43:865–871. doi: 10.1055/s-0031-1291333. [DOI] [PubMed] [Google Scholar]
- 57.Dahdaleh FS, Calva-Cerqueira D, Carr JC, et al. Comparison of clinicopathologic factors in 122 patients with resected pancreatic and ileal neuroendocrine tumors from a single institution. Ann Surg Oncol. 2012;19:966–972. doi: 10.1245/s10434-011-1997-4. [DOI] [PubMed] [Google Scholar]
- 58.Kim MJ, Choi DW, Choi SH, et al. Surgical strategies for non-functioning pancreatic neuroendocrine tumours. Br J Surg. 2012;99:1562–1568. doi: 10.1002/bjs.8892. [DOI] [PubMed] [Google Scholar]
- 59.Bilimoria KY, Talamonti MS, Tomlinson JS, et al. Prognostic score predicting survival after resection of pancreatic neuroendocrine tumors: analysis of 3851 patients. Ann Surg. 2008;247:490–500. doi: 10.1097/SLA.0b013e31815b9cae. [DOI] [PubMed] [Google Scholar]
- 60.Goh BKP, Chow PKH, Tan Y-M, et al. Validation of five contemporary prognostication systems for primary pancreatic endocrine neoplasms: results from a single institution experience with 61 surgically treated cases. ANZ J Surg. 2011;81:79–85. doi: 10.1111/j.1445-2197.2010.05403.x. [DOI] [PubMed] [Google Scholar]
- 61.Wang S-E, Su C-H, Kuo Y-J, et al. Comparison of functional and nonfunctional neuroendocrine tumors in the pancreas and peripancreatic region. Pancreas. 2011;40:253–259. doi: 10.1097/MPA.0b013e3181f94cc4. [DOI] [PubMed] [Google Scholar]
- 62.Tsutsumi K, Ohtsuka T, Mori Y, et al. Analysis of lymph node metastasis in pancreatic neuroendocrine tumors (PNETs) based on the tumor size and hormonal production. J Gastroenterol. 2012;47:678–685. doi: 10.1007/s00535-012-0540-0. [DOI] [PubMed] [Google Scholar]
- 63.Bettini R, Boninsegna L, Mantovani W, et al. Prognostic factors at diagnosis and value of WHO classification in a mono-institutional series of 180 non-functioning pancreatic endocrine tumours. Ann Oncol. 2008;19:903–908. doi: 10.1093/annonc/mdm552. [DOI] [PubMed] [Google Scholar]
- 64.Fischer L, Kleeff J, Esposito I, et al. Clinical outcome and long-term survival in 118 consecutive patients with neuroendocrine tumours of the pancreas. Br J Surg. 2008;95:627–635. doi: 10.1002/bjs.6051. [DOI] [PubMed] [Google Scholar]
- 65.Casadei R, Ricci C, Pezzilli R, et al. Are there prognostic factors related to recurrence in pancreatic endocrine tumors? Pancreatology. 2010;10:33–38. doi: 10.1159/000217604. [DOI] [PubMed] [Google Scholar]
- 66.Boninsegna L, Panzuto F, Partelli S, et al. Malignant pancreatic neuroendocrine tumour: lymph node ratio and Ki67 are predictors of recurrence after curative resections. Eur J Cancer. 2012;48:1608–1615. doi: 10.1016/j.ejca.2011.10.030. [DOI] [PubMed] [Google Scholar]
- 67.Martin-Perez E, Capdevila J, Castellano D, et al. Prognostic factors and long-term outcome of pancreatic neuroendocrine neoplasms: Ki-67 index shows a greater impact on survival than disease stage. The large experience of the Spanish National Tumor Registry (RGETNE) Neuroendocrinology. 2013;98:156–168. doi: 10.1159/000355152. [DOI] [PubMed] [Google Scholar]
- 68.Kang CM, Kim KS, Choi JS, et al. Experiences with nonfunctioning neuroendocrine neoplasms of the pancreas. Dig Surg. 2005;22:453–458. doi: 10.1159/000092011. [DOI] [PubMed] [Google Scholar]
- 69.Vagefi P, Razo O, Deshpande V, et al. Evolving patterns in the detection and outcomes of pancreatic neuroendocrine neoplasms. Arch Surg. 2007;142:347–354. doi: 10.1001/archsurg.142.4.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bloomston M, Muscarella P, Shah MH, et al. Cytoreduction results in high perioperative mortality and decreased survival in patients undergoing pancreatectomy for neuroendocrine tumors of the pancreas. J Gastrointest Surg. 2006;10:1361–1370. doi: 10.1016/j.gassur.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 71.Bahra M, Jacob D, Pascher A, et al. Surgical strategies and predictors of outcome for malignant neuroendocrine tumors of the pancreas. J Gastroenterol Hepatol. 2007;22:930–935. doi: 10.1111/j.1440-1746.2007.04893.x. [DOI] [PubMed] [Google Scholar]
- 72.Nguyen SQ, Angel LUZP, Divino CM, Schluender S, Schluender S. Surgery in Malignant Pancreatic Neuroendocrine Tumors. J Surg Oncol. 2007;96:397–403. doi: 10.1002/jso.20824. [DOI] [PubMed] [Google Scholar]
- 73.Schurr PG, Strate T, Rese K, et al. Aggressive surgery improves long-term survival in neuroendocrine pancreatic tumors: an institutional experience. Ann Surg. 2007;245:273–281. doi: 10.1097/01.sla.0000232556.24258.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bonney GK, Gomez D, Rahman SH, et al. Results following surgical resection for malignant pancreatic neuroendocrine tumours: a single institutional experience. JOP. 2008;9:19–25. [PubMed] [Google Scholar]
- 75.Bettini R, Partelli S, Boninsegna L, et al. Tumor size correlates with malignancy in nonfunctioning pancreatic endocrine tumor. Surgery. 2011;150:75–82. doi: 10.1016/j.surg.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 76.Sellner F, Thalhammer S, Stättner S, et al. TNM stage and grade in predicting the prognosis of operated, non-functioning neuroendocrine carcinoma of the pancreas–a single-institution experience. J Surg Oncol. 2011;104:17–21. doi: 10.1002/jso.21889. [DOI] [PubMed] [Google Scholar]
- 77.Wang D, Zhang D, Qiu M, et al. Prognostic factors and survival in patients with neuroendocrine tumors of the pancreas. Tumour Biol. 2011;32:697–705. doi: 10.1007/s13277-011-0170-9. [DOI] [PubMed] [Google Scholar]
- 78.Arvold ND, Willett CG, Fernandez-del Castillo C, et al. Pancreatic neuroendocrine tumors with involved surgical margins: prognostic factors and the role of adjuvant radiotherapy. Int J Radiat Oncol Biol Phys. 2012;83:e337–e343. doi: 10.1016/j.ijrobp.2011.12.068. [DOI] [PubMed] [Google Scholar]
- 79.Tsuchikawa T, Hirano S, Tanaka E, et al. Multidisciplinary treatment strategy for advanced pancreatic neuroendocrine tumors-a single center experience. Hepatogastroenterology. 2012;59:2623–2656. doi: 10.5754/hge12116. [DOI] [PubMed] [Google Scholar]
- 80.Cherenfant J, Stocker SJ, Gage MK, et al. Predicting aggressive behavior in nonfunctioning pancreatic neuroendocrine tumors. Surgery. 2013;154:785–791. doi: 10.1016/j.surg.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 81.Chu QD, Hill HC, Douglass HO, Jr, et al. Predictive factors associated with long-term survival in patients with neuroendocrine tumors of the pancreas. Ann Surg Oncol. 2002;9:855–862. doi: 10.1007/BF02557521. [DOI] [PubMed] [Google Scholar]
- 82.Winter JM, Cameron JL, Campbell KA, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg. 2006;10:1199–1210. doi: 10.1016/j.gassur.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 83.Ito H, Abramson M, Ito K, et al. Surgery and staging of pancreatic neuroendocrine tumors: a 14-year experience. J Gastrointest Surg. 2010;14:891–898. doi: 10.1007/s11605-010-1173-3. [DOI] [PubMed] [Google Scholar]
- 84.Pomianowska E, Gladhaug IP, Grzyb K, et al. Survival following resection of pancreatic endocrine tumors: importance of R-status and the WHO and TNM classification systems. Scand J Gastroenterol. 2010;45:971–979. doi: 10.3109/00365521003782363. [DOI] [PubMed] [Google Scholar]
- 85.Tsujie M, Nakamori S, Miyamoto A, et al. Risk factors of pancreatic fistula after pancreaticoduodenectomy-patients with low drain amylase level on postoperative day 1 are safe from developing pancreatic fistula. Hepatogastroenterology. 2012;59:2657–2660. doi: 10.5754/hge12098. [DOI] [PubMed] [Google Scholar]
- 86.Addeo P, Delpero JR, Paye F, et al. Pancreatic fistula after a pancreaticoduodenectomy for ductal adenocarcinoma and its association with morbidity: a multicentre study of the French Surgical Association. HPB. 2014;16:46–55. doi: 10.1111/hpb.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Butturini G, Marcucci S, Molinari E, et al. Complications after pancreaticoduodenectomy: the problem of current definitions. J Hepatobiliary Pancreat Surg. 2006;13:207–211. doi: 10.1007/s00534-005-1035-7. [DOI] [PubMed] [Google Scholar]
- 88.Bu MW, Friess H, Wagner M, et al. Pancreatic fistula after pancreatic head resection. Br J Surg. 2000;87:883–889. doi: 10.1046/j.1365-2168.2000.01465.x. [DOI] [PubMed] [Google Scholar]
- 89.Lee SH, Kang CM, Hwang HK, et al. Minimally invasive RAMPS in well-selected left-sided pancreatic cancer within Yonsei criteria: long-term (>median 3 years) oncologic outcomes. Surg Endosc. 2014;28:2848–2855. doi: 10.1007/s00464-014-3537-3. [DOI] [PubMed] [Google Scholar]
- 90.Hu M, Zhao G, Wang F, et al. Laparoscopic versus open distal splenopancreatectomy for the treatment of pancreatic body and tail cancer: a retrospective, mid-term follow-up study at a single academic tertiary care institution. Surg Endosc. 2014;28:2584–2591. doi: 10.1007/s00464-014-3507-9. [DOI] [PubMed] [Google Scholar]
- 91.Magge D, Gooding W, Choudry H, et al. Comparative effectiveness of minimally invasive and open distal pancreatectomy for ductal adenocarcinoma. JAMA Surg. 2013;148:525–531. doi: 10.1001/jamasurg.2013.1673. [DOI] [PubMed] [Google Scholar]
- 92.Rehman S, John SKP, Lochan R, et al. Oncological feasibility of laparoscopic distal pancreatectomy for adenocarcinoma: a single-institution comparative study. World J Surg. 2014;38:476–483. doi: 10.1007/s00268-013-2268-2. [DOI] [PubMed] [Google Scholar]
- 93.Diener MK, Seiler CM, Rossion I, et al. Efficacy of stapler versus hand-sewn closure after distal pancreatectomy (DISPACT): a randomised, controlled multicentre trial. Lancet (Lond Engl) 2011;377:1514–1522. doi: 10.1016/S0140-6736(11)60237-7. [DOI] [PubMed] [Google Scholar]
- 94.Brient C, Regenet N, Sulpice L, et al. Risk factors for postoperative pancreatic fistulization subsequent to enucleation. J Gastrointest Surg. 2012;16:1883–1887. doi: 10.1007/s11605-012-1971-x. [DOI] [PubMed] [Google Scholar]
- 95.Atema J, Jilesen A, Busch O, et al. Pancreatic fistulae after pancreatic resections for neuroendocrine tumours compared with resections for other lesions. HPB. 2015;17:38–45. doi: 10.1111/hpb.12319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Addeo P, Delpero JR, Paye F et al (2013) Pancreatic fistula after a pancreaticoduodenectomy for ductal adenocarcinoma and its association with morbidity: a multicentre study of the French Surgical Association. HPB 16:1–10 [DOI] [PMC free article] [PubMed]
- 97.Malleo G, Crippa S, Butturine G, et al. Delayed gastric emptying after pylorus-preserving pancreaticoduodenectomy: validation of International Study Group of Pancreatic Surgery classification and analysis of risk factors. HPB. 2010;12:610–618. doi: 10.1111/j.1477-2574.2010.00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.El Nakeeb A, Askr W, Mahdy Y, et al. Delayed gastric emptying after pancreaticoduodenectomy. Risk factors, predictors of severity and outcome. A single center experience of 588 cases. J Gastrointest Surg. 2015;19:1093–1100. doi: 10.1007/s11605-015-2795-2. [DOI] [PubMed] [Google Scholar]
- 99.Welsch T, Borm M, Degrate L, et al. Evaluation of the International Study Group of Pancreatic Surgery definition of delayed gastric emptying after pancreatoduodenectomy in a high-volume centre. Br J Surg. 2010;97(7):1043–1050. doi: 10.1002/bjs.7071. [DOI] [PubMed] [Google Scholar]
- 100.Romano G, Agrusa A, Galia M, et al. Whipple’s pancreaticoduodenectomy: surgical technique and perioperative clinical outcomes in a single center. Int J Surg. 2015;26:S68–S71. doi: 10.1016/j.ijsu.2015.06.062. [DOI] [PubMed] [Google Scholar]
- 101.Kawaguchi Y, Fuks D, Nomi T, et al. Laparoscopic distal pancreatectomy employing radical en bloc procedure for adenocarcinoma: technical details and outcomes. Surgery. 2015;157:1106–1112. doi: 10.1016/j.surg.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 102.Sauvanat A, Boher J, Paye F, et al. Severe jaundice increases early severe morbidity and decreases long-term survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. J Am Coll Surg. 2015;221:380–389. doi: 10.1016/j.jamcollsurg.2015.03.058. [DOI] [PubMed] [Google Scholar]
- 103.De Wilde R, Besselink M, van der Tweel I, et al. Impact of nationwide centralization of pancreaticoduodenectomy on hospital mortality. Br J Surg. 2012;99:404–410. doi: 10.1002/bjs.8664. [DOI] [PubMed] [Google Scholar]
- 104.Yoshioka R, Yasungaga H, Hasagawa K, et al. Impact of hospital volume on hospital mortality, length of stay and total costs after pancreaticoduodenectomy. Br J Surg. 2014;101:523–529. doi: 10.1002/bjs.9420. [DOI] [PubMed] [Google Scholar]
- 105.McPhee JT, Hill JS, Whalen GF, et al. Perioperative mortality for pancreatectomy: a national perspective. Ann Surg. 2007;246:246–253. doi: 10.1097/01.sla.0000259993.17350.3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Okabayashi T, Kobayashi M, Nishimori I, et al. Risk factors, predictors and prevention of pancreatic fistula formation after pancreatoduodenectomy. J Hepatobiliary Pancreat Surg. 2007;14:557–563. doi: 10.1007/s00534-007-1242-5. [DOI] [PubMed] [Google Scholar]
- 107.Yang Y-M, Tian X-D, Zhuang Y, et al. Risk factors of pancreatic leakage after pancreaticoduodenectomy. World J Gastroenterol. 2005;11:2456–2461. doi: 10.3748/wjg.v11.i16.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hüttner FJ, Koessler-Ebs J, Hackert T, et al. Meta-analysis of surgical outcome after enucleation versus standard resection for pancreatic neoplasms. Br J Surg. 2015;102:1026–1036. doi: 10.1002/bjs.9819. [DOI] [PubMed] [Google Scholar]