Abstract
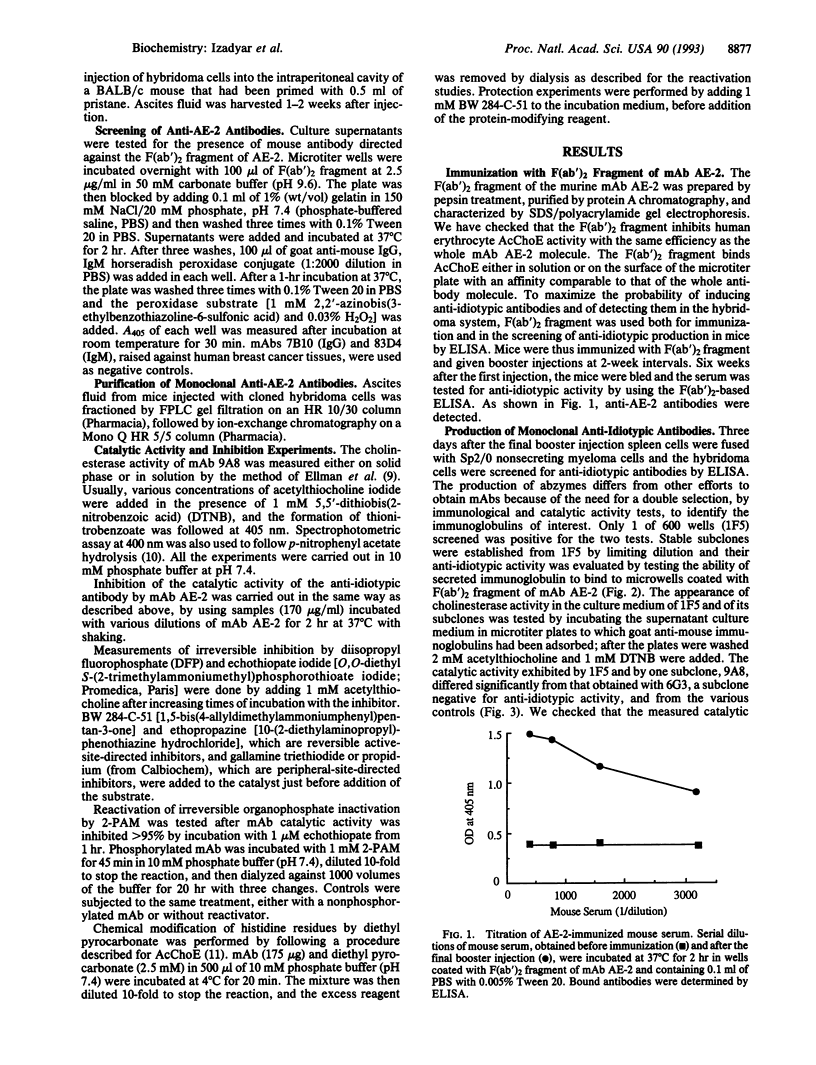
Monoclonal antibody 9A8 was selected by immunizing mice with AE-2, a monoclonal antibody directed against the active site of acetylcholinesterase. In accordance with the idiotypic network theory, monoclonal anti-idiotypic antibody 9A8 displayed internal-image properties of the original immunogen, the acetylcholinesterase active site. Hydrolysis of acetylthiocholine and related esters of thiocholine by 9A8 follows saturation kinetics and kinetic parameters were determined. The hydrolytic activity is characterized by a lowered kcat value (81 s-1) and an increased Km value (0.6 mM) when compared with the original enzyme. However, the rate acceleration (kcat/kuncat = 4.15 x 10(8) remains higher than for the esterase activities usually described for catalytic antibodies directed against transition-state analogs. The 9A8 activity exhibits a relaxation of specificity toward both substrates and inhibitors. This specificity does not correspond to a known enzymatic activity. The anti-idiotypic approach should be valuable for producing different structural and functional copies of the same enzyme active site. This should allow further insights into structure-activity relationships. Furthermore, use of chemically modified enzymes as immunogens may result in anti-idiotypic antibodies with catalytic activities not found in the native enzymes.
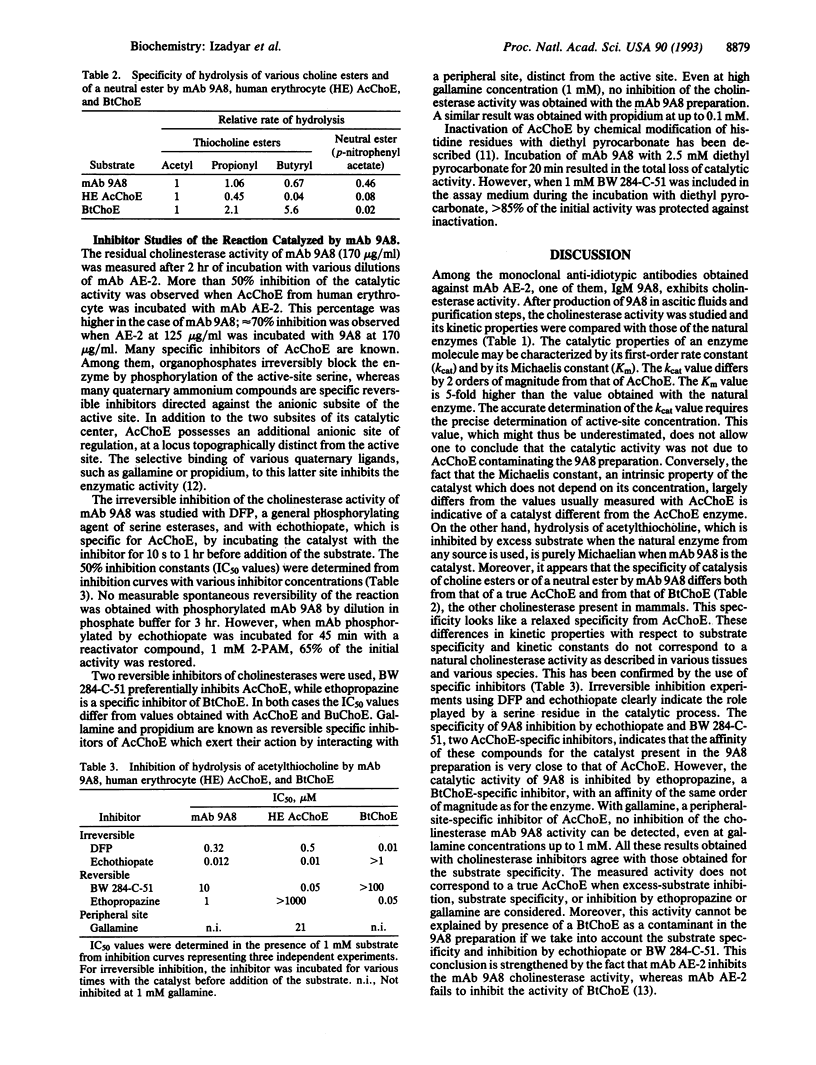
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bona C. A., Kang C. Y., Kohler H., Monestier M. Epibody: the image of the network created by a single antibody. Immunol Rev. 1986 Apr;90:115–127. doi: 10.1111/j.1600-065x.1986.tb01480.x. [DOI] [PubMed] [Google Scholar]
- Changeux J. P. Responses of acetylcholinesterase from Torpedo marmorata to salts and curarizing drugs. Mol Pharmacol. 1966 Sep;2(5):369–392. [PubMed] [Google Scholar]
- ELLMAN G. L., COURTNEY K. D., ANDRES V., Jr, FEATHER-STONE R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961 Jul;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Fambrough D. M., Engel A. G., Rosenberry T. L. Acetylcholinesterase of human erythrocytes and neuromuscular junctions: homologies revealed by monoclonal antibodies. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1078–1082. doi: 10.1073/pnas.79.4.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia K. C., Desiderio S. V., Ronco P. M., Verroust P. J., Amzel L. M. Recognition of angiotensin II: antibodies at different levels of an idiotypic network are superimposable. Science. 1992 Jul 24;257(5069):528–531. doi: 10.1126/science.1636087. [DOI] [PubMed] [Google Scholar]
- Joron L., Izadyar L., Friboulet A., Remy M. H., Pancino G., Roseto A., Thomas D. Antiidiotypic antibodies exhibiting an acetylcholinesterase abzyme activity. Ann N Y Acad Sci. 1992 Nov 30;672:216–223. doi: 10.1111/j.1749-6632.1992.tb32682.x. [DOI] [PubMed] [Google Scholar]
- Köhler H., Kaveri S., Kieber-Emmons T., Morrow W. J., Müller S., Raychaudhuri S. Idiotypic networks and nature of molecular mimicry: an overview. Methods Enzymol. 1989;178:3–35. doi: 10.1016/0076-6879(89)78003-4. [DOI] [PubMed] [Google Scholar]
- Lerner R. A., Benkovic S. J., Schultz P. G. At the crossroads of chemistry and immunology: catalytic antibodies. Science. 1991 May 3;252(5006):659–667. doi: 10.1126/science.2024118. [DOI] [PubMed] [Google Scholar]
- Majumdar R., Balasubramanian A. S. Chemical modification of acetylcholinesterase from eel and basal ganglia: effect on the acetylcholinesterase and aryl acylamidase activities. Biochemistry. 1984 Aug 28;23(18):4088–4093. doi: 10.1021/bi00313a012. [DOI] [PubMed] [Google Scholar]
- Nisonoff A. Idiotypes: concepts and applications. J Immunol. 1991 Oct 15;147(8):2429–2438. [PubMed] [Google Scholar]
- Olson C. E., Chhajlani V., August J. T., Schmell E. D. Novel allosteric sites on human erythrocyte acetylcholinesterase identified by two monoclonal antibodies. Arch Biochem Biophys. 1990 Mar;277(2):361–367. doi: 10.1016/0003-9861(90)90591-l. [DOI] [PubMed] [Google Scholar]
- Paul S., Volle D. J., Beach C. M., Johnson D. R., Powell M. J., Massey R. J. Catalytic hydrolysis of vasoactive intestinal peptide by human autoantibody. Science. 1989 Jun 9;244(4909):1158–1162. doi: 10.1126/science.2727702. [DOI] [PubMed] [Google Scholar]
- Pollack S. J., Jacobs J. W., Schultz P. G. Selective chemical catalysis by an antibody. Science. 1986 Dec 19;234(4783):1570–1573. doi: 10.1126/science.3787262. [DOI] [PubMed] [Google Scholar]
- Rosenberry T. L. Catalysis by acetylcholinesterase: evidence that the rate-limiting step for acylation with certain substrates precedes general acid-base catalysis. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3834–3838. doi: 10.1073/pnas.72.10.3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuster A. M., Gololobov G. V., Kvashuk O. A., Bogomolova A. E., Smirnov I. V., Gabibov A. G. DNA hydrolyzing autoantibodies. Science. 1992 May 1;256(5057):665–667. doi: 10.1126/science.1585181. [DOI] [PubMed] [Google Scholar]
- Sorensen K., Brodbeck U., Rasmussen A. G., Norgaard-Pedersen B. An inhibitory monoclonal antibody to human acetylcholinesterases. Biochim Biophys Acta. 1987 Mar 18;912(1):56–62. doi: 10.1016/0167-4838(87)90247-0. [DOI] [PubMed] [Google Scholar]
- Sussman J. L., Harel M., Frolow F., Oefner C., Goldman A., Toker L., Silman I. Atomic structure of acetylcholinesterase from Torpedo californica: a prototypic acetylcholine-binding protein. Science. 1991 Aug 23;253(5022):872–879. doi: 10.1126/science.1678899. [DOI] [PubMed] [Google Scholar]
- Taub R., Greene M. I. Functional validation of ligand mimicry by anti-receptor antibodies: structural and therapeutic implications. Biochemistry. 1992 Aug 25;31(33):7431–7435. doi: 10.1021/bi00148a001. [DOI] [PubMed] [Google Scholar]
- Tramontano A., Janda K. D., Lerner R. A. Catalytic antibodies. Science. 1986 Dec 19;234(4783):1566–1570. doi: 10.1126/science.3787261. [DOI] [PubMed] [Google Scholar]
- Wolfe A. D. The monoclonal antibody AE-2 modulates fetal bovine serum acetylcholinesterase substrate hydrolysis. Biochim Biophys Acta. 1989 Aug 31;997(3):232–235. doi: 10.1016/0167-4838(89)90192-1. [DOI] [PubMed] [Google Scholar]



