Abstract
Salinity-affected and heavy metal-contaminated soils limit the growth of glycophytic plants. Identifying genes responsible for superior tolerance to salinity and heavy metals in halophytes has great potential for use in developing salinity- and Cd-tolerant glycophytes. The objective of this study was to identify salinity- and Cd-tolerance related genes in seashore paspalum (Paspalum vaginatum), a halophytic perennial grass species, using yeast cDNA expression library screening method. Based on the Gateway-compatible vector system, a high-quality entry library was constructed, which contained 9.9 × 106 clones with an average inserted fragment length of 1.48 kb representing a 100% full-length rate. The yeast expression libraries were screened in a salinity-sensitive and a Cd-sensitive yeast mutant. The screening yielded 32 salinity-tolerant clones harboring 18 salinity-tolerance genes and 20 Cd-tolerant clones, including five Cd-tolerance genes. qPCR analysis confirmed that most of the 18 salinity-tolerance and five Cd-tolerance genes were up-regulated at the transcript level in response to salinity or Cd stress in seashore paspalum. Functional analysis indicated that salinity-tolerance genes from seashore paspalum could be involved mainly in photosynthetic metabolism, antioxidant systems, protein modification, iron transport, vesicle traffic, and phospholipid biosynthesis. Cd-tolerance genes could be associated with regulating pathways that are involved in phytochelatin synthesis, HSFA4-related stress protection, CYP450 complex, and sugar metabolism. The 18 salinity-tolerance genes and five Cd-tolerance genes could be potentially used as candidate genes for genetic modification of glycophytic grass species to improve salinity and Cd tolerance and for further analysis of molecular mechanisms regulating salinity and Cd tolerance.
Keywords: seashore paspalum, yeast, cDNA library, salinity-tolerance, Cd-tolerance
Introduction
Fresh water availability for agricultural and horticultural irrigation has decreased, whereas there has been an increased use of recycled and effluent water for irrigation, which contains a high content of salinity and heavy metals that leads to soil salinization and heavy metal contamination (Uddin et al., 2011; Azevedo et al., 2012; Pessarakli, 2014). Salinity and heavy metals, such as cadmium (Cd), accumulation can be detrimental for plant growth and development (DalCorso et al., 2010; Chen et al., 2015a). Functional characterization of genes conferring plant tolerance to salinity or Cd will help to further understand the molecular mechanisms controlling abiotic stress tolerance. Global gene expression analysis of salt-tolerant Arabidopsis thaliana suggests that many salt-related genes are also associated with other abiotic and biotic stress responses (Chan et al., 2011). Furthermore, identification of salt- or Cd-related genes is important for developing plant germplasm with improved salinity or Cd tolerance in salinity-affected or Cd-contaminated areas.
Full-length cDNA Over-eXpressor (FOX) gene hunting system has been recently used in various plant species to detect and identify novel genes associated with stress tolerance through heterologous overexpression of full-length cDNA libraries in microorganisms or in the model plant Arabidopsis (Eswaran et al., 2010; Higuchi et al., 2011; Sakurai et al., 2011). Yeast has been utilized for functional assays or screening of plant stress-tolerance genes due to the simplicity as single cells, rapid growth, and the availability of mutants sensitive to various abiotic stresses (Qiu, 2012). Several Cd-tolerance related genes, including aquaporin and protease inhibitors and metallothionein, were identified from rice (Oryza sativa) with FOX-gene hunting system, as those genes could restore Cd tolerance in Cd-sensitive yeast mutants (Wang et al., 2012). Few studies reported that phytochelatin synthase gene (PCS) could be related to Cd-detoxification (Brunetti et al., 2011). However, the genes underlying Cd tolerance are largely unknown. Thirty-two full length salinity-tolerance related genes from physic nut (Jatropha curcas) and sixteen salinity-tolerance related genes from zoysiagrass (Zoysia matrella) were isolated with the yeast FOX-gene hunting system (Eswaran et al., 2010; Chen et al., 2015b). OsMPG1 and OsKAT1 were also identified in rice and confirmed for salinity tolerance (Obata et al., 2007; Kumar et al., 2012). Genes related to salinity tolerance are numerous (Gupta and Huang, 2014), and the most studied genes include those serving functions in salinity exclusion (i.e., Salinity Overly Sensitive 1, SOS1; Zhu et al., 2007; Yang et al., 2009), salinity compartmentalization (i.e., vacuolar H+-pyrophosphatase, VP; Chen et al., 2015a), and osmotic adjustment (i.e., pyrroline-5-carboxylate synthetase, P5CS; Hur et al., 2004). Salinity survival-related mechanisms, particularly for halophytic plant species that adapt to chronically severe salinity conditions, are yet to be completely understood and deserve further exploration.
Seashore paspalum (Paspalum vaginatum) is a halophytic perennial grass species which is known for its superb Cd and salinity tolerance, and it has been mainly used as a turfgrass in salinity-affected areas and for phytoremediation in Cd-contaminated areas (Chen et al., 2006; Wang, 2010; Liu et al., 2012; Uddin et al., 2012). Previous studies showed that seashore paspalum can grow well in soil solution with up to 48 dS m−1 of salinity and 254 mg Cd kg−1, whereas most glycophytic grass species cannot tolerate to such high levels of salinity or Cd (Flowers and Colmer, 2008; Wang, 2010; Uddin et al., 2012). Exploring the genes associated with both salinity and Cd tolerances in this unique perennial grass species will have a great potential for further understanding the molecular mechanisms involved in regulating salinity and Cd tolerances and for genetic modification of glycophytic grass species to improve salinity and Cd tolerances. The objective of this study was to identify salinity- and Cd-tolerance related genes in seashore paspalum using yeast cDNA expression library screening or FOX-gene hunting method. Stress-related genes are often induced by their respective stress conditions (Song et al., 2012; Chen et al., 2013, 2014). Therefore, qRT-PCR analysis was performed to detect the expression level of these candidate genes cloned from seashore paspalum under salinity- or Cd-stress conditions.
Materials and methods
Plant materials, growth conditions, and treatments
Plants were propagated from stolons of seashore paspalum “SeaIsle 2000” plants. The stolons were cut into 4–5 cm segments including two nodes of each segment and hydroponically cultured in plantstic containers (23 cm length × 23 width × 18 cm depth) filled with half-strength Hoagland's nutrient solution (Hoagland and Arnon, 1950). The nutrient solution was changed weekly to ensure adequate nutrition.
After roots and shoots were generated from the stolons, plants were transferred to half-strength Hoagland's nutrient solution containing 1 mM CdCl2 for 48 h to impose Cd treatment. For the salinity treatment, roots were exposed to lower salinity levels (100 and 200 mM NaCl for 2 h each) to avoid salinity shock and then to 250 mM NaCl for 48 h in the half-strength Hoagland's nutrient solution. After Cd or salinity treatment, the whole plant including leaves, stems, and roots (~9.5 g fresh weight, mixture combining NaCl and Cd-treated plants) was collected from both NaCl and Cd treatments, frozen in liquid nitrogen, and then stored in a freezer at –80°C for library construction. In addition, the ion contents (K, Na, Ca, Fe, and Cd) of leaves and roots after 24 h of Cd or salinity treatment were detected using an inductively coupled plasma mass spectrometer (ICP-MS), which exhibits significant increase of endogenous toxic elements (Na and Cd) and decrease of other essential elements (K, Fe, and Ca) under exogenous salinity or cadmium treatment (Table S1). Leaves and roots were separately sampled at 0, 1, 3, 6, 24, or 48 h of 250 mM salinity or 1 mM Cd treatment, immediately frozen in liquid nitrogen, and stored at –80°C for qRT-PCR analysis of candidate gene expression. Each treatment was replicated in three hydroponic containers representing three biological replicates and each replicate with multiple plants, providing sufficient tissue samples for analysis.
The experiment was conducted in a climate-controlled room (Jinan, Shandong, China) with 14 hd-1 photoperiod, photosynthetically active radiation of 850 μmol photons m−2 s−1, day/night temperature of 28/25°C and relative humidity of 60%.
cDNA expression library construction and quality assays
The total RNA from roots, stems, and leaves of seashore paspalum was extracted using Trizol RNA Kit (Invitrogen), and mRNA was purified with Dynabeads® mRNA Purification Kit (Invitrogen). The integrity and purity of total RNA extracted from the plants were detected by 1% Agarose gel electrophoresis and Nucleic Acid Analyzer (Thermo nanodrop, USA), and purified mRNA was subjected to the same analysis.
The cDNA entry library was constructed using the SuperScript® Full Length cDNA Library Construction Kit II, including reverse transcription with primer Biotin-attB2-Oligo(dT), RNase I treatment and first-strand cDNA enrichment with Cap antibody, double-stranded cDNA synthesis and fractionation followed by attB1-adapter connection, BP reaction with cDNA and pDONR/Zeo vector, and ElectroMAX™ DH10B™ T1 transformation by Electroporation. Thousand-fold diluted library Bacilli were cultured overnight on solid medium (LB + 50 mg L−1 Zeocin) and the clones were counted. Twenty-four clones were selected for PCR reaction performed with the following pDONR/Zeo vector universal primer pair (F1/R1:TCCCAGTCACGACGTTGTAAAACGACGGCCAGTCTT/AGAGCTGCCAGGAAACAGCTATGACCATGTAATACGACTC). Sequencing was performed on 32 selected clones.
The mixed plasmids were isolated with PureLink® 96 Plasmid Purification System (Invitrogen), and an LR gateway reaction was performed with yeast expression vector pDEST52 (Invitrogen). The reaction products were transformed into ElectroMAX™ DH10B™ T1 competent cells, creating the expression library. A similar confirmation of the library was performed as above using the pDEST52 vector universal PCR primer pair F2/R2: TAATACGACTCACTATAGGG/ATCGAGACCGAGGAGAGG. The pDEST52 expression library plasmids were separated with PureLink® 96 Plasmid Purification System (Invitrogen), and then for yeast screening.
Yeast transformation and gene mining
Cd-sensitive mutant yeast strain ycf1 (his3Δ1; leu2Δ0; met15Δ0; ura3Δ0; YDR135c::kanMX4) was obtained from Euroscarf (Y04069, University of Frankfurt, Germany). PDEST52 expression library plasmids were transformed to G19 salinity-sensitive mutant cell (ena1-4 yeast mutant) or Cd-sensitive mutant ycf1 using PEG-lithium acetate-based transformation protocols (Clontech). Transformed plasmids were then plated on SD solid medium with 500 mM NaCl or 100 μM CdCl2 lacking uracil and histidine, and placed at 30°C for 3–7 days until single clones appeared. Single clones that survived treatment with either 500 mM NaCl or 100 μM CdCl2 were selected and cultured overnight, and then streaked with different dilution production (102, 103, 104, and 105) on control as well as the salinity- or Cd-selection plates. Growth of transformed clones with PDEST52 expression library plasmids was compared with G19 or ycf1 cells transformed with the pDEST52 empty vector on the same salinity or Cd medium plates. The yeast plasmid of validated salinity-tolerant clones was extracted and transformed to TOP10 E. coli cells for sequencing (Invitrogen). Sequence analysis was performed following BLASTX (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Expression vector reconstruction and stress-tolerance validation
To avoid the salinity- or Cd-tolerance achieved from co-transformation with multiple different genes in yeast, we reconstructed the ORF sequence of each screened gene into the yeast expression vector and retransformed to yeast cells to confirm their functionality. The full-length ORFs were PCR amplified with primer pairs including the enzyme-digestion connector and then digested for further connecting to the same digested entry vector pENTR1A. The pENTR1A constructs were then subjected to LR recombination reactions with pDEST52 expression vectors. The constructs were retransformed into yeast and then salinity- and Cd-tolerance were verified using the above method. The transformed and non-transformed yeast cells were cultured in Petri dishes containing 300 or 500 mM NaCl for salinity treatment and 100 μM Cd for Cd treatment.
qRT-PCR analysis of expression levels of candidate salinity- or Cd-tolerance genes under salinity or cadmium stress
The samples of leaves or roots sampled at 0, 1, 3, 6, 24, or 48 h of 250 mM salinity or 1 mM Cd treatment were used separately for RNA extraction using the RNApure reagent (Yuanpinghao Biology, Tianjing), and cDNA first strand was produced using PrimeScript RT reagent Kit with gDNA Eraser (TaKaRa, Dalian) following the manufacturer's instructions. A 10-fold dilution of cDNA was used for qRT-PCR reaction as template. Primer pairs of 18 candidate salinity-tolerance genes, five candidate Cd-tolerance genes, and a reference gene Elongation factor-1α (SpEF1α, GenBank accession number: KU049721) were produced following Primer Premier 5.0 software, as a principle of primer length 18–25 bp, GC content 40–60%, melting temperature 55–65°C, and amplicon lengths 80–300 bp. qRT-PCR detection was performed using a LightCycler 480 II instrument (Roche, Switzerland), and a reaction of a 15 μL mixture containing 7.5 μL 2X SYBR I master (Roche, Switzerland), 5 μL of diluted cDNA, 0.4 μL each primer (10 μM), and 1.7 μL ddH2O. The reaction procedure included an initial 10-min denaturation of 95°C, 40 cycles amplification (95°C for 15 s, 58°C for 15 s, and 72°C for 30 s), and then melting curves were produced at 60–95°C. Gene relative expression level was calculated following the 2−ΔΔCt method. Three biological replicates were used for significance detection by one-way analysis of variance (ANOVA) using a statistical program (SAS9.0, Cary, NC).
Results
Seashore paspalum cDNA library construction
RNA concentration and the quantity extracted from the whole plant of seashore paspalum (leaves, roots, and stems) were 905 ng μL−1 and 543 μg, respectively, and the A260/A280 value was 2.03 (Figure 1A). The mRNA exhibited excellent quality (Figure 1B) with a A260/A280 ratio of 2.19, and total mRNA concentration and quantity was ~76.5 ng μL−1 and 22 μg, respectively.
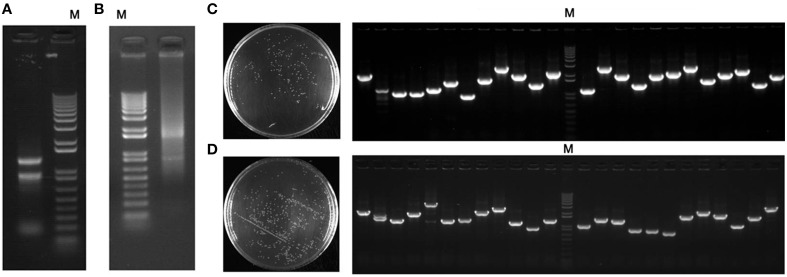
Figure 1.
Expression library procedure of seashore paspalum. (A) Analysis and detection of total RNA extracted from roots, stems, and leaves of seashore paspalum by 1% Agarose gel electrophoresis; (B) Analysis and detection of mRNA purified from total RNA extracted from roots, stems, and leaves of seashore paspalum by 1% Agarose gel electrophoresis and spectrophotometer (NanoDrop2000, Thermo Scientific, Massachusetts, USA); (C) titer and inserted fragment detection of entry cDNA library; (D) titer and inserted fragment detection of expression cDNA library. M represents invitrogen 1 kb plus ladder.
The total library included 9.9 × 106 clones, which represent most of the genes expressed in seashore paspalum (Figure 1C). The inserted fragment length of 24 selected clones ranged from 0.8 to 2.5 kb with an average size of 1.48 kb. Thirty-two selected clones were sequenced for detection of full-length rate (Table 1). BLASTX analysis showed that ATG start codon was found in all 32 sequences (NCBI accession number: KT203458–KT203489), and 5′UTR length ranged from 10 to 580 bp, with the average size of 105 bp (Table 1). This data demonstrated that full-length ratio of cDNA entry library was 100% and could be further used for the construction of expression library. The analysis of the pDEST52 expression library showed that the library capacity was 2 × 107 and average recombination size was 1.54 kb (Figure 1D).
Table 1.
5′UTR sequence analysis of 32 randomly selected clones in entry library of seashore paspalum.
| No. | Accession number | Prediction protein | Upstream sequence of Start codon ATG |
|---|---|---|---|
| 1 | KT203458 | Aspartic proteinase oryzasin-1-like [Setaria italica] | ACGGTTTCTGCCTTTGTCTGATCGCAACCATGGGA |
| 2 | KT203459 | S-Adenosylmethionine decarboxylase [Sorghum bicolor] | GCTTCTTGTTGCCGAAGAATTTCCTCCCCCTC GCCGCCGCCGCCGCCGCTCGTCTCGCTGAGAG AAGCTAACTGGGCCGGGGAACTCGCTTGTCTC TAGGGAGAAGAGGATCCTTCCCGCTGGTCTCA GCCCGGGGTCTGCTGCGTCGGAAGGCGGTGAA GGGGGGAGATTCGTGAGATCGTTTCCGGATCA CGCGTGCGCGCTCGGGAATTTCGGGGGTATCC ACACATAGCTTCGTCGAATTGAAATTGATGTA CTAATGGAGTCCAAGGGTGGCAAGAAGTCTAG CAGTAGTCGTTCCATGATGTATGAAGCTCCCC TTGGCTACAGCATTGAGGACGTTCGACCTGCT GGAGGCGTGAAGAAGTTCCAGTCTGCTGCTTA CTCCAACTGCGCGAAGAAGCCATCCTGATATC CCTTTGGGCTTCCCTTTCCTAGTAGTATAGGA TTTCTTTTCTAACGCTTTGATTCTGACCAATC TCTCTGGCCTGCTGCTTCCTGATAATCAACCA GTTCCCCAGTCTTGCTCTCTGCACTCCTCCCT CCATCTCTGGCATCGTGTGCCGATTCACCTGC TTCA ATGGCT |
| 3 | KT203460 | Homeobox-leucine zipper protein HOX1-like [Setaria italica] | CCTCCTCATTGCCCACGCCGAGACCGTTTCTT TCTCTGCAACCAGCGGCGGGCTCCGGTCTGGA GTAATCGTCGTCAGTGGAGATGATG |
| 4 | KT203461 | Palmitoyl-protein thioesterase 1-like [Setaria italica] | GTCCCGACCCGAGAGCGAGAGGAAGAGAAGAG GGGCTGCTGCCTGCTGGATCGAGTTGTTGGAG TGGAATCGGACCCCGGCCGCCGGAATCCGACC CCGATGCGG |
| 5 | KT203462 | 60S Ribosomal protein L14-1-like isoform X2 [Setaria italica] | CTCGCCGTCTCGCTCGCAGCCGCTTGCCGCCG CCGCCGCTCGCCTCTTCGACCGCCACGAGGATGCCG |
| 6 | KT203463 | Hypothetical protein OsI_03805 [Oryza sativa Indica Group] | CAACAACGGCCAGCTGAGCTGAGCCGAGCCGA GCCGAGCGCACGCAGCTCGTTCGTCCTCGTCC TCGATCGATCGGCCTAGCTATTAGCTCCTTCC TGCCGCCATACCATTACCATATGAGGTAGCGC AGGCGCAGGAGCCGCCGGCGGGGCTGGAAGGT GAGGTGGAGAGAGATATAGAGAAGGATCGGCC GGCCGGTACGGTAGAGGTGCGTGGTCTGGTCA GGCTCCGGGCGGGATCGATCATATCGAGATGGAG |
| 7 | KT203464 | ABC-type Co2+ transport system, permease component [Zea mays] | GCGCTCGTCGCCGTCGTCTTGAGCCGTGAGAC GACACCAAGATAAACATGGCC |
| 8 | KT203465 | Glutathione transferase [Zea mays] | ACAGCAAGCATCCTGCACGTTCGCAAGCTCTC TCTCGCACAGGGCACAGAGGAGGAAGGAAGGA GATCGAGGTCGAGGCCATGGCG |
| 9 | KT203466 | 40S ribosomal protein S7-like [Setaria italica] | CTCCTCCTCGAGCGCAGGGTCCGGCGGCGAAG GGAAGGCAAGATGTAC |
| 10 | KT203467 | Nicotianamine synthase1 [Zea mays] | GTTGTCGAGCACTTGCCACTCTTGATCGAGCT AAAGCCTAAAGACATCTCATCCGCTGCGTCGT CGTCCCTAGCTCATCTTCCCAAGTCCAACCGT AGAAAGTACTACAGCTGCCATGGAG |
| 11 | KT203468 | Transcription factor bHLH96-like [Setaria italica] | ACTGCATCCACGCCGGCCGGCCGGTGATCGAG CCGCCGCTCTAGTGCTGCTTACTACTCTCTCT CGTTCCCGCCGCGTCCTCCTCGAGTAGGCCGG TGATCGATTCTCGCGTGCCGCCATGGCG |
| 12 | KT203469 | Remorin [Zea mays] | CAGCAACAGCTAAGCTTCGCCCACCAGACAGC AACAGCATCATGGCT |
| 13 | KT203470 | Uncharacterized protein LOC101767282 [Setaria italica] | ATCAGCTGGAAATCGATCGATTCTTACCAAAA TCCAGCATCTACACACCTCACAACTTCACAAG ATCCTCCTTTCTTCTTCTTCTTCTTCTTCCTT CCCTGCTGCTGCTGCTGCTGCTGGTCACCAGT CACCACCCTCATCATTTCTTCCCCGGCAGCCT ATAACCTTCCTGCCTGCCTTCCCTGCCAGGCC ACCCAAGCCTCCAAGAGATAGATCATATATTG ATGGAT |
| 14 | KT203471 | Serine/Threonine protein phosphatase superfamily protein [Zea mays] | CCCCCTCCCTCCTTCCCACCTCTCGTTCTCTC CCCCTCTTCTCCACTTCCGTCCTCACGCGCGC GCGCGCGTCCAGATCTAGGGTTCCATCCGCGG CCAAGATGAGC |
| 15 | KT203472 | Chaperone protein dnaJ 8 [Setaria italica] | AAACCAAAGGCACCTCTCCAGTTCTCCACTTC TCCTCCCGTAGCGCGCGCTCTCTCGGACACAC AAGTCGCTTGCTTTGCTTCGCGCTCCTGGTGC TCGTTGCTTTCGATCCTCTCCGGCCGGTGATC TCGCTCGCTCGCGGCGGTGCTAGCTGTGGAAATGGCC |
| 16 | KT203473 | Probable sodium/metabolite cotransporter BASS2, chloroplastic-like [Setaria italica] | CCGGCGCTTTCCACGGGCTTCGGAAGCCACAA CTTTCCGCATCTCGTGCTTTGCCTCCTAGGTT CCTCCGCATGGCG |
| 17 | KT203474 | GTP-binding nuclear protein Ran-A1 [Zea mays] | AATTCTAGGGTTTTGCTGCTCCCTGTGCTCTG AAGCCCGCATCGCCGCATCCGGCGAG CTCCTCTCGGGCGACCGAAATGGCG |
| 18 | KT203475 | 30S Ribosomal protein S10, chloroplastic-like isoform X2 [Setaria italica] | ACGCGGCAAACGCCACTGCTCCTCTCCTCATC CTCTTCCTCTTCCCCGCCTCCCTCGCC TCGCCATGGCC |
| 19 | KT203476 | Uncharacterized protein LOC101762337 [Setaria italica] | ACATCACATACATGCATGTAGGCTCACAAAAA CCCTAGTCGCAGCACCTTGCTGGCATGGCT |
| 20 | KT203477 | Phosphoenolpyruvate carboxylase 1 [Aegilops tauschii] | GCCTCGCTCAGCAGCAAAAACACGCGGCCCTT GCTCTTGCTTCGTTCTCGCTTCCCGCCCCGCC ATGGCG |
| 21 | KT203478 | Chlorophyll a-b binding protein M9, chloroplastic-like [Setaria italica] | CTCTTCTGCAGAGTATAGTGTAACAGTAGACC AGCAGTGCAATGGCG |
| 22 | KT203479 | Catalytic/Hydrolase [Zea mays] | CTCCTCTCTCGCATCTGGTTCGGAGAGCTCTC CTGAAGCCTCGAGACTCGTCTCGCCAGCCGCC TGCTCCGCCTCCGCCCCGGCTCTGCCGCTGCC TGCGCTAGCTCTCGCATTGCGCCCCGCGAAGG CCTGAGCTGCTAGCTAGCTCCTGCTCCTTCTT CACCCCGCACACACAGTTCCGCGCGCCATGGAG |
| 23 | KT203480 | Nuclear cap-binding protein subunit 2-like isoform X4 [Setaria italica] | CACACCCACAATGGCG |
| 24 | KT203481 | Copper transporter [Triticum aestivum] | GCTCCCCACCACCACCAAGCACCTGCCTTTAC CCCTCTCCCCCCTCCCAAGAGGCCAGGAGGAC GAGGAGCCCGGCGCCGGCGTGCCAGCCCTGCC AGTGCCGCGCGGATCCGCCGCCGGCGCGCGGC CGTCGCTCTCCCGGCTCGACGCAGCAGGTGAC GCCAACATGGCG |
| 25 | KT203482 | Cytochrome P450 CYP78A53 [Zea mays] | ATCTGATCAAACAATCGAACCCGTGACCACCG CTCCTTGCTTCTCTGCTGGTGGTCTTAACTCT TCTTCTTCTTCCCACTCGTCAGTGCCATTCCA CACGACCGGACCACCATATGGCG |
| 26 | KT203483 | DRE-binding protein 3 [Zea mays] | CAAGCACAACTCAAGCAGCAGCAGCAGCAGAC AGCCACTCAGCTAGGCTAAAGCAATCGTTCCC CAGGGCGATTCAAGAACGAACAAGATGTGT |
| 27 | KT203484 | Non-specific lipid-transfer protein 2-like [Setaria italica] | GGGCATCCTCGATCGATCAGTTCCTCACTAGC TGCTAGTACTCATCATCACTCGCCCGCGATGACG |
| 28 | KT203485 | Tonoplast dicarboxylate transporter-like [Setaria italica] | CCCGCAGCGCTGAGCGCAGCGACGGTTCTGTT GCTCCGGTTTCCTCGGCCTCGGCCATGGAC |
| 29 | KT203486 | Peroxidase 5-like [Setaria italica] | GCGAGTAGCGACACGGGGTCTCCACTTTGCGA TTGCAACTGCAGCCGCGCGCCGGATTAGCGGC TAAGCAAGCCATCCCAGGGGACAGAGCTCCAG GCACTCAAGCAGCCAGCCACAGCCCACACAGC CAGGGATATGGAA |
| 30 | KT203487 | Malate dehydrogenase, cytoplasmic [Zea mays] | ATTTGGTCGACGCCTCCAAAGCCTCTCCCAAA CCTCCGCTTCCAGAACCTCCTCGAAGCTCCCC GCCACAGCCTCCACCCGCTCCGATGGCG |
| 31 | KT203488 | Putative signal peptide peptidase family protein [Zea mays] | CGCGGCTCTGCGCCATTCTCGAAGGTTCAACG GGCAATGGCG |
| 32 | KT203489 | Cysteine proteinase 1-like [Setaria italica] | CCGGAAAAAAAATCTAGCTCGACTCTGTCACC TCAACATGGCT |
Yeast transformation and clone selection
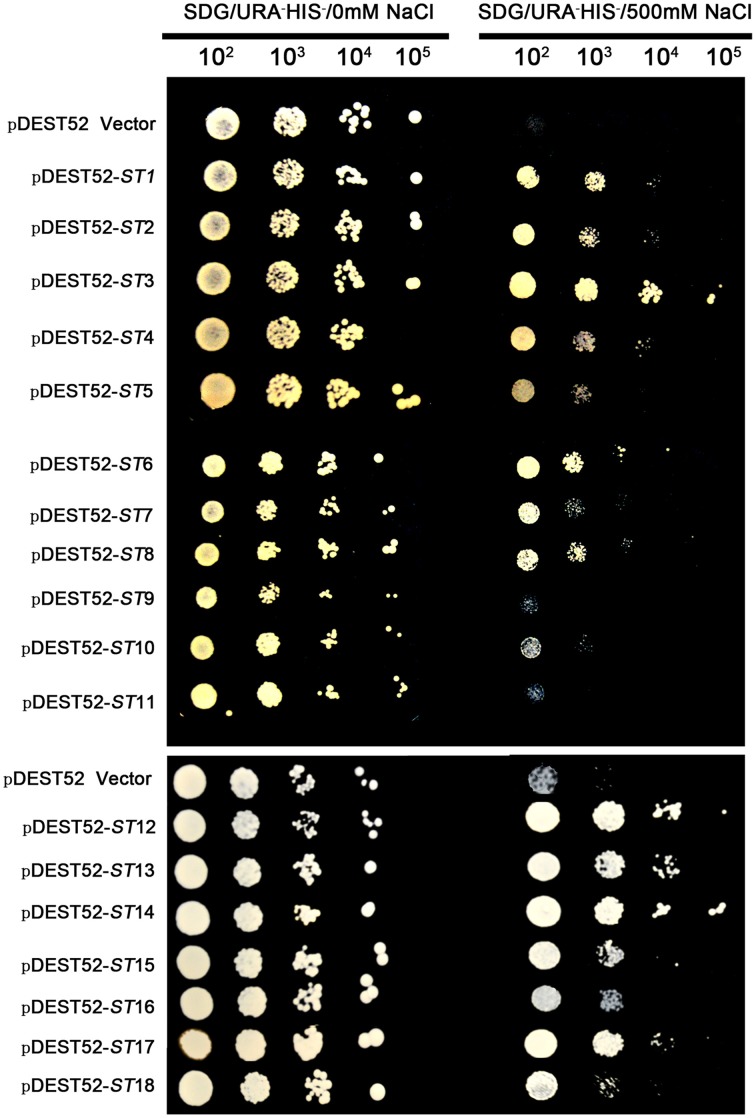
The pDEST52 expression library plasmids were transformed into salinity-sensitive G19 or Cd-sensitive ycf1-competent yeast cells. Among yeast cells transformed with pDEST52 expression library plasmids, 32 clones survived 250 mM NaCl treatment and 20 survived 1 mM Cd treatment. Through sequencing of these clones, 18 candidate salinity-tolerance genes and five candidate Cd-tolerance genes were identified. The yeast transformed with one of the 18 salinity-tolerance genes or the five Cd-tolerance genes exhibited more rapid growth in the culture medium treated with NaCl or Cd compared to the yeast transformed with the pDEST52 empty vector (Figures 2, 3, 4A). Yeast growth did not show differences between those transformed with the candidate genes from seashore paspalum and those with the pDEST52 empty vector under non-stress conditions.
Figure 2.
Salinity-tolerance confirmation of 18 clones via library screening of seashore paspalum. The overnight culture yeast were, respectively, diluted 102, 103, 104, and 105, and then the 5 μl dilution yeast grow on SDG with or without NaCl plates for 5 days.
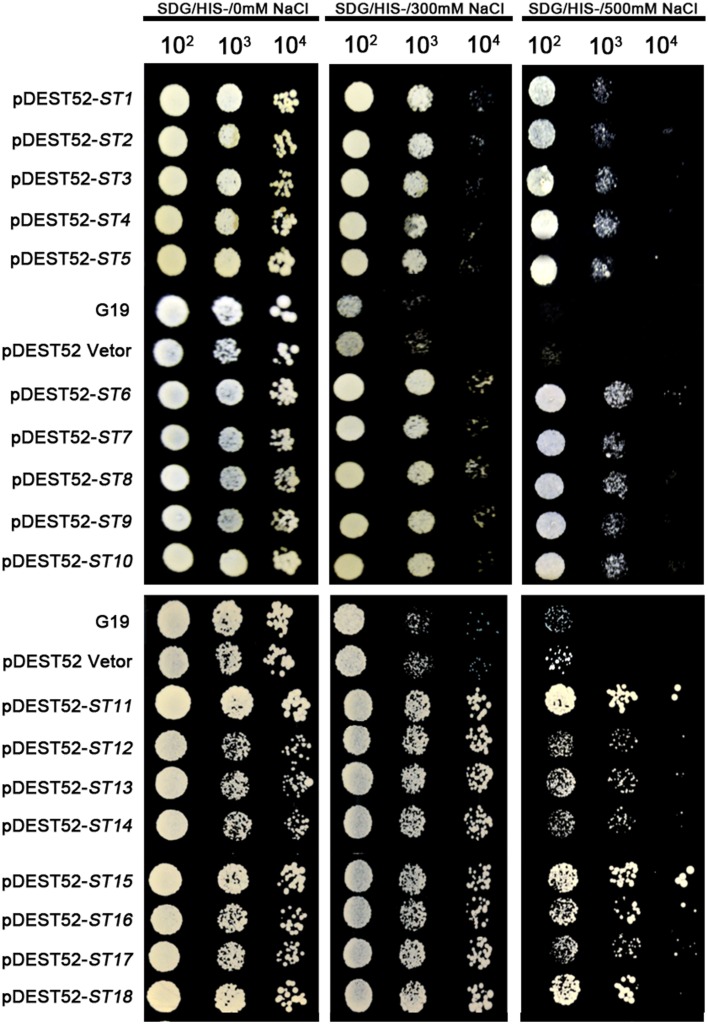
Figure 3.
Salinity-tolerance confirmation of retransformed yeast with 18 single genes of seashore paspalum. The overnight culture yeast were, respectively, diluted 102, 103, 104, and 105, and then the 5 μl dilution yeast grow on SDG with or without NaCl plates for 5 days.
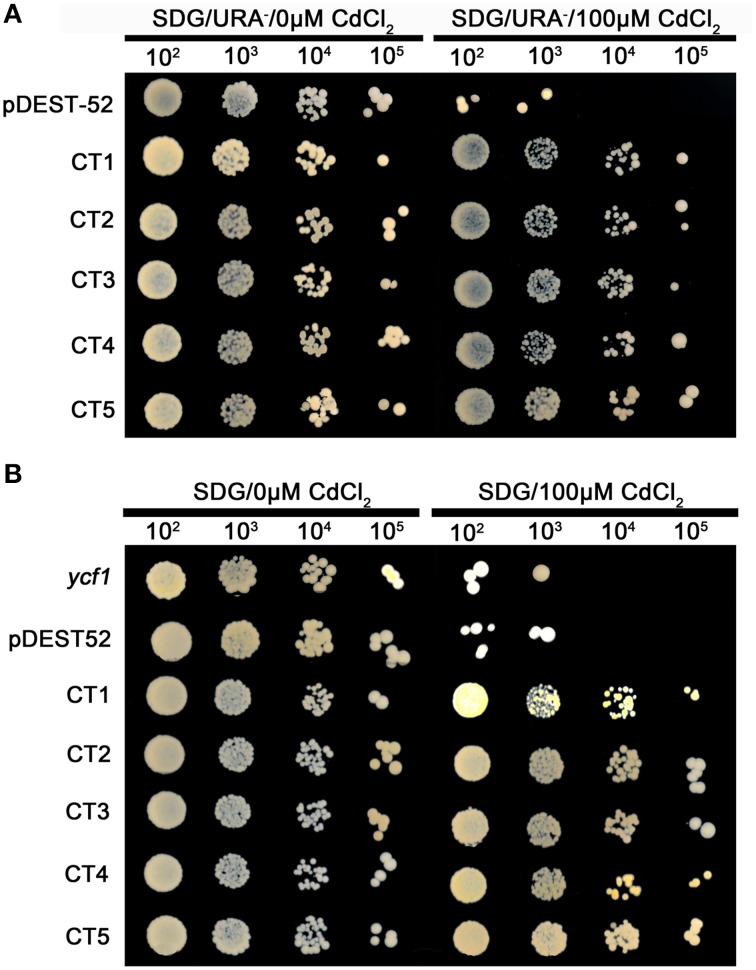
Figure 4.
(A) Cd-tolerance confirmation of five clones via library screening of seashore paspalum; (B) Cd-tolerance confirmation of retransformed yeast with five single genes of seashore paspalum. The overnight culture yeast were, respectively, diluted 102, 103, 104 and 105, and then the 5 μl dilution yeast grow on SDG with or without Cd plates for 5 days.
Gene sequence and functional analysis
The results of BLASTX analysis of cDNA sequences in the salinity- or Cd-tolerant clones were shown in Table 2 (NCBI accession number: KT203435–KT203457). The proteins encoded by 18 genes from the salinity-tolerant clones were found to be involved in iron transporter (ST2, ST12), photosynthetic metabolism (ST3, 4, 5), protein modification enzymes (ST6, 15), antioxidant metabolism (ST8, 9, 10), heat shock protein (ST7), vesicle-associated protein (ST11), Nop14-like family protein (ST13), choline-phosphate cytidylyltransferase (ST14), IQ-DOMAIN 14-like protein (ST16), metacaspase-5-like protein (ST17), BTB/POZ domain-containing protein (ST18), and a function-unknown protein (ST1). Five genes from Cd-tolerant clones were identified as phytochelatins synthase (CT1, 2), cytochrome P450 (CT3), a heat shock transcription factor (CT4), and UDP-glucose pyrophosphorylase (CT5).
Table 2.
Sequence analysis and function prediction of 18 salinity-tolerant and five Cd-tolerant candidate genes screened from cDNA expression library of seashore paspalum.
| Accession number | cDNA size (bp/aa) | Predicted function | |
|---|---|---|---|
| SALINITY-TOLERANT CLONES | |||
| ST1 | KT203435 | 961/227 | Uncharacterized protein (UP) |
| ST2 | KT203436 | 1599/370 | Iron-regulated transporter (IRT) |
| ST3 | KT203437 | 686/138 | Photosystem I reaction center subunit psaK (PSAK) |
| ST4 | KT203438 | 1105/268 | Chlorophyll a/b-binding protein (LHCB) |
| ST5 | KT203439 | 887/172 | Early light-induced protein (Elip) |
| ST6 | KT203440 | 1099/263 | 14-3-3-Like protein (14-3-3) |
| ST7 | KT203441 | 948/153 | Class 1 HSP (HSP) |
| ST8 | KT203442 | 1552/378 | Cysteine synthase (CS) |
| ST9 | KT203443 | 1370/310 | Aldo-ketoreductase (AKR) |
| ST10 | KT203444 | 1270/246 | L-Ascorbate peroxidase 2 (APX) |
| ST11 | KT203445 | 1199/225 | Vesicle-associated protein33 family (VAP33) |
| ST12 | KT203446 | 1390/342 | Iron-phytosiderophore transporter yellow stripe 1 (YS) |
| ST13 | KT203447 | 2214/501 | Nop14-like family protein (NOP14) |
| ST14 | KT203448 | 1327/293 | Choline-phosphate cytidylyltransferase B (CCT) |
| ST15 | KT203449 | 2527/655 | Leucine-rich repeat receptor-like protein kinase (LRR) |
| ST16 | KT203450 | 2094/559 | Protein IQ-DOMAIN 14-like (IQ14) |
| ST17 | KT203451 | 1551/415 | Metacaspase-5-like (MCP) |
| ST18 | KT203452 | 1379/336 | BTB/POZ domain-containing protein (BTB) |
| CADMIUM-TOLERANT CLONES | |||
| CT1 | KT203453 | 2080/499 | Phytochelatins synthase (PCS1) |
| CT2 | KT203454 | 1858/507 | Phytochelatins synthase (PCS2) |
| CT3 | KT203455 | 1808/534 | Cytochrome P450 (CYP450) |
| CT4 | KT203456 | 1647/435 | HSFA4a |
| CT5 | KT203457 | 1785/474 | UDP-glucose pyrophosphorylase (UGP) |
Confirmation and qRT-PCR analysis of candidate genes for salinity and Cd tolerance
Different plasmids in the library carrying different genes could be incorporated into a single yeast cell. To avoid that possibility, we reconstructed the single gene vector (the ORF primer shown in Table 3) and separately transformed them into yeast cells for an independent test of each individual gene (Figures 3, 4B). All transformed yeast cells exhibited better growth than untransformed yeast with empty vectors under 300 or 500 mM NaCl, or 100 μM Cd (Figures 3, 4B).
Table 3.
Primer pairs of 18 salinity-tolerant and five Cd-tolerant candidate genes for PCR amplification of full-length ORF region from seashore paspalum.
| Gene | Primer sequences 5′–3′ (ORF-F/ORF-R) |
|---|---|
| ST1/UP | GTCGACATGCTCCTGAGGAGCAAGCCT/ GATATCTCGAGGACAGCTCCTGTGCCT |
| ST2/IRT | GGATCCGGATGTCGTCTTCGCAGGCA/ GATATCTCGCCCACTTGGCCATGAC |
| ST3/PSAK | GTCGACATGGCTTCCCAGCTCTCCGCCG/ GATATCTGCCGATGATCTGGTCGAGCG |
| ST4/LHCB | GTCGACATGGCGGCTCAGGCTCTCCTCT/ GATATCTGTGGAACTTGAGGCTGGTGA |
| ST5/Elip | GTCGACATGGCAGCCACGGTGATGG/ GATATCTCACGTTGACGAATGGCGCG |
| ST6/14-3-3 | GAATTCGCATGGCGGGGCAGCAGA/ GATATCTCTGCTCATCCTCAGGCTT |
| ST7/HSP | GTCGACATGTCGCTCGTGAGGCGCAGCA/ GATATCTGCCAGAGATTTCAATCGCCTT |
| ST8/CS | GTCGACATGGAGAGGATGCTGACGAGGT/ GCGGCCGCGAGTCCACTGGCACTGGTTCCA |
| ST9/AKR | GTCGACATGGCGAGGCACTTCATGCTCAA/ GATATCTAAGTTCGCCATCCCAGAGGTCCT |
| ST10/APX | GGATCCGGATGGCGAAGGCCTACCC/ GCGGCCGCGACCCCAATTCAGAAAGTT |
| ST11/VAP33 | GTCGACATGACCACCGCCGACTCC/ GATATCTTCTCTTCATGAAGAACCCT |
| ST12/YS1 | GGATCCGGATGGGAGACGGTATGTACCA/ GCGGCCGCGAGGTTCCAGGTGTGAACTT |
| ST13/NOP14 | GTCGACATGTCTGCTAGGGACTGGGA/ GATATCTCCGCCTCCTTTTTCTGCCCT |
| ST14/CCT | GTCGACATGGCGCGCGTGTCCAATGCCA/ GATATCTGGCTGCCACCACTTCCTGCA |
| ST15/LRR | GTCGACATGGGCGGCGCGGGCGCCG/ GATATCTCGCGGAGTGGGAGCTGGGGA |
| ST16/IQ14 | GGATCCGGATGGGTAAGAAGGGAAACT/ GCGGCCGCGACTTGAAGGGCCTGCCGAG |
| ST17/MCP | GGATCCGGATGGGGGCGAAGCGCGCGGT/ GCGGCCGCGAGCATATGAAAGCCACATCG |
| ST18/BTB | GGATCCGGATGAACAGCGGTGGCGGCGG/ GCGGCCGCGATATGGGCTTCCAGACACCGA |
| CT1/PCS1 | GTCGACATGGCGGCGGCGGCGCCGT/ GATATCTAGGGAATGGTGGCACAAGAT |
| CT2/PCS2 | GTCGACATGGCGGCGGCCGTGGCGT/ GATATCTGCATTGCTGCTTAGATGATG |
| CT3/CYP450 | GTCGACATGATCGTTCTTGGAGAAGC/ GATATCTTATAGCCCTGAGCTTGATC |
| CT4/HSFA4a | GGATCCGGATGGAGGCGGGCGGCGGG/ GCGGCCGCGAGGTTTTCTCTGCCGAGGT |
| CT5/UGP | GGATCCGGATGGCCGCTGCCGCCGCCG/ GCGGCCGCGAAAGATCCTCAGGGCCATT |
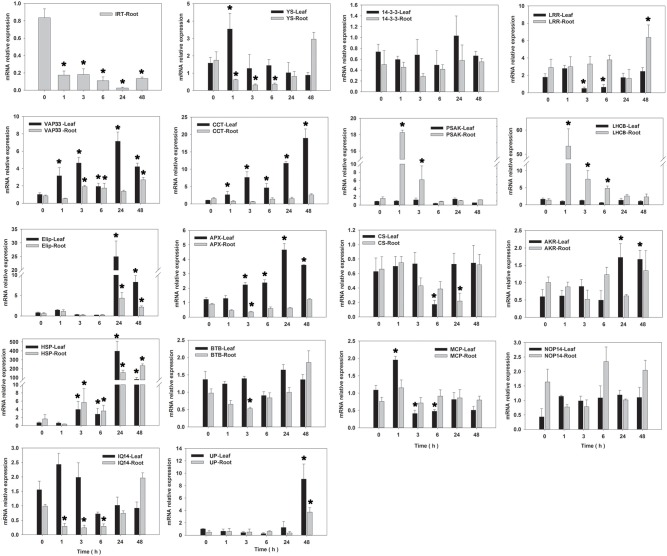
qRT–PCR analysis (primer shown in Table 4) was performed for determination of the expression level of 18 candidate salinity-tolerance genes under salinity stress conditions (Figure 5) or five candidate Cd-tolerance genes under Cd treatment (Figure 6) in two tissues (leaves and roots) of seashore paspalum. Under salinity stress, IRT was not detected in leaves, whereas its expression level decreased in roots. YS expression level did not change significantly in leaves during salinity treatment but decreased in roots within 24 h of salinity stress and then increased after 48 h compared to the non-stress conditions. 14-3-3 and NOP14 transcript levels in leaves and roots were not altered by salinity stress. The expression of AKR in leaves and LRR in roots was induced at 24 h of salinity stress. LHCB and PSAK of roots and MCP of leaves exhibited up-regulation of expression at 1 h and thereafter down-regulated by salinity stress. CS expression in leaves or roots was declined under salinity stress at 6 or 24 h and then returned to the untreated level. Salinity stress significantly promoted the expression of HSP, VAP33, Elip, and UP in leaves and roots, and APX and CCT in leaves. The expression of BTB in roots at 3 h, IQ14 in leaves and roots at 6 h, and LRR in leaves at 3 h were all reduced under salinity stress.
Table 4.
qRT-PCR primer pairs of 23 candidate tolerance genes in seashore paspalum.
| Gene | Primer sequences 5′–3′ (RT-F/RT-R) | Amplicon length (bp) |
|---|---|---|
| ST1/UP | CTCCCAGGAAGAAAGGCTG/GACGGTGATGAGGACAAGC | 185 |
| ST2/IRT1 | CTCCAGCTCCTCTCCTTCCT/CCGTCGGTTCTCCGTTTT | 100 |
| ST3/PSAK | ACGACGCTGATGCTGTTCG/ACCTGCTTGGCCCTTGATG | 297 |
| ST4/LHCB | GTCAACAACAACGTGCTCACC/GCATCTCGATCGCTTCCA | 141 |
| ST5/Elip | CTGGAACGGACGATTCG/CGCAGACGCAGTGTTTTTGA | 156 |
| ST6/14-3-3 | TGGTGGCGATGAAATGAA/AAGGCTACCCCAAGGGTTA | 102 |
| ST7/HSP | AGGACAAGAACGACAAGTGG/TGGACCAAGGACCATCAGTA | 256 |
| ST8/CS | GGGAGCGATACTTGTCTTCTG/CGGTGCCCTGTTTAGTTCTC | 104 |
| ST9/AKR | CCGCAGAGCGTTTACAAGA/CCACGATCCAATATGACAGG | 245 |
| ST10/APX | TTCCGCCCGCTTGTT/ATCCCCCCCCTTCTTTAG | 237 |
| ST11/VAP33 | CGAGACATCAGCAGGCAA/GCACACAGAGAACACGTACAATC | 171 |
| ST12/YS | GAGGCTCGTGCCACTACCAA/TGACTCCTTGACTGTGTCACACAT | 298 |
| ST13/NOP14 | CAAGGGAAAGGGCAGAAAAA/AGCAGCTCGCATTAAGGACA | 170 |
| ST14/CCT | GAGCAAAGGAATAAGCACTGG/CGATCATAACGGATACCCAAA | 287 |
| ST15/LRR | ATTACTGCTGCTGCTAGGTTCT/GTGTATTTGGCCTCCTTTTTCT | 194 |
| ST16/IQ14 | TGTGGTTTGGTGTGGGTTTC/CGCATGTACGGTTGTTCGAT | 159 |
| ST17/MCP | TGAATGCCTGAATCACTGGG/AGCAAAAAGCGTAACCACC | 125 |
| ST18/BTB | ACCTACCTGTTTGTGTTTGGG/TGTTAGCTTTACTTTGATGGCC | 194 |
| CT1/PCS1 | TATGTACCGCTCATCCCGA/GAAACGACGAATAACCTCTCTT | 106 |
| CT2/PCS2 | TCTACGGCGGCAACTCTAT/CCACTCTCTCACAACTTTTCTCTTC | 161 |
| CT3/CYP450 | TATACTCATGCGCCCAGCC/AACAGCCATCAGCAAACCT | 112 |
| CT4/HSFA4a | AACAGATGACAGAAAAGATGGG/CAGGAGCAAAGATATGATACAC | 150 |
| CT5/UGP | CCGAGGAAATGTCATAGGAGC/CAAAAACAAGTACAGACGCACC | 147 |
| SpEF1α | GCGGACTGTGCTGTGCTTATC/AGTGGTGGCATCCATCTTGTT | 153 |
Figure 5.
qRT-PCR analysis of 18 salinity-tolerant candidate genes in roots and leaves of seashore paspalum under salinity stress at 0, 1, 3, 6, 24 and 48 h. *Represent significant difference (p < 0.05) under salt stress comparing to CK (0 h).
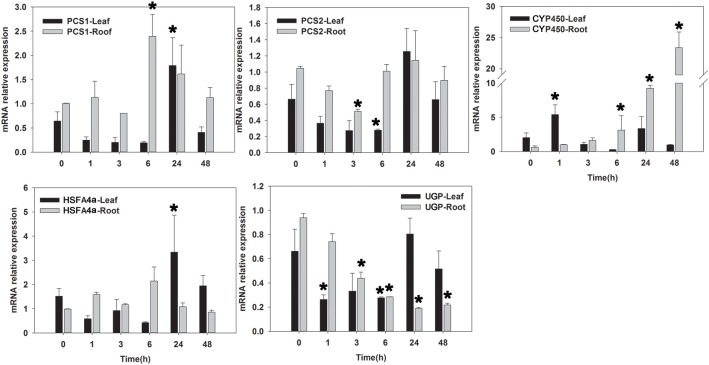
Figure 6.
qRT-PCR analysis of five Cd-tolerant candidate genes in roots and leaves of seashore paspalum under Cd stress at 0, 1, 3, 6, 24, and 48 h. *Represent significant difference (p < 0.05) under cadmium stress comparing to CK (0 h).
Under Cd-stress conditions, the expression of PCS1 and PCS2 in leaves decreased within 6 h and was up-regulated at 24 h. PCS1 in roots showed significant up-regulation at 6 h, whereas the expression of PCS2 in roots decreased at 6 h of Cd treatment and then returned to untreated levels. CYP450 expression in leaves and roots increased at 1 and 24 h of Cd treatment, respectively. The expression of HSFA4a in leaves decreased at 1 h and up-regulated after 24 h, whereas HSFA4a expression in roots was up-regulated at 6 h. Cd-stress significantly reduced the expression of UGP in roots within 48 h, whereas UGP expression in leaves decreased at 6 h of Cd treatment and then returned to the untreated level by 24 h.
Discussion
Candidate genes and their biological functions associated with salinity tolerance in seashore paspalum
By screening the cDNA library of seashore paspalum using the FOX-gene hunting method in yeast, 18 salinity-tolerance related genes were identified, and most of them have not been previously reported in relation to salinity tolerance, which could be novel salinity-tolerance genes contributing to superior salinity tolerance in the halophytic grass species. These genes were found to exhibit putative functions in iron transport (IRT and YS), protein modification (14-3-3 and LRR), vesicle-associated protein (VAP33), photosynthetic metabolism (PSAK, LHCB, and Elip), antioxidant metabolism (CS, AKR, and APX), heat shock protein (HSP), Nop14-like family protein (NOP14), choline-phosphate cytidylyltransferase (CCT), IQ-DOMAIN 14-likeprotein (IQ14), metacaspase-5-like protein (MCP), and BTB/POZ domain-containing protein (BTB). The biological functions of the aforementioned 18 genes in relation to salinity tolerance are discussed below.
Iron transport
Iron is an essential microelement, which is important for plant growth and development (Bashir et al., 2013). In Arabidopsis, iron can be assimilated through three steps: Fe3+ solubilization and chelation, reduction of Fe3+ to Fe2+, and Fe2+ uptake by divalent Fe transporter IRT1 (Brumbarova et al., 2015). In addition, in grass plants, such as rice, apart from the IRT1 pathway, Fe3+ can be directly absorbed by the yellow stripe-like (YSL) family of ferric iron transporters (Bashir et al., 2013). Arabidopsis plants lost viability in mutants with loss of function of irt1, which implies that IRT1 plays critical roles in iron uptake in plants (Varotto et al., 2002; Vert et al., 2002). However, both the iron transporters, IRT1 and YSL, have not been previously reported in relation to salinity tolerance of plants. A recent study with exogenous iron treatment reported that iron uptake could ameliorate salinity injury in tomato (Solanum lycopersicum; Ghasemi et al., 2014), whereas another study found salinity stress significantly reduced iron absorption of roots in rice (Abbas et al., 2015). In this study, IRT1 expresion level decreased in roots of seashore paspalum under salinity stress, suggesting that salinity could interupt iron transport. Furthermore, yeast cells containing either IRT1 or YSL cloned from seashore paspalum exposed to salinity stress exhibited improvement in growth in the culture medium containing NaCl. Therefore, it could be postulated that IRT1 and YSL could play positive roles in salinity tolerance of seashore paspalum. The biochemical and molecular mechanisms of IRT1- and YSL-mediated salinity tolerance in plants are currently unknown, which deserve further investigation in future research.
Protein modification
Plant responses to salinity stress involve protein modifications in different components of phosphorylation cascades. 14-3-3 proteins are a family of conserved regulatory proteins involved in phosphorylation cascades in higher plants (Smith et al., 2011). Previous studies showed that 14-3-3 family proteins could negatively regulate freezing tolerance through repressing CBF expression (Catala et al., 2014) and salinity tolerance by reducing SOS2 kinase activity (Zhou et al., 2014), and positively regulate drought tolerance in Arabidopsis (He et al., 2015). In grass plants, 14-3-3-mediated abiotic stress tolerance has not been well documented. Leucine-rich repeat receptor kinases (LRR-RKs) are the largest sub-family of transmembrane receptor kinases in plants, and the regulated pathway of LRR-RKs is involved in brassinosteroids signal, wound response, and stem cell maintenance (Torii, 2004; Ogawa et al., 2008). It has recently been reported that a LRR-RKs family member from rice named Leaf Panicle 2 (LP2) could interact with aquaporin proteins and negatively influence drought tolerance (Wu et al., 2015). Overexpression of another member of the LRR-RKs family, OsGIRL1, led to hypersensitive responses in plants to salinity stress and heat stress (Park et al., 2014). In our study, yeast cells transformed with either a 14-3-3 gene or a LRR-RKs gene cloned from seashore paspalum subjected to salinity stress exhibited better survival than those with the empty vectors. Our results indicated that a 14-3-3 family member and a LRR-RKs member of seashore paspalum could positively regulate salinity tolerance, which is in disagreement with what was previously found in Arabidopsis or rice. One potential reason of the discrepancy from previous reports is that the 14-3-3 identified from seashore paspalum in our study was a novel or different member of 14-3-3 family from previously reported Arabidopsis and rice (Chen et al., 2006; Zhou et al., 2014). However, qPCR analysis did not detect changes in the expression level of 14-3-3 during salinity stress, thereby suggesting possible posttranslational modification regulated by salinity. The function of 14-3-3 and LRR-RK cloned from seashore paspalum regulating salinity tolerance deserves further confirmation.
Vesicle traffic pathway
Vesicle traffic is necessary for cell metabolism, growth, and development in plants and depends on a superfamily of proteins named soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptors (SNAREs; Sutter et al., 2006). A previous study indicated that AtVAM3 (belong to the Qa-SNARE protein family) could be induced under salinity treatment and an atvam3 mutant displayed better tolerance to salinity stress (Hamaji et al., 2009). A recent study showed that Arabidopsis Qc-SNARE gene AtSFT12 positively participated in salinity tolerance and Na+ accumulation in vacuoles (Tarte et al., 2015). In Arabidopsis, VAP33 family proteins (SNARE-associated elements) can interact with a sterol-binding protein ORP3a and regulate the transport of sterols from the endoplasmic reticulum to the plasma membrane (Saravanan et al., 2009). However, to our knowledge, VAP33-mediated salinity tolerance in plants has not been previously reported. In this study, a VAP33 family gene was found to be potentially involved in the improvement of salinity tolerance through its heterologous expression in yeast and in the up-regulation of this gene in both leaves and roots of seashore paspalum in response to salinity stress. However, the regulatory mechanisms of VAP33 for salinity tolerance are unknown.
Phospholipid biosynthesis
Phospholipids are a major component of most eukaryotic cell membranes and critical for cell functions in plants (Lin et al., 2014). Phosphatidylcholine (PC) is an important phospholipid and synthesized largely via the CDP–choline pathway, which can be converted to phosphatidic acid (PA) by phospholipase D (PLD; Craddock et al., 2015). Several previous studies indicated that PA and PLD participated in drought and salinity tolerance (McLoughlin et al., 2013; Wang et al., 2014). However, whether upstream pathways of PC regulate salinity tolerance remains unclear. In this study, a gene encoding an upstream rate-limiting enzyme CTP:Phosphorylcholine Cytidylyltransferase (CCT) for PC synthesis was identified in yeast transformed with the gene from seashore paspalum subjected to salinity stress, and its gene expression level was also significantly up-regulated under salinity stress condition. Our results suggested that CCT could play roles in regulating plant tolerance to salinity stress as an upstream component of phospholipid metabolism.
Photosynthesis
The two photosystem complexes (PS I and PS II) located in thylakoid membranes of higher plants are essential components of light reactions in photosynthesis involved in electron transport (Chen and Xue, 2012). In this study, PSAK (a member of the PS I complex), LHCB (Light-harvesting chlorophyll a/b-binding protein belonging to the photosystem II complex), and Elip (early light-induced protein) isolated from seashore paspalum were up-regulated in response to salinity stress. To our knowledge, PSAK has not been reported to be related to salinity stress. In Arabidopsis, LHCB are required for stomatal response to abscisic acid (Xu et al., 2012). LHCB gene was identified as a candidate drought-related locus through QTL analysis in pearl millet (Pennisetum glaucum; Sehgal et al., 2012). Elip is proposed to serve as protection of the photosynthetic apparatus from high light stress, and the overexpression of MfELIP from Medicago sativa subsp. facilitates improved plant tolerance to several abiotic stresses, such as freezing, chilling, osmotic stress, and high light (Zhuo et al., 2013). The up-regulation of PSAK, LHCB, and Elip by salinity stress found in this study suggested that the superior salinity tolerance of seashore paspalum could be associated with the positive regulation of proteins involved in the light reactions of photosynthesis, PSAK, LHCB, and Elip, under salinity stress.
Antioxidant defense and metabolism
Abiotic stresses lead to oxidative damages that can be alleviated by antioxidant enzymes, such as ascorbate peroxidase (APX; Chen et al., 2015a). APX-overexpression enhanced abiotic stresses tolerance of tall fescue (Lolium arundinaceum; Lee et al., 2007). Cysteine synthase (CS) is responsible for the final step in biosynthesis of cysteine, which produces glutathion (GSH) that also play roles in scavenging of reactive oxygen species (Noji et al., 2001). CS-overexpression increased plant tolerance to oxidative stress in tobacco (Nicotiana tabacum; Noji et al., 2001). The aldo-keto reductase (AKR) superfamily is a large enzyme group of NADP-dependent oxidoreductases with numerous roles in metabolism, and Arabidopsis transformed with PpAKR1 of peach tree (Prunus persica) increased in salinity tolerance (Kanayama et al., 2014). The improved salinity tolerance of the salinity-sensitive yeast cells through transformation of APX, CS, and ARK from seashore paspalum suggested that antioxidant defense and metabolism regulated by those genes could play roles in the superior salinity tolerance in seashore paspalum.
Other pathways
Plant heat shock proteins (HSPs) serve important roles in responses to adverse environmental conditions (Timperio et al., 2008). Overexpression of a heat shock protein ThHSP18.3 from Tamarix hispida confers stress tolerance to salinity, drought, and heavy metals in yeast (Gao et al., 2012). Our results of HSP expression in yeast in relation to salinity tolerance are in agreement with the previous study, suggesting the postive roles of HSP in plant tolerance to salinity stress.
BTB/POZ domain-containing proteins are interaction partners with the Cullin component of the E3 ubiquitin ligase complex, and up-regulated expressions of BTB/POZ lead to up-regulation of various protease genes (Aulakh et al., 2014), whereas no reports of BTB/POZ was related to salinity tolerance. Plant metacaspases (MCPs), a family of cysteine proteases structurally related to caspases, play a positive regulatory role in biotic and abiotic stress-induced programmed cell death (Watanabe and Lam, 2011). Our data indicated that MCP screened by yeast might participate in salinity tolerance of seashore paspalum.
In addition, in this study, three novel unknown function genes such as Nop14-like family protein (NOP14), protein IQ-DOMAIN 14-like (IQ14), and uncharacterized protein (UP) were identified in seashore paspalum, and their biological functions and potential roles in regulating salinity tolerances deserve further analysis.
Candidate genes for Cd tolerance identified from seashore paspalum
Five genes from Cd-tolerant clones were found with biological functions including phytochelatins synthase (PCS1, PCS2), cytochrome P450 (CYP450), a heat shock transcription factor (HSFA4a), and UDP-glucose pyrophosphorylase (UDP).
Phytochelatin synthase (PCS) is a key rate-limiting enzyme in the synthesis of phytochelatin, which can form stable complexes with heavy metals that are subsequently transported into the vacuoles (Brunetti et al., 2011). Tall fescue plants overexpressing reed (Phragmites australis) PaPCS exhibited improved tolerance to Cd (Zhao et al., 2014). Our study found two PCS family genes from seashore paspalum, indicating the involvement of PCS in Cd tolerance for the halophytic grass species.
Cytochrome P450s (CYP450) are among the largest protein coding gene families in plant genomes, catalyzing several secondary metabolism including indole alkaloids synthesis (Irmler et al., 2000) and triterpenoid saponins synthesis (Seki et al., 2015). Previous studies indicated that the accumulation of triterpene saponins of Quillaja brasiliensis leaves might regulate abiotic stress tolerances (de Costa et al., 2013), and triterpene saponins could regulate ROS and NO production and then induce the metallothioneins synthesis, which is related to Cd tolerance (Balestrazz et al., 2011). The heavy metal chromium induced CYP450 family gene expressions in radish (Raphanus sativus) roots (Xie et al., 2015). A recent study found that a cytochrome P450 member named OsDSS1 was involved in drought stress responses in rice (Tamiru et al., 2015), but CYP450-mediated Cd tolerance has not been well documented. In this study, Cd-promoted CYP450 expression in seashore paspalum suggested that CYP450 could play roles in Cd tolerance via secondary metabolism pathway such as indole alkaloids synthesis and triterpenoid saponins synthesis.
Heat shock factors are principal regulators of plant responses to several abiotic stresses (Perez-Salamo et al., 2014). In a screen for wheat (Triticum aestivum) genes that confer Cd tolerance to Cd-hypersensitive yeast strain identified TaHSFA4a, a gene which overexpression improved Cd tolerance in rice (Shim et al., 2009). It has been shown that AtHSFA4a of Arabidopsis confers enhanced tolerance to salinity and oxidative agents (Perez-Salamo et al., 2014). A TaHSFA4a homolog from seashore paspalum was also identified via yeast screening for Cd tolerance in this study, implying the potential roles of HSFA4a in Cd tolerance of seashore paspalum.
UDP-glucose pyrophosphorylase (UGPase) is a sugar-metabolizing enzyme that catalyzes a reversible reaction of UDP-glucose and pyrophosphate from glucose-1-phosphate and UTP (Payyavula et al., 2014). UGPase functions in cell wall biosynthesis and vegetative growth improvement (Li et al., 2014a,b; Payyavula et al., 2014). Although little is known of how UGPase is related to Cd tolerance, UGP gene clones from seashore paspalum subjected to Cd stress improved yeast tolerance to Cd through heterogenous expression, indicating that UGP-involved sugar metabolism could facilitate plant tolerance to Cd.
Conclusions
This study identified 18 salinity-tolerance and five Cd-tolerance genes in seashore paspalum through full-length cDNA library screening in yeast and confirmation of gene expression in seashore paspalum exposed to salinity or Cd treatment using qPCR analysis. Major functional categories of the salinity-tolerance genes included iron transport, vesicle traffic, protein modification, phospholipid biosynthesis, photosynthetic metabolism, and antioxidant protection, as well as several other pathways. Cd tolerance of seashore paspalum could be related to genes for metal chelation, secondary metabolism, stress regulatory factors, and sugar metabolism. Most of those candidate genes identified in the halophytic seashore paspalum in this study are novel genes, which have not been previously reported to be related to salinity or Cd tolerance. However, the biochemical and molecular mechanisms of how these novel genes regulate salinity and Cd tolerance are unknown. Further analysis of the biological and molecular functions of those novel genes could provide further insights into survival mechanisms of halophytes to severe salinity and Cd stress, and those genes could serves as useful target genes for genetic modification of glycophytic plants for improved stress tolerance.
Author contributions
YC, CC, ZY, and BH conceived the study and designed the experiments. YC, CC, ZT, and JL performed the experiments. YC and CC analyzed the data with suggestions by ZY, LZ, and BH. YC, CC, and BH wrote the manuscript. All authors read and approved the final manuscript.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Dr. Huazhong Shi (Department of Chemistry and Biochemistry, Texas Tech University) for providing yeast strains G19 (Δena1::HIS3::ena4). This work was supported by the program of the China Postdoctoral Science Foundation (2014M551612) and Jiangsu Postdoctoral Science Foundation (1302018B).
Supplementary material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2016.00102
References
- Abbas G., Saqib M., Akhtar J., Haq M. (2015). Interactive effects of salinity and iron deficiency on different rice genotypes. J. Plant Nutr. Soil Sci. 178, 306–311. 10.1002/jpln.201400358 [DOI] [Google Scholar]
- Aulakh S. S., Veilleux R. E., Dickerman A. W., Tang G., Flinn B. S. (2014). Characterization and RNA-seq analysis of underperformer, an activation-tagged potato mutant. Plant Mol. Biol. 84, 635–658. 10.1007/s11103-013-0159-4 [DOI] [PubMed] [Google Scholar]
- Azevedo R. A., Gratão P. L., Monteiro C. C., Carvalho R. F. (2012). What is new in the research on cadmium-induced stress in plants? Food Energy Secur. 1, 133–140. 10.1002/fes3.10 [DOI] [Google Scholar]
- Balestrazz A., Macovei A., Tava A., Avato P., Raimondi E., Carbonera D. (2011). Unraveling the response of plant cells to cytotoxic saponins: role of metallothionein and nitric oxide. Plant Signal. Behav. 6, 516–519. 10.4161/psb.6.4.14746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashir K., Nozoye T., Ishimaru Y., Nakanishi H., Nishizawa N. K. (2013). Exploiting new tools for iron bio-fortification of rice. Biotechnol. Adv. 31, 1624–1633. 10.1016/j.biotechadv.2013.08.012 [DOI] [PubMed] [Google Scholar]
- Brumbarova T., Bauer P., Ivanov R. (2015). Molecular mechanisms governing Arabidopsis iron uptake. Trends Plant Sci. 20, 124–133. 10.1016/j.tplants.2014.11.004 [DOI] [PubMed] [Google Scholar]
- Brunetti P., Zanella L., Proia A., De Paolis A., Falasca G., Altamura M. M., et al. (2011). Cadmium tolerance and phytochelatin content of Arabidopsis seedlings over-expressing the phytochelatin synthase gene AtPCS1. J. Exp. Bot. 62, 5509–5519. 10.1093/jxb/err228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catala R., Lopez-Cobollo R., Mar Castellano M., Angosto T., Alonso J. M., Ecker J. R., et al. (2014). The Arabidopsis 14-3-3 protein RARE COLD INDUCIBLE 1A links low-temperature response and ethylene biosynthesis to regulate freezing tolerance and cold acclimation. Plant Cell 26, 3326–3342. 10.1105/tpc.114.127605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan Z., Grumet R., Loescher W. (2011). Global gene expression analysis of transgenic, mannitol-producing and salt tolerant Arabidopsis thaliana indicates widespread changes in expression of abiotic- and biotic-stress related genes. J. Exp. Bot. 62, 4787–4803. 10.1093/jxb/err130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F., Li Q., Sun L., He Z. (2006). The rice 14-3-3 gene family and its involvement in responses to biotic and abiotic stress. DNA Res. 13, 53–63. 10.1093/dnares/dsl001 [DOI] [PubMed] [Google Scholar]
- Chen X., Xue H. (2012). Primary reaction and release oxygen, Chapter 14, in Plant Physiology and Molecular Biology (Beijing: Higher Education Press; ), 234–246. [Google Scholar]
- Chen Y., Jiang J., Chang Q., Gu C., Song A., Chen S., et al. (2014). Cold acclimation induces freezing tolerance via antioxidative enzymes, proline metabolism and gene expression changes in two chrysanthemum species. Mol. Biol. Rep. 41, 815–822. 10.1007/s11033-013-2921-8 [DOI] [PubMed] [Google Scholar]
- Chen Y., Jiang J., Song A., Chen S., Shan H., Luo H., et al. (2013). Ambient temperature enhanced freezing tolerance of Chrysanthemum dichrum CdICE1 Arabidopsis via miR398. BMC Biol. 11:121. 10.1186/1741-7007-11-121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Li L., Zong J., Chen J., Guo H., Guo A., et al. (2015a). Heterologous expression of the halophyte Zoysia matrella H+-pyrophosphatase gene improved salinity tolerance in Arabidopsis thaliana. Plant Physiol. Biochem. 91, 49–55. 10.1016/j.plaphy.2015.04.004 [DOI] [PubMed] [Google Scholar]
- Chen Y., Zong J., Tan Z., Li L., Hu B., Chen C., et al. (2015b). Systematic mining of salinity-tolerant genes in halophyte-Zoysia matrella through cDNA expression library screening. Plant Physiol. Biochem. 89, 44–52. 10.1016/j.plaphy.2015.02.007 [DOI] [PubMed] [Google Scholar]
- Craddock C. P., Adams N., Bryant F. M., Kurup S., Eastmond P. J. (2015). PHOSPHATIDIC ACID PHOSPHOHYDROLASE regulates phosphatidylcholine biosynthesis in arabidopsis by phosphatidic acid-mediated activation of CTP:PHOSPHOCHOLINE CYTIDYLYLTRANSFERASE activity. Plant Cell 27, 1251–1264. 10.1105/tpc.15.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DalCorso G., Farinati S., Furini A. (2010). Regulatory networks of cadmium stress in plants. Plant Signal. Behav. 5, 663–667. 10.4161/psb.5.6.11425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Costa F., Yendo A. C., Fleck J. D., Gosmann G., Fett-Neto A. G. (2013). Accumulation of a bioactive triterpene saponin fraction of Quillaja brasiliensis leaves is associated with abiotic and biotic stresses. Plant Physiol. Biochem. 66, 56–62. 10.1016/j.plaphy.2013.02.003 [DOI] [PubMed] [Google Scholar]
- Eswaran N., Parameswaran S., Sathram B., Anantharaman B., Kumar G. R. K., Tangirala S. J. (2010). Yeast functional screen to identify genetic determinants capable of conferring abiotic stress tolerance in Jatropha curcas. BMC Biotechnol. 10:23. 10.1186/1472-6750-10-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flowers T. J., Colmer T. D. (2008). Salinity tolerance in halophytes. New Phytol. 179, 945–963. 10.1111/j.1469-8137.2008.02531.x [DOI] [PubMed] [Google Scholar]
- Gao C., Jiang B., Wang Y., Liu G., Yang C. (2012). Overexpression of a heat shock protein (ThHSP18.3) from Tamarix hispida confers stress tolerance to yeast. Mol. Biol. Rep. 39, 4889–4897. 10.1007/s11033-011-1284-2 [DOI] [PubMed] [Google Scholar]
- Ghasemi S., Khoshgoftarmanesh A. H., Afyuni M., Hadadzadeh H. (2014). Iron(II)-amino acid chelates alleviate salinity-stress induced oxidative damages on tomato grown in nutrient solution culture. Sci. Hortic. 165, 91–98. 10.1016/j.scienta.2013.10.037 [DOI] [Google Scholar]
- Gupta B., Huang B. (2014). Mechanism of salinity tolerance in plants: physiological, biochemical, and molecular characterization. Int. J. Genomics 2014:701596. 10.1155/2014/701596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaji K., Nagira M., Yoshida K., Ohnishi M., Oda Y., Uemura T., et al. (2009). Dynamic aspects of ion accumulation by vesicle traffic under salinity stress in Arabidopsis. Plant Cell Physiol. 50, 2023–2033. 10.1093/pcp/pcp143 [DOI] [PubMed] [Google Scholar]
- He Y., Wu J., Lv B., Li J., Gao Z., Xu W., et al. (2015). Involvement of 14-3-3 protein GRF9 in root growth and response under polyethylene glycol-induced water stress. J. Exp. Bot. 66, 2271–2281. 10.1093/jxb/erv149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi M., Kondou Y., Ichikawa T., Matsui M. (2011). Full-length cDNA overexpressor gene hunting system (FOX hunting system). Methods Mol. Biol. 678, 77–89. 10.1007/978-1-60761-682-5_7 [DOI] [PubMed] [Google Scholar]
- Hoagland D. R., Arnon D. I. (1950). The water-culture method for growing plants without soil. Calif. Agric. Exp. Stn. Circ. 347, 1–32. [Google Scholar]
- Hur J., Jung K. H., Lee C. H., An G. H. (2004). Stress-inducible OsP5CS2 gene is essential for salinity and cold tolerance in rice. Plant Sci. 167, 417–426. 10.1016/j.plantsci.2004.04.009 [DOI] [Google Scholar]
- Irmler S., Schröder G., St-Pierre B., Crouch N. P., Hotze M., Schmidt J., et al. (2000). Indole alkaloid biosynthesis in Catharanthus roseus: new enzyme activities and identification of cytochrome P450 CYP72A1 as secologanin synthase. Plant J. 24, 797–804. 10.1046/j.1365-313x.2000.00922.x [DOI] [PubMed] [Google Scholar]
- Kanayama Y., Mizutani R., Yaguchi S., Hojo A., Ikeda H., Nishiyama M., et al. (2014). Characterization of an uncharacterized aldo-keto reductase gene from peach and its role in abiotic stress tolerance. Phytochemistry 104, 30–36. 10.1016/j.phytochem.2014.04.008 [DOI] [PubMed] [Google Scholar]
- Kumar R., Mustafiz A., Sahoo K. K., Sharma V., Samanta S., Sopory S. K., et al. (2012). Functional screening of cDNA library from a salinity tolerant rice genotype Pokkali identifies mannose-1-phosphate guanyl transferase gene (OsMPG1) as a key member of salinity stress response. Plant Mol. Biol. 79, 555–568. 10.1007/s11103-012-9928-8 [DOI] [PubMed] [Google Scholar]
- Lee S. H., Ahsan N., Lee K. W., Kim D. H., Lee D. G., Kwak S. S., et al. (2007). Simultaneous overexpression of both Cu/Zn superoxide dismutase and ascorbate peroxidase in transgenic tall fescue plants confers increased tolerance to a wide range of abiotic stresses. J. Plant Physiol. 164, 1626–1638. 10.1016/j.jplph.2007.01.003 [DOI] [PubMed] [Google Scholar]
- Li N., Wang L., Zhang W., Takechi K., Takano H., Lin X. (2014a). Overexpression of UDP-glucose pyrophosphorylase from Larix gmelinii enhances vegetative growth in transgenic Arabidopsis thaliana. Plant Cell Rep. 33, 779–791. 10.1007/s00299-013-1558-3 [DOI] [PubMed] [Google Scholar]
- Li Y., Fan C., Xing Y., Yun P., Luo L., Yan B., et al. (2014b). Chalk5 encodes a vacuolar H(+)-translocating pyrophosphatase influencing grain chalkiness in rice. Nat. Genet. 46, 398–404. 10.1038/ng.2923 [DOI] [PubMed] [Google Scholar]
- Lin F., Qu Y., Zhang Q. (2014). Phospholipids: molecules regulating cytoskeletal organization in plant abiotic stress tolerance. Plant Signal. Behav. 9:e28337. 10.4161/psb.28337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Du H., He X., Huang B., Wang Z. (2012). Identification of differentially expressed salinity-responsive proteins in roots of two perennial grass species contrasting in salinity tolerance. J. Plant Physiol. 169, 117–126. 10.1016/j.jplph.2011.08.019 [DOI] [PubMed] [Google Scholar]
- McLoughlin F., Arisz S. A., Dekker H. L., Kramer G., de Koster C. G., Haring M. A., et al. (2013). Identification of novel candidate phosphatidic acid-binding proteins involved in the salinity-stress response of Arabidopsis thaliana roots. Biochem. J. 450, 573–581. 10.1042/BJ20121639 [DOI] [PubMed] [Google Scholar]
- Noji M., Saito M., Nakamura M., Aono M., Saji H., Saito K. (2001). Cysteine synthase overexpression in tobacco confers tolerance to sulfur-containing environmental pollutants. Plant Physiol. 126, 973–980. 10.1104/pp.126.3.973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata T., Kitamoto H. K., Nakamura A., Fukuda A., Tanaka Y. (2007). Rice shaker potassium channel OsKAT1 confers tolerance to salinity stress on yeast and rice cells. Plant Physiol. 144, 1978–1985. 10.1104/pp.107.101154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa M., Shinohara H., Sakagami Y., Matsubayashi Y. (2008). Arabidopsis CLV3 peptide directly binds CLV1 ectodomain. Science 319, 294. 10.1126/science.1150083 [DOI] [PubMed] [Google Scholar]
- Park S., Moon J. C., Park Y. C., Kim J. H., Kim D. S., Jang C. S. (2014). Molecular dissection of the response of a rice leucine-rich repeat receptor-like kinase (LRR-RLK) gene to abiotic stresses. J. Plant Physiol. 171, 1645–1653. 10.1016/j.jplph.2014.08.002 [DOI] [PubMed] [Google Scholar]
- Payyavula R. S., Tschaplinski T. J., Jawdy S. S., Sykes R. W., Tuskan G. A., Kalluri U. C. (2014). Metabolic profiling reveals altered sugar and secondary metabolism in response to UGPase overexpression in Populus. BMC Plant Biol. 14:265. 10.1186/s12870-014-0265-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Salamo I., Papdi C., Rigo G., Zsigmond L., Vilela B., Lumbreras V., et al. (2014). The heat shock factor A4A confers salinity tolerance and is regulated by oxidative stress and the mitogen-activated protein kinases MPK3 and MPK6. Plant Physiol. 165, 319–334. 10.1104/pp.114.237891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessarakli M. (2014). Handbook of Plant and Crop Physiology, 3rd Edn. Edited by Pessarakli M. (Revised and Expanded). Boca Raton, FL: CRC Press; Taylor & Francis Publishing Group. [Google Scholar]
- Qiu Q. S. (2012). Plant and yeast NHX antiporters: roles in membrane trafficking. J. Integr. Plant Biol. 54, 66–72. 10.1111/j.1744-7909.2012.01097.x [DOI] [PubMed] [Google Scholar]
- Sakurai T., Kondou Y., Akiyama K., Kurotani A., Higuchi M., Ichikawa T., et al. (2011). RiceFOX: a database of Arabidopsis mutant lines overexpressing rice full-length cDNA that contains a wide range of trait information to facilitate analysis of gene function. Plant Cell Physiol. 52, 265–273. 10.1093/pcp/pcq190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saravanan R. S., Slabaugh E., Singh V. R., Lapidus L. J., Haas T., Brandizzi F. (2009). The targeting of the oxysterol-binding protein ORP3a to the endoplasmic reticulum relies on the plant VAP33 homolog PVA12. Plant J. 58, 817–830. 10.1111/j.1365-313X.2009.03815.x [DOI] [PubMed] [Google Scholar]
- Sehgal D., Rajaram V., Armstead I. P., Vadez V., Yadav Y. P., Hash C. T., et al. (2012). Integration of gene-based markers in a pearl millet genetic map for identification of candidate genes underlying drought tolerance quantitative trait loci. BMC Plant Biol. 12:9. 10.1186/1471-2229-12-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki H., Tamura K., Muranaka T. (2015). P450s and UGTs: key players in the structural diversity of triterpenoid saponins. Plant Cell Physiol. 56, 1463–1471. 10.1093/pcp/pcv062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim D., Hwang J. U., Lee J., Lee S., Choi Y., An G., et al. (2009). Orthologs of the class A4 heat shock transcription factor HsfA4a confer cadmium tolerance in wheat and rice. Plant Cell 21, 4031–4043. 10.1105/tpc.109.066902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. J., Daut J., Schwappach B. (2011). Membrane proteins as 14-3-3 clients in functional regulation and intracellular transport. Physiology 26, 181–191. 10.1152/physiol.00042.2010 [DOI] [PubMed] [Google Scholar]
- Song A., Lu J., Jiang J., Chen S., Guan Z., Fang W., et al. (2012). Isolation and characterisation of Chrysanthemum crassum SOS1, encoding a putative plasma membrane Na(+)/H(+) antiporter. Plant Biol. 14, 706–713. 10.1111/j.1438-8677.2011.00560.x [DOI] [PubMed] [Google Scholar]
- Sutter J. U., Campanoni P., Blatt M. R., Paneque M. (2006) Setting SNAREs in a different wood. Traffic 7, 627–638. 10.1111/j.1600-0854.2006.00414.x [DOI] [PubMed] [Google Scholar]
- Tamiru M., Undan J. R., Takagi H., Abe A., Yoshida K., Undan J. Q., et al. (2015). A cytochrome P450, OsDSS1, is involved in growth and drought stress responses in rice (Oryza sativa L.). Plant Mol. Biol. 88, 85–99. 10.1007/s11103-015-0310-5 [DOI] [PubMed] [Google Scholar]
- Tarte V., Seok H. Y., Woo D. H., Le D., Tran H., Baik J. W., et al. (2015). Arabidopsis Qc-SNARE gene AtSFT12 is involved in salinity and osmotic stress responses and Na+ accumulation in vacuoles. Plant Cell Rep. 34, 1127–1138. 10.1007/s00299-015-1771-3 [DOI] [PubMed] [Google Scholar]
- Timperio A. M., Egidi M. G., Zolla L. (2008). Proteomics applied on plant abiotic stresses: role of heat shock proteins (HSP). J. Proteomics 71, 391–411. 10.1016/j.jprot.2008.07.005 [DOI] [PubMed] [Google Scholar]
- Torii K. U. (2004) Leucine-rich repeat receptor kinases in plants: structure, function, and signal transduction pathways. Int. Rev. Cytol. 234, 1–46. 10.1016/s0074-7696(04)34001-5. [DOI] [PubMed] [Google Scholar]
- Uddin K., Juraimi A. S., Ismail M. R., Hossain A., Othman R., Abdul Rahim A. (2012). Physiological and growth responses of six turfgrass species relative to salinity tolerance. Sci. World J. 2012:905468. 10.1100/2012/905468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin K., Juraimi A. S., Ismail M. R., Othman R., Rahim A. A. (2011). Relative salinity tolerance of warm season turfgrass species. J. Environ. Biol. 32, 309–312. [PubMed] [Google Scholar]
- Varotto C., Maiwald D., Pesaresi P., Jahns P., Salamini F., Leister D. (2002). The metal ion transporter IRT1 is necessary for iron homeostasis and efficient photosynthesis in Arabidopsis thaliana. Plant J. 31, 589–599. 10.1046/j.1365-313X.2002.01381.x [DOI] [PubMed] [Google Scholar]
- Vert G., Grotz N., Dedaldechamp F., Gaymard F., Guerinot M. L., Briat J. F., et al. (2002). IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell 14, 1223–1233. 10.1105/tpc.001388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Ding B., Guo Y., Li M., Chen S., Huang G., et al. (2014). Overexpression of a wheat phospholipase D gene, TaPLDalpha, enhances tolerance to drought and osmotic stress in Arabidopsis thaliana. Planta 240, 103–115. 10.1007/s00425-014-2066-6 [DOI] [PubMed] [Google Scholar]
- Wang J., Gao Y., Li J., Yang S. G., Wu K. Q., Zhang M. (2012). Construction of a yeast expression cDNA library and screening heavy metal related-genes in rice. Guangdong Agr. Sci. 13, 143–145. [Google Scholar]
- Wang K. (2010). The Stress Responses and Tolerance Thresholds to Soil Lead, Cadmium and Zinc Contamination in Centipedegrass and Seashore Paspalum. Master's Degree Thesis, Shanghai Jiaotong University. [Google Scholar]
- Watanabe N., Lam E. (2011). Arabidopsis metacaspase 2d is a positive mediator of cell death induced during biotic and abiotic stresses. Plant J. 66, 969–982. 10.1111/j.1365-313X.2011.04554.x [DOI] [PubMed] [Google Scholar]
- Wu F., Sheng P., Tan J., Chen X., Lu G., Ma W., et al. (2015) Plasma membrane receptor-like kinase leaf panicle 2 acts downstream of the DROUGHT SALINITY TOLERANCE transcription factor to regulate drought sensitivity in rice. J. Exp. Bot. 66, 271–281. 10.1093/jxb/eru417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y., Ye S., Wang Y., Xu L., Zhu X. W., Yang J. L., et al. (2015). Transcriptome-based gene profiling provides novel insights into the characteristics of radish root response to Cr stress with next-generation sequencing. Front. Plant Sci. 6:202. 10.3389/fpls.2015.00202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y. H., Liu R., Yan L., Liu Z. Q., Jiang S. C., Shen Y. Y., et al. (2012). Light-harvesting chlorophyll a/b-binding proteins are required for stomatal response to abscisic acid in Arabidopsis. J. Exp. Bot. 63, 1095–1106. 10.1093/jxb/err315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q., Chen Z. Z., Zhou X. F., Yin H. B., Li X., Xin X. F., et al. (2009). Overexpression of SOS (Salinity Overly Sensitive) genes increases salinity tolerance in transgenic Arabidopsis. Mol. Plant 2, 22–31. 10.1093/mp/ssn058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Xu J., Li Q., Li S., Wang P., Xiang F. (2014). Cloning and characterization of a Phragmites australis phytochelatin synthase (PaPCS) and achieving cd tolerance in tall fescue. PLoS ONE 9:e103771. 10.1371/journal.pone.0103771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H. P., Lin H. X., Chen S., Becker K., Yang Y. Q., Zhao J. F., et al. (2014). Inhibition of the Arabidopsis salinity overly sensitive pathway by 14-3-3 proteins. Plant Cell 26, 1166–1182. 10.1105/tpc.113.117069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Fu X., Koo Y. D., Zhu J. K., Jenney F. E., Jr., Adams M. W., et al. (2007). An enhancer mutant of Arabidopsis salt overly sensitive 3 mediates both ion homeostasis and the oxidative stress response. Mol. Cell. Biol. 27, 5214–5224. 10.1128/MCB.01989-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo C., Cai J., Guo Z. (2013). Overexpression of Early Light-Induced Protein (ELIP) Gene from Medicago sativa ssp. falcata increases tolerance to abiotic stresses. Agron. J. 105, 1433–1440. 10.2134/agronj2013.0155 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.