Abstract
The underlying genetic etiology of rhabdomyolysis remains elusive in a significant fraction of individuals presenting with recurrent metabolic crises and muscle weakness. Using exome sequencing, we identified bi-allelic mutations in TANGO2 encoding transport and Golgi organization 2 homolog (Drosophila) in 12 subjects with episodic rhabdomyolysis, hypoglycemia, hyperammonemia, and susceptibility to life-threatening cardiac tachyarrhythmias. A recurrent homozygous c.460G>A (p.Gly154Arg) mutation was found in four unrelated individuals of Hispanic/Latino origin, and a homozygous ∼34 kb deletion affecting exons 3–9 was observed in two families of European ancestry. One individual of mixed Hispanic/European descent was found to be compound heterozygous for c.460G>A (p.Gly154Arg) and the deletion of exons 3–9. Additionally, a homozygous exons 4–6 deletion was identified in a consanguineous Middle Eastern Arab family. No homozygotes have been reported for these changes in control databases. Fibroblasts derived from a subject with the recurrent c.460G>A (p.Gly154Arg) mutation showed evidence of increased endoplasmic reticulum stress and a reduction in Golgi volume density in comparison to control. Our results show that the c.460G>A (p.Gly154Arg) mutation and the exons 3–9 heterozygous deletion in TANGO2 are recurrent pathogenic alleles present in the Latino/Hispanic and European populations, respectively, causing considerable morbidity in the homozygotes in these populations.
Main Text
Several inherited muscle disorders have been described in association with rhabdomyolysis with inherent failure of energy production.1 Inborn errors of metabolism affecting glycogenolysis and fatty acid β oxidation are known to cause an increased susceptibility to rhabdomyolysis. Carnitine palmitoyltransferase II deficiency (CPT II [MIM: 600649]) is a common cause of inherited exercise-induced myoglobinuria,2 in addition to other disorders of fatty acid oxidation such as long chain 3-hydroxy acyl-coenzyme A dehydrogenase (LCHAD) deficiency (MIM: 609016)3 and very long chain acyl-CoA dehydrogenase (VLCAD) deficiency (MIM: 201475).4 Defects in the ryanodine receptor, due to RYR1 (MIM: 180901) mutations, can cause muscle rigidity and rhabdomyolysis secondary to a sudden rise in free sarcoplasmic calcium when affected individuals are exposed to general anesthesia.5 Recessive mutations in LPIN1 (MIM: 605518) are yet another cause, leading to severe, recurrent, and early-onset rhabdomyolysis, triggered by febrile illness or exercise.6 Primary mitochondrial disorders are a rare cause of rhabdomyolysis, reported in sporadic cases with mutations in mitochondrial DNA genes encoding cytochrome b, cytochrome c oxidase (COX) subunits, and transfer RNAs.7
Recurrent rhabdomyolysis can be fatal in severe cases, with an associated mortality rate of 8%–10%.8, 9 The mortality is even greater when accompanied by acute renal failure10 and cardiac arrhythmia due to hyperkalemia.11 Identification of genes underlying rhabdomyolysis has significantly enhanced our understanding of its molecular mechanisms and in several instances facilitated prevention of recurrent episodes in susceptible individuals.12, 13 However, despite considerable advances in molecular diagnostics, the etiology of rhabdomyolysis remains unknown in about half of pediatric cases.2 Whole-exome sequencing is increasingly utilized for the diagnostic evaluation of genetic disorders and is a powerful tool for molecular characterization of heterogeneous clinical entities.14, 15 Here we describe variants in the transport and Golgi organization 2 homolog (Drosophila) gene, TANGO2, identified by whole-exome sequencing in 12 subjects from 9 unrelated families who shared clinical features of episodic muscle weakness with recurrent rhabdomyolysis, intellectual disability, and seizures.
All subjects were ascertained from 4,438 consecutive individuals referred to the Exome Laboratory at the Baylor Miraca Genetics Laboratories from November 2011 to December 2014, with the exception of proband 5, who was primarily enrolled in the Baylor Hopkins Center for Mendelian Genomics (BHCMG) research study. All subjects were subsequently recruited in the BHCMG study and related protocols approved by the Institutional Review Board of Baylor College of Medicine. Informed consents were obtained in all families. Five families were of reported Hispanic/Latino origin, two of European ancestry, one of mixed Hispanic/Latino/European ethnicity, and one of Middle Eastern Arab descent.
Clinical whole-exome sequencing (WES) was completed in the Exome Laboratory at Baylor Miraca Genetics Laboratories and sequencing and data analyses were conducted as previously described,16 targeting ∼20,000 genes, including the coding and untranslated region (UTR) exons. Samples were also analyzed by a cSNP-array (Illumina HumanExome-12 v1 array) for quality-control assessment of exome data, as well as for detecting large copy-number variants (CNVs) and regions of absence of heterozygosity (AOH). CNVs were additionally characterized after comparing the normalized depth of coverage from 4,334 clinical WES samples. Exon deletions were called using a dual normalization approach consisting of principal-component analysis (PCA)-XHMM intermediates and RPKM (read per kilobase per million reads) read depth values.17 The former normalizes raw read depth values via PCA, which were then centered by exon target on a Z-score scale, whereas the latter was normalized at the sample level. By intersecting two orthogonal normalization approaches (target based and sample based), we expected an enhancement in signal and reduction in noise. In both methods, exons with GC and low-complexity biases were removed from the analysis. Complete homozygous and hemizygous exon deletions were called using a strict filtering criterion that must include exon target Z-scores that are less than −2 and the RPKM is equal or near 0. Long-range PCR (LR-PCR) reactions were performed to amplify the predicted junction fragments in the breakpoint regions in TANGO2 deletion carriers according to the manufacturer’s specifications (Takara Bio). The junction fragment of the exons 3–9 deletion was amplified by LR-PCR with primers (forward) 5′-CGTGGAAGCAGAGGAGGAAGGTATTTA-3′ and (reverse) 5′-ATAAAGAACGCGTGTGCTCAACTGTCT-3′. The junction fragment of the exons 4–6 deletion was amplified with primers (forward) 5′-TGTGCCACTGTCTTAGTTCGTTTGTGT-3′ and (reverse) 5′-TCTTCTGTCATCAAAAGTCCCTGGTGT-3′. Breakpoint analyses of junction fragments and haplotype studies were conducted for TANGO2 exonic CNVs.
Subject 1, born to non-consanguineous parents of Hispanic ancestry, presented at 1 year of age with a febrile seizure. She began to exhibit developmental regression in the following 3 years with recurrent seizures, losing the ability to ambulate and speak. Prior to her acute presentation at 4 years, she had a 3-day history of fever. This was followed by a right focal seizure, apnea, and unresponsiveness. On hospital admission, she was noted to have myoglobinuria with urine myoglobin of 94 ng/ml (normal range 10–65 ng/ml), serum creatine phosphokinase (CPK) of 205,000 U/l (normal range 75–230 U/l), elevated aspartate aminotransferase (AST) of 1,618 U/l (normal range 15–50 U/l), alanine aminotransferase (ALT) of 571 U/l (normal range 10–25 U/l), ammonia of 122 μmol/l (normal range 22–48 μmol/l), and hypoglycemia (blood glucose 30 mg/dl; normal range 70–110 mg/dl). She was mechanically ventilated while in the intensive care unit. Brain magnetic resonance imaging (MRI) showed mild diffuse atrophy of the left cerebral hemisphere. Ophthalmology evaluation showed intermittent exotropia. Echocardiogram and electrocardiogram (ECG) were unremarkable. Metabolic studies after clinical stabilization were normal including acylcarnitine profile, urine organic acids, plasma/cerebrospinal fluid (CSF) lactate levels, and serum ammonia. Physical examination was relevant for microcephaly (FOC 47.5 cm; <5th percentile for age), myopathic facies with drooling (Figure 1), increased tone in all extremities, and brisk deep tendon reflexes (DTRs) throughout with positive Babinski sign.
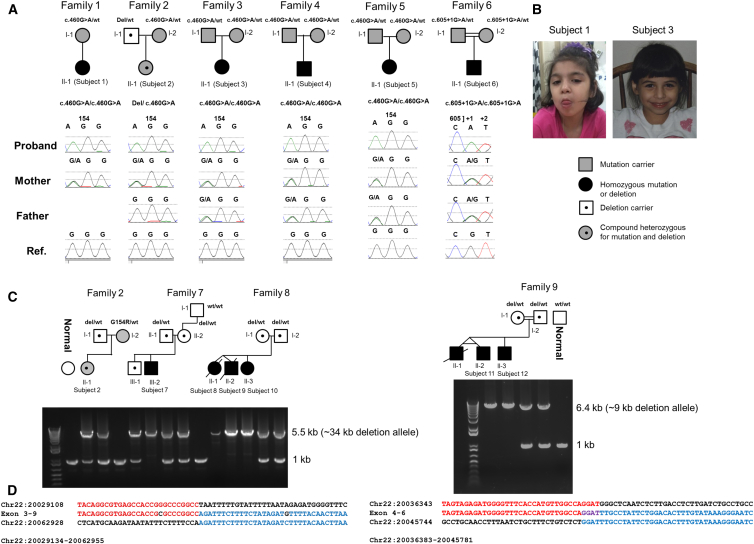
Figure 1.
Nine Pedigrees with TANGO2 Mutations
(A) Chromatograms are shown for families of Hispanic ancestry with the recurrent c.460G>A (p.Gly154Arg) mutation and c.605+1G>A mutation.
(B) Early childhood photos of subjects 1 and 3.
(C) Long-range PCR data confirm the exons 3–9 deletion in carrier parents and in subjects of European descent. Data are also shown for the consanguineous family 9 of Middle Eastern origin, having a smaller exons 4–6 deletion. In both gel pictures, the upper band represents the presence of the breakpoint junction for the deletion and the lower band (1 kb) represents the non-deleted allele.
(D) Breakpoint junction sequences for the exons 3–9 deletion and the exons 4–6 deletion are depicted under each gel picture, respectively. The breakpoint junction sequence is aligned with reference sequences with the red/blue color change indicating transition between proximal and distal reference sequences.
Subject 2 of mixed Hispanic/Latino and European origin presented at 6 months of age with metabolic acidosis (pH of 7.0; base deficit of 20 mEq/l) and lactic acidemia of 15 mmol/l (normal range 0.2–2.0 mmol/l). Urinalysis showed ketonuria. Serum ammonia was mildly elevated to 138 μmol/l. She presented with Kussmaul breathing and was resuscitated with fluids and treated with intravenous carnitine. She was noted to have a prolonged QTc interval and developed premature ventricular contractions (PVCs) transitioning into ventricular tachycardia (VT) that was successfully treated with amiodarone. Acylcarnitine profile showed a primary peak of C14:1 in addition to C14:2, C16, C18, and some hydroxy derivatives. The C14:1 completely normalized after intravenous glucose therapy. Etiologic evaluations, including DNA sequencing of ACADVL for VLCAD deficiency, muscle histopathology, quantitative mtDNA studies in muscle, and mitochondrial genome sequencing for common point mutations and deletions, were normal. She began walking unassisted around 15 months of age and presented to the hospital again at 16 months of age with weakness and gait abnormality after a 1-week history of sore throat and vomiting. She was normoglycemic, with CPK of 1,126 U/l, AST of 158 U/L, mildly elevated ammonia of 71 μmol/l, and lactate of 7.0 mmol/l. After resolution of this episode, she presented again at 5 years of age after a viral illness; this time with lethargy, lower extremity weakness, myoglobinuria, CPK of 287,230 U/l, hypoglycemia (glucose of 17 mg/dl), and elevated transaminases (AST of 7,618 U/l and ALT of 2,564 U/l). Her lactate was 2.0 mmol/l. Aldolase level was 595 U/l (normal range 2.7–8.8 U/l). Renal function tests remained stable throughout admission. Evaluation after resolution of her acute episode showed myopathic face with drooling, dysarthric speech, and ataxia.
Subject 3 was 27 years old, was of Hispanic/Latino origin, and had a history of multiple episodes of acute altered mental status, elevated CPK, increased transaminases, and ketotic hypoglycemia. Her first episode was at 5 months of age, when she arrived comatose in the emergency room with metabolic acidosis. Her CPK was elevated at 8,000 U/l. She had at least two additional episodes of rhabdomyolysis, with highest reported CPK of 22,000 U/l. During her most severe presentation at age 24 years, she had a concurrent urinary tract infection. During her subsequent hospitalization, she was noted to have torsade de pointes. Her clinical course was complicated by respiratory and multiorgan failure. She was noted to have multiple episodes of ventricular fibrillation, requiring placement of an automatic implantable cardioverter-defibrillator (ICD). She also was maintained on Nadolol therapy. Her additional diagnoses were epilepsy, bilateral sensorineural hearing loss, and intellectual disability. Muscle biopsy showed atrophic muscle fibers, suggestive of neuropathic process. She was able to ambulate and communicate with short phrases. Her physical examination was significant for height and weight below the 5th percentile for age (weight 42.9 kg, height 147 cm), head circumference below 5th percentile for age (51.4 cm), myopathic facies with persistent drooling, and wide-based gait.
Subject 4, also of Hispanic/Latino origin, was reported to have uncoordinated gait after 2 years of age. He had a diagnosis of intellectual disability. He presented at 8 years of age with an acute episode of disorientation, ataxic gait, weakness in lower extremities, and dysarthria. His lactate and glucose were found to be normal; ammonia was elevated to 111 μmol/l. CPK was elevated to 16,674 U/l. Urine was negative for myoglobin. TSH level was increased to 30.8 μIU/ml (range 0.32–5.00 μIU/ml), T3 was 166 ng/dl (range 90–260 ng/dl), and T4 was 5.2 μg/dl (range 6.4–13.3 μg/dl). Urine organic acids were normal. He also developed a prolonged QTc interval (579 ms) during hospitalization. A Brugada pattern was noted on one ECG (Figure S1). Muscle biopsy showed normal respiratory chain studies. Mitochondrial genome sequencing for common point mutations and deletions were normal. Brain MRI study showed mild cerebellar volume loss. His speech continued to be slurred when evaluated at 12 years. His physical examination was significant for myopathic facies, brisk DTRs with sustained clonus, and impaired gait.
The clinical description of the remaining eight individuals is provided in the Supplemental Data and summarized in Tables 1 and S1. Ten out of 12 subjects presented with acute rhabdomyolysis, their first episode between 5 months and 8 years of age, with highest CPK levels ranging from 16,674–287,230 U/l. Neurodevelopmental problems were observed in 100% of the affected individuals by early childhood, with muscle weakness, gait abnormality, or poor coordination reported in most individuals prior to the acute presentation of myoglobinuria. Seizures were present in 9/12 subjects. The acute clinical presentation ranged from profound muscle weakness, ataxia, and/or disorientation to a comatose state frequently precipitated by an acute illness. In subject 7, rhabdomyolysis and hypoglycemia occurred after implementation of a ketogenic diet for the treatment of seizures. During these metabolic crises, hypoglycemia (range 6–30 mg/dl), hyperlactacidemia (range 2–17 mm/l), and mild hyperammonemia (range 71–138 μmol/l) were repeatedly observed. Elevated transaminases were also noted, indicative of muscle injury. Acylcarnitine profiles during acute episodes showed elevated C14:1 in at least three individuals (subjects 2, 6, and 10); subject 10 additionally had elevated C10 species during the acute episode. Subject 11 had elevated propionyl-carnitine (C3) and C10 species on evaluation. Life-threatening cardiac tachyarrhythmia presented as torsade de pointes or ventricular tachycardia in 4/12 (33%) individuals. Intermittent prolonged QTc interval ranging from 462 to 579 ms was seen in 6/12 (50%) subjects (Figure S1), which often reverted to normal between episodes. Brugada pattern was observed only in subject 4 (Figure S1). Subjects 3, 5, and 12 required placement of an ICD as prophylaxis for their increased propensity toward serious cardiac arrhythmias. Metabolic abnormalities typically normalized outside the critical period of crises in these individuals, although some continued with mildly elevated CPK levels. Mitochondrial studies (both molecular and biochemical, including electron transport chain analysis) and muscle biopsies were essentially normal, although mild histological abnormalities such as acute neurogenic atrophy were observed in a minority. Structural brain abnormalities were frequently present (7/10), mostly reflective of varying degrees of cerebral atrophy or volume loss. Gait disturbances, dysarthria, and myopathic facies were observed in most individuals outside the crisis episodes. Hypothyroidism was diagnosed in 4/12 subjects. Two young subjects in family 8 died at 2 years of age. The oldest individual in our study is currently alive at 27 years of age.
Table 1.
Clinical Features of Individuals with Bi-allelic TANGO2 Mutations
|
Family 1 |
Family 2 |
Family 3 |
Family 4 |
Family 5 |
Family 6 |
Family 7 |
Family 8 |
Family 8 |
Family 8 |
Family 9 |
Family 9 |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Subject 1 |
Subject2 |
Subject 3 |
Subject 4 |
Subject 5 |
Subject 6 |
Subject 7 |
Subject 8 |
Subject 9 |
Subject 10 |
Subject 11 |
Subject 12 |
|
| II-1 | II-1 | II-1 | II-1 | II-1 | II-1 | III-2 | II-1 | II-2 | II-3 | II-2a | II-3 | |
| Age at first presentation | 4 years | 6 months | 5 months | 8 years | 7 years | 1 year | 18 months | 9 months | 1 year | 3.5 months | 2 years | 4 years |
| Current age | 6 years | 6 years | 27 years | 14 years | 17 years | 3 years | 9 years | deceased (2 years) | deceased (22 months) | 11 months | 11 years | 8 years |
| Gender | female | female | female | male | female | male | male | female dizygotic twin | male dizygotic twin | female | male monozygotic twin | male |
| Ethnicity | Hispanic | Hispanic/European | Hispanic | Hispanic | Hispanic | Hispanic | European | European | European | European | Arab | Arab |
| Parental relationship | non-consanguineous | non-consanguineous | non-consanguineous | non-consanguineous | non-consanguineous | consanguineous | non-consanguineous | non-consanguineous | non-consanguineous | non-consanguineous | consanguineous | consanguineous |
| Nucleotide change | c.460G>A | c.460G>A | c.460G>A | c.460G>A | c.460G>A | c.605+1G>A | – | – | – | – | – | – |
| Amino acid change | p.Gly154Arg | p.Gly154Arg | p.Gly154Arg | p.Gly154Arg | p.Gly154Arg | – | – | – | – | – | – | – |
| Intragenic deletion | – | exons 3–9 del | – | – | – | – | exons 3–9 del | exons 3–9 del | exons 3–9 del | exons 3–9 del | exons 4–6 del | exons 4–6 del |
| Mutation | homozygous | compound heterozygous | homozygous | homozygous | homozygous | homozygous | homozygous | homozygous | homozygous | homozygous | homozygous | homozygous |
| Highest CPK | 205,000 U/l | 287,230 U/l | 22,000 U/l | 16,674 U/l | 60,000 U/l | 103 U/l | 258,000 U/l | 97,500 U/l | 17,900 U/l | 248 U/l | >100,000 U/l | 54,258 U/l |
| Rhabdomyolysis | yes | yes | yes | yes | yes | no | yes | yes | yes | no | yes | yes |
| Glucose | hypoglycemia | hypoglycemia | hypoglycemia | normoglycemia | hypoglycemia | hypoglycemia | hypoglycemia | hypoglycemia | hypoglycemia | hypoglycemia | normoglycemia | normoglycemia |
| Ammonia | ↑ | ↑ | normal | ↑ | ND | ↑ | ↑ | normal | ↑ | ↑ | ↑ | ↑ |
| Lactate | ND | ↑ | ↑ | normal | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ |
| Seizures | yes | no | yes | yes | yes | no | yes | yes | yes | no | yes | yes |
| Intellectual disability | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes |
| ECG/arrhythmia | normal | prolonged QTc interval, ventricular tachycardia | ventricular tachycardia requiring ICD placement | prolonged QTc interval, Brugada pattern | prolonged QTc interval, torsade de pointes, requiring ICD placement | ND | normal | prolonged QTc interval | bradycardia and premature ventricular contractions and junctional rhythm; prolonged QTc interval | normal | normal | prolonged QTc interval, torsade de pointes progressing to ventricular tachycardia, requiring ICD placement |
Abbreviations are as follows: ND, not determined; ICD, automatic implantable cardioverter-defibrillator.
Monozygotic twin of subject 11 had a very similar presentation, but passed away at 7 years of age before molecular confirmation could be obtained.
Prior to clinical WES, the differential diagnoses for many of these individuals included VLCAD deficiency (MIM: 201475), acute recurrent myoglobinuria (MIM: 268200), carnitine palmitoyltransferase II deficiency (MIM: 600649), glutaric acidemia IIB (MIM: 231680), and mitochondrial disorders. Nevertheless, after WES, the sequence analyses of the associated genes, ACADVL (MIM: 609575), LPIN1 (MIM: 605518), CPT2 (MIM: 600650), and ETFB (MIM: 130410), did not identify any contributing variants. Although no deleterious mutations were found in the known nuclear-encoded mitochondrial genes or in other genes related to the described phenotype in these subjects, a recurrent apparently homozygous variant c.460G>A (p.Gly154Arg) in exon 7 of TANGO2 (GenBank: NM_152906.5 transcript) was identified in five out of nine unrelated families (Figures 1 and 2). Four of these subjects (1, 3, 4, and 5) were of Hispanic/Latino origin and subject 2 was of mixed Latino/European ancestry. Sanger sequencing confirmed the homozygous variant in probands 1, 3, 4, and 5 and validated the presence of heterozygous alleles in each parent for probands 3, 4, and 5. For subject 1, the mother was confirmed to be heterozygous and the father was unavailable for further studies. Coding single-nucleotide polymorphism (cSNP) data showed no evidence of deletion or uniparental isodisomy affecting the region, strongly suggesting that the proband carried two copies of the c.460G>A (p.Gly154Arg) allele, one inherited from each parent.
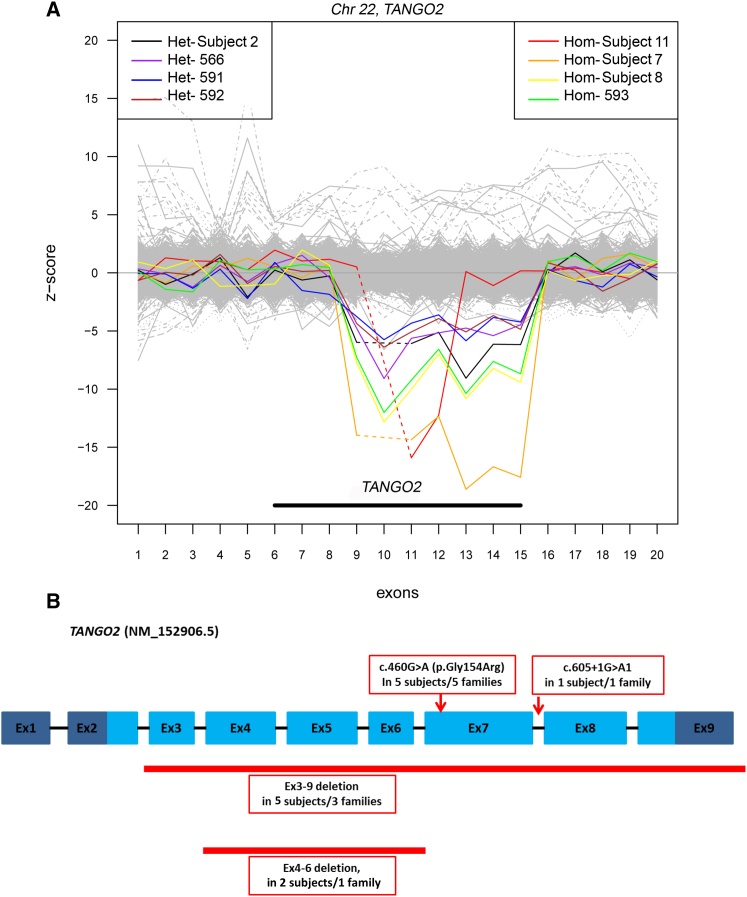
Figure 2.
TANGO2-Specific CNV Detection from the Clinical Exome Data and the Spectrum of Mutations in Nine Unrelated Families
(A) Target Z-score of PCA-normalized read depths for exon targets of TANGO2. Samples with heterozygous or homozygous deletions are shown in various colors on the left and right, respectively. All samples with deletions are depicted with read depths (RD) well below the other samples (gray) (n = 4,334 total samples). Samples with homozygous deletions have RD lower than heterozygous deletions and contain exons with reads per kilobase of transcript per million mapped reads (RPKM) values equal or near 0. Individuals with exons 3–9 homozygous deletions (∼34 kb) and heterozygous deletions are shown in this figure, as subjects 2, 7, and 8. Subject 11 with the smaller exons 4–6 homozygous deletion is represented in red.
(B) TANGO2 transcript (GenBank: NM_152906.5) with mutations and deletions found in our study cohort. Light blue regions represent the coding exons, dark blue regions represent 5′ and 3′ untranslated regions, and black bars represent introns (not to scale). The locations of c.460G>A (p.Gly154Arg) and c.605+1G>A variants are shown. The ∼34 kb deletion that encompasses exons 3 through 9 and the ∼9 kb deletion that includes exons 4 through 6 are shown.
For subject 2, only the maternal sample was noted to be heterozygous for the c.460G>A (p.Gly154Arg) variant the father of European ancestry was found to have normal sequencing results (Figure 1). Considering that a cryptic copy-number loss within TANGO2 could potentially elucidate the observed segregation results in this family, we compared the normalized depth of coverage from more than 4,000 clinical WES samples with that from individual subjects and identified an apparent intragenic deletion of exons 3–9 (∼34 kb) in subject 2 (Figure 2A). Long-range PCR validated the presence of the heterozygous deletion, inherited from her father, confirming that she was compound heterozygous for the TANGO2 mutation. We subsequently identified the same ∼34 kb deletion as homozygous alleles in two additional families of European descent (families 7 and 8) (Figures 1 and 2).
Of the two families with known consanguinity, proband 6 (family 6) was found to have a homozygous splice site variant in intron 7, c.605+1G>A, and subjects 11 and 12 (family 9) were shown to be homozygous for a smaller intragenic deletion CNV (∼9 kb), which encompassed exons 4–6 of TANGO2 (Figures 1 and 2). We then investigated whether the findings in subjects with the recurrent c.460G>A (p.Gly154Arg) homozygous mutation and exons 3–9 deletion were potentially due to undetermined consanguinity. The cSNP array data and exome data did not detect any absence of heterozygosity (AOH) regions greater than 5 Mb in subjects from families 1, 3, 4, 5, 7, or 8, all of whom were homozygous for either the recurrent c.460G>A (p.Gly154Arg) mutation or the ∼34 kb CNV deletion; conversely, the total AOH region in proband 6 from family 6 was about 46.3 Mb, including 9.7 Mb of AOH encompassing TANGO2, while the total AOH in subjects 11 and 12 from family 9 was ∼212.5 Mb, including 13.3 Mb of AOH encompassing TANGO2. Our genomic data support the reported consanguineous relationships in families 6 and 9.
The analysis of the ∼350 nt region surrounding the breakpoint junctions in families 2, 7, and 8 with the exons 3–9 deletion showed 20 variants differing from the reference genome (Figure S2), including 12 variants not observed in the public databases. These variants were shared by all heterozygous parents and subjects carrying the deletion, implicating this as a likely single ancestral variant in the European population. For individuals with the c.460G>A (p.Gly154Arg) mutation, a lack of common segregating variants on the cSNP array in the immediate 500 kb region across TANGO2 obscured the identification of a common ancestral haplotype; however, subjects 1, 3, and 4 shared the same homozygous haplotype over a longer 1.4 Mb region that traverses TANGO2 and includes 23 segregating variants (Figure S3). These data suggest a distant shared ancestry among these families. This extended haplotype was not observed in subject 5, however, suggesting either recurrent mutation at this CpG site or that there has still been sufficient time since the variant arose for the effect of recombination on the original ancestral haplotype to be observed. A higher density of segregating markers in the intervening region could potentially help to resolve this question.
The minor allele frequency (MAF) of the c.460G>A (p.Gly154Arg) allele in control subjects has been reported to be 0.26% (14/5,306 alleles) in the Hispanic/Latino population, 0.0026% (1/38,266 alleles) in Europeans, and 0% in African Americans, with an overall frequency of 0.02% (16/69,044 alleles) in all populations in the database from the Exome Aggregation Consortium (ExAC; n = 61,486 exomes; accessed February 2015). No homozygotes have been reported for this variant in this database or other databases including the 1000 Genomes (release 20110521), dbSNP134, and NHLBI GO Exome Sequencing Project (accessed February 2015). The rare c.605+1G>A splice site mutation has been reported in ExAC with a MAF of 0.25% (1/396 alleles) in the Latino population and an overall MAF of 0.03% (6/18,796 alleles, no homozygotes found) in all populations.
We then investigated the frequency of the exons 3–9 deletion of TANGO2 in large population-based studies and interrogated whether homozygotes were identified in these studies. Shaikh et al.18 reported heterozygous loss affecting the same region as the ∼34 kb deletion in 3/1,320 white Europeans, with an allele frequency of 0.11% (3/2,640 alleles). Similarly, for the same deletion, Coe et al.19 reported a population frequency of 17/13,347 samples (MAF 0.06%, 17/26,694 alleles), including 8,822 of presumed European ethnicity (ARIC and Wellcome Trust Case Control Consortium, WTCCC2 project samples from National Blood Donors). Ethnicity was available for only 11 of the 17 individuals with the heterozygous deletion, and all the 11 individuals were of European origin, corresponding to a European-specific MAF of at least 0.062% (11/17,644 alleles). No homozygous deletions were reported in these large control datasets. The ∼9 kb deletion identified from subjects of Arab origin in this study was not identified in the public control datasets.
The gross deletions and the c.605+1G>A mutation affecting the canonical splice donor site at the exon 7/intron 7 boundary of TANGO2 (GenBank: NM_152906.5 transcript, total nine exons) were interpreted as loss-of-function (LOF) pathogenic variants based on variant interpretation guidelines from the American College of Medical Genetics and Genomics (ACMG).20 The c.460G>A (p.Gly154Arg) substitution affects a glycine residue that is highly conserved in species from fish to primates (Figure S4). The substitution of glycine to arginine at this site is predicted to alter the tertiary structure and probably be damaging by in silico prediction programs (Figure S5).
The function of TANGO2 is unknown; however, in previous studies, depletion in Drosophila S2 tissue culture cells was observed to cause fusion of the Golgi with the ER.21 Because of this known association, we sought to evaluate the structure of the Golgi apparatus in the fibroblasts from subject 5 with homozygous c.460G>A (p.Gly154Arg) TANGO2 mutation. Evaluation of Golgi apparatus and TANGO2 were performed by fluorescence microscopy as previously described.22 Subject fibroblasts were allowed to adhere to poly-L-lysine-coated slides overnight at 37°C, 5% CO2, which were then washed in PBS and permeabilized with Cytofix/Cytoperm reagent (BD). Cells were then stained with 1 μg/ml polyclonal rabbit IgG αTANGO2 (Abcam) for 1 hr. Cells were washed and then stained with α-Rabbit Alexa Fluor-647 and WGA Alexa Fluor-488 for 30 min. Slides were mounted with prolong gold containing DAPI and allowed to cure overnight. Slides were then visualized with a Leica SP8 TCS STED microscope (Leica Microsystems).22 In brief, excitation was performed using a tunable white light laser and emission was detected by HyD detectors. Settings were adjusted based on single-color controls and independent channels were acquired sequentially to limit fluorescence contamination. Microscopy images were analyzed with Volocity software. Intensity of signal was thresholded based on 3 SDs above the mean intensity. Objects less than 0.1 μm3 were excluded. All values were exported to GraphPad Prism v.6 for graphing and statistical analysis. Statistical tests were performed with the Student’s t test. Differences were considered significant at a p < 0.05. ER was identified by staining with ER tracker red (Life Technologies) and Golgi was evaluated by intracellular staining with wheat germ agglutinin (WGA) conjugated with Alexa-488 (Molecular Probes). Images were then acquired on Amnis Image Stream Mark II (EMD Millipore) and analyzed with IDEAS software package. Both ER (14.4 ± 1.2 μm2 p.Gly154Arg, 65 ± 3.5 μm2 control, p < 0.0001) and Golgi (34.4 ± 7.5 μm3 p.Gly154Arg, 246 ± 60 μm3 control, p < 0.01) showed decreased size in the mutant fibroblasts, when compared to control (Figures 3A and 3C). The combined data shown are the mean ± SD.
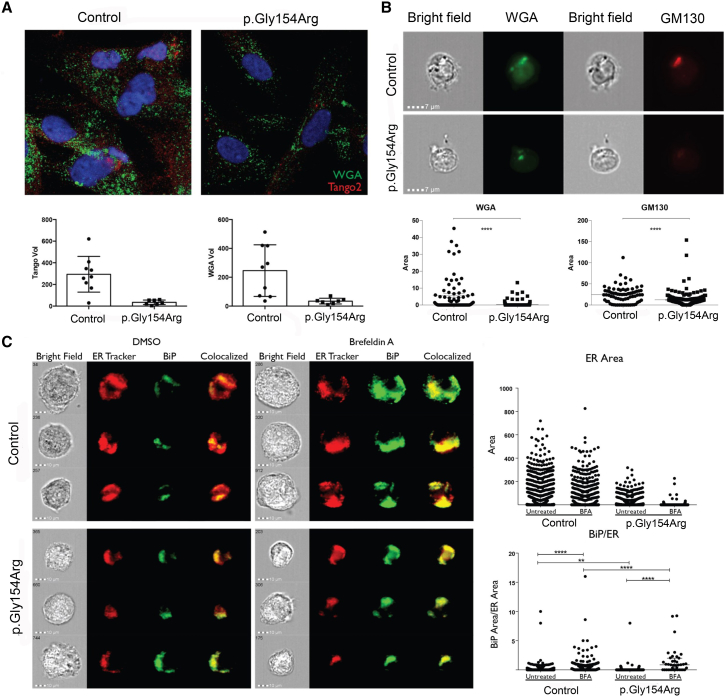
Figure 3.
c.460G>A (p.Gly154Arg) TANGO2 Mutant Cells Show Decreased ER and Golgi Area and Increased ER Stress
(A) Fibroblasts from healthy control and subject 5 were intracellularly stained with wheat germ agglutinin (WGA), a marker of trans Golgi and anti-TANGO2. The volumes of the resulting structures of both WGA (34.4 ± 7.5 μm3 p.Gly154Arg, 246 ± 60 μm3 control, p < 0.01) and TANGO2 (34.9 ± 7.8 μm3 p.Gly154Arg, 294 ± 55 μm3 control, p < 0.01) were found to be significantly lower in the subject fibroblasts by Student’s t test.
(B) BLCLs from healthy control and subject 4 were intracellularly stained with WGA and anti-GM130. The area of the resulting structures was found to be significantly smaller in affected BLCLs than from healthy controls (Student’s t test p < 0.0001) for both WGA and GM130.
(C) Fibroblasts from healthy control and subject 5 were intracellularly stained with ER Tracker red and anti-BiP and treated either with DMSO or Brefeldin A (BFA), an inhibitor of the ER Ca2+ ATPase. The volume of the resulting structure of the ER was found to be lower in the subject fibroblasts by Student’s t test (14.4 ± 1.2 μm2 p.Gly154Arg, 65 ± 3.5 μm2 control, p < 0.0001), whereas the level of BiP within the ER was significantly increased when normalized to ER after treatment with brefeldin A (1.5 ± 0.4 μm2 p.Gly154Arg, 0.4 ± 0.05 μm2 control, p < 0.0001).
We then used EBV-transformed B-lymphoblastoid cell lines (BLCLs) from another individual, subject 4 with homozygous c.460G>A (p.Gly154Arg) mutation, and evaluated the Golgi by intracellular staining with WGA, as well as cis Golgi marker, anti-GM130 antibody (Abcam cat# ab31561, RRID: AB_2115328). The area of the resulting structures was found to be significantly smaller in subject-derived BLCLs than from healthy control (WGA staining: control 5.76 ± 1.2 μm3 [n = 74], p.Gly154Arg 0.35 ± 0.1 μm3 [n = 190]; GM130 staining: control 24.4 ± 2.3 μm3 [n = 74], p.Gly154Arg 12.2 ± 1.2 μm3 [n = 190], Student’s t test p < 0.0001) (Figure 3B). Using anti-TANGO2 (anti-C22orf25) antibody (Abcam cat# ab87576, RRID: AB_2040650), the TANGO2 volume was found to be reduced in the subject fibroblasts compared to controls (34.9 ± 7.8 μm3 p.Gly154Arg, 294 ± 55 μm3 control; p < 0.01) (Figure 3A).
Given the role of the Golgi in vesicular trafficking and the delicate balance that must be maintained, we evaluated the level of ER stress in the affected fibroblast tissue in subject 5. To induce ER stress, the fibroblasts were treated with low levels of Brefeldin A (BFA), an inhibitor of the ER Ca2+ ATPase, and the expression of ER stress sensor, immunoglobulin heavy chain binding protein (BiP), was measured by staining with rabbit polyclonal anti BiP (Anti-GRP78 BiP antibody; Abcam cat# ab21685, RRID: AB_2119834) followed with anti-rabbit IgG Alexa 488 (Life Technologies). We found that whereas ER-associated BiP levels were similar in subject-derived fibroblasts and control (0.04 ± 0.02 μm2 p.Gly154Arg, 0.1 ± 0.02 μm2 control), when treated with brefeldin A, the BiP levels were increased in the affected cells (1.5 ± 0.4 μm2 p.Gly154Arg normalized to ER, 0.4 ± 0.05 μm2 control; p < 0.0001) (Figure 3C). Taken together, these data suggest that disruptions in TANGO2 result in an imbalance in the vesicular pathway.
We describe nine families with a Mendelian disorder characterized by recurrent rhabdomyolysis and susceptibility to cardiac arrhythmias. The various homozygous and compound heterozygous genetic alterations including both point mutations (SNVs) and copy-number variants (multi-exon deletion CNVs ranging from 9 to 34 kb in size) in TANGO2 in these unrelated families support a common etiology of a progressive neurologic disorder characterized by intellectual disability and intermittent rhabdomyolysis. The overlap in phenotype with disorders of fatty acid oxidation and mitochondrial energy metabolism with associated lactic acidosis and hypoglycemia led to extensive relevant biochemical and molecular studies in several of these children. Sudden tachyarrhythmias are known to occur with disturbance of fatty acid oxidation even in the absence of cardiomyopathy; this is thought to be secondary to the accumulation of arrhythmogenic intermediary metabolites of fatty acids, such as long-chain acylcarnitines.23, 24 The basis of arrhythmia in individuals with TANGO2 mutation is unknown at present. It is also unclear whether this phenotype is integral to the functions of TANGO2 in cardiac tissue or a consequence of acute myocyte breakdown with rhabdomyolysis. It is important to note that 2 of the 12 individuals (subjects 8 and 9) and the monozygotic twin of subject 11 (family 9) died during acute crises, underscoring the need for urgent molecular diagnosis of this life-threatening disorder.
Transport and Golgi organization (TANGO) proteins, first discovered in RNA-mediated interference screens in Drosophila, play an important role in cargo loading of newly synthesized secretory proteins in the ER. TANGO1, a transmembrane receptor ER protein, binds to collagen VII in the lumen of the ER, facilitating its loading into coat protein complex II (COPII) carriers on the cytoplasmic sites.25, 26 Much less is known about TANGO2, found to be localized to the Golgi and cytoplasm. The gene is within the critical region of the 22q11.2 locus, commonly deleted in the DiGeorge/Velocardiofacial syndrome (MIM: 188400). The encoded protein has a highly conserved DUF833 domain, of unknown function. Previous studies have shown that depletion of Tango2 causes fusion of the Golgi with the ER in Drosophila.21 We hypothesized that perturbed vesicular Golgi/ER transport from TANGO2 mutations could result in similar ER and Golgi disruption and increased ER stress contributing to disease mechanism. We found that the size of Golgi was decreased, both in the fibroblasts derived from subject 5 and lymphoblasts originating from subject 4. We also found that the BiP levels were enhanced in the mutant fibroblasts with higher expression when normalized to ER, indicating an increased level of ER stress. It remains to be seen whether the Golgi organization is affected in other individuals with the deletion genotype. The functional analysis in these subjects provide a framework for future experiments in individuals with multi-exonic TANGO2 deletions. Golgi fragmentation is known to be a common hallmark of neurodegenerative diseases including Parkinson, Alzheimer,27 and amyotrophic lateral sclerosis (ALS).28 Further studies would ultimately define whether ER-Golgi trafficking is inhibited with protein aggregation, as observed in these neurodegenerative diseases.
Our study shows that the c.460G>A (p.Gly154Arg) allele in TANGO2 is enriched in the Hispanic-Latino population and can cause significant morbidity in homozygotes in this population. This mutation is exclusively found in the heterozygous state in controls, with a minor allele frequency of 0.26% in the Hispanic/Latino population. Hardy-Weinberg expectations for homozygotes for the mutant allele in the Hispanic/Latino population would be approximately 1 in every 150,000 individuals. The allele frequency of the exons 3–9 deletion from previous studies, predominantly including Europeans,18, 29 is 0.06%–0.1%. On the basis of the estimated frequency of this deletion allele in heterozygous state, homozygosity would be expected to occur (at most) in 1 in every 1,000,000 individuals (given Hardy-Weinberg expectations). Our study shows that the exons 3–9 deletion of TANGO2 occurs on a common genetic background with shared haplotype in the European deletion carriers. The disease burden due to exon-specific deletion in non-Europeans remains to be ascertained.
Accumulating evidence has revealed disease susceptibility associated with low-frequency variants that are often specific to populations of distinct ancestries.30, 31 This was initially highlighted with the discovery of low-frequency missense variants in PCSK9 (MIM: 607786) associated with reduced low-density lipoprotein (LDL) cholesterol levels and reduced coronary heart disease risk in distinct populations.32 In our previous study, we reported a single-exon deletion copy-number variant within TM4SF20 (MIM: 615404) enriched in the Southeast Asian population (allele frequency of ∼1%) causing increased susceptibility to early language delay and cerebral white matter hyperintensities (MIM: 615432).33 The complex admixture of persons of Hispanic ancestry makes it difficult to invoke a specific population genetic mechanism for the commonality of the c.460G>A (p.Gly154Arg) allele from the available data, although a founding event seems more likely than other inferences. Advances in sequencing technology have led to a substantial improvement in the annotation of genomic variants among global population samples; thus, our understanding of the impact of low-frequency population-specific deleterious alleles of different variant types (SNV or CNV) is only beginning to take shape. This is particularly true for population-specific alleles deleterious only in a homozygous state, such as observed for the c.460G>A (p.Gly154Arg) mutation and exons 3–9 TANGO2 deletion in the Hispanic/Latino and European populations, respectively. Understanding the population genetic events driving such observations in their respective populations represents a fertile area for further study.
Acknowledgments
We thank the families for their participation and collaboration. The study was supported in part by the US National Human Genome Research Institute (NHGRI)/National Heart Lung and Blood Institute (NHLBI) Grant No. U54HG006542 to the Baylor-Hopkins Center for Mendelian Genomics. J.R.L. is a paid consultant for Regeneron Pharmaceuticals, holds stock ownership in 23andMe and Lasergen, Inc., and is a co-inventor on United States and European patents related to molecular diagnostics. The Department of Molecular and Human Genetics at Baylor College of Medicine derives revenue from molecular genetic testing offered at the Baylor Miraca Genetics Laboratories.
Published: January 21, 2016
Footnotes
Supplemental Data include clinical description of subjects 5–12, five figures, and one table and can be found with this article online at http://dx.doi.org/10.1016/j.ajhg.2015.12.008.
Contributor Information
Seema R. Lalani, Email: seemal@bcm.edu.
Yaping Yang, Email: yapingy@bcm.edu.
Accession Numbers
The ClinVar accession numbers for the DNA variant data reported in this paper are SCV000245441, SCV000245442, SCV000245443, and SCV000245444.
Web Resources
The URLs for data presented herein are as follows:
1000 Genomes, http://browser.1000genomes.org
Baylor Miraca Genetics Laboratories, Whole Genome Laboratory, https://www.bcm.edu/research/medical-genetics-labs/wholegenomelab
ExAC Browser, http://exac.broadinstitute.org/
NHLBI Exome Sequencing Project (ESP) Exome Variant Server, http://evs.gs.washington.edu/EVS/
OMIM, http://www.omim.org/
Supplemental Data
References
- 1.Scalco R.S., Gardiner A.R., Pitceathly R.D., Zanoteli E., Becker J., Holton J.L., Houlden H., Jungbluth H., Quinlivan R. Rhabdomyolysis: a genetic perspective. Orphanet J. Rare Dis. 2015;10:51. doi: 10.1186/s13023-015-0264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tonin P., Lewis P., Servidei S., DiMauro S. Metabolic causes of myoglobinuria. Ann. Neurol. 1990;27:181–185. doi: 10.1002/ana.410270214. [DOI] [PubMed] [Google Scholar]
- 3.Tyni T., Pihko H. Long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency. Acta Paediatr. 1999;88:237–245. doi: 10.1080/08035259950169954. [DOI] [PubMed] [Google Scholar]
- 4.Straussberg R., Harel L., Varsano I., Elpeleg O.N., Shamir R., Amir J. Recurrent myoglobinuria as a presenting manifestation of very long chain acyl coenzyme A dehydrogenase deficiency. Pediatrics. 1997;99:894–896. doi: 10.1542/peds.99.6.894. [DOI] [PubMed] [Google Scholar]
- 5.Dlamini N., Voermans N.C., Lillis S., Stewart K., Kamsteeg E.J., Drost G., Quinlivan R., Snoeck M., Norwood F., Radunovic A. Mutations in RYR1 are a common cause of exertional myalgia and rhabdomyolysis. Neuromuscul. Disord. 2013;23:540–548. doi: 10.1016/j.nmd.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Zeharia A., Shaag A., Houtkooper R.H., Hindi T., de Lonlay P., Erez G., Hubert L., Saada A., de Keyzer Y., Eshel G. Mutations in LPIN1 cause recurrent acute myoglobinuria in childhood. Am. J. Hum. Genet. 2008;83:489–494. doi: 10.1016/j.ajhg.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olpin S.E., Murphy E., Kirk R.J., Taylor R.W., Quinlivan R. The investigation and management of metabolic myopathies. J. Clin. Pathol. 2015;68:410–417. doi: 10.1136/jclinpath-2014-202808. [DOI] [PubMed] [Google Scholar]
- 8.Cervellin G., Comelli I., Lippi G. Rhabdomyolysis: historical background, clinical, diagnostic and therapeutic features. Clin. Chem. Lab. Med. 2010;48:749–756. doi: 10.1515/CCLM.2010.151. [DOI] [PubMed] [Google Scholar]
- 9.Bagley W.H., Yang H., Shah K.H. Rhabdomyolysis. Intern. Emerg. Med. 2007;2:210–218. doi: 10.1007/s11739-007-0060-8. [DOI] [PubMed] [Google Scholar]
- 10.de Meijer A.R., Fikkers B.G., de Keijzer M.H., van Engelen B.G., Drenth J.P. Serum creatine kinase as predictor of clinical course in rhabdomyolysis: a 5-year intensive care survey. Intensive Care Med. 2003;29:1121–1125. doi: 10.1007/s00134-003-1800-5. [DOI] [PubMed] [Google Scholar]
- 11.Slater M.S., Mullins R.J. Rhabdomyolysis and myoglobinuric renal failure in trauma and surgical patients: a review. J. Am. Coll. Surg. 1998;186:693–716. doi: 10.1016/s1072-7515(98)00089-1. [DOI] [PubMed] [Google Scholar]
- 12.Pichler K., Scholl-Buergi S., Birnbacher R., Freilinger M., Straub S., Brunner J., Zschocke J., Bittner R.E., Karall D. A novel therapeutic approach for LPIN1 mutation-associated rhabdomyolysis--the Austrian experience. Muscle Nerve. 2015;52:437–439. doi: 10.1002/mus.24749. [DOI] [PubMed] [Google Scholar]
- 13.Roe C.R., Yang B.Z., Brunengraber H., Roe D.S., Wallace M., Garritson B.K. Carnitine palmitoyltransferase II deficiency: successful anaplerotic diet therapy. Neurology. 2008;71:260–264. doi: 10.1212/01.wnl.0000318283.42961.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abreu A.P., Dauber A., Macedo D.B., Noel S.D., Brito V.N., Gill J.C., Cukier P., Thompson I.R., Navarro V.M., Gagliardi P.C. Central precocious puberty caused by mutations in the imprinted gene MKRN3. N. Engl. J. Med. 2013;368:2467–2475. doi: 10.1056/NEJMoa1302160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor R.W., Pyle A., Griffin H., Blakely E.L., Duff J., He L., Smertenko T., Alston C.L., Neeve V.C., Best A. Use of whole-exome sequencing to determine the genetic basis of multiple mitochondrial respiratory chain complex deficiencies. JAMA. 2014;312:68–77. doi: 10.1001/jama.2014.7184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Y., Muzny D.M., Reid J.G., Bainbridge M.N., Willis A., Ward P.A., Braxton A., Beuten J., Xia F., Niu Z. Clinical whole-exome sequencing for the diagnosis of mendelian disorders. N. Engl. J. Med. 2013;369:1502–1511. doi: 10.1056/NEJMoa1306555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fromer M., Moran J.L., Chambert K., Banks E., Bergen S.E., Ruderfer D.M., Handsaker R.E., McCarroll S.A., O’Donovan M.C., Owen M.J. Discovery and statistical genotyping of copy-number variation from whole-exome sequencing depth. Am. J. Hum. Genet. 2012;91:597–607. doi: 10.1016/j.ajhg.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaikh T.H., Gai X., Perin J.C., Glessner J.T., Xie H., Murphy K., O’Hara R., Casalunovo T., Conlin L.K., D’Arcy M. High-resolution mapping and analysis of copy number variations in the human genome: a data resource for clinical and research applications. Genome Res. 2009;19:1682–1690. doi: 10.1101/gr.083501.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coe B.P., Witherspoon K., Rosenfeld J.A., van Bon B.W., Vulto-van Silfhout A.T., Bosco P., Friend K.L., Baker C., Buono S., Vissers L.E. Refining analyses of copy number variation identifies specific genes associated with developmental delay. Nat. Genet. 2014;46:1063–1071. doi: 10.1038/ng.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richards S., Aziz N., Bale S., Bick D., Das S., Gastier-Foster J., Grody W.W., Hegde M., Lyon E., Spector E., ACMG Laboratory Quality Assurance Committee Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bard F., Casano L., Mallabiabarrena A., Wallace E., Saito K., Kitayama H., Guizzunti G., Hu Y., Wendler F., Dasgupta R. Functional genomics reveals genes involved in protein secretion and Golgi organization. Nature. 2006;439:604–607. doi: 10.1038/nature04377. [DOI] [PubMed] [Google Scholar]
- 22.Watkin L.B., Jessen B., Wiszniewski W., Vece T.J., Jan M., Sha Y., Thamsen M., Santos-Cortez R.L., Lee K., Gambin T., Baylor-Hopkins Center for Mendelian Genomics COPA mutations impair ER-Golgi transport and cause hereditary autoimmune-mediated lung disease and arthritis. Nat. Genet. 2015;47:654–660. doi: 10.1038/ng.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonnet D., Martin D., Villain E., Jouvet P., Rabier D., Brivet M., Saudubray J.M., Pascale De Lonlay Arrhythmias and conduction defects as presenting symptoms of fatty acid oxidation disorders in children. Circulation. 1999;100:2248–2253. doi: 10.1161/01.cir.100.22.2248. [DOI] [PubMed] [Google Scholar]
- 24.Choong K., Clarke J.T., Cutz E., Pollit R.J., Olpin S.E. Lethal cardiac tachyarrhythmia in a patient with neonatal carnitine-acylcarnitine translocase deficiency. Pediatr. Dev. Pathol. 2001;4:573–579. doi: 10.1007/s10024001-0101-7. [DOI] [PubMed] [Google Scholar]
- 25.Saito K., Chen M., Bard F., Chen S., Zhou H., Woodley D., Polischuk R., Schekman R., Malhotra V. TANGO1 facilitates cargo loading at endoplasmic reticulum exit sites. Cell. 2009;136:891–902. doi: 10.1016/j.cell.2008.12.025. [DOI] [PubMed] [Google Scholar]
- 26.D’Arcangelo J.G., Stahmer K.R., Miller E.A. Vesicle-mediated export from the ER: COPII coat function and regulation. Biochim. Biophys. Acta. 2013;1833:2464–2472. doi: 10.1016/j.bbamcr.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fan J., Hu Z., Zeng L., Lu W., Tang X., Zhang J., Li T. Golgi apparatus and neurodegenerative diseases. Int. J. Dev. Neurosci. 2008;26:523–534. doi: 10.1016/j.ijdevneu.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 28.Gonatas N.K., Stieber A., Mourelatos Z., Chen Y., Gonatas J.O., Appel S.H., Hays A.P., Hickey W.F., Hauw J.J. Fragmentation of the Golgi apparatus of motor neurons in amyotrophic lateral sclerosis. Am. J. Pathol. 1992;140:731–737. [PMC free article] [PubMed] [Google Scholar]
- 29.Cooper G.M., Coe B.P., Girirajan S., Rosenfeld J.A., Vu T.H., Baker C., Williams C., Stalker H., Hamid R., Hannig V. A copy number variation morbidity map of developmental delay. Nat. Genet. 2011;43:838–846. doi: 10.1038/ng.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kurki M.I., Gaál E.I., Kettunen J., Lappalainen T., Menelaou A., Anttila V., van ’t Hof F.N., von Und Zu Fraunberg M., Helisalmi S., Hiltunen M. High risk population isolate reveals low frequency variants predisposing to intracranial aneurysms. PLoS Genet. 2014;10:e1004134. doi: 10.1371/journal.pgen.1004134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lupski J.R., Belmont J.W., Boerwinkle E., Gibbs R.A. Clan genomics and the complex architecture of human disease. Cell. 2011;147:32–43. doi: 10.1016/j.cell.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen J.C., Boerwinkle E., Mosley T.H., Jr., Hobbs H.H. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N. Engl. J. Med. 2006;354:1264–1272. doi: 10.1056/NEJMoa054013. [DOI] [PubMed] [Google Scholar]
- 33.Wiszniewski W., Hunter J.V., Hanchard N.A., Willer J.R., Shaw C., Tian Q., Illner A., Wang X., Cheung S.W., Patel A. TM4SF20 ancestral deletion and susceptibility to a pediatric disorder of early language delay and cerebral white matter hyperintensities. Am. J. Hum. Genet. 2013;93:197–210. doi: 10.1016/j.ajhg.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.