Abstract
Microcystins are toxic molecules produced by cyanobacterial blooms due to water eutrophication. Exposure to microcystins is a global health problem because of its association with various other pathological effects and people all over the world are exposed to microcystins on a regular basis. Evidence shows that microcystin-LR (MC-LR) may adversely affect the immune system, but its specific effects on immune functions are lacking. In the present review, immunotoxicological effects associated with MC-LR in animals, humans, and in vitro models have been reported. Overall, the data shows that chronic exposure to MC-LR has the potential to impair vital immune responses which could lead to increased risk of various diseases including cancers. Studies in animal and in vitro models have provided some pivotal understanding into the potential mechanisms of MC-LR related immunotoxicity suggesting that further investigation, particularly in humans, is required to better understand the relationship between development of disease and the MC-LR exposure.
1. Introduction
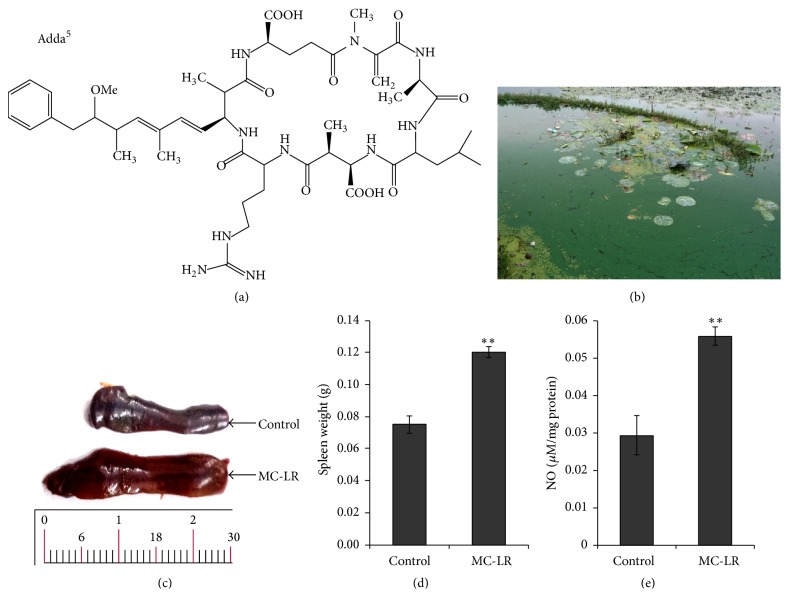
The frequent occurrence of cyanobacterial blooms with increasing water eutrophication has become a worldwide concern. Cyanobacterial blooms are often coupled with the production of different ranges of bioactive and toxic metabolites with microcystins (MCs) being the most widely studied [1]. More than 90 microcystin isoforms have been detected, among which microcystin-leucine arginine (MC-LR, Figure 1(a)) is the most abundant and the most toxic variant of microcystin [2]. MC-LR is a potential carcinogen for animal and humans, and the International Agency for Research on Cancer has classified MC-LR as a possible human carcinogen due to its potential carcinogenic activity via inhibition of protein phosphatases, which leads to the hyperphosphorylation of cellular proteins [3]. The provisional guideline set by the World Health Organization for MC-LR in drinking water is 1 μg/L, but the concentration of MCs in many water bodies is far beyond that guideline; for example, in Sagar lake water (India, Figure 1(b)) MC-LR was found to be 0.67 μg/mL [4]. MC-LR, which is a well-known hepatotoxin, also induces damage in other organs as was supported by evidence of kidney impairment, gastrointestinal disorder, reproductive toxicity, immune intruders, and embryo toxicity [5–7]. There are reports which suggest that microcystin can alter the immune system through several mechanisms like lymphocyte proliferation reduction, modulation of phagocytic activity, adaptation of natural killer cell activity, and disturbance of cytokine synthesis [8, 9]. This lethal effect of MCs on human being and livestock considerably depends on the stimulation of their immune system; thus MCs alter the immunomodulatory activities [10]. In this review, an attempt has been made to figure out the information regarding conditions and mechanisms of immunotoxic activity of MCs.
Figure 1.
Structure of microcystin-LR (a) and Microcystis aeruginosa bloom in Sagar lake water (b) and MC-LR treatment of mice for 14 days shows splenomegaly (c) and causes a significant increase in the weight of spleen (d) and NO level in spleen (e). Values represent mean ± SD, where n = 3. ∗∗ p < 0.01 (control versus MC-LR treated mice).
2. Role of Hematological Parameters in Microcystin Mediated Immunotoxicity
Cyanobacteria produce a range of bioactive and toxic metabolites, which have been reported to bioaccumulate in aquatic food chains and have been reported to impact human health indirectly due to the presence of toxins in edible fish [11]. Various documented toxic effects of microcystins include chronic hepatocarcinogenicity and oxidative stress as well as modulations of hepatological parameters and immunosuppression via inhibition of IFN production and synthesis of cytokines [11]. Thus the immune system is prone to exposure and is sensitive to toxic agents. Previous studies have observed thrombocytopenia (platelet deficiency) in animals treated with MCs or cyanobacteria bloom extracts entirely containing MCs [12, 13]. Early investigations of mice treated with MCs have found thrombocytopenia, pulmonary thrombi, and hepatic congestion and it has been reported that rats treated with an acute dose of MC-LR (125 μg/kg, i.p.) showed a significant decrease in WBC and mean corpuscular volume and a significant increase in platelets [12, 14]. Palikova et al. observed that mice fed with different concentration of cyanobacterial bloom extract for 28 days showed significant differences in RBC count, hematocrit value, MCH, MCV, and MCHC in comparison with the control group [11]. Previous results from the comet assay in mice leukocytes have shown that MC-LR (37.5 μg/kg bw/day, i.p.) induced a 2-fold transient increase in the level of DNA breaks after 30 min exposure [15]. In vivo studies by Kujbida et al. suggested that topical application of MC-LR (1000 nM) for 4 hours to male rats caused an improvement of the number of rolling and adhered leukocytes in the endothelium of postcapillary mesenteric venules [16].
Yuan et al. reported that, after the administration of MC-LR (50 μg/kg bw), when blood was collected at 0, 1, and 3 hours and with 12 μg/kg bw, when blood was collected at 0, 1, 3, 12, 24, 48, and 168 hours, respectively, significant increase in plasma white blood cells was observed [17]. Takahashi et al. reported that rats treated with MC-LR (100 and 200 μg/kg, i.p.) for one hour showed dose dependent reductions in leukocyte count, erythrocyte count, hemoglobin (Hb) concentration, coagulation parameters, and hematocrit (Ht) [13]. Recently we investigated the effect of MC-LR (15 μg/kg bw, i.p.) for 14 days on mice and observed that the treatment caused a significant elevation of hemoglobin and RBC, whereas it significantly declined WBC (Table 1, p < 0.005). Grabow et al. revealed that erythrocytes exposed to MC-LR had significant morphological changes [18]. Incubation of human erythrocytes with MC-LR concentrations of 1–1000 nM for 1, 6, 12, and 24 hours resulted in hemolysis and echinocytes and conversion of oxyhemoglobin to methemoglobin and a decrease in membrane fluidity [19]. Further in the treated erythrocytes activities of glutathione reductase and superoxide dismutase declined, while ROS and lipid peroxidation increased. Zhou et al. reported that when mice were exposed to MC-LR at the doses of 0.5, 2, and 8 μg/kg bw every 48 h for 30 days, prominent decrease in RBC, Hb, and Ht was observed as compared to control [20].
Table 1.
Hematological parameters in blood of control and MC-LR treated mice for 14 days.
| Hematological parameters | Control | Microcystin-LR |
|---|---|---|
| RBC (106/mm3) | 7.25 ± 0.72 | 3.12 ± 0.12∗∗ |
| WBC (103/mm3) | 4.21 ± 0.29 | 5.73 ± 0.26∗∗ |
| Hb (g/dL) | 9.21 ± 0.25 | 6.23 ± 0.48∗∗ |
Values are mean ± SD (n = 3), ∗∗ p < 0.01 (control versus MC-LR treated groups).
3. Microcystins Activate Neutrophils and Macrophages
Neutrophils play significant role in regulation of cancer development and spontaneous tumorigenesis [21]. Activated neutrophils release ROS and play an important role in host defense system and removal of debris but they can also cause damage and injury to tissues [22]. Neutrophils have been reported to be involved in the liver injury induced by MC-LR [23]. Kujbida et al. had shown that MC-LR and [Asp3]-MC-LR increase migration of human neutrophils and ROS formation and its killing capacity [24]. Treatment of both rat and human neutrophils with MC-LA and MC-LR (1 and 1000 nM) for 24 hours has been observed to cause loss of membrane integrity as well an increase in percentage of cells with fragmented DNA in rats whereas in humans an increase in neutrophil viability and decrease in percentage of cells with fragmented DNA was observed [25]. Previous reports have suggested that MCs have a chemotactic effect [16, 26] and can attract neutrophil as well as enhance their migration, as the cells are induced to produce additional amounts of chemokine [16]. In vitro studies have shown that all the three MCs cause neutrophil chemotaxis by increasing intracellular calcium levels [25].
Macrophages play an important role in immunity and foreign particles like microorganisms, macromolecules, and injured or apoptotic tissues [27] and other antigens are phagocytosed by macrophages [28]. Stimulated macrophages produce a number of enzymes, NO, and chemokines like IL-1β, TNFα, and GM-CSF for the primary protection of the host [29, 30]. In our studies, we have observed that concentration of NO in spleen increases significantly in mice treated with MC-LR (15 μg/kg bw, i.p.) for 14 days (Figure 1(e)). Alterations in the level of cytokines or chemokines are considered as a marker of immunomodulation. Shen et al. examined the function of MCs on the phagocytosis of peritoneal cells in mice exposed to sublethal doses (16, 32, 64 mg/kg bw, i.p.) for 14 days and observed that MCs reduced phagocytic index of peritoneal phagocyte [31]. Microcytins also produced the inhibition of lipopolysaccharide induced lymphocyte proliferation and the dose dependent decrease of the numbers of antibody forming cells in mice that were immunized by using T-dependent antigen sheep red blood cells. In vitro studies of mice macrophages incubated with MC-LR at dose of 1, 10, 100, and 1000 nmol/L for 24 hours were studied by Chen et al. during which he observed the downregulation of NO production and mRNA levels of iNOS, IL-1β, and TNFα in peritoneal macrophages [32].
4. Microcystins Alter and Activate Lymphocyte
Using human and chicken peripheral blood lymphocytes treated with 1, 10, and 25 μg/mL for 12, 24, 48, and 72 hours, Lankoff et al. showed that MC-LR influences the production of IL-2 and IL-6 and decreases the proliferation of T as well as B lymphocytes [33]. Human lymphocytes pretreated with MC-LR (1 μg/mL) have been reported to increase the level of DNA damage as a function of time which might be due to apoptosis [33]. When mouse splenocytes were exposed to 7.5 μg/mL of MC-LR for 4 and 24 hours, apoptosis was observed only in B cells whereas T cells were not affected [34]. We have also observed that treatment of mice with MC-LR (15 μg/kg bw) for 14 days results in splenomegaly and significant increase in weight of spleen (Figures 1(c) and 1(d)) and cotreatment with nitrate was observed to potentiate this MC-LR induced toxicity in mice [35]. Flow cytometric analysis of nonstimulated lymphocytes treated with MC-LR (7.5 μg/mL) for 4 and 24 hours has shown that MC-LR induces apoptosis in the B cell subpopulation via the B cell antigen receptor pathway, but not in T cells [34].
When mice were treated with three doses of MCs equivalent (4.97, 9.94, and 19.88 μg/kg bw/i.p.) for 14 days, it was found that B cell was more susceptible, maybe due to the depression of B cell surface markers or B cell growth cytokines or their receptors by MCs [31]. Mice exposed at four doses of 7, 12, 24, and 36 mg/kg body weight for 8 hours resulted in a significant decrease of mRNA levels of TNFα, IL-1β (proinflammatory cytokines), and IL-4, IL-2, and IL-10 (Th1/Th2 related cytokines), while IL-6 level was unaffected [36]. In mice, prolonged exposure of MC-LR has been reported to cause DNA damage and inhibition of proliferation of bone marrow cells and changes of hematopoietic factors, which is an indicator of severe damage in bone marrow cells [20].
5. Conclusions
MC-LR is one of the most toxic cyanotoxins that has been extensively studied. However there are only few studies elucidating the possible connection between observed immunomodulating activities of MCs or other cyanotoxins. In the present review, an attempt has been made to comprehensively address the impact of MC-LR toxicity on immune system. In this paper, we have mainly described the in vitro and in vivo effect of MC-LR on both acute and chronic immune system of mice and an attempt has been made to describe the possible pathways and molecules which might be implicated in MC-LR induced immunotoxicity in mice.
Acknowledgments
Yaqoob Lone thanks Dr. Harisingh Gour Central University, Sagar, for fellowship. This work was financially supported by a project from UGC Faculty Research Promotion Scheme (F.30-12/2014/BSR) and SERB (SERB/LS-816/2013), Govt. of India, sanctioned to Raj Kumar Koiri. The authors are grateful to Department of Zoology, Dr. Harisingh Gour Central University, Sagar, for providing infrastructural facilities and financial support.
Abbreviations
- GM-CSF:
Granulocyte macrophage colony-stimulating factor
- Hb:
Hemoglobin
- Ht:
Hematocrit
- IL:
Interleukin
- iNOS:
Inducible nitric oxide synthase
- MCH:
Mean cell hemoglobin
- MCHC:
Mean corpuscular hemoglobin concentration
- MC-LR:
Microcystin-LR
- MCs:
Microcystins
- MCV:
Mean corpuscular volume
- RBC:
Red blood cells
- ROS:
Reactive oxygen species
- TNF:
Tumor necrosis factor
- WBC:
White blood cells.
Conflict of Interests
The authors declare that there is no conflict of interests.
References
- 1.Welker M., von Döhren H. Cyanobacterial peptides—nature's own combinatorial biosynthesis. FEMS Microbiology Reviews. 2006;30(4):530–563. doi: 10.1111/j.1574-6976.2006.00022.x. [DOI] [PubMed] [Google Scholar]
- 2.Dietrich D., Hoeger S. Guidance values for microcystins in water and cyanobacterial supplement products (blue-green algal supplements): a reasonable or misguided approach? Toxicology and Applied Pharmacology. 2005;203(3):273–289. doi: 10.1016/j.taap.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Fan H., Cai Y., Xie P., et al. Microcystin-LR stabilizes c-myc protein by inhibiting protein phosphatase 2A in HEK293 cells. Toxicology. 2014;319(1):69–74. doi: 10.1016/j.tox.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 4.Lone Y., Koiri R. K., Bhide M. An overview of the toxic effect of potential human carcinogen Microcystin-LR on testis. Toxicology Reports. 2015;2:289–296. doi: 10.1016/j.toxrep.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beasley V. R., Cook W. O., Dahlem A. M., Hooser S. B., Lovell R. A., Valentine W. M. Algae intoxication in livestock and waterfowl. The Veterinary Clinics of North America: Food Animal Practice. 1989;5(2):345–361. doi: 10.1016/s0749-0720(15)30980-4. [DOI] [PubMed] [Google Scholar]
- 6.Zhang X., Xie P., Wang W., et al. Dose-dependent effects of extracted microcystins on embryonic development, larval growth and histopathological changes of southern catfish (Silurus meridionalis) Toxicon. 2008;51(3):449–456. doi: 10.1016/j.toxicon.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Zhou Y., Yuan J., Wu J., Han X. The toxic effects of microcystin-LR on rat spermatogonia in vitro. Toxicology Letters. 2012;212(1):48–56. doi: 10.1016/j.toxlet.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Cooper M. A., Fehniger T. A., Caligiuri M. A. The biology of human natural killer-cell subsets. Trends in Immunology. 2001;22(11):633–640. doi: 10.1016/S1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 9.Vitale M., Bassini A., Secchiero P., et al. NK-active cytokines IL-2, IL-12, and IL-15 selectively modulate specific protein kinase C (PKC) isoforms in primary human NK cells. The Anatomical Record. 2002;266(2):87–92. doi: 10.1002/ar.10039. [DOI] [PubMed] [Google Scholar]
- 10.Yea S. S., Kim H. M., Oh H.-M., Paik K.-H., Yang K.-H. Microcystin-induced down-regulation of lymphocyte functions through reduced IL-2 mRNA stability. Toxicology Letters. 2001;122(1):21–31. doi: 10.1016/s0378-4274(01)00339-3. [DOI] [PubMed] [Google Scholar]
- 11.Palikova M., Ondrackova P., Mares J., et al. In vivo effects of microcystins and complex cyanobacterial biomass on rats (Rattus norvegicus var. alba): changes in immunological and haematological parameters. Toxicon. 2013;73:1–8. doi: 10.1016/j.toxicon.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 12.Slatkin D. N., Stoner R. D., Adams W. H. Atypical pulmonary thrombosis caused by a toxic cyanobacterial peptide. Science. 1983;220(4604):1383–1385. doi: 10.1126/science.6407109. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi O., Oishi S., Watanabe M. F. Defective blood coagulation is not causative of hepatic haemorrhage induced by microcystin-LR. Pharmacology & Toxicology. 1995;76(4):250–254. doi: 10.1111/j.1600-0773.1995.tb00138.x. [DOI] [PubMed] [Google Scholar]
- 14.Ravindran J., Kumar D., Lakshmanarao P. V. Protective effect of rifampicin on microcystin-LR induced physiological and haematological changes in rats. Journal of Cell and Tissue Research. 2011;11(1):2451–2458. [Google Scholar]
- 15.Dias E., Louro H., Pinto M., et al. Genotoxicity of microcystin-LR in in vitro and in vivo experimental models. BioMed Research International. 2014;2014:9. doi: 10.1155/2014/949521.949521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kujbida P., Hatanaka E., Vinolo M. A. R., et al. Microcystins -LA, -YR, and -LR action on neutrophil migration. Biochemical and Biophysical Research Communications. 2009;382(1):9–14. doi: 10.1016/j.bbrc.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 17.Yuan G., Xie P., Zhang X., et al. In vivo studies on the immunotoxic effects of microcystins on rabbit. Environmental Toxicology. 2012;27(2):83–89. doi: 10.1002/tox.20615. [DOI] [PubMed] [Google Scholar]
- 18.Grabow W. O. K., Du Randt W. C., Prozesky O. W., Scott W. E. Microcystis aeruginosa toxin: cell culture toxicity, hemolysis and mutagenicity assays. Applied and Environmental Microbiology. 1982;43(6):1425–1433. doi: 10.1128/aem.43.6.1425-1433.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sicińska P., Bukowska B., Michałowicz J., Duda W. Damage of cell membrane and antioxidative system in human erythrocytes incubated with microcystin-LR in vitro. Toxicon. 2006;47(4):387–397. doi: 10.1016/j.toxicon.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 20.Zhou W., Zhang X., Xie P., Liang H., Zhang X. The suppression of hematopoiesis function in Balb/c mice induced by prolonged exposure of microcystin-LR. Toxicology Letters. 2013;219(2):194–201. doi: 10.1016/j.toxlet.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 21.De Visser K. E., Eichten A., Coussens L. M. Paradoxical roles of the immune system during cancer development. Nature Reviews Cancer. 2006;6(1):24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 22.Jaeschke H., Gores G. J., Cederbaum A. I., Hinson J. A., Pessayre D., Lemasters J. J. Mechanisms of hepatotoxicity. Toxicological Sciences. 2002;65(2):166–176. doi: 10.1093/toxsci/65.2.166. [DOI] [PubMed] [Google Scholar]
- 23.US EPA. DC. EPA/600/R-06/139. Washington, DC, USA: US Environmental Protection Agency; 2006. Toxicological reviews of cyanobacterial toxins: microcystins LR, RR, YR and LA (external review draft) [Google Scholar]
- 24.Kujbida P., Hatanaka E., Campa A., Colepicolo P., Pinto E. Effects of microcystins on human polymorphonuclear leukocytes. Biochemical and Biophysical Research Communications. 2006;341(1):273–277. doi: 10.1016/j.bbrc.2005.12.177. [DOI] [PubMed] [Google Scholar]
- 25.Kujbida P., Hatanaka E., Campa A., Curi R., Farsky S. H. P., Pinto E. Analysis of chemokines and reactive oxygen species formation by rat and human neutrophils induced by microcystin-LA, -YR and -LR. Toxicon. 2008;51(7):1274–1280. doi: 10.1016/j.toxicon.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 26.Hernández M., Macia M., Padilla C., Del Campo F. F. Modulation of human polymorphonuclear leukocyte adherence by cyanopeptide toxins. Environmental Research. 2000;84(1):64–68. doi: 10.1006/enrs.2000.4080. [DOI] [PubMed] [Google Scholar]
- 27.Takizawa F., Tsuji S., Nagasawa S. Enhancement of macrophage phagocytosis upon iC3b deposition on apoptotic cells. FEBS Letters. 1996;397(2-3):269–272. doi: 10.1016/s0014-5793(96)01197-0. [DOI] [PubMed] [Google Scholar]
- 28.Ichinose M., Asai M., Imai K., Sawada M. Enhancement of phagocytosis in mouse macrophages by Pituitary Adenylate Cyclase Activating Polypeptide (PACAP) and related peptides. Immunopharmacology. 1995;30(3):217–224. doi: 10.1016/0162-3109(95)00025-o. [DOI] [PubMed] [Google Scholar]
- 29.Kawagishi C., Kurosaka K., Watanabe N., Kobayashi Y. Cytokine production by macrophages in association with phagocytosis of etoposide-treated P388 cells in vitro and in vivo. Biochimica et Biophysica Acta (BBA)—Molecular Cell Research. 2001;1541(3):221–230. doi: 10.1016/s0167-4889(01)00158-6. [DOI] [PubMed] [Google Scholar]
- 30.Mongan L. C., Jones T., Patrick G. Cytokine and free radical responses of alveolar macrophages in vitro to asbestos fibres. Cytokine. 2000;12(8):1243–1247. doi: 10.1006/cyto.2000.0700. [DOI] [PubMed] [Google Scholar]
- 31.Shen P. P., Zhao S. W., Zheng W. J., Hua Z. C., Shi Q., Liu Z. T. Effects of cyanobacteria bloom extract on some parameters of immune function in mice. Toxicology Letters. 2003;143(1):27–36. doi: 10.1016/s0378-4274(03)00110-3. [DOI] [PubMed] [Google Scholar]
- 32.Chen T., Zhao X., Liu Y., Shi Q., Hua Z., Shen P. Analysis of immunomodulating nitric oxide, iNOS and cytokines mRNA in mouse macrophages induced by microcystin-LR. Toxicology. 2004;197(1):67–77. doi: 10.1016/j.tox.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 33.Lankoff A., Krzowski Ł., Glab J., et al. DNA damage and repair in human peripheral blood lymphocytes following treatment with microcystin-LR. Mutation Research. 2004;559(1-2):131–142. doi: 10.1016/j.mrgentox.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 34.Teneva I., Mladenov R., Popov N., Dzhambazov B. M. Cytotoxicity and apoptotic effects of microcystin-LR and anatoxin-a in mouse lymphocytes. Folia Biologica. 2005;51(3):62–67. [PubMed] [Google Scholar]
- 35.Lone Y., Koiri R. K., Bhide M. Nitrate enhances microcystin-LR induced toxicity in mice. Austin Journal of Molecular and Cellular Biology. 2015;2(1):p. 1006. [Google Scholar]
- 36.Shi Q., Cui J., Zhang J., Kong F. X., Hua Z. C., Shen P. P. Expression modulation of multiple cytokines in vivo by cyanobacteria blooms extract from Taihu Lake, China. Toxicon. 2004;44(8):871–879. doi: 10.1016/j.toxicon.2004.08.010. [DOI] [PubMed] [Google Scholar]