Abstract
Background
Obstructive sleep apnea (OSA) is a very common disorder (prevalence 2–7% in women, 7–14% in men). It impairs the quality of life and increases mortality. Conservative treatment with continuous positive airway pressure is highly effective, but patient compliance is variable. Surgical treatments are controversial, as only a few are supported by evidence from controlled clinical trials.
Method
Adult patients with OSA, CPAP intolerance, and oropharyngeal obstruction were included in the trial. All underwent polysomnography (PSG) and were randomly allotted to one of two groups. Patients in the treatment group underwent tonsillectomy with uvulopalatopharyngoplasty (TE-UPPP) within one month. All patients had a follow-up PSG at three months, and all PSGs were evaluated in blinded fashion. The primary outcome variable was the apnea-hypopnea index (AHI) as determined by PSG. Other outcome variables were subjective symptoms (daytime sleepiness, quality of life), complications, and patient satisfaction.
Results
42 patents were included in the trial (23 in the treatment group, 19 in the control group). The baseline AHI was 35.7 ± 19.4/hr in the control group and 33.7 ± 14.6/hr in the treatment group. The corresponding figures at 3 months were 28.6 ± 19.4/hr in the control group and 15.4 ± 14.1/hr in the treatment group (p = 0.036). The intervention also led to significant improvement in daytime sleepiness and in snoring, according to the patients’ and their bed partners’ assessment. 97% of the patients who underwent surgery were satisfied with the outcome. 65% of them needed no further treatment for OSA.
Conclusion
TE-UPPP significantly improved apnea/hypopnea, daytime sleepiness, and snoring compared to control (i.e., no) treatment. It is a safe and effective treatment for OSA.
Obstructive sleep apnea is a common disorder. Its reported prevalence is between 7 and 14% among men and 2 and 7% among women (1). It has also been established as an independent risk factor for a number of cardiovascular diseases, and it increases the risk of myocardial infarction and stroke (2); an increase in mortality of up to fourfold has also been described (3– 4). In addition, one cohort study determined the population-based mortality of 380 participants depending on the presence and severity of obstructive sleep apnea, based on outpatient four-channel examination (5). There were 22 deaths among the 285 participants without obstructive sleep apnea and six deaths among the 18 participants with severe obstructive sleep apnea; this corresponds to an adjusted risk of 6.24. In addition to models of fluid shift (6) and neuronal degeneration, various other functional factors are accepted as causes of sleep apnea (7– 9). However, anatomical factors are also particularly relevant to its pathophysiology (10– 12).
Airway collapse may occur at any level in the upper airways, but by far the most common location is the oropharynx (13).
Until continuous positive airway pressure (CPAP) treatment was introduced in the late 1980s, tonsillectomy with uvulopalatopharyngoplasty (TE-UPPP) as developed by Ikematsu and modified by Fujita, together with tracheotomy, was one of the few available treatments (14, 15).
In the 1980s TE-UPPP had quickly become widespread (16– 17). However, with the introduction of CPAP treatment, which controlled trials showed to be highly effective (18), surgery became less popular. Despite various technical improvements, 5 to 50% of patients either do not accept CPAP or cease using it during their first week of treatment (19). Information on longer-term compliance in the literature is highly heterogeneous, even concerning the definition of the word “compliance” itself. If compliance is defined as use for more than four hours per night, between 29 and 83% of patients, depending on the study, are classed as noncompliant (20). A recent review of the complex issues surrounding compliance is provided by Weaver (21). It is not uncommon for noncompliance to result in longer untreated periods, which on average reduce treatment efficacy (20, 22, 23). All this explains the need for other treatment strategies.
The S3 guideline of the German Sleep Society (DGSM, Deutsche Gesellschaft für Schlafforschung und Schlafmedizin) concludes that the majority of surgical treatments for obstructive sleep apnea cannot currently be recommended (24). Some of the reasons for this are that often only trials with a low level of evidence are available for surgical procedures, and that they rarely go beyond uncontrolled case series (25). Numerous trials highlight the efficacy and safety of TE-UPPP in both the short and the long term (26– 28), but despite their impressive conclusions the trials often fail to meet the requirements of evidence-based medicine.
The aim of this research was therefore to conduct a randomized controlled trial on the efficacy and safety of TE-UPPP as a treatment for obstructive sleep apnea.
Methods
The trial was conducted at the Department of Otorhinolaryngology, Head and Neck Surgery, Sleep Disorders Center, University Hospital Mannheim and the Department of Otorhinolaryngology, University Hospital Klinikum rechts der Isar, Technische Universität München, Munich.
Trial registration and ethical approval
The Medical Ethics Committee II, Faculty of Medicine, Ruprecht Karl University of Heidelberg, voted to approve trial conduct (ref. no. 2009–325N-MA). The Ethics Committee of the Faculty of Medicine of TUM School of Medicine, Munich, concurred with this decision. The trial was also registered in a WHO primary register under number DRKS00000549.
Inclusion and exclusion criteria
This prospective, randomized controlled trial involved patients of both sexes aged between 18 and 65 years, who were enrolled between 2010 and 2014. Inclusion criteria were obstructive sleep apnea confirmed by polysomnography (PSG) with an Apnea–Hypopnea Index (AHI) above 15, according to the second edition of the International Classification of Sleep Disorders valid at that time (8), and tonsillar hypertrophy with velopharyngeal obstruction confirmed by clinical examination. A further very important inclusion criterion was rejection of or poor compliance with ventilation therapy and an explicit wish on the part of the patient for a different approach (second-line therapy). All enrolled patients had tried CPAP treatment without success for at least one night. Because of the large number of patients who coped well with CPAP treatment, it is not possible to draw a CONSORT diagram for this trial.
The most important exclusion criteria were body mass index above 34 kg/m2, increased anesthetic risk according to the criteria of the American Society of Anesthesiologists (ASA), specifically ASA class above III (29), and other relevant types of obstruction or significant malformations of the facial skeleton confirmed by clinical examination.
Trial conduct
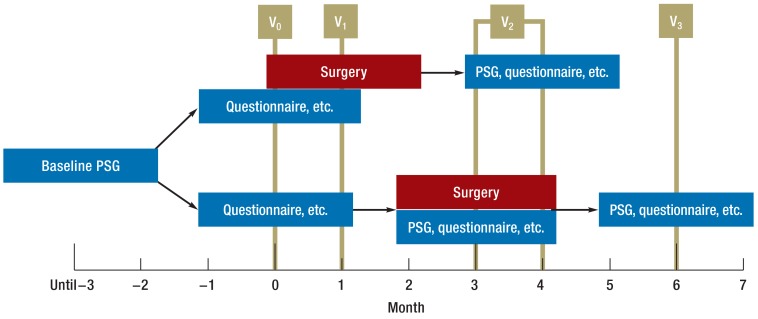
Patients were randomized to the treatment arm or the control arm. Patients in the treatment arm underwent TE-UPPP within one month after inclusion. Patients in the control arm initially received no treatment and underwent repeat polysomnography again after three months, then underwent TE-UPPP. In the control group, target parameters were measured again three months later, following surgery. Visit 2 for the treatment group could therefore be up to one month later than for the control group.
The trial’s primary and secondary endpoints were measured at three points in time (visits 0 to 2). AHI (total number of complete cessations of breathing [apneas] and reduced respiratory airflows [hypopneas] per hour of sleep) was selected as the primary target parameter, as it is one of the best researched parameters for defining the severity of obstructive sleep apnea (8). In addition, it is used in the majority of studies on the increase in mortality and morbidity (2).
Secondary parameters are listed in Table 1. Successful surgery was defined, according to the criteria of Sher et al., as a more than 50% reduction in AHI to a final value under 20 per hour (30). Figure 1 illustrates the study procedure.
Table 1. Primary and secondary endpoints of the trial*.
| Parameter | Group | n | Visit 0 | Visit 2 | Ø difference | 95% CI | p-value | ||
|---|---|---|---|---|---|---|---|---|---|
| AHI | Control | 16 | 35.7 ± 19.4 | 28.6 ± 19.3 | −7.2 | −25.7 | −11.0 | } | 0.036 |
| Treatment | 18 | 33.7 ± 14.5 | 15.4 ± 14.1 | −18.4 | −15.2 | 0.9 | |||
| Total | 34 | 34.7 ± 16.8 | 21.6 ± 17.8 | −12.8 | −18.0 | −7.5 | |||
| ESS | Control | 15 | 10.2 ± 4.9 | 9.6 ± 5.2 | −0.6 | −2.5 | 1.3 | } | 0.010 |
| Treatment | 20 | 10.6 ± 4.4 | 6.2 ± 2.9 | −4.4 | −6.4 | −2.3 | |||
| Total | 35 | 10.4 ± 4.5 | 7.7 ± 4.3 | −2.5 | −3.9 | −1.1 | |||
| Snoring score: patient’s assessment | Control | 14 | 5.4 ± 2.7 | 5.2 ± 2.5 | −0.2 | −2.0 | 1.6 | } | 0.001 |
| Treatment | 15 | 6.1 ± 2.3 | 1.5 ± 1.8 | −4.5 | −6.1 | −3 | |||
| Total | 29 | 5.8 ± 2.5 | 3.3 ± 2.8 | −2.4 | −3.5 | −1.2 | |||
| Snoring score: bed partner’s assessment | Control | 9 | 7.4 ± 1.1 | 6.7 ± 2.4 | −0.8 | −2.9 | 1.3 | } | 0.001 |
| Treatment | 16 | 8.3 ± 1.2 | 3.0 ± 2.7 | −5.4 | −7.0 | −3.7 | |||
| Total | 25 | 8.0 ± 1.2 | 4.3 ± 3.1 | −3.1 | −4.4 | −1.8 | |||
| AI | Control | 15 | 27.9 ± 22.7 | 23.0 ± 19.8 | −5.0 | −13.5 | 3.5 | } | 0.10 |
| Treatment | 16 | 22.3 ± 16.0 | 13.6 ± 22.6 | −8.7 | −23.0 | 5.6 | |||
| Total | 31 | 25.0 ± 19.4 | 18.1 ± 21.5 | −6.9 | −15.0 | 1.2 | |||
| HI | Control | 15 | 7.0 ± 7.3 | 5.2 ± 8.6 | −1.8 | −5.7 | 2.1 | } | 0.79 |
| Treatment | 16 | 11.0 ± 9.5 | 6.7 ± 8.1 | −4.3 | −10.7 | 2.1 | |||
| Total | 31 | 9.0 ± 8.6 | 6.0 ± 8.2 | −3.1 | −6.7 | 0.6 | |||
| RDI | Control | 16 | 36.7 ± 19.0 | 28.7 ± 19.4 | −8.1 | −16.5 | 0.3 | } | 0.27 |
| Treatment | 17 | 34.3 ± 14.8 | 21.8 ± 21.8 | −12.5 | −25.6 | 0.5 | |||
| Total | 33 | 35.5 ± 16.7 | 25.1 ± 20.7 | −10.3 | −17.9 | –2.8 | |||
| RERA | Control | 16 | 24.5 ± 23.5 | 24.5 ± 22.5 | 0.0 | −8.7 | 8.7 | } | 0.117 |
| Treatment | 16 | 22.3 ± 25.2 | 14.3 ± 20.2 | −8.0 | −13.9 | –2.1 | |||
| Total | 32 | 23.4 ± 24.0 | 19.4 ± 21.6 | −4.0 | −9.1 | 1.0 | |||
| Ø SpO2 | Control | 16 | 90.8 ± 5.1 | 91.7 ± 4.6 | 0.9 | −0.6 | 2.5 | } | 0.136 |
| Treatment | 17 | 89.1 ± 7.1 | 92.9 ± 5.5 | 3.8 | 0.2 | 7.4 | |||
| Total | 33 | 89.9 ± 6.2 | 92.3 ± 5.0 | 2.4 | 0.5 | 4.3 | |||
| min. SpO2 | Control | 16 | 79.3 ± 9.4 | 83.4 ± 8.7 | 4.0 | −0.3 | 8.3 | } | 0.946 |
| Treatment | 17 | 79.4 ± 9.8 | 83.7 ± 7.1 | 4.2 | −1.5 | 10 | |||
| Total | 33 | 79.4 ± 9.4 | 83.5 ± 7.8 | 4.1 | 0.6 | 7.6 | |||
| SpO2T90 | Control | 16 | 11.6 ± 19.3 | 10.2 ± 18.2 | −0.8 | −2.9 | 1.3 | } | 0.079 |
| Treatment | 17 | 7.8 ± 9.2 | 1.6 ± 2.6 | −5.4 | −7.0 | –3.7 | |||
| Total | 33 | 9.7 ± 14.9 | 5.8 ± 13.3 | −3.1 | −4.4 | –1.8 | |||
*Case number, mean, standard deviation, and mean difference are stated with 95% confidence intervals. The mean difference is based on estimated marginal means. The “p-value” column gives the significance of the variance analysis of the interaction “time × group.” Calculation of p-values for AI, HR, RDI, and SPO2T90 is based on log-transformed variables.95% CI: 95% confidence interval; AHI: Apnea–Hypopnea Index; AI: Apnea Index; ESS: Epworth Sleepiness Scale; HI: Hypopnea Index; Surgery; RDI: Respiratory Disturbance Index; RERA: Respiratory effort-related arousal; SPO 2 #: Oxygen saturation; SPO 2 T 90 : Percentage of total sleep time with oxygen saturation below 90%
Figure 1.
Diagram of trial procedure. Bottom: control group; top: treatment group. PSG: Polysomnogram
Polysomnography
EEGs were recorded and evaluated according to the definitions established by Rechtschaffen and Kales, which were valid when the trial began (31). Respiratory events were scored according to the 2007 procedures and definitions of the American Academy of Sleep Medicine (AASM) (32). Hypopnea was defined as a 30% decrease in airflow with 4% desaturation; PSGs were evaluated by an investigator blinded to the trial procedures.
Daytime sleepiness was documented using the Epworth Sleepiness Scale (ESS) (33), a self-assessment daytime sleepiness questionnaire. Quality of life and functional impact of sleep were documented using questionnaires (RAND-SF36, FOSQ) (34, 35) and a visual analog scale on snoring (assessed by the patient and the patient’s bed partner). At the end of the follow-up period all patients were asked about their satisfaction with the surgery they had undergone.
Surgical procedure
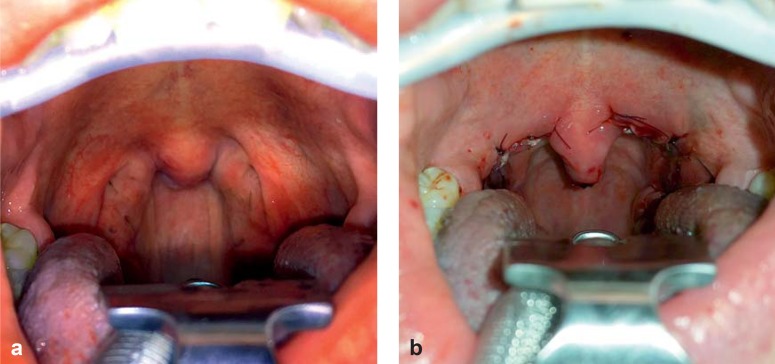
After cold steel tonsillectomy using general anesthesia, uvulopalatopharyngoplasty according to the modifications by Pirsig was performed Figure 2 shows a typical preoperative and postoperative surgical site. Tonsil size was determined immediately following surgery using volume displacement. Complications occurring during inpatient stay, particularly hemorrhages, were recorded by type and severity. TE-UPPP was performed by three different surgeons at the Mannheim trial site and by one in Munich.
Figure 2.
Typical preoperative and postoperative site in tonsillar hypertrophy:
a) Preoperative
b) Postoperative. Resorbable sutures used to reconfigure the oropharynx are clearly visible.
Statistics
Before the beginning of the trial, a sample size estimation was performed on the basis of previous studies and the expected surgical improvement in sleep medicine parameters. Differences between groups were analyzed using variance analysis with repeat measurement (visit 0 versus visit 2). Parameters with nonnormal distribution (AI, HI, RDI, and SPO2T90) were approximated using logarithmic transformation. Rank correlation coefficients (Kendall’s tau-b) were used to examine the correlation between tonsil size and BMI on the one hand and changes in target parameters on the other. p-values ≤ 0.05 were considered significant. Standard deviation was indicated by the “±” symbol. All analyses (intention-to-treat analyses) were performed using SPSS 22 (IBM Corporation, Armonk, USA) and R, an open-source framework for statistical calculation.
Results
Data on the included patients is shown in Table 2. In the control group, 16 patients underwent TE-UPPP following visit 2 and also underwent all postoperative examinations three months after surgery. One patient in the control group left the trial after visit 0 as he wanted to be treated closer to his home.
Table 2. Clinical data of all enrolled patients.
| Parameter | Control group | Treatment group | Total |
|---|---|---|---|
| n | 19 | 23 | 42 |
| Age (years) | 38.4 ± 8.5 | 36.6 ± 12.5 | 37.4 ± 10.7 |
| BMI (kg/m2) | 29.2 ± 3.1 | 28.5 ± 3.4 | 28.8 ± 3.2 |
| Women (%) | 11 | 0 | 5 |
| Tonsil size (mL) | 9.5 ± 1.7 | 12.7 ± 4.5 | 11.7 ± 4.1 |
BMI: Body mass index
There was no statistically significant difference between the groups in terms of the mean time between visit 0 and visit 2: this was 4.4 ± 1.0 months in the treatment group and 3.6 ± 1.4 months in the control group.
Primary endpoints
At visit 0 there was no statistically significant difference between the groups in terms of the Apnea–Hypopnea Index (AHI), the most important respiratory parameter in polysomnography (p >0.05) (Table 1).
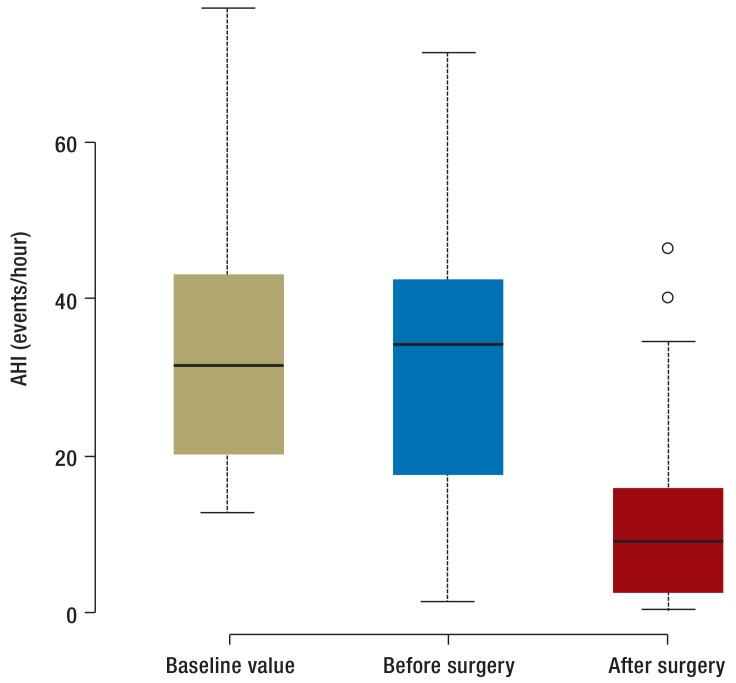
At visit 2, AHI in the control group was largely unchanged, while in the treatment group it had fallen significantly (Figure 3). AHI was reduced in more than 90% of the 31 patients who had undergone surgery, and surgery was defined as successful in 20 of these cases. Of the 31 patients whose obstructive sleep apnea status could be evaluated following surgery, 11 still had an AHI above 15 per hour or an AHI above 5 per hour with ESS score above 10. These 11 patients therefore still had obstructive sleep apnea.
Figure 3.
Box-and-whisker plot showing change in Apnea–Hypopnea Index (AHI) at V0, V2, and V3. Whisker length indicates interquartile range; box length indicates doubled interquartile range. Outliers are marked. “Baseline value” indicates V0 for the control group. “Before surgery” denotes V2 for the control group and V0 for the treatment group. “After surgery” denotes V3 for the control group and V2 for the treatment group. Intra-individual changes in this parameter are shown in eFigure 1.
Secondary endpoints
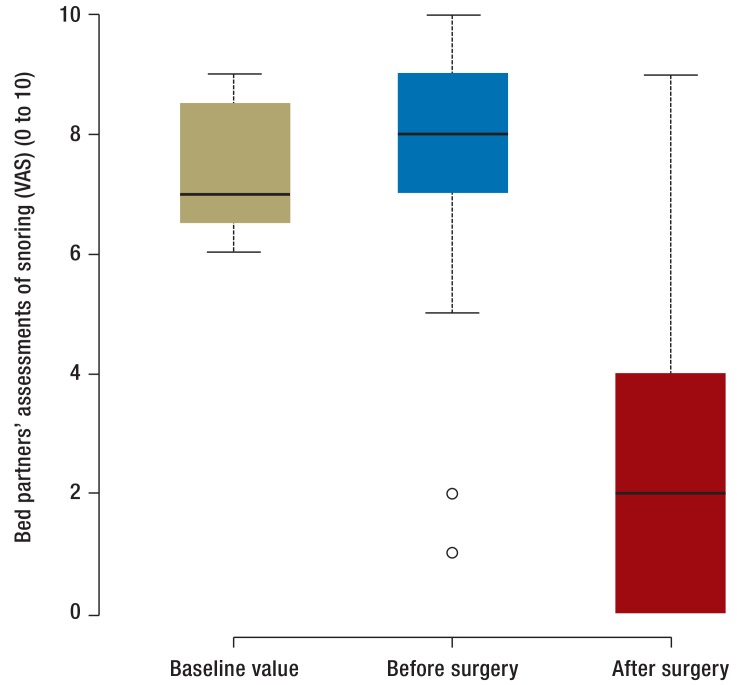
There were improvements in daytime sleepiness and in snoring following surgery, with a statistically significant difference between the groups for these parameters. Detailed values for the secondary endpoints are shown in Table 1. Figure 4 shows a graphical representation of bed partners’ assessment of patients’ snoring. In terms of this parameter, there was no statistically significant difference between the patients in the control group who underwent surgery and those in the treatment group who underwent surgery.
Figure 4.
Box-and-whisker plot showing change in snoring score as assessed by bed partner at V0, V2, and V3. Whisker length indicates interquartile range; box length indicates doubled interquartile range. Outliers are marked. “Baseline value” indicates V0 for the control group. “Before surgery” denotes V2 for the control group and V0 for the treatment group. “After surgery” denotes V3 for the control group and V2 for the treatment group. Intra-individual changes in this parameter are shown in eFigure 2.
Correlation analysis
With the exception of one statistically significant correlation between reduction in snoring sounds according to bed partners’ assessments and tonsil size (τb = –0.49, ρ = 0.50, p = 0.05), there were no significant correlations.
Complications
Two of the 39 patients who underwent surgery suffered postoperative hemorrhages on days 4 and 11 following surgery, respectively. Repeat surgery was required in one case. Of the 39 patients who underwent surgery, 38 were satisfied with the outcome after the end of their treatment.
Discussion
This trial aimed to examine the efficacy of TE-UPPP in a randomized controlled trial in patients with obstructive sleep apnea who had not tolerated CPAP. The results showed TE-UPPP to be superior in terms of reduction in respiratory events, daytime sleepiness, severity of snoring, and individual aspects of sleep-related quality of life. Postoperative complications were rare and were manageable in all cases. Almost all patients were satisfied at the end of their treatment.
Primary endpoints
One of the most important findings of this trial is the statistically significant, clinically relevant reduction in the Apnea–Hypopnea Index (AHI) in the group who had undergone TE-UPPP when compared to the control group. The 54% reduction in AHI in the treatment group after three months, versus 12% in the control group, is broadly in line with data published earlier from another trial site (37). However, data from a meta-analysis comparing 15 cohort studies in which UPPP was performed showed only a 33% reduction in AHI (38). Successful surgery as defined by Sher’s 1996 criteria was achieved in more than 70% of the patients enrolled in the trial. This success rate is towards the upper middle range of the rates found in trials published to date (30).
The 12% reduction in AHI in the control group is probably due to regression to the mean. This is the phenomenon by which, after an extreme measurement which may have led to a patient’s enrolment in the trial, the next measurement lies closer to the mean.
Another parameter that differed significantly between the treatment group and the untreated control group was self-assessment and bed partner’s assessment of snoring on the visual analog scale. While bed partners of patients in the control group gave a score of 8 out of a possible 10, for example, they gave only 3.1 out of 10 following TE-UPPP. This reduction in snoring sounds, which was found in all patients but one, is greater than previously published findings on TE-UPPP (39).
Correlation analysis
This trial did not confirm the correlation between tonsil size and clinical outcome, as captured by AHI, postulated in some of the literature (40). The findings presented in this article and those published for other trials contradict the common view that patients with smaller tonsils would not benefit from TE-UPPP (27, 28). However, the results of this correlation analysis must be interpreted critically, as only patients with significant oropharyngeal obstruction were enrolled in the trial. In particular, extrapolation of these results to very small tonsil sizes does not seem possible. Large tonsils, however, do seem to be a reliable predictor of a reduction in snoring, even though in this trial correlation analysis was performed for the treatment group only and therefore corresponds only to the level of a cohort study.
Comparing TE-UPPP and CPAP
Many papers indicate that CPAP treatment can generally reduce the Apnea–Hypopnea Index to below 5 per hour. However, these trials examine only the effects of CPAP as shown by polysomnography, often under laboratory conditions. In everyday circumstances, limited compliance means there are often longer periods without treatment; these should be taken into account when considering treatment efficacy. Numerous papers published more recently have therefore addressed the correlation between reduction in Apnea–Hypopnea Index and individual CPAP device usage habits (22). In a 2011 study, a baseline Apnea–Hypopnea Index of 35.6 ± 22.1 per hour, almost equal to the baseline Apnea–Hypopnea Index in this trial, was reduced only to 11.9 per hour in a population of regular CPAP users when compliance was taken into account (23). It seems necessary to take available compliance data into account when comparing the efficacy of different treatments. A direct randomized comparison between TE-UPPP and CPAP treatment, as has been published for maxillary surgery (maxillomandibular advancement), for example, is not yet available but would be desirable both scientifically and for the purposes of clinical practice (e1).
Trial strengths and weaknesses
The main strength of the trial described here is its two-center randomized design, which substantially reduces the risk of unwanted influences and selection biases. All polysomnograms were evaluated by hand by investigators blinded to the trial procedures, according to international standards.
The main limitation of the trial is its three-month follow-up period, which is not long enough to evaluate long-term efficacy. However, for ethical reasons longer follow-up for the untreated control group did not seem justifiable. Nevertheless, all patients should be recruited into a follow-up study in order to uncover potential adverse long-term effects.
In summary, TE-UPPP has been shown to be safe and effective in patients who are of normal weight or only slightly overweight and who have obstructive sleep apnea and oropharyngeal obstruction (64.5% of patients required no further treatment following surgery). It should therefore be more strongly emphasized in future guidelines.
Key Messages.
TE-UPPP reduced the Apnea–Hypopnea Index in 90% of patients.
TE-UPPP was shown to be superior to an untreated control group in terms of respiratory events as described by the Apnea–Hypopnea Index, daytime sleepiness, and snoring.
The complication rate of surgery was low: 2 of the 39 patients who underwent surgery suffered postoperative hemorrhages.
Obstructive sleep apnea requiring treatment remained after surgery in 35.5% of patients.
TE-UPPP was shown to be safe and effective in this population.
Acknowledgments
Translated from the original German by Caroline Shimakawa-Devitt, M.A.
Footnotes
Conflict of interest statement
PD Dr. Sommer has received consultancy fees, reimbursement of conference fees and travel and accommodation expenses, fees for preparing scientific continuing professional development or other events, funding for research he himself initiated, and fees for conducting clinical studies on related subjects from Neuwirth Medical, ImThera Medical, Thorax Medical, Fisher & Paykel Healthcare, Heinen & Löwenstein, Medtronic, Revent Medical, MedEl, Philips, Meda Pharma, Inspire Medical, and Nyxoah.
Prof. Stuck has received financial support for research and consultancy and speaker’s fees from Aspire Medical, Fisher & Paykel, Healthcare, Celon AG Medical Instruments, Olympus, Sutter Medizintechnik, Fachlabor Dr. W. Klee, Neuwirth Medical Products, Philips Healthcare, Heinen & Löwenstein, Alaxo GmbH, Tomed Dr. Toussaint GmbH, MEDA Pharma Gmbh, Inspire Medical, 3NT Medical, and PCI Biotech. He is a speaker for the Surgical Methods of Therapy Task Group of the German Sleep Society (DGSM, Deutsche Gesellschaft für Schlafforschung und Schlafmedizin).
Dr. Heiser receives fees for training events, research fees, and expenses from Impire Medical Systems, Heinen & Löwenstein, Sutter Medizintechnik, and Neuwirth Medical Products.
Dr. Maurer has received lecture fees from GlaxoSmithKline, Heinen & Löwenstein, Inspire Medical, Medtronic, Novartis, Olympus, Revent Medical, Resmed, Sissel Novacare, and Weinmann Medizintechnik. He has received reimbursement of travel expenses from Heinen & Löwenstein, Inspire Medical, Medel, Philipps, and Revent Medical. He has received funding for clinical studies from ImThera, Inspire Medical, Neuwirth Medical, Nyxoah, Revent Medical, and Sissel Novacare. He has received financial support paid into third-party accounts from ImThera, Inspire Medical, Medel, Neuwirth Medical, Nyxoah, Philipps, Revent Medical, and Sissel Novacare. He has received research funding from Medel. He has received funding for writing papers on related subjects by Inspire Medical and ImThera Medical. He has received reimbursement of conference fees from Olympus.
Prof. Hörmann, Dr. Gahleitner and Dipl. rer. soc. Herr declare that no conflict of interest exists.
References
- 1.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 2.Wang X, Ouyang Y, Wang Z, Zhao G, Liu L, Bi Y. Obstructive sleep apnea and risk of cardiovascular disease and all-cause mortality: a meta-analysis of prospective cohort studies. International Journal of Cardiology. 2013;169:207–214. doi: 10.1016/j.ijcard.2013.08.088. [DOI] [PubMed] [Google Scholar]
- 3.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 4.Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071–1078. [PMC free article] [PubMed] [Google Scholar]
- 5.Marshall NS, Wong KK, Liu PY, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep. 2008;31:1079–1085. [PMC free article] [PubMed] [Google Scholar]
- 6.White LH, Motwani S, Kasai T, Yumino D, Amirthalingam V, Bradley TD. Effect of rostral fluid shift on pharyngeal resistance in men with and without obstructive sleep apnea. Respir Physiol Neurobiol. 2014;192:17–22. doi: 10.1016/j.resp.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Heiser C, Zimmermann I, Sommer JU, Hormann K, Herr RM, Stuck BA. Pharyngeal chemosensitivity in patients with obstructive sleep apnea and healthy subjects. Chemical Senses. 2013;38:595–603. doi: 10.1093/chemse/bjt031. [DOI] [PubMed] [Google Scholar]
- 8.American Academy of Sleep Medicine. 2nd edition. Westchester, Ill.: American Academy of Sleep Medicine; 2005. The international classification of sleep disorders : diagnostic and coding manual. [Google Scholar]
- 9.Friberg D, Ansved T, Borg K, Carlsson-Nordlander B, Larsson H, Svanborg E. Histological indications of a progressive snorers disease in an upper airway muscle. Am J Respir Crit Care Med. 1998;157:586–593. doi: 10.1164/ajrccm.157.2.96-06049. [DOI] [PubMed] [Google Scholar]
- 10.Owens RL. An integrative model of physiological traits can be used to predict obstructive sleep apnea and response to non positive airway pressure therapy. Sleep. 2015;38:961–970. doi: 10.5665/sleep.4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hudgel DW, Mulholland M, Hendricks C. Neuromuscular and mechanical responses to inspiratory resistive loading during sleep. Journal of Applied Physiology. 1987;63:603–608. doi: 10.1152/jappl.1987.63.2.603. [DOI] [PubMed] [Google Scholar]
- 12.Ryan CM, Bradley TD. Pathogenesis of obstructive sleep apnea. Journal of Applied Physiology. 2005;99:2440–2450. doi: 10.1152/japplphysiol.00772.2005. [DOI] [PubMed] [Google Scholar]
- 13.Boudewyns AN, Van de Heyning PH, De Backer WA. Site of upper airway obstruction in obstructive apnoea and influence of sleep stage. Eur Respir J. 1997;10:2566–2572. doi: 10.1183/09031936.97.10112566. [DOI] [PubMed] [Google Scholar]
- 14.Fujita S, Conway W, Zorick F, Roth T. Surgical correction of anatomic azbnormalities in obstructive sleep apnea syndrome: uvulopalatopharyngoplasty. Otolaryngol Head Neck Surg. 1981;89:923–934. doi: 10.1177/019459988108900609. [DOI] [PubMed] [Google Scholar]
- 15.Kuhlo W, Doll E, Franck MC. Erfolgreiche Behandlung eines Pickwick-Syndroms durch eine Dauertrachealkanüle. DMW. 1969;94:1286–1290. doi: 10.1055/s-0028-1111209. [DOI] [PubMed] [Google Scholar]
- 16.Zorick F, Roehrs T, Conway W, Fujita S, Wittig R, Roth T. Effects of uvulopalatopharyngoplasty on the daytime sleepiness associated with sleep apnea syndrome. Bull Eur Physiopathol Respir. 1983;19:600–603. [PubMed] [Google Scholar]
- 17.Fujita S, Conway WA, Zorick FJ, et al. Evaluation of the effectiveness of uvulopalatopharyngoplasty. Laryngoscope. 1985;95:70–74. doi: 10.1288/00005537-198501000-00017. [DOI] [PubMed] [Google Scholar]
- 18.Giles TL, Lasserson TJ, Smith BJ, White J, Wright J, Cates CJ. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2006;25 doi: 10.1002/14651858.CD001106.pub2. [DOI] [PubMed] [Google Scholar]
- 19.Engleman HM, Wild MR. Improving CPAP use by patients with the sleep apnoea/hypopnoea syndrome (SAHS) Sleep Medicine Reviews. 2003;7:81–99. doi: 10.1053/smrv.2001.0197. [DOI] [PubMed] [Google Scholar]
- 20.Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proceedings of the American Thoracic Society. 2008;5:173–178. doi: 10.1513/pats.200708-119MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weaver TE. Don’t start celebrating-CPAP adherence remains a problem. Journal of clinical sleep medicine: JCSM. official publication of the American Academy of Sleep Medicine. 2013;9:551–552. doi: 10.5664/jcsm.2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ravesloot MJ, de Vries N, Stuck BA. Treatment adherence should be taken into account when reporting treatment outcomes in obstructive sleep apnea. Laryngoscope. 2014;124:344–345. doi: 10.1002/lary.24302. [DOI] [PubMed] [Google Scholar]
- 23.Stuck BA, Leitzbach S, Maurer JT. Effects of continuous positive airway pressure on apnea-hypopnea index in obstructive sleep apnea based on long-term compliance. Sleep Breath. 2012;16:467–471. doi: 10.1007/s11325-011-0527-8. [DOI] [PubMed] [Google Scholar]
- 24.Mayer G. Leitlinie S3: Nicht erholsamer Schlaf/Schlafstörungen. Somnologie - Schlafforschung und Schlafmedizin. 2009;1:4–16. [Google Scholar]
- 25.Sommer JU, Maurer JT, Hormann K, Stuck BA. Kontrollierte Studien in der operativen Therapie der OSA - Möglichkeiten und Grenzen. HNO. 2012;60:294–299. doi: 10.1007/s00106-011-2468-8. [DOI] [PubMed] [Google Scholar]
- 26.Franklin KA, Anttila H, Axelsson S, et al. Effects and side-effects of surgery for snoring and obstructive sleep apnea-a systematic review. Sleep. 2009;32:27–36. [PMC free article] [PubMed] [Google Scholar]
- 27.Lundkvist K, Januszkiewicz A, Friberg D. Uvulopalatopharyngoplasty in 158 OSAS patients failing non-surgical treatment. Acta Otolaryngol. 2009;129:1280–1286. doi: 10.3109/00016480802654380. [DOI] [PubMed] [Google Scholar]
- 28.Browaldh N, Friberg D, Svanborg E, Nerfeldt P. 15-year efficacy of uvulopalatopharyngoplasty based on objective and subjective data. Acta Otolaryngol. 2011;131:1303–1310. doi: 10.3109/00016489.2011.616912. [DOI] [PubMed] [Google Scholar]
- 29.American Society of Anesthesiologists. New Classification of Physical Status. Anesthesiology. 1963;24 [Google Scholar]
- 30.Sher AE, Schechtman KB, Piccirillo JF. The efficacy of surgical modifications of the upper airway in adults with obstructive sleep apnea syndrome. Sleep. 1996;19:156–177. doi: 10.1093/sleep/19.2.156. [DOI] [PubMed] [Google Scholar]
- 31.Rechtschaffen A, Kales AA. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. NIH Publication. 1968 doi: 10.1046/j.1440-1819.2001.00810.x. [DOI] [PubMed] [Google Scholar]
- 32.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the scoring of sleep and associated events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. JCSM. 2012;8:597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 34.Hays RD, Sherbourne CD, Mazel RM. The RAND 36-Item Health Survey 10 . Health Economics. 1993;2:217–227. doi: 10.1002/hec.4730020305. [DOI] [PubMed] [Google Scholar]
- 35.Weaver TE, Laizner AM, Evans LK, et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep. 1997;20:835–843. [PubMed] [Google Scholar]
- 36.Pirsig W, Schäfer J, Yildiz F, Nagel J. Uvulopalatopharyngoplastik ohne Komplikationen: eine Modifikation nach Fujita. Laryngorhinootologie. 1989;68:585–590. doi: 10.1055/s-2007-998408. [DOI] [PubMed] [Google Scholar]
- 37.Browaldh N, Nerfeldt P, Lysdahl M, Bring J, Friberg D. SKUP3 randomised controlled trial: polysomnographic results after uvulopalatopharyngoplasty in selected patients with obstructive sleep apnoea. Thorax. 2013;68:846–853. doi: 10.1136/thoraxjnl-2012-202610. [DOI] [PubMed] [Google Scholar]
- 38.Caples SM, Rowley JA, Prinsell JR, et al. Surgical modifications of the upper airway for obstructive sleep apnea in adults: a systematic review and meta-analysis. Sleep. 2010;33:1396–1407. doi: 10.1093/sleep/33.10.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hofmann T, Schwantzer G, Reckenzaun E, Koch H, Wolf G. Radiofrequency tissue volume reduction of the soft palate and UPPP in the treatment of snoring. Eur Arch Otorhinolaryngol. 2006;263:164–170. doi: 10.1007/s00405-005-0959-5. [DOI] [PubMed] [Google Scholar]
- 40.Friedman M, Ibrahim H, Bass L. Clinical staging for sleep-disordered breathing. Otolaryngol Head Neck Surg. 2002;127:13–21. doi: 10.1067/mhn.2002.126477. [DOI] [PubMed] [Google Scholar]
- e1.Vicini C, Dallan I, Campanini A, et al. Surgery vs ventilation in adult severe obstructive sleep apnea syndrome. Am J Otolaryngol. 2010;31:14–20. doi: 10.1016/j.amjoto.2008.09.002. [DOI] [PubMed] [Google Scholar]