Abstract
Objective
Toll like receptors (TLRs) are one of the main components of the innate im- mune system. It has been reported that expression of these receptors are altered in the female reproductive tract (FRT) during menstrual cycle. Here we used a fallopian tube epithelial cell line (OE-E6/E7) to evaluate the effect of two sex hormones in modulating TLR expression.
Materials and Methods
In this experimental study, initially TLR gene expression in OE- E6/E7 cells was evaluated and compared with that of fallopian tube tissue using quanti- tative real time-polymerase chain reaction (qRT-PCR) and immunostaining. Thereafter, OE-E6/E7 cells were cultured with different concentrations of estradiol and progesterone, and combination of both. qRT-PCR was performed to reveal any changes in expression of TLR genes as a result of hormonal treatment.
Results
TLR1-10 genes were expressed in human fallopian tube tissue. TLR1-6 genes and their respective proteins were expressed in the OE-E6/E7 cell line. Although estradiol and progesterone separately had no significant effect on TLR expression, their combined treatment altered the expression of TLRs in this cell line. Also, the pattern of TLR expres- sion in preovulation (P), mensturation (M) and window of implantation (W) were the same for all TLRs with no significant differences between P, M and W groups.
Conclusion
These data show the significant involvement of the combination of es- tradiol and progesterone in modulation of TLR gene expression in this human fal- lopian tube cell line. Further experiments may reveal the regulatory mechanism and signalling pathway behind the effect of sex hormones in modulating TLRs in the hu- man FRT.
Keywords: Estradiol, Progesterone, Fallopian Tube, Toll Like Receptors
Introduction
Infection within the upper regions of the female reproductive tract (FRT), particularly the fallopian tubes, can have serious consequences such as chronic pelvic inflammation, infertility and pregnancy complications (1, 2). For example, sexually transmitted diseases (STDs) that infect the upper regions of the FRT are a major worldwide health problem (3, 4). Approximately 8% of females annually develop pelvic inflammatory disease (PID) and after re-infection, this risk increases by 40-70% (5) . It is thus of paramount importance that infectious agents are quickly recognised and removed from the upper parts of the FRT. Characterization of the defense systems present within the FRT will assist the development of effective therapies or vaccination strategies against STDs.
The innate immune system is the first line of defense against infection. This system is able to identify what is foreign or non-self and produces adequate responses that lead to the pathogens being suppressed. Toll like receptors (TLRs) are a family of pattern recognition receptors that recognise pathogen-associated molecular patterns (PAMP) and constitute a major part of the innate immune system (6, 7). Until now, eleven members of this receptor family have been discovered in humans. Of these, TLR1–9 are conserved between human and mouse. Using various methods including ectopic expression of mammalian cDNA in cell lines, some of the activating ligands of TLRs have been discovered. Each individual TLR is known to detect molecules (ligands) from varying classes of microbial agents (8, 9). Some TLRs like TLR2 and its associated receptors TLR1 and TLR6 mainly react against Gram-positive bacteria by detecting molecules from mycobacteria and Gram-positive bacteria (10-12). Some PAMP, like lipoteichoic acid (LTA), can be detected only by TLR2 (11).
In contrast, TLR1 associates with TLR2 to recognize triacylated lipoproteins (13), whereas TLR2 together with TLR6 detects diacylated lipoproteins and peptidoglycans (14-16). TLR4 recognises lipopolysaccharides (LPS) which are present in Gram-negative bacteria (17-19). TLR5 recognises bacterial flagellin (20). Other TLRs mainly react against viruses. For example, TLR3 recognises RNA from double stranded RNA viruses (21, 22). Also, TLR7 and 8 recognise RNA from single stranded RNA viruses and antiviral compounds such as imidazoquinolines (16, 19, 23), whilst TLR9 recognises unmethylated CpG DNA found richly in prokaryotic genomes and DNA viruses (19, 24). Like TLR1 and TLR6, TLR10 is another TLR2-associated receptor and also highly homologous to TLR2, however, the function of this TLR is not completely understood (25-27). In addition, it was revealed that the TLR pathway has physiological relevance to human fertility TLR by playing roles in ovulation, sperm capacitation, fertilization and pregnancy (28-31). They were also shown to play a role in the pathophysiology of relevant disorders including endometriosis and poor ovarian response (32, 33).
Several studies have investigated the presence and the role of TLRs in the male and FRTs (34-43). Furthermore, the expression of TLRs in endometrial cell lines have been shown by Abussahoud et al. (44). It is evident that sex hormones modulate cells and their immune response potential, but varies throughout the FRT (45). Reports by us and others have demonstrated the existence of TLRs in FRT and the cycle-dependent expression of TLRs in the endometrium (34, 46). The effect of estradiol has also been showed in endometrial cells in several studies (47-49).
The cycle-dependent expression of TLRs in FRT implicates a role for sex hormones in regulation of TLR function in FRT. Here we used a fallopian tube epithelial cell line (OE-E6/E7) (50) to investigate the role of two sex hormones (estradiol and progesterone) in modulating TLR expression in the fallopian tube. This cell line has characteristics of human fallopian tube epithelial cells, including morphology, receptor expression and hormonal responses (50). Thereafter, the effect of the sex hormones and their combination as well as their antagonists on expression of TLR1-10 in fallopian tube cells was investigated. This was done by testing TLR whether TLR expression is altered in the presence of sex hormones.
Materials and Methods
Fallopian tube tissue collection
This investigation was an experimental study approved by the Royan Institute Ethics Committee. Informed written consent was obtained prior to the collection of tissue samples. Human fallopian tube tissues were collected from 9 patients undergoing total abdominal hysterectomy for benign gynaecological conditions. The mean age of the women taking part in the study was 42 (range of 33-56) years with all in the secretory phase of their menstrual cycle. For genomic studies, fallopian tube tissue samples were immediately placed in RNAlater (Ambion, UK) and stored for 24 hours at 4˚C followed by immersion and storage in liquid nitrogen until the time of processing.
Antibodies and peptides
Antibodies and peptides used in the experiments were obtained from Santa Cruz Biotechnology Inc. (USA). These were goat polyclonal antibodies specific for N-terminal domains of TLR1, TLR2, TLR3, TLR5 and TLR6, and a goat polyclonal antibody specific for the C-terminal domain of TLR4. Blocking peptides specific for the respective antibodies were used to detect non-specific staining.
Immunostaining
For immunostaining, OE-E6/E7 cells were cultured in four well chamber slides. They were cultured at 37˚C in Dulbecco’s Modified Eagle MediumF12 (DMEM-F12) culture medium (Invitrogen, UK) supplemented with 1% penicillin and streptomycin (Sigma-Aldrich, UK), 10% fetal calf serum (FCS, Invitrogen, USA) and L-glutamine (Invitrogen, USA) in 5% CO2 atmosphere. At confluency, the slides were washed five times with Ca2+ and Mg2+ free phosphate buffered saline (PBS, Gibco, USA), fixed with 5% formalin and stored at 4˚C until use.
Formalin-fixed slides were washed in PBS and then stained using a Vectastain Elite ABC peroxidase kit (Vector Laboratories Ltd, UK). In addition, to avoid non-specific binding, an avidin/ biotin blocking kit (Vector) was used. Briefly, slides were blocked for 1 hour at room temperature in PBS solution containing 0.2% v/v horse serum and 25% v/v avidin supplied in the blocking kit. The block was subsequently removed and slides were incubated for 2 hours at room temperature with primary antibody at an appropriate dilution using antibody diluent media (Dakocytomation Ltd, UK) and 250 ml biotin per ml of diluted antibody. Binding was then visualized by incubating the slide with peroxidase substrate 3-amino-9-ethylcarbazole (AEC) (Vector) for 10 minutes, washed in distilled water for 3 minutes and counterstained in 10% haematoxylin for 10 minutes. Slides were finally washed in tap water for 2 minutes and mounted with Aquamount (VWR International, UK).
Optimal staining was achieved by incubating slides with different concentrations of TLR antibodies (TLR1, TLR2, TLR3, TLR4, TLR5, TLR6 with 4, 4, 10, 10, 4 and 10 μg/ml respectively). Negative control sections were obtained by blocking the primary antibody with the corresponding specific peptide. Immunostained sections were examined using an Olympus BH2 microscope (Olympus, UK).
Toll like receptors expression in OE-E6/E7 cells
TLR expression in the OE-E6/E7 cell line was investigated and compared to fallopian tube tissue samples. OE-E6/E7 cells were cultured at 37˚C in DMEM (F12) supplemented with 1% penicillin and streptomycin, 10% FCS and L-glutamine in 5% CO2 atmosphere. At confluency, cells were washed with Ca2+ and Mg2+ free PBS and then harvested using trypsin-Ethylenediaminetetraacetic acid (EDTA, Invitrogen, USA) pelleted by centrifugation at 300 g for 5 minutes. One ml of TRIreagent (Sigma, UK) was added onto the pellet (5×106 cells). Thereafter, total RNA from pelleted cells was extracted following the standard protocol supplied by the manufacturer.
On the day of the experiment, fallopian tube tissues were removed from RNAlater and homogenised in 3 ml of TRI reagent using an Ultra-Turrax homogenizer for 2 minutes following the standard protocol supplied by the manufacturer.
Total RNA obtained from OE-E6/E7 cells and fallopian tube tissue samples (by using chloroform and isopropanol) were then treated with DNase I (DNA-freeTM, Ambion, USA) to remove genomic DNA contamination from the samples. First strand cDNA was synthesized by reverse transcription using oligodT primers (Metabion, Germany) and SuperScript II (200 U/μl, Invitrogen, USA). RT controls were prepared without the enzyme (nonreverse- transcribed controls).
Polymerase chain reaction (PCR) was performed using prepared cDNA, Platinum Blue PCR Super Mix (Invitrogen, USA) and primers from Metabion (Table 1). The amplification was run for 40 cycles under the following conditions: initial heat at 95˚C for 30 secends, 59˚C to 65˚C for 30 seconds and final annealing at 72˚C for 30 seconds. All experiments included reverse transciptase (RT) controls as well as negative controls (no cDNA). PCR products were visualised on a 1.2 % agarose gel. All amplified PCR products were sequenced to confirm the identity of the amplified product.
Table 1.
Sequence of primers used in this study
| Genes | Primer (5ˊ-3ˊ) | Annealingtemperatue (C) | Accession no. | Product size(bp) | Refrence |
|---|---|---|---|---|---|
| TLR1 | F: GGGTCAGCTGGACTTCAGA | 63 | Gene Bank: U88540.1 | 250 | 43 |
| R: AAAATCCAAATGCAGGAACG | |||||
| TLR2 | F: TCGGAGTTCTCCCAGTTCTCT | 60 | Gene Bank: NM_003264.3 | 175 | 43 |
| R: TCCAGTGCTTCAACCCACAA | |||||
| TLR3 | F: GTATTGCCTGGTTTGTTAATTGG | 60 | Gene Bank:NM_003265.2 | 156 | 43 |
| R: AAGAGTTCAAAGGGGGCACT | |||||
| TLR4 | F: TGATGTCTGCCTCGCGCCTG | 60 | Gene Bank:NM-138554.3 | 98 | 32 |
| R: AACCACCTCCACGCAGGGCT | |||||
| TLR5 | F: CACCAAACCAGGGATGCTAT | 60 | Gene Bank: NM_003268.5 | 111 | 43 |
| R: CCTGTGTATTGATGGGCAAA | |||||
| TLR6 | F: GCCACCATGCTGGTGTTGGCT | 60 | Gene Bank: NM-006068.4 | 101 | 43 |
| R: CGCCGAGTCTGGGTCCACTG | |||||
| TLR7 | F: CCTTGAGGCCAACAACATCT | 63 | Gene Bank: NM_016562.3 | 285 | 43 |
| R: GTAGGGACGGCTGTGACATT | |||||
| TLR8 | F: CTTCGATACCTAAACCTCTCTAGCAC | 60 | Gene Bank: NM_138636.4 | 90 | 43 |
| R: AAGATCCAGCACCTTCAGATGA | |||||
| TLR9 | F: TTCCCTGTAGCTGCTGTCC | 60 | Gene Bank: NM_017442.3 | 207 | 43 |
| R: ACAGCCAGTTGCAGTTCACC | |||||
| TLR10 | F: TGCCCACCACAATCTCTTCCATGA | 60 | Gene Bank: NM-030956.3 | 184 | 43 |
| R: AGCAGCTCGAAGGTTTGCCCA | |||||
| β-actin | F: CAAGATCATTGCTCCTCCTG | 60 | Gene Bank: NM-001101 | 90 | 43 |
| R: ATCCACATCTGCTGGAAGG | |||||
| GAPDH | F: CTCATTTCCTGGTATGACAACGA | 60 | Gene Bank: NM_002046.4 | 122 | 43 |
| R: CTTCCTCTTGTGCTCTTGCT | |||||
TLR; Toll like receptor.
Cell culture in the presence of sex hormones
To investigate the effect of estradiol and progesterone on TLR expression in the OE-E6/E7 cell line, OE-E6/E7 cells were cultured again in triplicates at 37˚C in DMEM (F12) culture medium and water soluble estradiol and progesterone (Sigma-Aldrich, UK) to reach the final concentrations of 0.1, 1, 10, 100 nM and 1, 10, 100, 1000 nM for estradiol and progesterone respectively. In addition, the following combinations of these two (final concentrations) were used in four groups of: control (C, without any additional treatment of sex hormones), menstruation (M, 1 nM progesterone and 0.1 nM estradiol), pre-ovulation (P, 6.5 nM progesterone and 1.5 nM estradiol) and window of implantation (W, 35 nM progesterone and 1 nM estradiol) in 5% CO2 atmosphere in 75 ml flasks for 24 hours in the absence of phenol red and serum. In the next step, the effect of sex hormone antagonists on TLR expression was evaluated. To do this, OE-E6/E7 cells were divided in two groups. One group was pre-treated for 2 hours with 1 μM ICI 182, 780 (fulvestrant, an estradiol antagonist, Sigma-Aldrich, UK) and the other pre-treated for 2 hours with 0.1 μM RU486 (mifepristone, a progesterone antagonist, Tocris, USA). After pre-treatment, both groups were treated again separately with a combination of estradiol and progesterone based on the four treatment groups (C, M, P and W) in 5% CO2 atmosphere in 75 ml flasks for 24 hours in the absence of phenol red and serum.
Cells were next treated for RNA isolation and cDNA synthesis following the same protocol mentioned above. qRT-PCR was performed using the cDNA prepared from the estradiol and progesterone treatment experiments, same primers as in table 1 and SYBR Green Jump Start (Sigma, UK) master mix (containing 10 μl SYBR Green, 7 μl Water, 1 μl of each primer and 1 μl cDNA). The PCR amplification was performed under the following conditions: 50 cycles of 95˚C for 30 seconds, 59˚C to 63˚C for 30 seconds and 72˚C for 30 seconds. All experiments included RT controls and negative controls (no cDNA). qRT-PCR was performed on a Mx3005P QPCR machine (Stratagene, Germany) and results were analyzed using MxPro QPCR software version 4.01. In preliminary experiments, the efficiency of the primer sets of each Q-PCR reaction was established. Variation in Beta-actin and GAPDH expression as two housekeeping genes was also tested.
The qRT-PCR data were analyzed using the comparative CT method (51). The fold change was calculated as FC=2-ΔΔCT.
The results were expressed as mean ± SEM. Significance testing was performed by one-way ANOVA with Tukey’s multiple comparison test. P<0.05 was considered significant.
Results
Reverse transcriptase-polymerase chain reaction
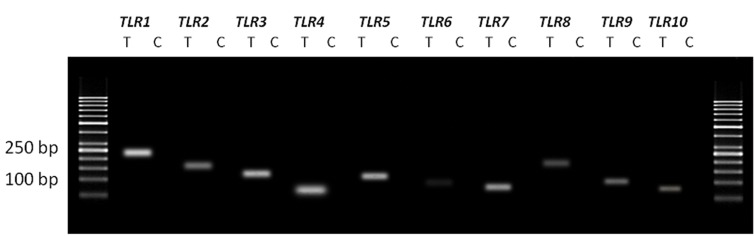
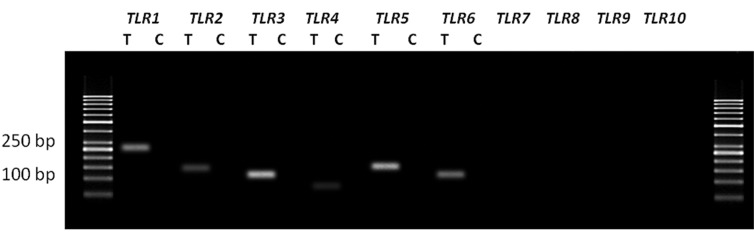
Figure 1 shows the results of RT-PCR of TLR1- 10 genes in the human fallopian tube tissue. Figure 2 shows the results of RT-PCR of TLR1-6 genes in OE-E6/E7 cells. Size of all amplified PCR products were as predicted and sequencing verified correct amplification of each gene. No product was amplified in negative control samples, indicating absence of genomic DNA contamination.
Fig.1.
The expression of TLR1-10 genes in the human fallopian tube tissue. Each pair of primers produced a specific product with the specific predicted size observed in the test (T) samples. C; Control samples (samples without using cDNA) and TLR; Toll like receptor.
Fig.2.
The expression of TLR1-6 genes in the human fallopian tube cell line (OE-E6/E7). Each pair of primers produced a specific product with the specific predicted size observed in the test (T) samples. C; Control samples (samples without using cDNA) and TLR; Toll like receptor.
Immunostaining
Formalin-fixed slides were used to study the distribution of TLR1-6 in OE-E6/E7 cells. Positive immunostaining for all six TLRs was observed with TLR1, 2, 4 and TLR6 displaying moderate staining in this epithelial cell line. However, strong staining for TLR3 and TLR5 was observed. The immunocytochemical localisation of TLR1-6 is shown in figure 3.
Fig.3.
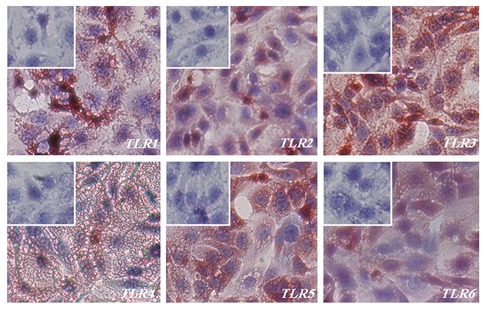
Immunohistochemical staining of TLR1-6 in the OE-E6/E7 cell line. Positive staining is red, negative staining is blue. Small inserts show blocking of anti TLR1-6 antibodies with respective specific peptides. TLR; Toll like receptor.
Quantitative polymerase chain reaction
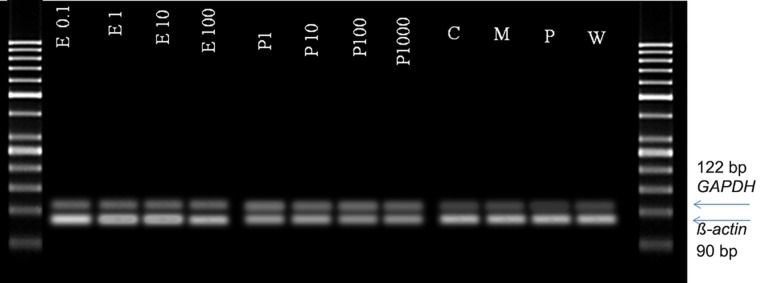
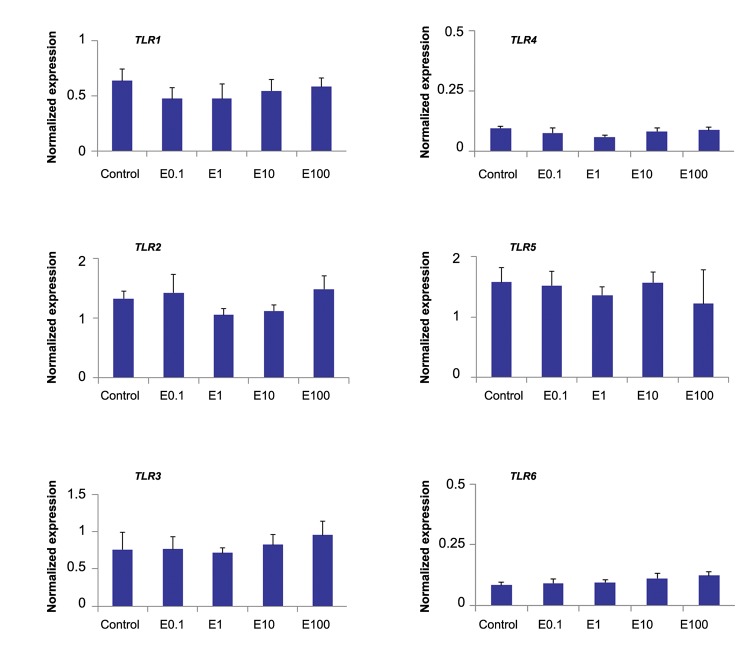
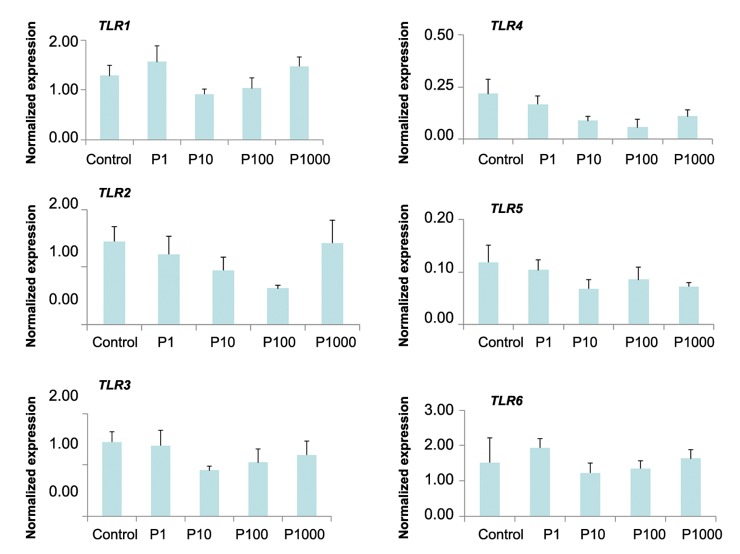
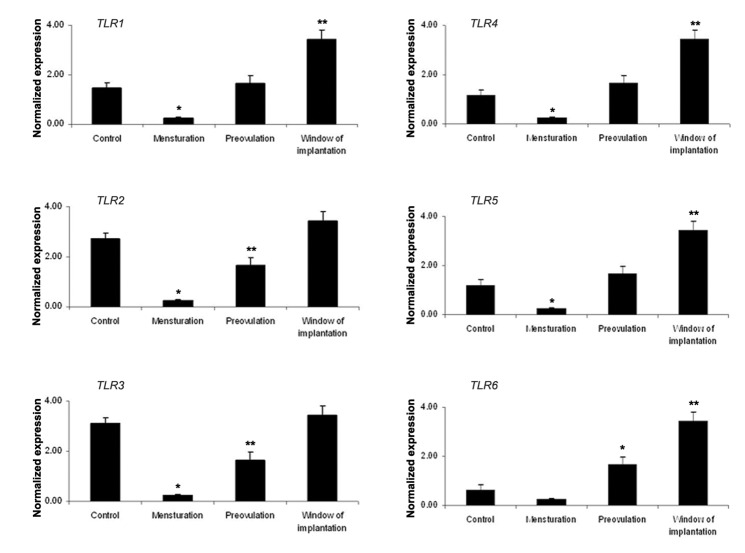
A stable expression of Human ß-actin and GAPDH genes with variable hormonal treatments was observed (Fig .4).The quantitative expression profiles of TLR1-6 genes in OE-E6/E7 cells treated with varying concentrations of estradiol and progesterone are shown in figures 5 and 6 respectively. The relative expression of TLR1-6 genes did not significantly differ in response to different concentrations of either estradiol or progesterone. The mean relative expression of TLR1-6 differed considerably between P, M, W and C groups (Fig .7). The Pattern of TLR expression in P, M and W were significantly different among P, M and W groups. Also, by using 1 μM of fulvestrant (estradiol antagonist) or 0.1 μM mifepristone (progesterone antagonist) 2 hours prior to the combined estradiol and progesterone treatment, the mean relative expression of TLR1-6 did not differ markedly in C, P, M and W groups (Figes.8, 9).
Fig.4.
The stable expression of GAPDH and β-actin in OE-E6/E7 under variable hormonal treatments. E; Estradiol, P; Progestrone, C; Control, M; Menstruation and W; Window of implantation.
Fig.5.
Expression of TLR1-6 in OE-E6/E7cultured with estradiol. Mean ± SEM of normalized expression values (with β-actin) for TLR1-6 genes in OE-E6/E7 cultured with different concentrations of estradiol (control: 0 nM, E 0.1: 0.1 nM, E1: 1 nM, E10: 10 nM and E100: 100 nM or 1 μM). No significant results were obtained. TLR; Toll like receptor.
Fig.6.
Expression of TLR1-6 in OE-E6/E7cultured with progesterone. Mean ± SEM of normalized expression values (with β-actin) for TLR1- 6 genes in OE-E6/E7 cultured with different concentrations of progesterone (control: 0 nM, P1: 1 nM, P10: 10 nM, P100: 100 nM and P1000: 1000 nM or 1 μM). No significant results were obtained. TLR; Toll like receptor.
Fig.7.
Expression of TLR1-6 in OE-E6/E7 cultured with a combination of estradiol and progesterone concentrations. Mean ± SEM of normalized expression values (with β-actin) for TLR1-6 genes in OE-E6/E7 cultured with a combination of estradiol and progesterone. Control (C, without any additional treatment of sex hormone), menstruation (M, 1 nM progesterone and 0.1 nM estradiol), pre-ovulation (P, 6.5 nM progesterone and1.5 nM estradiol) and window of implantation (W, 35 nMprogesterone and 1 nM estradiol) (control: 0 nM, P1: 1 nM, P10: 10 nM, P100: 100 nM and P1000: 1000 nM or 1 μM). Star denotes statistically significant differences. TLR; Toll like receptor.
Fig.8.
Expression of TLR1-6 in OE-E6/E7 precultured with fulvestrant, then cultured with a combination of estradiol and progesterone concentrations. Mean ± SEM of normalized expression values (with β-actin) for TLR1-6 genes in OE-E6/E7 pre-cultured with fulvestrant for 2 hours, then cultured with a combination of estradiol and progesterone in 4 groups (C, M, P, W). No significant results were obtained. TLR; Toll like receptor.
Fig.9.
Expression of TLR1-6 in OE-E6/E7 pre-cultured with mifepristone and cultured with a combination of estradiol and progesterone in 4 groups (C, M, P, W). Mean ± SEM of normalized expression values (with β-actin) for TLR1-6 genes. No significant results were obtained. TLR; Toll like receptor.
Discussion
Epithelial cells are the first layer of defense against pathogens ascending FRT. Considering their role in protecting the tract from the external environment, TLRs are expected to be present in this tissue. Several studies have investigated the presence and the role of TLRs in FRT especially within the fallopian tube (34,42,52). Previously, Pioli et al. (39), showed the presence of TLR1-6 in the FRT as well as the accessory molecule CD14 and the molecular adapter MyD88 both of which are needed for TLR signalling. They also observed expression of TLR1-6 at the transcript level in fallopian tubes. In agreement with this report, we previously showed the localization of TLR1-6 in the fallopian tube using immunohistochemistry (36). Both observations are in agreement with our findings in this study that demonstrate the presence of TLR1-6 transcripts in human fallopian tube tissues and cell line. The expression of TLR4 protein and transcript has been detected in human endometrial epithelial cells, stromal cells (38) and fallopian tube stromal fibroblasts (53). However, TLR4 expression was not detected in fallopian tube epithelial cells by Itoh et al. (53). In another study Hart et al. (42), reported the expression of TLR7–9 in the fallopian tube, uterine, endometrium, cervix and ectocervix, while TLR10 expression was restricted to the fallopian tube.
In this study, although expression of TLR1-10 was detected in human fallopian tube tissues, expression of only TLR1-6 was detected in OE-E6/ E7 cells. This may be due to difference in features of the fallopian tube tissue and this specific cell line. Fallopian tube tissue contains an epithelial layer, stroma and capillaries supplying blood. Within the epithelial layer of the fallopian tube tissue, some of the cells are ciliated and some are known as secretory cells. The OE-E6/E7 cell line is only an isolated fallopian tube epithelial layer cell line. Therefore, the expression of TLR genes and their level of expression in this cell line is likely to be different compared with the original tissue consisting of different types of cells. The other potential explanation for the absence of expression of some TLRs in OE-E6/E7 cells may be due to differences between immortalised cell lines and their original parental primary cells. It is known that cell lines may undergo changes due to the process of immortalization. However, the characteristics of this immortalized cell line have been compared to the parental human fallopian tube epithelium in several investigations. For example, human oviduct-specific glycoprotein, estradiol receptors and cytokeratin molecules have been found to be produced in both primary fallopian tube cells and OE-E6/E7 cells (50,54,55).
The FRT environment is under the control of sex hormones during the menstrual cycle. Sex hormones not only regulate anatomical and histological characteristics of this tract (56,57), they are also involved in the influx and localization of immune cells in this tract (58,60). For example, uterine natural killer (uNK) cells are found in the human uterus in large numbers and are spread throughout the endometrium with increasing numbers as the menstrual cycle progresses (61,62). In addition, the adaptive immune system is also influenced by the changing levels of sex hormones during the menstrual cycle. Antigen presentation has been shown to be suppressed in response to increasing concentrations of estradiol (63,64). Sex hormones including estradiol and progesterone tightly control the distribution of macrophages within the endometrium (65,68). Another study demonstrated that estradiol, which is secreted by the ovary during the menstrual cycle, modulates epithelial cells and other immune cells in FRT directly and indirectly to regulate a wide range of immune functions specific to each site in FRT (69). Nasu and Narahara (49) also showed that antigen-presenting cells in the uterus and vagina are responsive to estradiol where antigen presentation as well as co-stimulatory molecule expression is inhibited by estradiol. Furthermore, they suggested that antigen-presenting cells in the uterus and vagina respond to selected TLR agonists with altered antigen presentation.
It is therefore likely that the action of TLRs is also modified by changing concentrations of estradiol and progesterone. Although all TLR molecules are expressed throughout the cycle, the majority of these genes are expressed at their lowest level during menstrual and proliferative stages of the cycle (34). At follicular stage of the cycle, while progesterone levels are at their lowest, estradiol levels are at their highest. This may indicate an inhibitory effect of estradiol and/or enhancing influence of progesterone on the expression of TLR molecules in FRT, especially in the endometrium. It is plausible that this alteration in TLR gene expression may even influence the function of TLRs in mediating innate immune responses in FRT. To test these hypotheses, we examined the effect of sex hormones on TLR expression in the OE-E6/ E7 cell line. We used various concentrations of estradiol and progesterone to determine the effects of these sex hormones on TLR expression. No significant alteration in the relative expression of TLR1-6 transcripts was observed in the fallopian tube cell line in response to different concentrations of estradiol and progesterone. These results agreed with the findings of Lesmeister et al. (47) who also showed that in vitro treatment of endometrial epithelial cell line (RL95-2) with 17betaestradiol did not have an effect on TLR3 transcript or protein expression. Also, in a recent study on human uterine epithelial cells and the ECC-1 uterine epithelial cell line, Nasu and Narahara (49) demonstrated that estradiol either alone or prior to treatment with poly (I:C), had no effect on the expression of interferon β (IFNβ) or interferonstimulated genes (ISG). However, another study showed that production and secretion of protective antimicrobials including human β defensin-2 (HBD2) and secretory leukocyte protease inhibitor (SLPI) are directly upregulated by estradiol. On the contrary, estradiol inhibited LPS and poly (I:C)induced secretion of macrophage inhibitory factor (MIF), interleukin 6 (IL-6) and IL-8 in primary uterine epithelial cells (70).
In the next step of our study, combined effect of estradiol and progesterone was evaluated. Expression of TLR1-6 was higher when the level of sex hormones in culture media was comparable to their concentration in serum during the window of implantation (group W) in the menstrual cycle. These results agreed with our previous findings where higher expression of TLRs was observed during the secretory phase in human endometrium (34,71). These findings demonstrated a stimulatory effect of co-presence of both sex hormones (M, P and W) on TLR1-6 expression.
A recent study reported the fluctuation of TLR responsiveness in peripheral blood throughout the menstrual cycle (72). In agreement with our data, during the follicular phase, lower levels of IL-6 and tumor necrosis factor (TNF)-α following stimulation with the TLR2 agonist, lower levels of IL-1β, IL-6 and TNF-α following stimulation with the TLR4 agonist LPS, and lower levels of IL-1β and TNF-α following stimulation of whole blood with the TLR5 agonist flagellin was observed when compared with the early luteal phase (72). Jorgenson et al. (46) also illustrated that TLR3 transcript level in primary endometrial epithelial cells is menstural cycle-dependent. They showed TLR3 was expressed throughout the menstrual cycle but at its highest during the secretory phase of the cycle. In another study, Yao et al. (73) reported that except TLR11, the expression of TLR1-10 is cycle-dependent in mouse. Whether the above mentioned findings are due to estradiol, progesterone or their combined effect on FRT epithelium remains to be deciphered.
When fulvestrant (estradiol antagonist) or mifepristone (progesterone antagonist) was used, the combined effect of estradiol and progesterone on TLR expression was inhibited. These data thus confirm our results that on the expression of TLR16 is not affected by progesterone and estradiol individually TLR1-6 but TLR1-6 under the synergistic/additive effect of the combination of estradiol and progesterone.
In performing this investigation, we took several precautionary measures. The effect of hormones in cell culture experiments was tested in the absence of phenol red and serum. Phenol red has estradiolic properties and serum may contain small molecules with estradiolic effects and if present, may hamper the results of experiments.
In in vitro culture systems and particularly in the presence of blood or serum samples, progesterone degrades quickly (74). To avoid early degradation of hormones during our cell culture experiments, we used water soluble estradiol and progesterone (Cyclodextrin-encapsulated 17β-estradiol and progesterone) which are stable and tested for cell culture applications. These compounds have been used in several similar investigations (75,79). Furthermore, our results clearly demonstrated that these compounds do not alter TLR gene expression in cultured cells.
It seems that the pattern of TLR expression under different concentrations of estradiol and progesterone (mimicking those of the menstrual cycle) is similar to the pattern of TLR expression in endometrial tissue during the menstrual cycle (34). On the other hand, higher expression of TLRs in the proliferative phase compared with menstruation as well as their higher expression in the secretory phase than other phases show that safety in the human fallopian tube at the time of ovulation or early embryo development is a key factor.
Future studies should be directed towards understanding the role of signalling pathways that enable estradiol and progesterone to modulate the expression and function of TLRs in FRT.
Conclusion
This study firmly points to the involvement of sex hormones in modulation of TLR gene expression in human fallopian tube cells. Further experiments should be undertaken to reveal the regulatory mechanism(s) and signalling pathway(s) responsible for the effect of sex hormones in modulating innate immunity in human FRT.
Acknowledgments
This study was financially supported by Royan Institute. We wish to thank Dr Kai-Fai Lee for his kind courtesy (providing us with the OE-E6/ E7 cell line) and Miss Arghavan Janan for skillful technical assistance. The authors declare that they have no conflict of interest.
References
- 1.McCormack WM. Pelvic inflammatory disease. N Engl J Med. 1994;330(2):115–119. doi: 10.1056/NEJM199401133300207. [DOI] [PubMed] [Google Scholar]
- 2.Witkin SS. Immunological aspects of genital chlamydia infections. Best Pract Res Clin Obstet Gynaecol. 2002;16(6):865–874. doi: 10.1053/beog.2002.0326. [DOI] [PubMed] [Google Scholar]
- 3.Cates W Jr. Priorities for sexually transmitted diseases in the late 1980s and beyond. Sex Transm Dis. 1986;13(2):114–117. [PubMed] [Google Scholar]
- 4.Piot P, Plummer FA, Mhalu FS, Lamboray JL, Chin J, Mann JM. AIDS: an international perspective. Science. 1988;239(4840):573–579. doi: 10.1126/science.3277271. [DOI] [PubMed] [Google Scholar]
- 5.Jiang J, Karimi O, Ouburg S, Champion CI, Khurana A, Liu G, et al. Interruption of CXCL13-CXCR5 axis increases upper genital tract pathology and activation of NKT cells following chlamydial genital infection. PloS One. 2012;7(11):e47487–e47487. doi: 10.1371/journal.pone.0047487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2(8):675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 7.Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17(1):1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 8.Janeway CA Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 9.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 10.Medzhitov R, Janeway C Jr. The Toll receptor family and microbial recognition. Trends Microbiol. 2000;8(10):452–456. doi: 10.1016/s0966-842x(00)01845-x. [DOI] [PubMed] [Google Scholar]
- 11.Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning CJ. Peptidoglycanand lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J Biol Chem. 1999;274(25):17406–17409. doi: 10.1074/jbc.274.25.17406. [DOI] [PubMed] [Google Scholar]
- 12.Underhill DM, Ozinsky A, Smith KD, Aderem A. Toll-like receptor-2 mediates mycobacteria-induced proinflammatory signaling in macrophages. Proc Natl Acad Sci USA. 1999;96(25):14459–14463. doi: 10.1073/pnas.96.25.14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takeuchi O, Sato S, Horiuchi T, Hoshino K, Takeda K, Dong Z, et al. Cutting edge: role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J Immunol. 2002;169(1):10–14. doi: 10.4049/jimmunol.169.1.10. [DOI] [PubMed] [Google Scholar]
- 14.Nakao Y, Funami K, Kikkawa S, Taniguchi M, Nishiguchi M, Fukumori Y, et al. Surface-expressed TLR6 participates in the recognition of diacylated lipopeptide and peptidoglycan in human cells. J Immunol. 2005;174(3):1566–1573. doi: 10.4049/jimmunol.174.3.1566. [DOI] [PubMed] [Google Scholar]
- 15.Ozinsky A, Underhill DM, Fontenot JD, Hajjar AM, Smith KD, Wilson CB, et al. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc Natl Acad Sci USA. 2000;97(25):13766–13771. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wetzler LM. The role of Toll-like receptor 2 in microbial disease and immunity. Vaccine. 2003;21(Suppl 2):S55–60. doi: 10.1016/s0264-410x(03)00201-9. [DOI] [PubMed] [Google Scholar]
- 17.Akashi S, Nagai Y, Ogata H, Oikawa M, Fukase K, Kusumoto S, et al. Human MD-2 confers on mouse Toll-like receptor 4 species-specific lipopolysaccharide recognition. Int Immunol. 2001;13(12):1595–1599. doi: 10.1093/intimm/13.12.1595. [DOI] [PubMed] [Google Scholar]
- 18.Nagai Y, Akashi S, Nagafuku M, Ogata M, Iwakura Y, Akira S, et al. Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat Immunol. 2002;3(7):667–672. doi: 10.1038/ni809. [DOI] [PubMed] [Google Scholar]
- 19.Pasare C, Medzhitov R. Toll-like receptors: linking innate and adaptive immunity. Microbes Infect. 2004;6(15):1382–1387. doi: 10.1016/j.micinf.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 20.Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410(6832):1099–1103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 21.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NFkappaB by Toll-like receptor 3. Nature. 2001;413(6857):732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 22.Kariko K, Ni H, Capodici J, Lamphier M, Weissman D. mRNA is an endogenous ligand for Toll-like receptor 3. J Biol Chem. 2004;279(13):12542–12550. doi: 10.1074/jbc.M310175200. [DOI] [PubMed] [Google Scholar]
- 23.Jurk M, Heil F, Vollmer J, Schetter C, Krieg AM, Wagner H, et al. Human TLR7 or TLR8 independently confer responsiveness to the antiviral compound R-848. Nat Immunol. 2002;3(6):499–499. doi: 10.1038/ni0602-499. [DOI] [PubMed] [Google Scholar]
- 24.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408(6813):740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 25.Flacher V, Bouschbacher M, Verronese E, Massacrier C, Sisirak V, Berthier-Vergnes O, et al. Human langerhans cells express a specific TLR profile and differentially respond to viruses and gram-positive bacteria. J Immunol. 2006;177(11):7959–7967. doi: 10.4049/jimmunol.177.11.7959. [DOI] [PubMed] [Google Scholar]
- 26.Hasan U, Chaffois C, Gaillard C, Saulnier V, Merck E, Tancredi S, et al. Human TLR10 is a functional receptor, expressed by B cells and plasmacytoid dendritic cells, which activates gene transcription through MyD88. J Immunol. 2005;174(5):2942–2950. doi: 10.4049/jimmunol.174.5.2942. [DOI] [PubMed] [Google Scholar]
- 27.Chuang T, Ulevitch RJ. Identification of hTLR10: a novel human Toll-like receptor preferentially expressed in immune cells. Biochim Biophys Acta. 2001;1518(1-2):157–161. doi: 10.1016/s0167-4781(00)00289-x. [DOI] [PubMed] [Google Scholar]
- 28.Growe RG, Luster MI, Fail PA, Lippes J. Quinacrineinduced occlusive fibrosis in the human fallopian tube is due to a unique inflammatory response and modification of repair mechanisms. J Reprod Immunol. 2013;97(2):159–166. doi: 10.1016/j.jri.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 29.Ghosh M, Schaefer TM, Fahey JV, Wright JA, Wira CR. Antiviral responses of human Fallopian tube epithelial cells to toll-like receptor 3 agonist poly(I:C) Fertil Steril. 2008;89(5 Suppl):1497–1506. doi: 10.1016/j.fertnstert.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koga K, Aldo PB, Mor G. Toll-like receptors and pregnancy: trophoblast as modulators of the immune response. J Obstet Gynaecol Res. 2009;35(2):191–202. doi: 10.1111/j.1447-0756.2008.00963.x. [DOI] [PubMed] [Google Scholar]
- 31.Amirchaghmaghi E, Taghavi SA, Shapouri F, Saeidi S, Rezaei A, Aflatoonian R. The role of Toll like receptors in pregnancy. Int J Fertil Steril. 2013;7(3):147–153. [PMC free article] [PubMed] [Google Scholar]
- 32.Taghavi SA, Ashrafi M, Mehdizadeh M, Karimian L, Joghataie M, Aflatoonian R. Tolllike receptors expression in follicular cells of patients with poor ovarian response. Int J Fertil Steril. 2014;8(2):183–192. [PMC free article] [PubMed] [Google Scholar]
- 33.Khan KN, Kitajima M, Fujishita A, Nakashima M, Masuzaki H. Toll-like receptor system and endometriosis. J Obstet Gynaecol Res. 2013;39(8):1281–1292. doi: 10.1111/jog.12117. [DOI] [PubMed] [Google Scholar]
- 34.Aflatoonian R, Tuckerman E, Elliott SL, Bruce C, Aflatoonian A, Li TC, et al. Menstrual cycle-dependent changes of Toll-like receptors in endometrium. Hum Reprod. 2007;22(2):586–593. doi: 10.1093/humrep/del388. [DOI] [PubMed] [Google Scholar]
- 35.Andersen JM, Al-Khairy D, Ingalls RR. Innate immunity at the mucosal surface: role of toll-like receptor 3 and toll-like receptor 9 in cervical epithelial cell responses to microbial pathogens. Biol Reprod. 2006;74(5):824–831. doi: 10.1095/biolreprod.105.048629. [DOI] [PubMed] [Google Scholar]
- 36.Fazeli A, Bruce C, Anumba DO. Characterization of Tolllike receptors in the female reproductive tract in humans. Hum Reprod. 2005;20(5):1372–1378. doi: 10.1093/humrep/deh775. [DOI] [PubMed] [Google Scholar]
- 37.Fichorova RN, Cronin AO, Lien E, Anderson DJ, Ingalls RR. Response to Neisseria gonorrhoeae by cervicovaginal epithelial cells occurs in the absence of Toll-like receptor 4-mediated signaling. J Immunol. 2002;168(5):2424–2432. doi: 10.4049/jimmunol.168.5.2424. [DOI] [PubMed] [Google Scholar]
- 38.Hirata T, Osuga Y, Hirota Y, Koga K, Yoshino O, Harada M, et al. Evidence for the presence of Toll-like receptor 4 system in the human endometrium. J Clin Endocrinol Metab. 2005;90(1):548–556. doi: 10.1210/jc.2004-0241. [DOI] [PubMed] [Google Scholar]
- 39.Pioli PA, Amiel E, Schaefer TM, Connolly JE, Wira CR, Guyre PM. Differential expression of Toll-like receptors 2 and 4 in tissues of the human female reproductive tract. Infect Immun. 2004;72(10):5799–5806. doi: 10.1128/IAI.72.10.5799-5806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pivarcsi A, Nagy I, Koreck A, Kis K, Kenderessy-Szabo A, Szell M, et al. Microbial compounds induce the expression of pro-inflammatory cytokines, chemokines and human beta-defensin-2 in vaginal epithelial cells. Microbes Infect. 2005;7(9-10):1117–1127. doi: 10.1016/j.micinf.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 41.Schaefer TM, Fahey JV, Wright JA, Wira CR. Innate immunity in the human female reproductive tract: antiviral response of uterine epithelial cells to the TLR3 agonist poly(I:C) J Immunol. 2005;174(2):992–1002. doi: 10.4049/jimmunol.174.2.992. [DOI] [PubMed] [Google Scholar]
- 42.Hart KM, Murphy AJ, Barrett KT, Wira CR, Guyre PM, Pioli PA. Functional expression of pattern recognition receptors in tissues of the human female reproductive tract. J Reprod Immunol. 2009;80(1-2):33–40. doi: 10.1016/j.jri.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saeidi S, Shapouri F, Amirchaghmaghi E, Hoseinifar H, Sabbaghian M, Sadighi Gilani MA, et al. Sperm protection in the male reproductive tract by Toll-like receptors. Andrologia. 2014;46(7):784–790. doi: 10.1111/and.12149. [DOI] [PubMed] [Google Scholar]
- 44.Aboussahoud W, Aflatoonian R, Bruce C, Elliott S, Ward J, Newton S, et al. Expression and function of Toll-like receptors in human endometrial epithelial cell lines. J Reprod Immunol. 2010;84(1):41–51. doi: 10.1016/j.jri.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 45.Li HWR, Liao SB, Chiu PCN, Tam WW, Ho JC, Ng EH, et al. Expression of adrenomedullin in human oviduct, its regulation by the hormonal cycle and contact with spermatozoa, and its effect on ciliary beat frequency of the oviductal epithelium. J Clin Endocrinol Metab. 2010;95(9):E18–E25. doi: 10.1210/jc.2010-0273. [DOI] [PubMed] [Google Scholar]
- 46.Jorgenson RL, Young SL, Lesmeister MJ, Lyddon TD, Misfeldt ML. Human endometrial epithelial cells cyclically express Toll-like receptor 3 (TLR3) and exhibit TLR3dependent responses to dsRNA. Hum Immunol. 2005;66(5):469–482. doi: 10.1016/j.humimm.2004.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lesmeister MJ, Jorgenson RL, Young SL, Misfeldt ML. 17Beta-estradiol suppresses TLR3-induced cytokine and chemokine production in endometrial epithelial cells. Reprod Biol Endocrinol. 2005;3:74–74. doi: 10.1186/1477-7827-3-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kawai T, Akira S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int Immunol. 2009;21(4):317–337. doi: 10.1093/intimm/dxp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nasu K, Narahara H. Pattern recognition via the Toll-like receptor system in the human female genital tract. Mediators Inflamm. 2010;2010:976024–976024. doi: 10.1155/2010/976024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee YL, Lee KF, Xu JS, Wang YL, Tsao SW, Yeung WS. Establishment and characterization of an immortalized human oviductal cell line. Mol Reprod Dev. 2001;59(4):400–409. doi: 10.1002/mrd.1046. [DOI] [PubMed] [Google Scholar]
- 51.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 52.Gregorczyk KP, Krzyzowska M. Innate immunity to infection in the lower female genital tract. Postepy Hig Med Dosw (Online) 2012;67:388–401. doi: 10.5604/17322693.1048816. [DOI] [PubMed] [Google Scholar]
- 53.Itoh H, Nasu K, Nishida M, Matsumoto H, Yuge A, Narahara H. Human oviductal stromal fibroblasts, but not oviductal epithelial cells, express Toll-like receptor 4: the sitespecific mucosal immunity of the human fallopian tube against bacterial infection. Am J Reprod Immunol. 2006;56(2):91–101. doi: 10.1111/j.1600-0897.2006.00389.x. [DOI] [PubMed] [Google Scholar]
- 54.Watters TM, Kenny EF, ONeill LA. Structure, function and regulation of the Toll/IL-1 receptor adaptor proteins. Immunol Cell Biol. 2007;85(6):411–419. doi: 10.1038/sj.icb.7100095. [DOI] [PubMed] [Google Scholar]
- 55.Ling L, Lee YL, Lee KF, Tsao SW, Yeung WS, Kan FW. Expression of human oviductin in an immortalized human oviductal cell line. Fertil Steril. 2005;84(Suppl 2):1095–1103. doi: 10.1016/j.fertnstert.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 56.Beier HM, Beier-Hellwig K. Molecular and cellular aspects of endometrial receptivity. Hum Reprod Update. 1998;4(5):448–458. doi: 10.1093/humupd/4.5.448. [DOI] [PubMed] [Google Scholar]
- 57.Classen-Linke I, Alfer J, Hey S, Krusche CA, Kusche M, Beier HM. Marker molecules of human endometrial differentiation can be hormonally regulated under in-vitro conditions as in-vivo. Hum Reprod Update. 1998;4(5):539–549. doi: 10.1093/humupd/4.5.539. [DOI] [PubMed] [Google Scholar]
- 58.von Rango U, Classen-Linke I, Kertschanska S, Kemp B, Beier HM. Effects of trophoblast invasion on the distribution of leukocytes in uterine and tubal implantation sites. Fertil Steril. 2001;76(1):116–124. doi: 10.1016/s0015-0282(01)01859-3. [DOI] [PubMed] [Google Scholar]
- 59.Spornitz UM. The functional morphology of the human endometrium and decidua. Adv Anat Embryol Cell Biol. 1992;124:1–99. doi: 10.1007/978-3-642-58099-4. [DOI] [PubMed] [Google Scholar]
- 60.Yeaman GR, Guyre PM, Fanger MW, Collins JE, White HD, Rathbun W, et al. Unique CD8+ T cell-rich lymphoid aggregates in human uterine endometrium. J Leukoc Biol. 1997;61(4):427–435. [PubMed] [Google Scholar]
- 61.Givan AL, White HD, Stern JE, Colby E, Gosselin EJ, Guyre PM, et al. Flow cytometric analysis of leukocytes in the human female reproductive tract: comparison of fallopian tube, uterus, cervix, and vagina. Am J Reprod Immunol. 1997;38(5):350–359. doi: 10.1111/j.1600-0897.1997.tb00311.x. [DOI] [PubMed] [Google Scholar]
- 62.Hunt JS. Immunologically relevant cells in the uterus. Biol Reprod. 1994;50(3):461–466. doi: 10.1095/biolreprod50.3.461. [DOI] [PubMed] [Google Scholar]
- 63.Qiu F, Cui Z. CD4+ T helper cell response is required for memory in CD8+ T lymphocytes induced by a poly(I:C)-adjuvanted MHC I-restricted peptide epitope. J Immunother. 2007;30(2):180–189. doi: 10.1097/01.cji.0000211330.61019.6f. [DOI] [PubMed] [Google Scholar]
- 64.Welters MJ, Bijker MS, van den Eeden SJ, Franken KL, Melief CJ, Offringa R, et al. Multiple CD4 and CD8 T-cell activation parameters predict vaccine efficacy in vivo mediated by individual DC-activating agonists. Vaccine. 2007;25(8):1379–1389. doi: 10.1016/j.vaccine.2006.10.049. [DOI] [PubMed] [Google Scholar]
- 65.Taylor KR, Trowbridge JM, Rudisill JA, Termeer CC, Simon JC, Gallo RL. Hyaluronan fragments stimulate endothelial recognition of injury through TLR4. J Biol Chem. 2004;279(17):17079–17084. doi: 10.1074/jbc.M310859200. [DOI] [PubMed] [Google Scholar]
- 66.Yu L, Wang L, Chen S. Endogenous Toll-like receptor ligands and their biological significance. J Cell Mol Med. 2010;14(11):2592–2603. doi: 10.1111/j.1582-4934.2010.01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Frantz S, Kelly RA, Bourcier T. Role of TLR-2 in the activation of nuclear factor kappaB by oxidative stress in cardiac myocytes. J Biol Chem. 2001;276(7):5197–5203. doi: 10.1074/jbc.M009160200. [DOI] [PubMed] [Google Scholar]
- 68.Wan T, Zhou X, Chen G, An H, Chen T, Zhang W, et al. Novel heat shock protein Hsp70L1 activates dendritic cells and acts as a Th1 polarizing adjuvant. Blood. 2004;103(5):1747–1754. doi: 10.1182/blood-2003-08-2828. [DOI] [PubMed] [Google Scholar]
- 69.Laflamme J, Akoum A, Leclerc P. Induction of human sperm capacitation and protein tyrosine phosphorylation by endometrial cells and interleukin-6. Mol Hum Reprod. 2005;11(2):141–150. doi: 10.1093/molehr/gah142. [DOI] [PubMed] [Google Scholar]
- 70.Rodriguez-Martinez H, Kvist U, Ernerudh J, Sanz L, Calvete JJ. Seminal plasma proteins: what role do they play? Am J Reprod Immunol. 2011;66(Suppl 1):11–22. doi: 10.1111/j.1600-0897.2011.01033.x. [DOI] [PubMed] [Google Scholar]
- 71.Aflatoonian R, Fazeli A. Toll-like receptors in female reproductive tract and their menstrual cycle dependent expression. J Reprod Immunol. 2008;77(1):7–13. doi: 10.1016/j.jri.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 72.Sioud M. Innate sensing of self and non-self RNAs by Tolllike receptors. Trends Mol Med. 2006;12(4):167–176. doi: 10.1016/j.molmed.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 73.Yao XD, Fernandez S, Kelly MM, Kaushic C, Rosenthal KL. Expression of Toll-like receptors in murine vaginal epithelium is affected by the estrous cycle and stromal cells. J Reprod Immunol. 2007;75(2):106–119. doi: 10.1016/j.jri.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 74.Vahdat F, Hurtgen JP, Whitmore HL, Seguin BE, Johnston SD. Decline in assayable progesterone in bovine plasma: effect of time, temperature, anticoagulant, and presence of blood cells. Am J Vet Res. 1981;42(3):521–522. [PubMed] [Google Scholar]
- 75.Gresack JE, Frick KM. Effects of continuous and intermittent estradiol treatments on memory in aging female mice. Brain Res. 2006;1115(1):135–147. doi: 10.1016/j.brainres.2006.07.067. [DOI] [PubMed] [Google Scholar]
- 76.Gresack JE, Frick KM. Post-training estradiol enhances spatial and object memory consolidation in female mice. Pharmacol Biochem Behav. 2006;84(1):112–119. doi: 10.1016/j.pbb.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 77.Karagenc L, Lane M, Gardner DK. Oestradiol, cyclodextrinencapsulated 17beta-oestradiol and the oestradiol solubilizer 2-hydroxypropyl-beta-cyclodextrin all impair preimplantation mouse embryo development. Reprod Biomed Online. 2004;9(3):280–286. doi: 10.1016/s1472-6483(10)62142-6. [DOI] [PubMed] [Google Scholar]
- 78.Koster F, Felberbaum R, Finas D, Wunsch K, Schulz C, Diedrich K, et al. Progesterone and estradiol enhance lipid mediated transfection of Sk-Br-3 mammalian cancer cells. Int J Mol Med. 2002;9(6):617–620. [PubMed] [Google Scholar]
- 79.Rucker B, Pochmann D, Furstenau CR, Carneiro-Ramos MS, Battastini AM, Barreto-Chaves ML, et al. Effects of steroid hormones on synaptosomal ectonucleotidase activities from hippocampus and cortex of adult female rats. Gen Comp Endocrinol. 2005;140(2):94–100. doi: 10.1016/j.ygcen.2004.10.008. [DOI] [PubMed] [Google Scholar]