Abstract
Objective
Many studies have been published on the antioxidative effects of boric acid (BA) and sodium borates in in vitro studies. However, the boron (B) concentrations tested in these in vitro studies have not been selected by taking into account the realistic blood B concentrations in humans due to the lack of comprehensive epidemiological studies. The recently published epidemiological studies on B exposure conducted in China and Turkey provided blood B concentrations for both humans in daily life and workers under extreme exposure conditions in occupational setting. The results of these studies have made it possible to test antioxidative effects of BA in in vitro studies within the concentra- tion range relevant to humans. The aim of this study was to investigate the protective ef- fects of BA against oxidative DNA damage in V79 (Chinese hamster lung fibroblast) cells. The concentrations of BA tested for its protective effect was selected by taking the blood B concentrations into account reported in previously published epidemiological studies. Therefore, the concentrations of BA tested in this study represent the exposure levels for humans in both daily life and occupational settings.
Materials and Methods
In this experimental study, comet assay and neutral red uptake (NRU) assay methods were used to determinacy to toxicity and genotoxicity of BA and hydrogen peroxide (H2O2).
Results
The results of the NRU assay showed that BA was not cytotoxic within the tested concentrations (3, 10, 30, 100 and 200 µM). These non-cytotoxic concentrations were used for comet assay. BA pre-treatment significantly reduced (P<0.05, one-way ANOVA) the DNA damaging capacity of H2O2 at each tested BA concentrations in V79 cells.
Conclusion
Consequently, pre-incubation of V79 cells with BA has significantly reduced the H2O2-induced oxidative DNA damage in V79 cells. The protective effect of BA against oxidative DNA damage in V79 cells at 5, 10, 50, 100 and 200 μM (54, 108, 540, 1080, and 2161 ng/ml B equivalents) concentrations was proved in this in vitro study.
Keywords: Boric Acid, Boron, Comet Assay, Oxidative DNA Damage
Introduction
Boron (B) is the fifth element with the symbol "B" in the periodic table. B does not exist as elemental form in the environment, whereas it is generally found as borates, borax, boric acid (BA), colemanite, ulexite, etc. BA and sodium borates are the most widely used B compounds in the industrial, agricultural, and medicinal products (1,3). The previously published studies are consistently pointed out that B is an essential element for plants and beneficial in certain concentrations also for humans (4,5). Accordingly, B supplementation has a beneficial effect on the bone mineral density, brain function, cognitive performance, regulation of the normal inflammatory response, and lipid levels in serum, as well as B can be protective against lipid peroxidation, oxidative stress, DNA damage and prostate cancer by inhibiting the prostate specific antigen (6,15). In spite of these well-known beneficial effects of B in humans, BA and sodium borates are classified as toxic to reproduction and development (Category 1B, H360DF) in the classification, labeling and packaging (CLP) regulation and included into the candidate list of substances of very high concern (16). This classification is mainly based on the results of experimental studies in animals. Accordingly no-observed-adverse-effectslevels (NOAELs) for B mediated toxic effects on the development and reproduction in rats were identified as 9.6 and 17.5 mg/kg/day, respectively (17).
Turkey possesses the largest B reserves in the world. As a natural consequence of this situation many people living in the south Marmara region around the B deposits and mining areas are exposed to high level of B (18,20). Therefore, the classification of BA and sodium borates in Category 1B (H360DF) has initiated public concern about the potential unfavorable effects of high level of B exposure in the people living in such residential areas. From this point of view, investigating the antioxidative or other beneficial effects of B compounds might be considered to have a lesser value. However, it should be kept in mind that in the CLP regulation of the chemicals are assigned to the hazard categories according to the hazard assessment procedure. It simply means that risk assessment have no value in assigning the chemicals to hazard categories in the CLP regulation. Therefore, certain levels of daily B intake (or exposure) might still be safe and beneficial. Indeed B mediated reprotoxic effects have not been proven in recently published major epidemiological studies conducted in China and Turkey (9,21,23). Both studies have concluded that human B exposures, even in the highest exposure cohorts, are too low to reach the blood (and target tissue) concentrations that would be required to exert adverse effects on reproductive functions (22,24). Moreover, protective effects of B exposure have also been reported on the sperm morphology, sperm motility and DNA integrity in the semen samples of manufacturing workers under the exposure conditions of the BA production plant in B andırma, Turkey (21,23). Consequently, the key parameter which determines the benefit and harm is the daily B intake level.
The present study aimed to investigate the protective effect of BA on oxidative DNA damage in V79 cells with BA concentrations relevant to humans. The B concentrations tested in this study are based on the blood B concentrations in humans reported in the recently published epidemiological studies in China and Turkey (19, 22). The potential DNA damaging effect of hydrogen peroxide (H2O2) was tested in V79 cells pre-incubated with increasing concentrations (5, 10, 50, 100 and 200 μM) of BA using the alkaline comet assay. The possible cytotoxic effects of BA were identified using the NRU.
Materials and Methods
This study was conducted in the laboratory of Ankara University Faculty of Pharmacy Department of Pharmaceutical Toxicology in 2013.
Chemicals
BA and H2O2 were purchased from Sigma-Aldrich (Germany). For cell culture, we used Dulbecco’s Modified Eagle’s Medium (DMEM, Biological Industries, Israel) and fetal calf serum (FCS, Sigma- Aldrich, Germany). Dimethyl sulfoxide (DMSO) was the product of Merck (Germany). The NR solution, normal melting point agarose (NMA) and low melting point agarose (LMA) were purchased from Sigma-Aldrich (Germany). Sodium chloride (NaCl), disodium ethylene diaminetetraacetic acid (Na2EDTA), Triton-X 100, Tris and sodium sarcosinate were purchased from Amresco (OH, USA). Ethidium bromide (EtBr) was purchased from Sigma-Aldrich (Germany) for fluorescent dying in the comet assay.
Setting the pre-treatment concentrations of boric acid
This experimental study is an original article conducted to determine the protective effect of BA against the H2O2-induced oxidative DNA damage in V79 cells that was tested at concentrations representing the blood B levels in humans. Accordingly the most recent epidemiological studies conducted in China and Turkey was comprehensively reviewed (19-23). The highest mean blood B concentrations reported in China and Turkey for the B exposed workers were 499.2 ± 790.6 ppb (20.4–3568.9) and 223.89 ± 69.49 ng/g (152.82–454.02), respectively, as shown in table 1. The extreme blood B concentrations determined in China surely reflects extreme daily B intake levels accompanied with poor hygienic conditions. Therefore, such a high level of blood B concentration seems not possible in western countries applying the standard risk management regulations in their workplaces. Nevertheless we decided to fix the upper concentration of BA at 200 μM (corresponds to 2163 ppb B) in investigating the protective role of BA against H2O2- induced DNA damage.
The mean blood B concentrations of the control workers reported in the above mentioned epidemiological studies were taken into consideration in deciding to the lowest test concentration for BA. The mean blood B concentration of the Chinese control group representing the sampling period of 2004 was comparable to the blood B concentration of the control group from the Turkish study (Table 1).
Table 1.
The blood boron (B) concentrations reported in the epidemiological studies conducted in China and Turkey
| Blood B concentrations (ppb) reported in the epidemiological study conducted in China | ||||
|---|---|---|---|---|
| Member | Control | Community comparison | Exposed | |
| Xing, 2008 (sampled in 2003) | 22.1 ± 6.7 (14.0–33.2) | - | 204.8 ± 356.8 (27.1–2003.5) | |
| Xing, 2008 (sampled in 2004) | 48.0 ± 23.9 (8.2–113.0) | 96.5 ± 90.8 (3.3–536) | 499.2 ± 790.6 (20.4–3568.9) | |
| Blood B concentrations (ng/g) reported in the epidemiological study conducted in Turkey | ||||
| Control | Low exposure | Medium exposure | High exposure | |
| Duydu, 2011 | <48.5 | 72.94 ± 15.43 (48.46–99.91) | 121.68 ± 15.62 (100.51–146.07) | 223.89 ± 69.49 (152.82–454.02) |
Mean ± SD, range in parenthesis. Community comparison are not working in the B industry but living in the B reach area.
On the other hand, Yazbeck et al. (25) reported a study on the correlation between B concentrations in drinking water and blood B concentrations in Northern France. According to this study, the mean blood B concentration was 123 ng/g, for the population living in municipalities with water B levels less than 0.3 mg/L. The current drinking water limits for B are 1 mg/L and 2.4 mg/L in the European Union (EU) Drinking Water Directive (98/83/EC) and World Health Organization (WHO) Guidelines for Drinking Water Quality 4th ed. (2011), respectively. Accordingly we decided to set the lowest B concentration to 5 μM (corresponds to 54 ppb B). Thus, the concentration range which we selected to study the protective effect of B against oxidative DNA damage was based on the results of the epidemiological studies. The BA concentrations that we used in this study and the corresponding B equivalents are compiled in table 2.
Cytotoxicity of BA in V79 cells was determined by means of the NRU assay as described previously (26, 27). Briefly, 1×104 cells were plated in 0.2 ml DMEM (with 10% FCS and 1% penicillin/streptomycin) per well in 96-well tissue-culture plates and allowed to attach and grow for 24 hours at 37˚C. BA (3, 10, 30, 100, and 200 μM) were then added to the cell culture medium. After 18 hours, the medium was replaced by fresh medium containing 50 μg/ml NR solution, and the incubation was continued for 3 hours at 37˚C. Thereafter, the medium was withdrawn, and cells were washed two times with phosphate buffered saline (PBS), and fixed with 0.2 mL glacial acetic acid/ water/ethanol (1:49:50, v/v/v) per well; the plates were shaken for 20 minutes to solubilize the NR. Then NR absorbance was measured at 540 nm (SpectraMax, Molecular Devices Inc., USA).
Table 2.
The boric acid concentrations used in pre-treatment of V79 cells
| Boric acid concentrations used in pre-treatment of V79 cells | |||||
|---|---|---|---|---|---|
| H3BO3(μM) | 5 | 10 | 50 | 100 | 200 |
| H3BO3, ppb (ng/ml) | 309 | 618 | 3090 | 6180 | 12360 |
| B equivalent, ppb (ng/ml) | 54 | 108 | 540 | 1080 | 2161 |
Molecular weight of H3BO3: 61.83 g/mol, atomic weight of B: 10.81 g/mol and conversion factor for equivalent dose of B: 0.1748.
Comet assay
The alkaline comet assay was based on the standard method as described earlier (28-30) with minor modifications. Initially, 5×104 V79 cells were seeded into 25 cm2 flasks containing DMEM with 10% FCS and cultured for 48 hours at 37˚C. The cells were pre-treated with BA at the concentrations of 5, 10, 50, 100 and 200 μM for 16 hours at 37˚C. Thereafter, the cells were treated with H2O2 at two concentrations (50 and 100 μM) for 1 hour at 37˚C. Afterwards the cells were harvested in appropriate manner and the cell suspensions (1-2×104 cells/50 μL) were mixed with 100 μL of LMA (0.5%, in PBS, Sigma- Aldrich, Germany). These final cell suspensions were rapidly pipetted onto the pre-coated slides with NMA (1%), allowed to spread using a cover slip, and maintained on an ice-cold flat tray for 5 minutes for solidification.
After removal of the cover slip, the slides were immersed into cold lysing solution (2.5 M NaCl,100 mM Na2EDTA, 10 mM Tris, and 1% sodium sarcosinate at pH=10) containing freshly added 1% Triton-X 100 and 10% DMSO and were left for at least 1 hour at 4˚C. The untreated cells, the cells treated with solely BA, and the cells treated with H2O2 were not immersed simultaneously into same lysing solution. The slides were removed from the lysing solution, drained, and placed side by side in a horizontal gel electrophoresis tank. The tank was filled with freshly prepared electrophoresis solution (1 mM Na2EDTA and 300 mM NaOH, pH=13). The time of alkali denaturation and electrophoresis (24 V, 300 mA) was 20 minutes each. Afterwards the slides were neutralized with tris buffer (0.4 M Tris, pH=7.5) and allowed to stand for 5 minutes in room temperature (the neutralization step was repeated 3 times). The slides were stained with 65 μL of EtBr (20 μg/mL), covered with a cover slip and analyzed within 3-4 hours. Slides were examined on a fluorescence microscope (Leica DM 1000, Germany) with the Comet Assay IV Software. The Images of 100 randomly selected cells were analyzed for each group. Tail % intensity was used as the measure of the DNA damage in V79 cells.
Statistical analysis
The SPSS (SPSS Inc., USA) for Windows Release 20.0 was used for all data analysis. The results from the comet assay were expressed as median, and the results of the tail intensities of the control and the treated groups were statistically compared using one-way ANOVA test. Post hoc analysis of group differences was performed by the Fisher’s least significant difference (LSD) test. The limit for statistical significance was fixed as P<0.05.
Results
Cytotoxicity assay
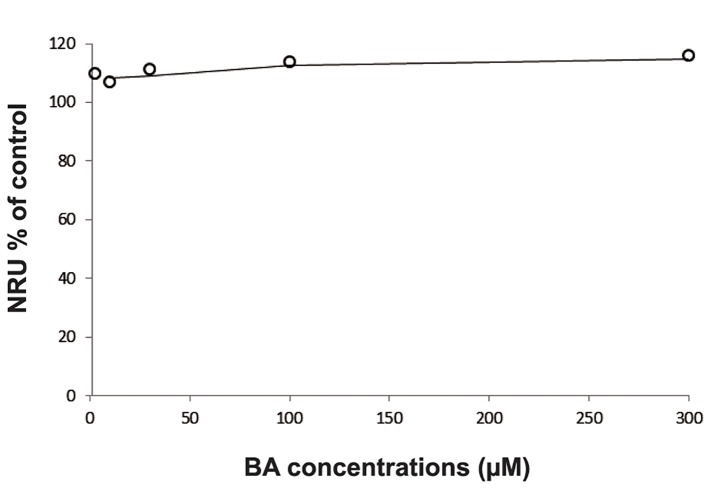
According to the results of the NRU assay BA was not cytotoxic within the tested concentrations (3, 10, 30, 100 and 200 µM). This concentration range covers the BA concentrations ( (5, 10, 50, 100 and 200 µM) tested for its protective effect against H2O2-mediated DNA damage in V79 cells. It proves that the comet assay was performed at non-cytotoxic concentrations (Fig .1).
Fig.1.
The NRU assay results of BA in V79 cells.
NRU; Ndeutral red uptake and BA; Boric acid.
Comet assay
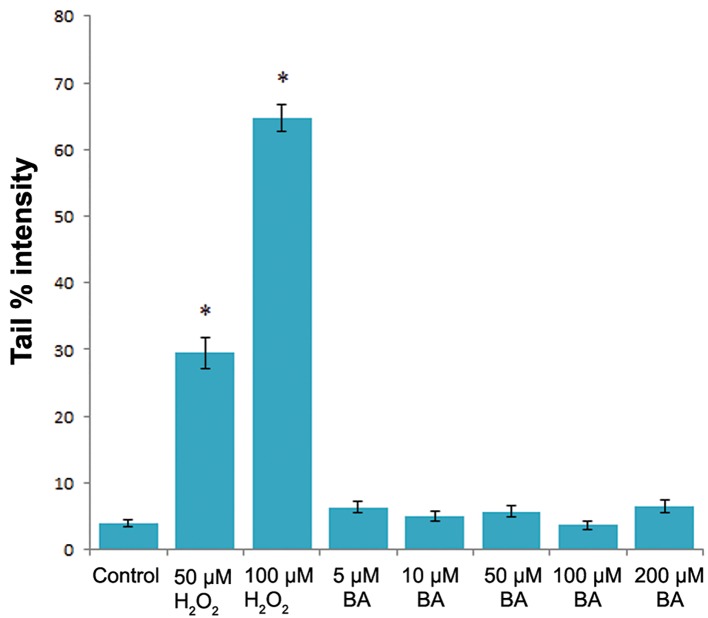
H2O2 was used as DNA-damaging agent in V79 cells. Both 50 and 100 μM H2O2 induced statistically significant (P<0.05, one-way ANOVA) DNA damage when compared with the control (Fig .2). The increasing concentrations of BA were also tested for its effects on the DNA integrity of V79 cells. However, statistically significant difference Protective Ef fect of BA on Oxidative DNA Damage in tail % intensity values between control and exposure groups were not determined (P>0.05, one-way ANOVA) as shown in figure 2. On the other hand BA pre-treatment significantly reduced (P<0.05, one-way ANOVA) the DNA damaging capacity of H2O2 at each tested BA concentrations in V79 cells (Fig .3,4).
Fig.2.
The level of the DNA damge in V79 cells treated with H2O2 and BA. The "tail % intensity" was used at the measure of the DNA damage.
*; Statistically significant (P˂0.05, one-way ANOVA) and BA; Boric acid.
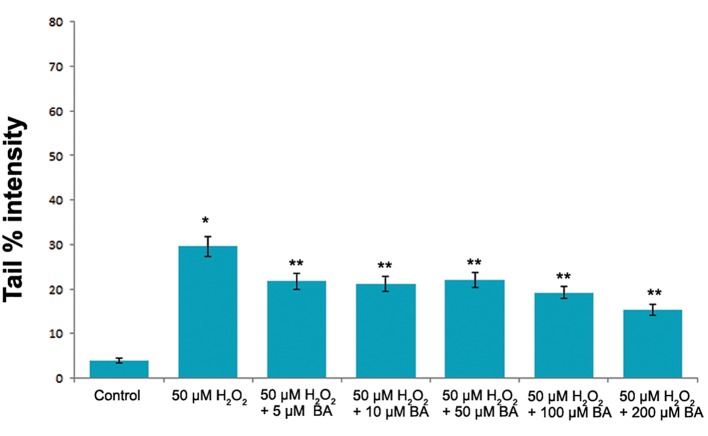
Fig.3.
The levels of the DNA damge in V79 cells induced by 50 µM H2O2. The DNA damage was significantly lower in V79 cells pre-induced with BA.
*; Significantly higher than the control (P˂0.05, one-way ANOVA), **; Significantly lower than the DNA damage induced by 50 µM H2O2(P˂0.05, one-way ANOVA) and BA; Boric acid.
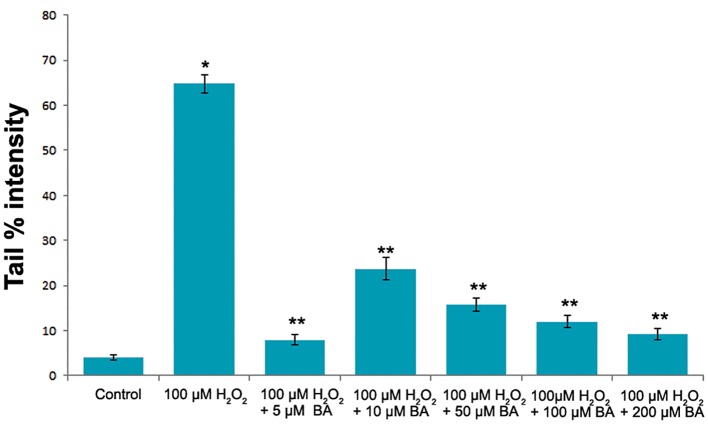
Fig.4.
The levels of the DNA damge in V79 cells induced by 100 µM H2O2. The DNA damage was significantly lower in V79 cells pre-induced with BA.
*; Significantly higher than the control (P˂0.05, one-way ANOVA), **; Significantly lower than the DNA damage induced by 100 µM H2O2(P˂0.05, one-way ANOVA) and BA; Boric acid.
Discussion
As is known, BA and sodium borates are classified as toxic to reproduction and development in the CLP regulation and included into the candidate list of the substances of very high concern (16). These classifications have raised the public concern about the daily B exposure levels in the population living around the B deposits and mining areas in Turkey. However, these effects have not been proven in recently published comprehensive epidemiological studies conducted in China and Turkey (19,24).
On the other hand, it should be kept in mind that the chemicals are assigned to the hazard categories according to hazard assessment procedure in the CLP regulation. It simply means that risk assessment have no value in assigning the chemicals to hazard categories. In essence, all chemicals which are toxic to reproduction and development have threshold concentrations to exert their unfavorable effects as it is for BA. Therefore, the daily B intake levels lower than the identified threshold level should be considered as safe and maybe beneficial. Indeed, the available studies show that B is essential for plants and also for some higher animals as frogs and zebrafish (31). Although the studies failed to prove the essentiality of B in humans, numerous beneficial effects of B have been reported in many published studies. B eneficial effects on the strength and trabecular microarchitecture of bone (32), on the human central nervous system (33,34), on the prostate cancer by taking into account the inverse association between dietary B intake and prostate cancer (35,36), on the functions of vitamin D, estrogen, thyroid hormone, insulin, and progesterone (31), on the antioxidant enzyme activities(14), and on reducing the incidence of arthritis (37) are some of the well documented special features of B. Additionally, unfavorable effects of the B deprivation have also been documented in some of the above mentioned studies.
All of these studies indicate the benefits of B at the dietary intake levels. Nowadays some beneficial effects have also been reported at higher exposure levels in occupational settings. The mean daily B exposure in the high exposure group in B andırma (Turkey) BA production plant was 14.45 ± 6.57 (3.32–35.62) mg/day (19). Although it depends on the use of some personal products and consumed food/water, the daily B intake is considered to be between 1-3 mg/day for humans in daily life (5). When this level of B intake is considered as normal, the daily B exposure in the above mentioned BA production plant might be considered as high. In spite of this high B exposure, some motility and morphology parameters of sperm samples collected from the exposure group were improving with increasing blood B concentrations (mean blood B concentration of high exposure group: 223.89 ± 69.49 ng/g) and the correlation between the dose response was statistically significant. Additionally the oxidative DNA damage in sperm cells was decreasing with increasing blood B concentrations in the same population and this association was also statistically significant (19, 21). These results support a dose dependent increase in the protection capacity of BA against the oxidative DNA damage in sperm cells of the workers employed in B andırma BA production plant. These results encouraged us to prove the protective effect of BA against H2O2-induced oxidative DNA damage at low and high concentrations reflecting the blood B concentrations of humans in daily life and occupational settings, respectively.
The lowest and highest BA concentrations used in the pre-incubation period of V79 cells were 5 and 200 µM which are corresponding to 54 and 2161 ng/ml B equivalents, respectively. These concentrations represent the blood B concentrations of control and high exposure workers in epidemiological studies conducted in China and Turkey (19,22).
The H2O2-induced oxidative DNA damage at both 50 and 100 µM concentrations were significantly reduced (P>0.05, one-way ANOVA) in V79 cells pre-incubated with 5, 10, 50, 100, and 200 µM (54, 108, 540, 1080, 2161 ng/ml B equivalents) BA concentrations. This result suggests a protective effect of BA against oxidative DNA damage at reasonable B exposure levels for humans in daily life or in occupational setting.
Conclusion
Consequently, in spite of the unfavorable effects of B in animal experiments at high doses, the daily B intake levels (at concentrations of lower than the threshold for reproductive and developmental toxicity) have beneficial effects in all tested living organisms including humans. Our study covers the daily B exposure as well as the occupational exposure conditions. The protective effect of BA against oxidative DNA damage has been demonstrated within these common and extreme exposure conditions. From this point of view, our results have supported the earlier studies on the antioxidant capacity of BA. However, further studies are needed to investigate the mechanism of the BA mediated protective effect against oxidative DNA damage.
Acknowledgments
This study was supported by Ankara University Research Fund ( Project Number: 12B3336007 ). There are no known conflicts of interest associated with this publication.
References
- 1.Becking GC, Chen BH. International Programme on Chemical Safety (IPCS) environmental health criteria on boron human health risk assessment. Biol Trace Elem Res. 1998;66(1-3):439–452. doi: 10.1007/BF02783154. [DOI] [PubMed] [Google Scholar]
- 2.WHO. Guidelines for Drinking-water Quality. Available from:http://apps.who.int/iris/bitstream/10665/44584/1/9789241548151_eng.pdf. (16 Aug 2015)
- 3.Toxicology review of boron and compounds. In support of summary information on the integrated risk information system (IRIS) National Center for Environmental Assessment; Available from:http://www.epa.gov/iris/toxreviews/0410tr.pdf. (16 Aug 2015) [Google Scholar]
- 4.Hunt CD. The biochemical effects of physiologic amounts of dietary boron in animal nutrition models. Environ Health Perspect. 1994;102(Suppl 7):35–43. doi: 10.1289/ehp.94102s735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dinca L, Scorei R. Boron in human nutrition and its regulations use. J Nutr Ther. 2013;2(1):22–29. [Google Scholar]
- 6.Meacham SL, Taper LJ, Volpe SL. Effects of boron supplementation on bone mineral density and dietary, blood, and urinary calcium, phosphorus, magnesium, and boron in female athletes. Environ Health Perspect. 1994;102(Suppl 7):79–82. doi: 10.1289/ehp.94102s779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devirian TA, Volpe SL. The physiological effects of dietary boron. Crit Rev Food Sci Nutr. 2003;43(2):219–231. doi: 10.1080/10408690390826491. [DOI] [PubMed] [Google Scholar]
- 8.Ince S, Kucukkurt I, Cigerci IH, Fatih Fidan A, Eryavuz A. The effects of dietary boric acid and borax supplementation on lipid peroxidation, antioxidant activity, and DNA damage in rats. J Trace Elem Med Biol. 2010;24(3):161–164. doi: 10.1016/j.jtemb.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Armstrong TA, Spears JW, Lloyd KE. Inflammatory response, growth, and thyroid hormone concentrations are affected by long-term boron supplementation in gilts. J Anim Sci. 2001;79(6):1549–1556. doi: 10.2527/2001.7961549x. [DOI] [PubMed] [Google Scholar]
- 10.Eren M, Kocao lu Guclu B, Uyanık F, Karabulut N. The effects of dietary boron supplementation on performance, carcass composition, and serum lipids in Japanese quail. J Anim Vet Adv. 2006;5(12):1105–1108. [Google Scholar]
- 11.Penland JG. Dietary boron, brain function, and cognitive performance. Environ Health Perspect. 1994;102(Suppl 7):65–72. doi: 10.1289/ehp.94102s765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallardo-Williams MT, Maronpot RR, Wine RN, Brunssen SH, Chapin RE. Inhibition of the enzymatic activity of prostatespecific antigen by boric acid and 3-nitrophenyl boronic acid. Prostate. 2003;54(1):44–49. doi: 10.1002/pros.10166. [DOI] [PubMed] [Google Scholar]
- 13.Hunt CD, Idso JP. Dietary boron as a physiological regulator of the normal inflammatory response: a review and current research progress. J Trace Elem Exp Med. 1999;12:221–233. [Google Scholar]
- 14.Turkez H, Geyikoglu F, Tatar A, Keles S, Ozkan A. Effects of some boron compounds on peripheral human blood. Z Naturforsch C. 2007;62(11-12):889–896. doi: 10.1515/znc-2007-11-1218. [DOI] [PubMed] [Google Scholar]
- 15.Singh NP, Mccoy MT, Tice RR, Schneder EL. A simple technigue for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175(1):184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 16.European Chemicals Agency (ECHA) Proposal for Harmonised Classification and Labelling. Substance name: boric acid. Available from:http://echa.europa.eu/documents/10162/13626/clh_report_boric_acid_en.pdf. (16 Aug 2015)
- 17.European Chemicals Agency(ECHA) Committee for Risk Assessment: opinion on new scientific evidence on theuse of boricacidandborates in photographic applications by consumers. Available from:https://echa.europa.eu/documents/10162/13641/rac_opinion_borates_20100429_en.pdf. (16 Aug 2015)
- 18.Korkmaz M, Sayli U, Sayli BS, Bakirdere S, Titretir S, Yavuz Ataman O, et al. Estimation of human daily boron exposure in a boron-rich area. Br J Nutr. 2007;98(3):571–575. doi: 10.1017/S000711450770911X. [DOI] [PubMed] [Google Scholar]
- 19.Duydu Y, Basaran N, Ustundag A, Aydin S, Undeger U, Ataman OYet al. Reproductive toxicity parameters and biological monitoring in occupationally and environmentally boron-exposed persons in Bandirma, Turkey. Arch Toxicol. 2011;85(6):589–600. doi: 10.1007/s00204-011-0692-3. [DOI] [PubMed] [Google Scholar]
- 20.Duydu Y, Basaran N, Bolt HM. Exposure assessment of boron in Bandırma boric acid production plant. J Trace Elem Med Biol. 2012;26(2-3):161–164. doi: 10.1016/j.jtemb.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Duydu Y, Basaran N, Ustundag A, Aydin S, Undeger U, Ataman OY, et al. Assessment of DNA integrity (COMET assay) in sperm cells of boron-exposed workers. Arch Toxicol. 2012;86(1):27–35. doi: 10.1007/s00204-011-0743-9. [DOI] [PubMed] [Google Scholar]
- 22.Xing X, Wu G, Wei F, Liu P, Wei H, Wang C, et al. Biomarkers of environmental and workplace boron exposure. J Occup Environ Hyg. 2008;5(3):141–147. doi: 10.1080/15459620701845132. [DOI] [PubMed] [Google Scholar]
- 23.Basaran N, Duydu Y, Bolt HM. Reproductive toxicity in boron exposed workers in Bandirma, Turkey. J Trace Elem Med Biol. 2012;26(2-3):165–167. doi: 10.1016/j.jtemb.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 24.Bolt HM, Basaran N, Duydu Y. Human environmental and occupational exposures to boric acid: Reconciliation with experimental reproductive toxicity data. J Toxicol Environ Health A. 2012;75(8-10):508–514. doi: 10.1080/15287394.2012.675301. [DOI] [PubMed] [Google Scholar]
- 25.Yazbeck C, Kloppmann W, Cottier R, Sahuquillo J, Debotte G, Huel G. Health impact evaluation of boron in drinking water: a geographical risk assessment in Northern France. Environ Geochem Health. 2005;27(5-6):419–427. doi: 10.1007/s10653-005-1796-6. [DOI] [PubMed] [Google Scholar]
- 26.Bonacker D, Stoiber T, Bohm KJ, Prots I, Wang M, Unger E, et al. Genotoxicity of inorganic lead salts and disturbance of microtubule function. Environ Mol Mutagen. 2005;45(4):346–353. doi: 10.1002/em.20100. [DOI] [PubMed] [Google Scholar]
- 27.Ustundag A, Behm C, Follmann W, Duydu Y, Degen GH. Protective effect of boric acid on leadand cadmium-induced genotoxicity in V79 cells. Arch Toxicol. 2014;88(6):1281–1289. doi: 10.1007/s00204-014-1235-5. [DOI] [PubMed] [Google Scholar]
- 28.Behm C, Degen GH, Follmann W. The Fusarium toxin enniatin B exerts no genotoxic activity, but pronounced cytotoxicity in vitro. Mol Nutr Food Res. 2009;53(4):423–430. doi: 10.1002/mnfr.200800183. [DOI] [PubMed] [Google Scholar]
- 29.Di Virgilio AL, Iwami K, Watjen W, Kahl R, Degen GH. Genotoxicity of the isoflavones genistein, daidzein and equol in V79 cells. Toxicol Lett. 2004;151(1):151–162. doi: 10.1016/j.toxlet.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Üstündağ A, Şimşek K, Ay H, Dündar K, Süzen S, Aydın A, et al. DNA integrity in patients undergoing hyperbaric oxygen (HBO) therapy. Toxicol In Vitro. 2012;26(7):1209–1215. doi: 10.1016/j.tiv.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 31.Nielsen FH, Meacham SL. Growing evidence for human health benefits of boron. J Evid Based Complementary Altern Med. 2011;16(3):169–180. [Google Scholar]
- 32.Nielsen FH, Stoecker BJ. Boron and fish oil have different beneficial effects on strength and trabecular microarchitecture of bone. J Trace Elem Med Biol. 2009;23(3):195–203. doi: 10.1016/j.jtemb.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 33.Penland JG. Quantitative analysis of EEG effects following experimental marginal magnesium and boron deprivation. Magnes Res. 1995;8(4):341–358. [PubMed] [Google Scholar]
- 34.Penland JG. The importance of boron nutrition for brain and psychological function. Biol Trace Elem Res. 1998;66(1-3):299–317. doi: 10.1007/BF02783144. [DOI] [PubMed] [Google Scholar]
- 35.Cui Y, Winton MI, Zhang ZF, Rainey C, Marshall J, De Kernion JB, et al. Dietary boron intake and prostate cancer risk. Oncol Rep. 2004;11(4):887–892. [PubMed] [Google Scholar]
- 36.Newnham RE. How boron is being used in medical practice. In: Goldbach HE, Brown PH, Rerkasem B, Thellier M, Wimmer MA, Bell RW, editors. Boron in plant and animal nutrition. 1st ed. NewYork: Kluwer Academic/Plenum; 2002. pp. 59–62. [Google Scholar]
- 37.Barranco WT, Kim DH, Stella SL Jr, Eckhert CD. Boric acid inhibits stored Ca2+ release in DU-145 prostate cancer cells. Cell Biol Toxicol. 2009;25(4):309–320. doi: 10.1007/s10565-008-9085-7. [DOI] [PubMed] [Google Scholar]