Abstract
Objective. RA predominantly affects post-menopausal women and is strongly associated with development of generalised osteoporosis. To find treatments that target both joint manifestations and osteoporosis in RA is desirable. The third generation of selective oestrogen receptor modulators (SERMs) [lasofoxifene (LAS) and bazedoxifene (BZA)] are new treatment options for post-menopausal osteoporosis. The aim of this study was to investigate the effects of LAS and BZA on arthritic disease and inflammation-associated bone loss using CIA in mice.
Methods. Female DBA/1 mice were ovariectomised and subjected to CIA as a model of post-menopausal RA. Mice received treatment with LAS, BZA, 17β-estradiol (E2) as reference or vehicle. Arthritis development was assessed and BMD was determined by peripheral quantitative CT of the femurs. Serologic markers of inflammation and cartilage destruction were analysed. Immune cells in lymph nodes were studied by flow cytometry.
Results. LAS and BZA reduced the clinical severity of arthritis as well as the grade of histologic synovitis and erosions on cartilage and bone. Moreover, SERMs protected against generalised bone loss in CIA by increasing trabecular BMD. Both SERMs decreased serum marker of cartilage destruction and LAS reduced serum IL-6 levels. SERMs did not alter Th17 cells in lymph nodes as E2 did.
Conclusion. The anti-osteoporotic drugs LAS and BZA were found to be potent inhibitors of joint inflammation and bone destruction in experimental arthritis. This study provides new important knowledge regarding the treatment regimen of post-menopausal women with RA who suffer from increased risk for osteoporosis.
Keywords: arthritis, selective oestrogen receptor modulators, oestrogen, osteoporosis
Rheumatology key messages
Lasofoxifene and bazedoxifene are potent inhibitors of experimental post-menopausal arthritis.
Lasofoxifene and bazedoxifene prevent osteoporosis during severe arthritic disease in mice.
Clinical trials are necessary to assess if post-menopausal RA patients could benefit from selective oestrogen receptor modulator treatment.
Introduction
RA is an autoimmune disease with a prevalence of 0.2–1% that is characterized by chronically inflamed joints [1]. The pathogenesis of RA involves both genetic and environmental factors that synergistically trigger an immune response towards oneself, however, the antigen remains unknown. Dendritic cells (DCs) and B cells present antigen on MHC class II molecules, express co-stimulatory molecules and produce cytokines, thereby activating T cells. Th17 cells are the major producers of IL-17 and constitute a key effector cell in autoimmune arthritis [2]. Conversely, Foxp3-expressing Tregs are essential to prevent autoimmunity [3]. Moreover, B cells produce autoantibodies in RA, including anti-collagen type II (anti-CII) and ACPA [4, 5].
Several types of skeletal manifestations are present in RA, including bone erosions, periarticular osteopenia and generalised osteoporosis with increased fracture risk [6–9]. About 50% of post-menopausal women with RA suffer from generalised osteoporosis, with multifactorial causes involving oestrogen deficiency, long-term glucocorticoid therapy and systemic inflammation [8, 10]. Bone is remodelled by bone-resorbing osteoclasts and bone-forming osteoblasts. Osteoclasts are differentiated from precursors of the monocyte/macrophage lineage in the presence of RANK ligand (RANKL) and M-CSF [11]. RANKL is produced by T and B lymphocytes, synovial fibroblasts and osteoblasts [12–14]. In addition, pro-inflammatory cytokines such as IL-17 and TNF contribute to excessive osteoclast formation and activation in RA [15, 16]. Furthermore, bone resorption in ovariectomy-induced osteoporosis is driven by TNF produced by T cells [17].
The female preponderance in RA (3:1) is an unresolved complex issue [18]. Obviously, attention should be paid to the impact of sex hormones. A substantial proportion of RA patients achieve disease remission during pregnancy [19]. Furthermore, early menopause is an independent predictor of RA and the incidence of RA accelerates after menopause [20, 21]. Treatment with oestrogen is protective in experimental arthritis [22, 23], while results from clinical studies with hormone replacement therapy of post-menopausal RA patients are inconclusive; however, reduced disease activity has been reported [24].
Selective oestrogen receptor modulators (SERMs) were developed in the search for a molecule that exhibits positive oestrogenic effects such as protection of bone, but is devoid of oestrogenic side effects such as breast and endometrial cancer. SERMs can act as either oestrogen receptor agonists or antagonists in a tissue-dependent manner. Raloxifene was the first SERM approved as a treatment of post-menopausal osteoporosis [25]. We have previously reported that raloxifene has anti-arthritic and bone-protective properties in an experimental model of post-menopausal RA–CIA in ovariectomised (OVX) mice [26, 27]. The third-generation SERMs include lasofoxifene (LAS) and bazedoxifene (BZA). LAS, the first SERM that prevents non-vertebral fractures, was approved 2009 in the European Union (EU) [28]. BZA is currently used in the EU for treatment of post-menopausal osteoporosis and is under late phases of registration in the USA. In high-risk fracture patients, BZA also protects from non-vertebral fractures [29].
The development of a new generation of SERMs with enhanced tissue specificity, more pronounced effects on bone and improved safety profiles sheds new light on the therapeutic potential of exogenous hormone-like compounds. Thus this study aimed to determine if the third-generation SERMs—LAS and BZA—can improve the severity and progression of arthritis as well as prevent osteoporosis in arthritic disease.
Materials and methods
The regional ethical review board in Gothenburg approved this study. Female DBA/1 mice (Taconic, Ry, Denmark) were kept in groups of seven to eight animals in each cage, under standard environmental conditions and fed with soy-free laboratory chow and tap water ad libitum. At 8–10 weeks of age, ovaries were removed under anaesthesia, through skin and peritoneal incisions, as described previously [30]. Mice were treated 5 days a week from the first signs of arthritis (day 18) with s.c. injections of 17β-estradiol-3-benzoate (E2, 1 μg/mouse/day; Sigma, St Louis, MO, USA), LAS (4 μg/mouse/day; Pfizer, New York, NY, USA) or BZA (24 μg/mouse/day; Pfizer). All substances were dissolved in inert oil (Recip, Årsta, Sweden) and control mice received oil only. Doses were chosen based on their capacity to protect from OVX-induced osteoporosis [31]. Body surface area calculations ensured that LAS and BZA doses used in mice were similar to human doses [28, 29, 32]. LAS and BZA were gifts from Pfizer.
Induction and evaluation of arthritis
Two weeks after ovariectomy, mice were immunized with 100 μg chicken CII (Sigma) in 0.1 M acetic acid and emulsified in an equal volume of Freund’s incomplete adjuvant (Sigma) supplemented with 0.5 mg/ml Mycobacterium tuberculosis H37 RA [Becton Dickinson (BD), Franklin Lakes, NJ, USA] (day 0). Each mouse received 100 μl emulsion injected s.c. at the base of the tail. Immunization was repeated after 28 days, without mycobacteria. Arthritis development was scored by examining mice every other day in a blinded manner regarding treatment groups. Arthritis severity was scored (0–3) for each paw, with a maximum of 12 points per mouse, determined as follows: 1 = swelling or erythema in one joint, 2 = swelling or erythema in two joints, 3 = severe swelling or erythema of more than two joints, or ankylosis of the entire paw. Mice were anaesthetized with ketamine (Pfizer) and medetomidine (Orion Pharma, Dhaka, Bangladesh), bled and killed by cervical dislocation. Sera were stored at −20°C. Paws were placed in 4% formaldehyde, decalcified and embedded in paraffin. Tissue sections were stained with eosin and haematoxylin. Synovitis and erosions were separately scored from 0 to 3 (0 = normal appearance, 1 = mild, 2 = moderate, 3 = severe synovitis and/or cartilage and bone erosions). A histopathological score was calculated by adding the scores from all evaluated joints in each animal.
Tissue collection and single cell preparation
Uterine wet weights were recorded. Bone marrow (BM) cells were harvested by flushing the cavity of one femur and one humerus with PBS. Lymph nodes draining the joints (subiliac, popliteal, sciatic, proper and accessory axillary) were dissected and mashed through a 70 μm nylon mesh filter and re-suspended in complete medium [phenol red-free RPMI 1640 (PAA Laboratories, Pasching, Austria) supplemented with 10% dextran-coated charcoal hormone-stripped FCS (Sigma) and 1% penicillin-streptomycin-l-glutamine solution (Sigma)]. Erythrocytes in BM were lysed by using Tris-buffered 0.83% NH4Cl solution. Cells were counted using an automated cell counter (Sysmex Europe, Nordenstedt, Germany).
Proliferation assay
Lymph node cells in complete medium [with 5 mM of 2-mercaptoethanol (Sigma)] were cultured at 2 × 105 cells per well in flat-bottomed 96-well plates (Nunc, Roskilde, Denmark) at 37°C and 5% CO2. The T cell mitogen concanavalin A (ConA; Sigma) was added at 1.25 μg/ml and control cells were cultured in medium without mitogen. All samples were set in triplicates. Thereafter 1 μCi [3H] thymidine (Perkin-Elmer, Waltham, MA, USA) per well was added for 21 h. Cells were harvested onto glass fibre filters and counted in a β-counter (Perkin-Elmer). Results are presented as a proliferation index (median of counts per minute in wells with ConA minus the median of counts per minute in control wells).
Flow cytometry
BM cells were stained with fluorochrome-conjugated anti-mouse antibodies for Gr-1, F4/80, M-CSFR/CD115 (Biolegend, San Diego, CA, USA) and CD11b (BD) to obtain pre-osteoclasts (CD11b+F480+Gr-1−M-CSFR+). Lymph node DCs and B cells were analysed by staining with antibodies for B220 (BD), MHC II, CD11c, CD8a and CD80 (Biolegend). DCs were defined as CD11chiCD8+ or CD11chiCD8− and B cells as B220+CD11c−. Staining of intracellular cytokines (IL-17) and transcription factors (Foxp3) was performed as described in detail elsewhere [30]. Th17 cells were defined as CD4+IL-17+ and Treg as CD4+Foxp3+CD25+. All cells were analysed in a FACS Canto II (BD) and data were processed in FlowJo version 8.8.6/10.0.5 (Three Star, Ashland, OR, USA). All analyses started with a singlet gate using FSC-H vs FSC-A, thereafter a lymphocyte gate or a live gate and, subsequently, gates for indicated populations.
Assessment of BMD
Femurs were placed in 4% formaldehyde for 2 days and thereafter stored in 70% ethanol until assessment of BMD. BMD was determined by performing a peripheral quantitative CT (pQCT) scan with Stratec pQCT XCT Research M software (version 5.4B; Norland, Fort Atkinson, WI, USA) at a resolution of 70 μm, as described previously [33]. Cortical BMD was determined with a mid-diaphyseal scan while trabecular BMD was determined with a metaphyseal scan, at a point 3% of the length of the femur from the growth plate. The inner 45% of the area was defined as the trabecular bone compartment. The precision (interassay variation) for pQCT was reported to be (in %CV) 2.0 (trabecular BMD) and 2.5 (cortical thickness).
Serum analysis
Serum levels of cartilage oligomeric matrix protein (COMP) were measured using the Animal COMP ELISA (AnaMar, Gothenburg, Sweden); levels of IL-6 were determined using the Quantikine ELISA mouse IL-6 (R&D Systems, Abingdon, UK); C-telopeptide of type I collagen (CTX-1) using the RatLaps (CTX-I) enzyme immunoassay (Immunodiagnostics Systems, Copenhagen, Denmark) and procollagen type I N-terminal propeptide (PINP) using the rat/mouse PINP enzyme immunoassay (Immunodiagnostics Systems, Copenhagen, Denmark) according to the manuals provided by the manufacturers. Anti-CII IgG antibodies in serum were quantified using an in-house ELISA, where low-binding 96-well plates (Nunc, Roskilde, Denmark) were coated with chicken CII (1 μg/ml) and blocked with 0.5% BSA (Sigma) in PBS. A pool of serum from CIA mice was serially diluted to obtain a standard curve where the top standard was set as 100 arbitrary units. Biotinylated F(ab′)2 fragments of goat anti-mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) was used as a secondary antibody. ExtrAvidin peroxidase (Sigma) was added, followed by tetramethylbenzidine substrate solution (Sigma) and 1 M H2SO4. Absorbance was measured with a SpectraMax Plus (Molecular Devices, Sunnyvale, CA, USA) at 450 nm for all assays. The precision (interassay variation) for ELISAs was reported by the manufacturers as (in %CV) 10.7–14.8 (CTX-I), 5.9–7.6 (COMP), 6.2–7.6 (IL-6) and 8.0–9.2 (PINP). For the in-house anti-CII IgG ELISA the interassay CV was 15.04%.
Statistical analysis
Statistical evaluations were performed using SPSS software 22.0.0.0 (IBM, Armonk, NY, USA) and GraphPad Prism version 6.0b (GraphPad Software, La Jolla, CA, USA). Normality was checked and logarithmic transformations were used when appropriate to ensure normal distribution of data. Treatment groups (E2, LAS and BZA) were compared with the vehicle group using analysis of variance. Analysis of covariance was used when adjustments for covariates were needed, that is, to adjust for day-to-day variation at termination. Dunnett’s post hoc test was used unless Levene’s test revealed unequal variances between the groups, then Dunnett’s post hoc test was used instead. The area under the curve (AUC) for severity and frequency of arthritis was calculated by the trapezoidal method. Scoring of the severity of arthritis and histopathological score was performed using an ordinal scale requiring non-parametric statistical evaluation, therefore the Kruskal–Wallis analysis of variance followed by Dunn’s post hoc test was used.
Results
LAS and BZA ameliorate arthritis in CIA
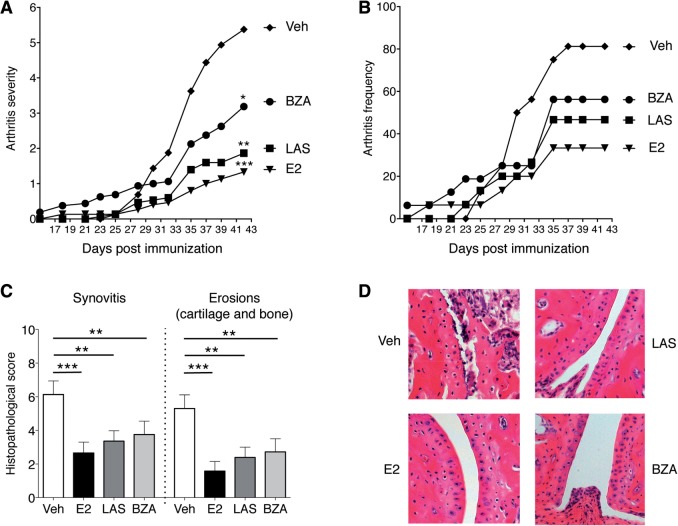
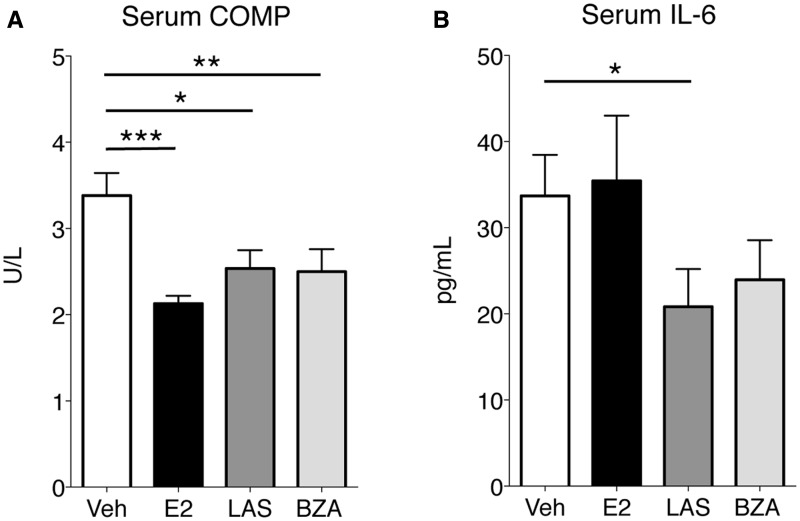
OVX mice were subjected to CIA and therapeutically treated 5 days per week with LAS, BZA, E2 or vehicle. Arthritis development was macroscopically assessed during the experiment. Comparison of the AUC of arthritis severity over time revealed that LAS was a potent inhibitor of arthritis, with a dramatic reduction in arthritis severity compared with vehicle (Fig. 1A). The inhibitory effect of BZA on arthritis was not as pronounced as for LAS, but was still significant compared with vehicle (Fig. 1A). As previously demonstrated, E2 treatment decreased arthritis severity compared with vehicle-treated mice (Fig. 1A). At termination (day 42), the mean arthritis frequency was highest in the vehicle group (81%), followed by BZA (56%), LAS (47%) and E2 (33%) (Fig. 1B). Similarly, the AUC for arthritis frequency over time was highest in the vehicle group, followed by SERMs and E2 (AUC for vehicle, 1009; BZA, 797; LAS, 600; E2, 471; Fig. 1B). Microscopic arthritis evaluation, specifying the degree of synovitis and erosions in cartilage and bone, showed decreased synovial inflammation and destruction of joints in all treatment groups compared with vehicle (Fig. 1C and D). Serum COMP was quantified as a measure of cartilage degradation, and all treatments decreased COMP (Fig. 2A). Furthermore, in order to assess systemic inflammation, the pro-inflammatory cytokine IL-6 was measured in serum. LAS decreased IL-6, and after BZA treatment, IL-6 levels appeared slightly reduced (non-significant), although levels were unaltered in mice treated with E2 (Fig. 2B). Uteri were weighed at termination as a measurement of classic oestrogen-related side effects. All treatments significantly increased uterine wet weights compared with vehicle [mean 16.8 mg (s.e.m. 1.2)], with the highest mean uterine weights in the E2-treated group [153.7 (6.5)], followed by LAS [68.9 (2.0)] and BZA [26.9 (2.7)]. To summarize, both SERMs efficiently inhibited the severity and progression of arthritis.
Fig. 1.
Macroscopic and microscopic arthritis development in CIA
OVX DBA/1 mice were subjected to CIA and treated with LAS, BZA, E2 or vehicle. (A and B) Macroscopic scoring of arthritis development (n = 15–16 mice/group). (A) Severity of arthritis, expressed as the mean. The area under curve was calculated for each treatment group. (B) Incidence of arthritis, presented as the mean. (C) Microscopic synovitis and erosions on bone and cartilage, presented as the median and interquartile range (n = 13–16 mice/group). Kruskal–Wallis analysis of variance and Dunn’s post hoc test were used in A and C. *P < 0.05, **P < 0.01, ***P < 0.001, compared with the vehicle group. (D) Representative histological photos of joints in the paws. BZA: bazedoxifene; E2: 17β-estradiol; LAS: lasofoxifene; OVX: ovariectomised; veh: vehicle.
Fig. 2.
Serum COMP and IL-6 levels
OVX DBA/1 mice were subjected to CIA and treated with LAS, BZA, E2 or vehicle. Serum concentrations of (A) COMP and (B) IL-6 were assessed by ELISA. Bars represent mean and s.e.m. Differences between treatments and vehicle were statistically analysed using analysis of variance and Dunnett’s post hoc test (A) or analysis of covariance with experiment day as the covariate and Dunnett’s post hoc test (B) on log data. n = 13–16 mice/group. *P < 0.05, **P < 0.01, ***P < 0.001. BZA: bazedoxifene; E2: 17β-estradiol; LAS: lasofoxifene; OVX: ovariectomised; veh: vehicle.
LAS and BZA preserve BMD in CIA mice
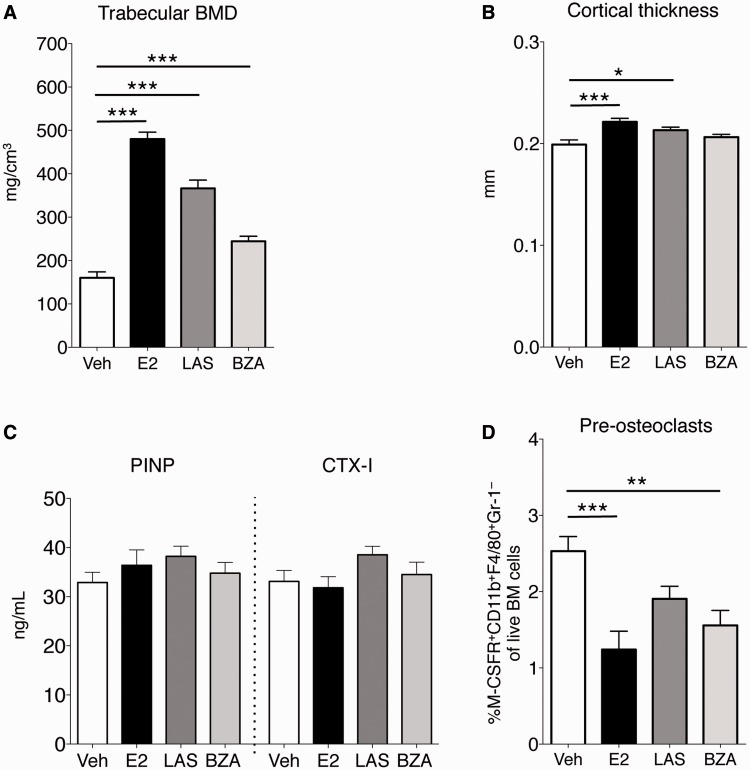
Femurs from CIA mice were analysed by pQCT. Treatment with E2, LAS and BZA protected against bone loss, illustrated by a dramatic increase in trabecular BMD in treatment groups compared with vehicle (Fig. 3A). Also, LAS and E2 increased cortical thickness, although BZA did not significantly affect cortical bone (Fig. 3B). Serum markers of bone formation (PINP) and bone resorption (CTX-1) were assessed, but no differences were found between the vehicle group and any of the treatment groups (Fig. 3C). BM cells were subjected to flow cytometry, and BZA and E2 reduced the percentage of pre-osteoclasts (CD11b+F4/80+Gr-1−M-CSFR+) in BM, and a tendency towards decreased pre-osteoclasts was seen after LAS treatment (Fig. 3D). In conclusion, LAS and BZA provided robust protection against generalised trabecular bone loss despite arthritic disease.
Fig. 3.
pQCT analysis of femurs
OVX DBA/1 mice were subjected to CIA and treated with LAS, BZA, E2 or vehicle. (A and B) Femurs were subjected to pQCT. (A) Trabecular BMD. (B) Cortical thickness. (C) Serum PINP and CTX-I, assessed by ELISA. (D) BM pre-osteoclasts were quantified using flow cytometry. Bars represent mean (s.e.m.). Differences between treatments and vehicle were analysed using analysis of variance and Dunnett’s post hoc test (A and B) or analysis of covariance with experiment day as the covariate and Dunnett’s post hoc test (C and D) on log data (D: CTX-I). n = 13–16 mice/group. *P < 0.05, **P < 0.01, ***P < 0.001. BM: bone marrow; BZA: bazedoxifene; E2: 17β-estradiol; LAS: lasofoxifene; OVX: ovariectomised; pQCT: peripheral quantitative CT; veh: vehicle.
SERMs regulate neither DCs nor T cells in CIA
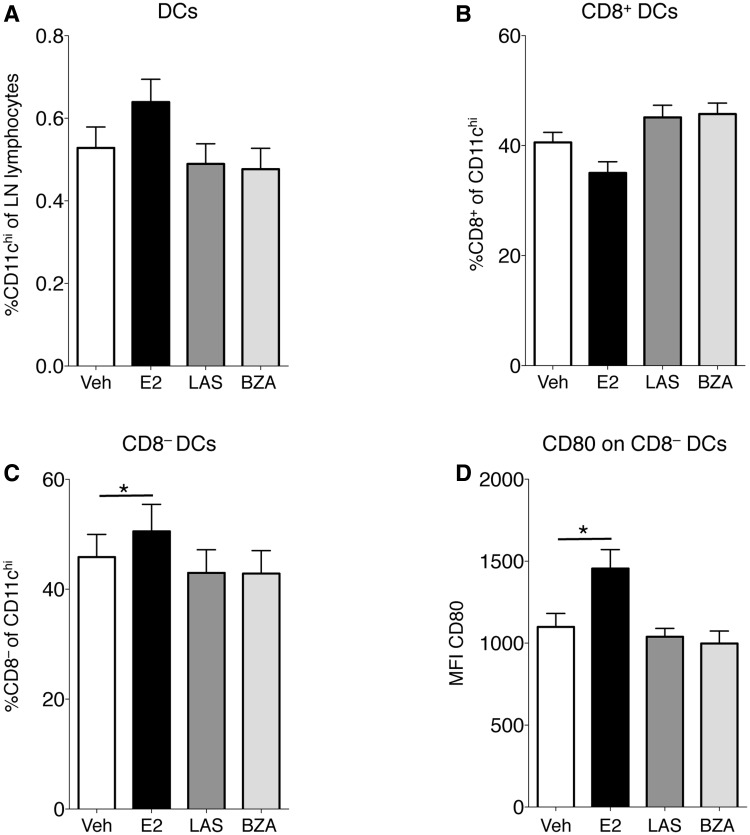
In order to investigate possible mechanisms for the anti-inflammatory effects of SERMs in arthritis, lymph nodes draining the joints were subjected to FACS analysis. DC subsets were studied and neither the frequencies of all CD11chi DCs nor CD11chiCD8+ DCs were significantly influenced by any of the treatments (Fig. 4A and B). Interestingly, CD11chiCD8− frequency was increased after E2 treatment, but not by any SERM (Fig. 4C). Furthermore, in order to assess the T cell–activating capacity of DCs, expression of the co-stimulatory molecule CD80 was analysed. The mean fluorescence intensity (MFI) of CD80 on CD8− DCs was significantly increased with E2 but remained unchanged with LAS and BZA (Fig. 4D).
Fig. 4.
Lymph node DC populations
OVX DBA/1 mice were subjected to CIA and treated with LAS, BZA, E2 or vehicle. Lymph node cells were analysed using flow cytometry and the percentage of (A) total DCs (CD11chi), (B) CD8+ DCs and (C) CD8− DCs are presented. (D) MFI of CD80 on CD8− DCs. Bars represent mean (s.e.m.). Differences between treatments and vehicle were analysed using ANOVA and Dunnett’s post hoc test (A, C and D) or analysis of covariance with experiment day as the covariate and Dunnett’s post hoc test (B). n = 13–16 mice/group. *P < 0.05. BZA: bazedoxifene; E2: 17β-estradiol; LAS: lasofoxifene; MFI: mean fluorescence intensity; OVX: ovariectomised; veh: vehicle.
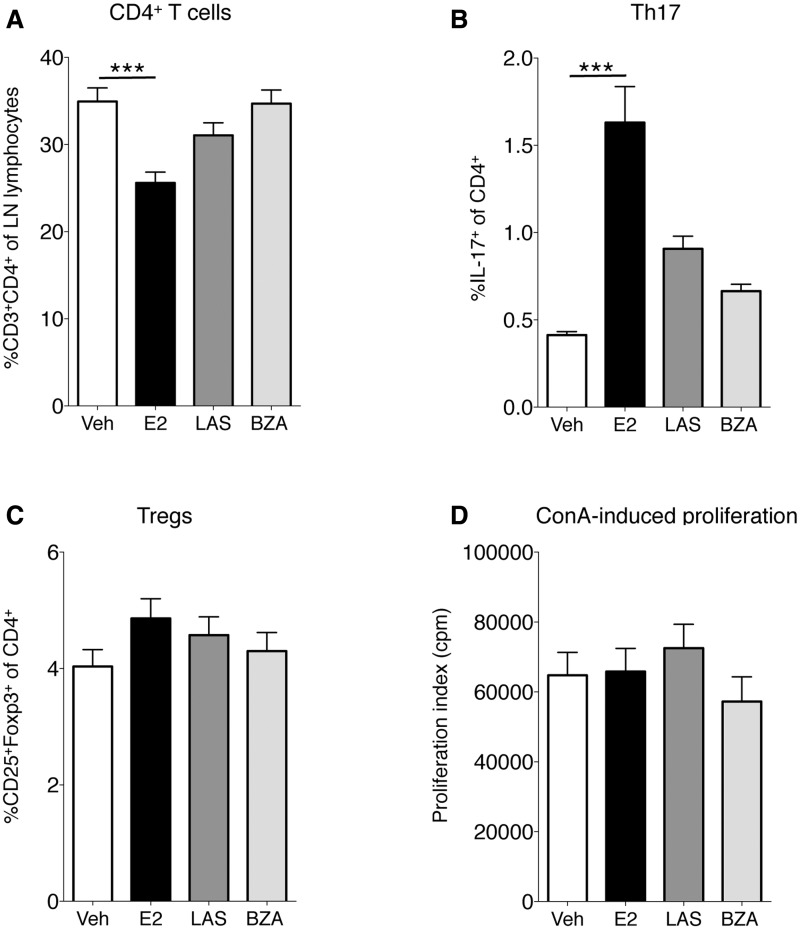
Th17 cells are pathogenic in RA, and we have recently shown that E2 regulates Th17 cell migration in experimental arthritis [30]. In that study, E2 increased Th17 cell frequency in lymph nodes and decreased Th17 cell levels in arthritic joints in CIA. Herein, CD4+ cells were generally decreased in lymph nodes after E2 treatment, but not by LAS or BZA (Fig. 5A). E2 increased lymph node Th17 cell frequency, however, LAS and BZA did not affect Th17 cells (Fig. 5B). No effect was seen on Treg frequency after treatment with E2 or SERMs (Fig. 5C). Finally, to assess functional T cell responses, an in vitro proliferation assay was performed. Lymph node cells were stimulated with the T cell mitogen ConA for 3 days. However, none of the treatments influenced the T cell proliferative activity in this assay (Fig. 5D).
Fig. 5.
Phenotypic analysis of Th cells and ConA-induced proliferation in lymph nodes
OVX DBA/1 mice were subjected to CIA and treated with LAS, BZA, E2 or vehicle. Lymph node cells were analysed using flow cytometry, defining (A) CD4+ cells (B) Th17 cells and (C) Tregs. (D) Lymph node cells were cultured with ConA and proliferation was measured by adding [3H]thymidine. Bars represent mean (s.e.m.). Differences between treatments and vehicle were analysed using analysis of covariance with experiment day as the covariate and Dunnett’s post hoc test (A, C and D) or analysis of variance and Dunnett’s T3 post hoc test (B). n = 13–16 mice/group. ***P < 0.001. ConA: concanavalin A; BZA: bazedoxifene; E2: 17β-estradiol; LAS: lasofoxifene; OVX: ovariectomised; veh: vehicle.
Effects of SERM on B cells in CIA
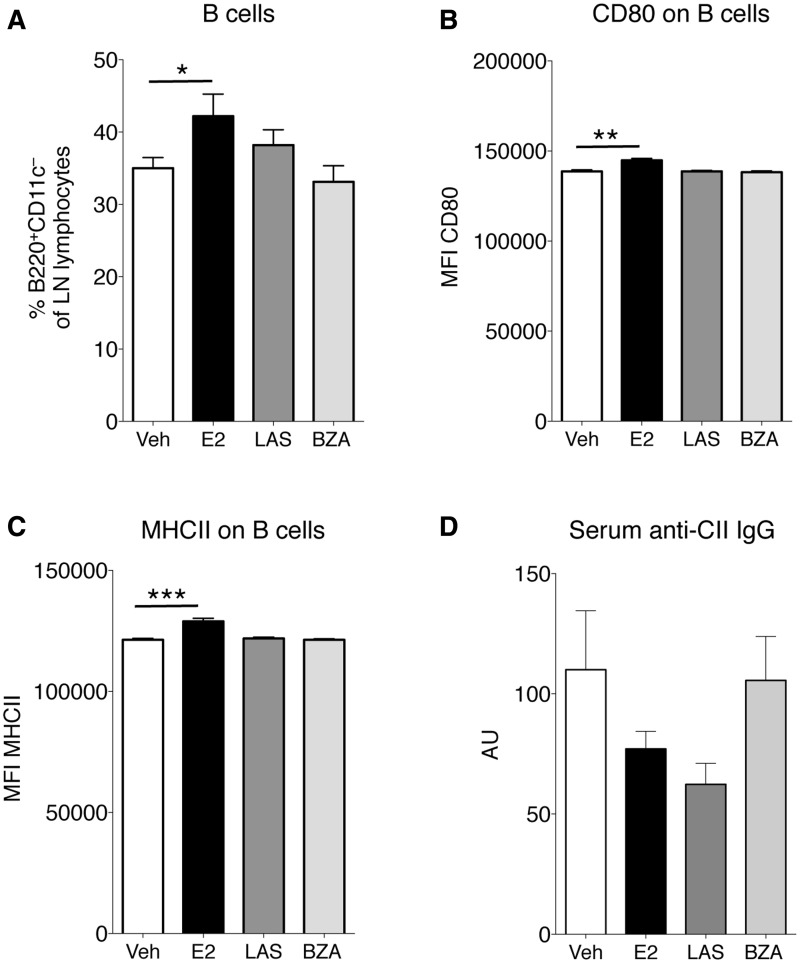
B cells were studied in lymph nodes of CIA mice and E2 increased the total B cell population; however, this population was unchanged after treatment with LAS or BZA (Fig. 6A). Phenotypic markers related to antigen presentation were also analysed on B cells. The expression of the co-stimulatory molecules CD80 (Fig. 6B) and MHC II (Fig. 6C) were increased after E2 treatment. Nevertheless, LAS and BZA had no effects on the expression of these molecules (Fig. 6B and C). Finally, serum levels of IgG antibodies directed towards CII were assessed, but none of the treatments significantly influenced levels of CII antibodies (Fig. 6D).
Fig. 6.
Analysis of lymph node B cells and serum CII antibodies
OVX DBA/1 mice were subjected to CIA and treated with LAS, BZA, E2 or vehicle. Lymph node cells were analysed using flow cytometry, defining (A) the percentage of B cells (B220+CD11c−), (B) MFI of CD80 on the B220+CD11c−CD80+ population and (C) MFI of MHC class II on the B220+CD11c−MHCII+ population. (D) Serum concentrations of IgG antibodies against collagen type II were assessed by ELISA. Bars represent mean (s.e.m.). Differences between treatments and vehicle were analysed using analysis of covariance with experiment day as the covariate and Dunnett’s post hoc test (A and C) or analysis of variance and Dunnett’s post hoc test (B and D). n = 13–16 mice/group. *P < 0.05, **P < 0.01, ***P < 0.001. BZA: bazedoxifene; E2: 17β-estradiol; LAS: lasofoxifene; MFI: mean fluorescence intensity; OVX: ovariectomised; veh: vehicle.
Discussion
Our study is the first to show that therapeutic treatment with the third-generation SERMs LAS and BZA potently inhibits arthritis. Importantly, these treatments also protect mice from osteoporosis during inflammatory disease.
CIA, a well-established model of human RA, contributed to development of several of the currently used biologic treatments, including TNF blockade and IL-1 receptor antagonists [34]. We have previously established that beyond oestrogen deficiency, inflammation contributes to bone loss in OVX mice with CIA [35]. The mice in the present study were treated therapeutically, which is, from a clinical point of view, the most relevant treatment regimen. Both SERMs potently inhibited arthritis; however, in the doses used in this study, LAS seemed to be more effective than BZA. While both SERMs exerted protective effects on trabecular bone, only LAS increased cortical thickness, in accordance with a previous study in castrated mice [36]. The finding that the third-generation SERMs have such a profound impact on joint synovitis, erosions and cartilage degradation in CIA make LAS and BZA promising candidates for future treatment of RA in post-menopausal women. We previously reported that the second-generation SERM raloxifene inhibits arthritis and bone loss in CIA, which after prophylactic treatment was associated with decreased serum levels of IL-6 and reduced spleen TNF mRNA levels [26]. Also in the present study, serum IL-6 was reduced after treatment with SERMs, although not reaching statistical significance for BZA.
We sought to define possible immunological targets underlying the anti-arthritic effects of LAS and BZA. In line with our recent study [30], E2 increased Th17 cells in the lymph nodes of CIA mice, an effect that was not observed after treatment with SERMs. The frequency of Tregs was not affected by either E2 or SERMs, however, the suppressive capacity of Tregs was not evaluated in this study. We have recently established that LAS and BZA completely lack effects on T lymphopoiesis in healthy mice and T cell–dependent inflammation in the skin delayed-type hypersensitivity model [37]. In this study, flow cytometry phenotyping of T cells from lymph nodes of CIA mice further indicates that SERMs do not affect T cells.
Both E2 and SERMs can regulate DCs in multiple ways [38]. In this study, we found that E2, but not SERMs, increased the CD8− DC population in lymph nodes of CIA mice and increased expression of the co-stimulatory molecule CD80 on these cells. Activated CD8− DCs induce a Th2 response [39]. Thus the E2-mediated increase of CD8− DCs is in line with the general view of E2 as an inducer of a Th2 shift, for example, during pregnancy [40].
B cells are crucial in the development of CIA [41]. The antigen-presenting capacity of lymph node B cells in CIA mice was studied and E2 increased expression of MHC II and CD80 on B cells, while LAS and BZA lacked these effects. However, the CD80-inducing effect of E2 is not likely involved in E2-mediated arthritis inhibition, since B cell CD80 expression has been demonstrated to be essential for arthritis development (in the proteoglycan-induced arthritis model) [42].
Antibodies against CII are undoubtedly pathogenic, since anti-CII antibody administration induces polyarthritis in mice [43]. However, none of the treatments significantly altered levels of anti-CII antibodies in CIA; nevertheless, both E2 and LAS reduced the levels of anti-CII antibodies ∼25%. Previous studies have shown that higher E2 doses than used herein inhibit anti-CII antibody levels in CIA [44]. We recently investigated the effects of LAS and BZA on B cells in healthy mice [31]. In that study, LAS and BZA suppressed BM B cell development, but at a later developmental stage than E2. Moreover, LAS and BZA lacked effects on antibody-producing cells, as opposed to E2. To summarize, results from this study suggest that SERMs do not primarily affect the frequency and phenotype of cells in the adaptive immune system or DCs in CIA. Hence we speculate that cytokine production—perhaps TNF production since its crucial in the pathogenesis of both ovariectomy-induced osteoporosis and arthritis—or other cells of the innate immune system might be regulated by SERMs, but further studies are required to define the mechanisms involved in the inhibitory effects of LAS and BZA in arthritis.
An increased risk of generalised osteoporosis and subsequent fractures are major concerns in chronic inflammatory diseases; in fact, RA patients have double the occurrence of fractures compared with matched controls [45]. Osteoporosis in RA patients is mainly treated with bisphosphonates, the most commonly used anti-osteoporotic drug, which have been shown to increase BMD in the lumbar spine and forearm in RA patients [46]. Moreover, denosumab (RANKL inhibition) increases spine and hip BMD in RA patients [47]. Both bisphosphonates and denosumab are more efficient in protecting against fractures in the general osteoporotic population compared with SERMs, but neither bisphosphonates nor denosumab is capable of reducing RA disease activity [46, 47].
In conclusion, the complex interaction between inflammation and bone in RA is an important target for therapy. The findings in our study establish that the anti-osteoporotic drugs BZA and LAS exhibit both anti-arthritic and bone-protective effects in experimental arthritis and are thus of great importance. Considering the large number of post-menopausal women with RA suffering from osteoporosis, we find it highly relevant to initiate clinical trials in order to evaluate the addition of a third-generation SERM to the treatment regimen of post-menopausal RA patients.
Acknowledgements
The authors wish to thank Anette Hansevi and Malin Erlandsson for excellent technical assistance, Christina Björklund and Margareta Rosenkvist for animal care, Anna E. Börjesson for collaboration on SERMs, Mattias N.D. Svensson for help with phenotypical analysis of DCs and Louise Grahnemo for help with the statistical analysis.
Funding: This work was supported by the Gothenburg Medical Society, COMBINE, the Swedish Research Council, King Gustav V’s 80 years’ Foundation, the Association Against Rheumatism, the Swedish Association for Medical Research and Sahlgrenska University Hospital, the Swedish Society of Medicine, the Wilhelm and Martina Lundgren Science Foundation, the Lars Hierta Foundation, the Magnus Bergvall Foundation, the Family Thöléns and Kristlers Foundation, the Ragnar Söderberg Foundation and the Åke Wiberg Foundation. The FACS Canto II instrument was bought thanks to generous support from the Inga-Britt and Arne Lundberg Foundation. Lasofoxifene and bazedoxifene were kind gifts from Pfizer.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1.Alamanos Y, Voulgari PV, Drosos AA. Incidence and prevalence of rheumatoid arthritis, based on the 1987 American College of Rheumatology criteria: a systematic review. Semin Arthritis Rheum 2006;36:182–8. [DOI] [PubMed] [Google Scholar]
- 2.Lubberts E. IL-17/Th17 targeting: on the road to prevent chronic destructive arthritis? Cytokine 2008;41:84–91. [DOI] [PubMed] [Google Scholar]
- 3.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol 2007;8:191–7. [DOI] [PubMed] [Google Scholar]
- 4.McInnes IB, Schett G. Mechanisms of disease the pathogenesis of rheumatoid arthritis. N Engl J Med 2011;365:2205–19. [DOI] [PubMed] [Google Scholar]
- 5.Cook AD, Rowley MJ, Mackay IR, Gough A, Emery P. Antibodies to type II collagen in early rheumatoid arthritis—correlation with disease progression. Arthritis Rheum 1996;39:1720–7. [DOI] [PubMed] [Google Scholar]
- 6.Stewart A, Mackenzie LM, Black AJ, Reid DM. Predicting erosive disease in rheumatoid arthritis. A longitudinal study of changes in bone density using digital X-ray radiogrammetry: a pilot study. Rheumatology 2004;43:1561–4. [DOI] [PubMed] [Google Scholar]
- 7.Scott DL. Prognostic factors in early rheumatoid arthritis. Rheumatology 2000;39(Suppl 1):24–9. [DOI] [PubMed] [Google Scholar]
- 8.Sinigaglia L, Nervetti A, Mela Q, et al. A multicenter cross sectional study on bone mineral density in rheumatoid arthritis. Italian Study Group on Bone Mass in Rheumatoid Arthritis. J Rheumatol 2000;27:2582–9. [PubMed] [Google Scholar]
- 9.Spector TD, Hall GM, McCloskey EV, Kanis JA. Risk of vertebral fracture in women with rheumatoid arthritis. BMJ 1993;306:558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forsblad d’Elia H, Larsen A, Waltbrand E, et al. Radiographic joint destruction in postmenopausal rheumatoid arthritis is strongly associated with generalised osteoporosis. Ann Rheum Dis 2003;62:617–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quinn JM, Elliott J, Gillespie MT, Martin TJ. A combination of osteoclast differentiation factor and macrophage-colony stimulating factor is sufficient for both human and mouse osteoclast formation in vitro. Endocrinology 1998;139:4424–7. [DOI] [PubMed] [Google Scholar]
- 12.Horwood NJ, Kartsogiannis V, Quinn JM, et al. Activated T lymphocytes support osteoclast formation in vitro. Biochem Biophys Res Commun 1999;265:144–50. [DOI] [PubMed] [Google Scholar]
- 13.Manabe N, Kawaguchi H, Chikuda H, et al. Connection between B lymphocyte and osteoclast differentiation pathways. J Immunol 2001;167:2625–31. [DOI] [PubMed] [Google Scholar]
- 14.Yasuda H, Shima N, Nakagawa N, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA 1998;95:3597–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobayashi K, Takahashi N, Jimi E, et al. Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. J Exp Med 2000;191:275–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kotake S, Udagawa N, Takahashi N, et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest 1999;103:1345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roggia C, Gao Y, Cenci S, et al. Up-regulation of TNF-producing T cells in the bone marrow: a key mechanism by which estrogen deficiency induces bone loss in vivo. Proc Natl Acad Sci USA 2001;98:13960–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oliver JE, Silman AJ. Why are women predisposed to autoimmune rheumatic diseases? Arthritis Res Ther 2009;11:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Man YA, Dolhain RJ, van de Geijn FE, Willemsen SP, Hazes JM. Disease activity of rheumatoid arthritis during pregnancy: results from a nationwide prospective study. Arthritis Rheum 2008;59:1241–8. [DOI] [PubMed] [Google Scholar]
- 20.Humphreys JH, Verstappen SM, Hyrich KL, et al. The incidence of rheumatoid arthritis in the UK: comparisons using the 2010 ACR/EULAR classification criteria and the 1987 ACR classification criteria. Results from the Norfolk Arthritis Register. Ann Rheum Dis 2013;72:1315–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pikwer M, Bergstrom U, Nilsson JA, Jacobsson L, Turesson C. Early menopause is an independent predictor of rheumatoid arthritis. Ann Rheum Dis 2012;71:378–81. [DOI] [PubMed] [Google Scholar]
- 22.Holmdahl R, Carlsten H, Jansson L, Larsson P. Oestrogen is a potent immunomodulator of murine experimental rheumatoid disease. Br J Rheumatol 1989;28(Suppl 1):54–8; discussion 69–71. [DOI] [PubMed] [Google Scholar]
- 23.Engdahl C, Jochems C, Windahl SH, et al. Amelioration of collagen-induced arthritis and immune-associated bone loss through signaling via estrogen receptor alpha, and not estrogen receptor beta or G protein-coupled receptor 30. Arthritis Rheum 2010;62:524–33. [DOI] [PubMed] [Google Scholar]
- 24.Forsblad d’Elia H, Larsen A, Mattsson LA, et al. Influence of hormone replacement therapy on disease progression and bone mineral density in rheumatoid arthritis. J Rheumatol 2003;30:1456–63. [PubMed] [Google Scholar]
- 25.Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA 1999;282:637–45. [DOI] [PubMed] [Google Scholar]
- 26.Jochems C, Islander U, Kallkopf A, et al. Role of raloxifene as a potent inhibitor of experimental postmenopausal polyarthritis and osteoporosis. Arthritis Rheum 2007;56:3261–70. [DOI] [PubMed] [Google Scholar]
- 27.Jochems C, Lagerquist M, Hakansson C, Ohlsson C, Carlsten H. Long-term anti-arthritic and anti-osteoporotic effects of raloxifene in established experimental postmenopausal polyarthritis. Clin Exp Immunol 2008;152:593–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cummings SR, Ensrud K, Delmas PD, et al. Lasofoxifene in postmenopausal women with osteoporosis. N Engl J Med 2010;362:686–96. [DOI] [PubMed] [Google Scholar]
- 29.Silverman SL, Chines AA, Kendler DL, et al. Sustained efficacy and safety of bazedoxifene in preventing fractures in postmenopausal women with osteoporosis: results of a 5-year, randomized, placebo-controlled study. Osteoporosis Int 2012;23:351–63. [DOI] [PubMed] [Google Scholar]
- 30.Andersson A, Stubelius A, Nurkkala-Karlsson M, et al. Estrogen regulates T helper 17 phenotype and localization in experimental autoimmune arthritis. Arthritis Res Therapy 2015;17:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bernardi A, Andersson A, Grahnemo L, et al. Effects of lasofoxifene and bazedoxifene on B cell development and function. Immun Inflamm Dis 2014:2:214–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J 2008;22:659–61. [DOI] [PubMed] [Google Scholar]
- 33.Windahl SH, Vidal O, Andersson G, Gustafsson JA, Ohlsson C. Increased cortical bone mineral content but unchanged trabecular bone mineral density in female ERβ−/− mice. J Clin Invest 1999;104:895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hegen M, Keith JC, Jr, Collins M, Nickerson-Nutter CL. Utility of animal models for identification of potential therapeutics for rheumatoid arthritis. Ann Rheum Dis 2008;67:1505–15. [DOI] [PubMed] [Google Scholar]
- 35.Jochems C, Islander U, Erlandsson M, et al. Osteoporosis in experimental postmenopausal polyarthritis: the relative contributions of estrogen deficiency and inflammation. Arthritis Res Ther 2005;7:R837–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borjesson AE, Farman HH, Engdahl C, et al. The role of activation functions 1 and 2 of estrogen receptor-α for the effects of estradiol and selective estrogen receptor modulators in male mice. J Bone Miner Res 2013;28:1117–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bernardi AI, Andersson A, Stubelius A, et al. Selective estrogen receptor modulators in T cell development and T cell dependent inflammation. Immunobiology 2015;220:1122–8. [DOI] [PubMed] [Google Scholar]
- 38.Nalbandian G, Kovats S. Understanding sex biases in immunity: effects of estrogen on the differentiation and function of antigen-presenting cells. Immunol Res 2005;31:91–106. [DOI] [PubMed] [Google Scholar]
- 39.Maldonado-Lopez R, De Smedt T, Pajak B, et al. Role of CD8α+ and CD8α− dendritic cells in the induction of primary immune responses in vivo. J Leukoc Biol 1999;66:242–6. [DOI] [PubMed] [Google Scholar]
- 40.Wilder RL. Hormones, pregnancy, and autoimmune diseases. Ann N Y Acad Sci 1998;840:45–50. [DOI] [PubMed] [Google Scholar]
- 41.Svensson L, Jirholt J, Holmdahl R, Jansson L. B cell-deficient mice do not develop type II collagen-induced arthritis (CIA). Clin Exp Immunol 1998;111:521–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Neill SK, Cao Y, Hamel KM, et al. Expression of CD80/86 on B cells is essential for autoreactive T cell activation and the development of arthritis. J Immunol 2007;179:5109–16. [DOI] [PubMed] [Google Scholar]
- 43.Nandakumar KS, Svensson L, Holmdahl R. Collagen type II-specific monoclonal antibody-induced arthritis in mice: description of the disease and the influence of age, sex, and genes. Am J Pathol 2003;163:1827–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jansson L, Olsson T, Holmdahl R. Estrogen induces a potent suppression of experimental autoimmune encephalomyelitis and collagen-induced arthritis in mice. J Neuroimmunol 1994;53:203–7. [DOI] [PubMed] [Google Scholar]
- 45.van Staa TP, Geusens P, Bijlsma JW, Leufkens HG, Cooper C. Clinical assessment of the long-term risk of fracture in patients with rheumatoid arthritis. Arthritis Rheum 2006;54:3104–12. [DOI] [PubMed] [Google Scholar]
- 46.Eggelmeijer F, Papapoulos SE, van Paassen HC, et al. Increased bone mass with pamidronate treatment in rheumatoid arthritis. Results of a three-year randomized, double-blind trial. Arthritis Rheum 1996;39:396–402. [DOI] [PubMed] [Google Scholar]
- 47.Cohen SB, Dore RK, Lane NE, et al. Denosumab treatment effects on structural damage, bone mineral density, and bone turnover in rheumatoid arthritis: a twelve-month, multicenter, randomized, double-blind, placebo-controlled, phase II clinical trial. Arthritis Rheum 2008;58:1299–309. [DOI] [PubMed] [Google Scholar]