Abstract
Recombinant surfactants present a new platform for stabilizing and targeting nanoparticle imaging agents. Superparamagnetic iron oxide nanoparticle-loaded micelles for MRI contrast are stabilized by an engineered variant of the naturally occurring protein oleosin and targeted using a Her2/neu affibody-oleosin fusion. The recombinant oleosin platform allows for simple targeting and the ability to easily swap of the ligand for numerous targets.
Keywords: Oleosin, MRI contrast, iron oxide, recombinant surfactant, targeting
Superparamagnetic iron oxide (SPIO) nanoparticles have gained interest for use as magnetic resonance contrast agents, with the ability to provide T2-weighted contrast enhancement for MR imaging applications.[1] Their strong contrast enhancing capabilities have rendered them useful for molecular imaging with various targeting molecules conjugated to their surfaces.[2] The ability to target nanoparticles has the potential to increase tumor accumulation, specificity, and therapeutic efficacy. The prerequisite for any targeted nanoparticle is the successful bioconjugation of ligands onto the nanoparticle surface. Many techniques to do so have low reaction efficiencies, require multiple conjugation steps, and often create products with poorly oriented antibodies. Developing amphiphilic recombinant proteins that can assemble on the surface of SPIO nanoparticles in a defined orientation would allow for the functionalization of particles during the formulation step. Moreover, the choice of targeting ligand and the physical-chemical properties of the hydrophilic block can be directly and very precisely modified through molecular biology.
To assemble targeted structures using a surfactant, we chose to engineer the naturally occurring protein oleosin.[3] Oleosin is a surfactant protein expressed in plant seeds with the native function of stabilizing fat reservoirs called oil bodies. The protein consists of three domains, a central hydrophobic domain flanked by two hydrophilic arms on the C- and N-termini.[3–4] The protein resembles a hairpin structure with a proline knot embedded in the central hydrophobic domain that forces a 180° turn.[5] Recombinant oleosin has been exploited for its surfactant nature in many biotechnology applications.[6]
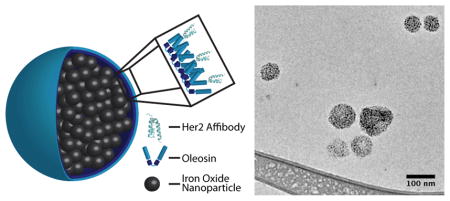
We have previously engineered oleosin to self-assembly into vesicles, fibers or sheets by creating a family of truncation mutants thereby varying the hydrophilic/hydrophobic ratio of the surfactant protein.[7] Further truncations of the hydrophobic block have led to soluble oleosin variants that spontaneously self-assemble in aqueous solution as a function of concentration.[8] These proteins can be engineered with exact peptide motifs for specific applications. We present here the engineering of oleosin variants with functional peptide domains that stabilize and target encapsulated SPIO nanoparticles for enhanced magnetic resonance imaging (Figure 1A).
Figure 1.
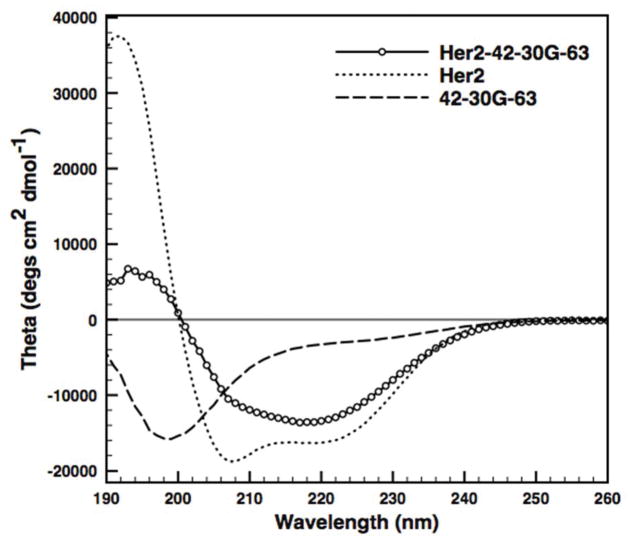
A) Cartoon depiction of Her2/neu targeted iron oxide nanoparticle micelles stabilized by oleosin. B) Protein purity is accessed to be >95% pure by SDS-PAGE (lane 1: Oleosin-30G(−), lane 2: Her2/neu-Oleosin-30G, lane 3: Her2/neu affibody). C) Circular dichroism indicates an unordered structure for the charged mutant Oleosin-30G(−). D) CD spectra for the fusion Her2/neu-Oleosin-30G show contributions from the helical Her2/neu affibody and the unordered Oleosin-30G. E) CDSSTR analysis of CD spectra shows increased helical structure in the fusion compared to Oleosin-30G indicating that the affibody is likely folded on the N-terminus of the oleosin mutant.
Two oleosin genes were engineered, one to stabilize the SPIO-loaded micelles and a second with a targeting ligand fused to the terminus of one of the hydrophilic domains of the protein. Previously it has been shown that oleosin can be engineered to stabilize various interfaces such as emulsion droplets[7] and bubbles.[9] In order to provide adequate repulsion between the micelles, we mutated the hydrophilic arms of oleosin-30G to be negatively charged. Negative nanoparticles have also been shown to limit nonspecific cell targeting.[10] We achieved this goal by altering all positive amino acids as well as any tyrosine residues in the hydrophilic arms to Q, N, D, or E depending on the location and local charge. The negative charge was spread evenly across the hydrophilic arms with an average negative amino acid every six residues. This variant is called Oleosin-30G(−). To directly target Her2/neu+ cancer cells, we fused a Her2/neu affibody[11] onto the N-terminus of the oleosin variant Oleosin-30G. This targeted variant is named Her2/neu-Oleosin-30G. Separately, the Her2/neu affibody was expressed as a soluble molecule for use as a competitive inhibitor in cell studies. Variants were made using standard molecular biology techniques and cloned into the expression vector pBamUK, which adds a 6-histidine tag on the C-terminus of the protein for immobilized metal affinity chromatography (IMAC). Oleosin variants were confirmed through DNA sequencing. Vectors were transformed into the Escherichia coli strain BL21 (DE3) for expression. Her2/neu-Oleosin-30G was insoluble and expressed in inclusion bodies whereas Oleosin-30G(−) was soluble. Variants were purified by IMAC. Protein yields were ~24 mg, ~80 mg, and ~65 mg of purified protein per liter of culture for Her2/neu-Oleosin-30G, Oleosin-30G(−), and Her2/neu respectively. SDS-PAGE indicates highly purified products after IMAC (Figure 1B). The band for Oleosin-30G(−) runs much higher than expected on the gel, likely due to its highly negative charge. Molecular weights were confirmed with MALDI-TOF (Figure 1S) (Oleosin-30G(−) expected: 14956, measured: 14958; Her2/neu-Oleosin-30G: expected: 21714, measured: 21713; Her2/neu expected: 7771, measured: 7773).
Protein secondary structure was elucidated with circular dichroism. The parent molecule Oleosin-30G is a highly unordered protein.[9] CD indicates that Oleosin-30G(−) remains unordered after the various mutations to the hydrophilic arms (Figure 1C). The secondary structure of Her2/neu-Oleosin-30G was investigated to ensure correct affibody folding as a fusion partner. The Her2/neu affibody is a highly helical protein (Figure 1D) and when fused to oleosin, the Her2/neu-Oleosin fusion displays structure from the helical affibody and the unordered oleosin backbone (Figure 1D). The spectra were fit with the CDSSTR analysis method using Dichroweb (Figure 1E).[12] The analysis shows clear helical structure in the fusion protein indicating that the affibody is likely folded in the fusion.
SPIO-loaded oleosin micelles were assembled through an emulsion method. SPIO nanoparticles solubilized in toluene were injected into solutions of protein in PBS. The emulsion was sonicated and the toluene was allowed to evaporate overnight at room temperature. This led to formation of micelles with tightly-packed SPIO within the hydrophobic core. SPIO-loaded oleosin micelles were purified using stepwise centrifugation.[13] Cryo-TEM of the each separation fraction indicated large aggregated particles were removed in pellet after low RCF spins and excess protein and small particles were removed in the supernatant of the high RCF spins (Figure S2). The mass ratio of the particles to the protein, the oil volume fraction, and the particle stabilization coat all modulate the formation of packed nanoclusters. The oil volume fraction and mass ratio of protein to iron was optimized. Previous studies used an oil volume fraction of 4.8% for particle formation and a 4:4 ratio of nanoparticle to surfactant (mg:mg).[13] We found that decreasing the volume fraction of toluene in the emulsion to 1.2% and increasing the protein concentration led to significant enhanced ordered clustering of the FeO particles. The optimal particles were created by injecting 50 μl of toluene containing 4 mg of SPIO-DDA coated nanoparticles into a 4 ml solution of protein in PBS at a concentration of 2 mg/ml (Figure S3).
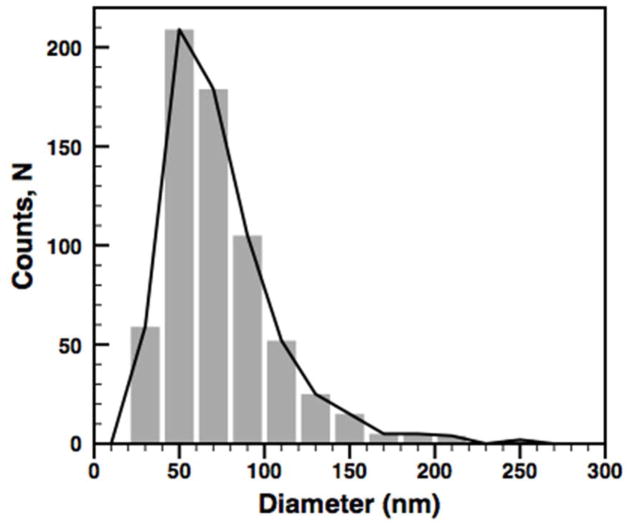
Dynamic light scattering of the purified particles show a single population with an average hydrodynamic diameter of 113 nm and low polydispersty (peak: 127 nm, PDI=0.104) (Figure 2A). Purified particles were imaged using cryogenic transmission electron microscopy (Cryo-TEM) (Figure 2B). The micrograph displays tightly packed SPIO nanoparticles and no visible excess protein on the particles. Particles from three independent batches were directly measured from micrographs and found to have an average diameter of 74 ± 33 nm (N=660 particles) (Figure 2C). As expected, the average diameter measured in micrographs is less than the hydrodynamic diameter measured by DLS. The DLS data are skewed to higher diameters due to increased intensity of scattering from larger particles.
Figure 2.
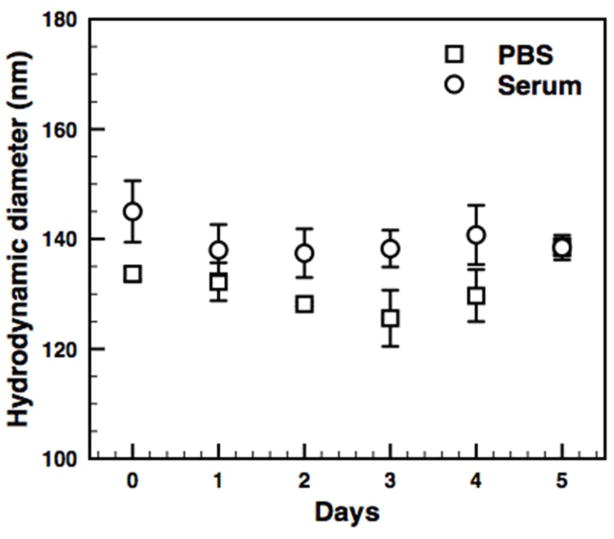
A) Dynamic light scattering reveals a monodisperse population of micelles with an average diameter of 113 nm (PDI=.104). B) Cryo-TEM micrograph of FeO micelles stabilized by Oleosin-30G(−) in PBS (pH 7.4). C) Particle size distribution measured directly from cryo-TEM images. The average particle size was found to be 74 ± 33 nm (Standard deviation, N=660 particles). This diameter is significantly lower than the hydrodynamic diameter from DLS due to the increased scattering from larger particles. D) Protein stabilized particles are stable over 5 days in buffer (1xPBS) and serum at 37°C as measured by DLS. E) Particles show high relaxivity with an R2 value of 154.9 ± 4.0 mM−1 s−1. F) The R1 value was found to be 4.47 ± 0.46 mM−1 s−1.
The surface charge of SPIO particles has been shown to have significant impact in the uptake by cells[1d]. Measurements of the zeta potential indicated a negative surface charge of −12.5 ± 1.7 mV in 1 x PBS. The high negative charge provides repulsive electrostatic interactions between the emulsion droplets during particle formation, which reduce aggregation. The particles show long-term stability in buffer (1 x PBS) and serum with no significant change in the hydrodynamic diameter over 5 days at 37°C (Figure 2D). The particles display extremely high relaxivity with an r2 value of 407.2 ± 4.0 s−1 mM−1 and an r1 value of 4.47 ± 0.46 s−1 mM−1. The potential cytotoxicity of the nanoparticles was assessed using an MTT assay. Over all concentrations tested, cell viability remained above 97% for the 4-hour incubation with particles (Figure 3A). The physical and magnetic properties of the oleosin-stabilized nanoparticles are summarized in Table 1.
Figure 3.
A) Particles show no metabolic inhibition based on the MTT assay between 25 and 150 μM after 4 hours of incubation at 37°C with NIH/3T3 cells. B) Functional evaluation of the Her2/neu SPIO-oleosin micelles conjugates. SPIO-oleosin and Her2/neu-SPIO-oleosin were incubated with either Her2/neu-positive and Her2/neu-negative cells in the presence and absence of excess free affibody. Free affibody served as a competitive inhibitor to confirm specific binding of the Her2/neu receptor. Relaxivity measurements of cells incubated with SPIO-oleosin micelles or Her2/neu-SPIO-oleosin micelles were acquired.
Table 1.
Physical and magnetic properties of oleosin stabilized nanoparticles
| Hydrodynamic diameter (nm) | 113 ± 36 |
| Number Diameter (nm) | 74 ± 33 |
| Zeta Potential, pH 7.4 (mV) | −12.5 ± 1.7 |
| r2 (mM−1 s−1) | 407.2 ± 4.0 |
| r1 (mM−1 s−1) | 4.47 ± 0.46 |
| r2/r1 | 91.1 |
Her2/neu+ targeted micelles were created by blending Her2/neu-Oleosin with Oleosin-30G(−) at 10% by weight in the PBS solutions (0.8 mg Her2/neu-Oleosin-30G: 7.2 mg Oleosin-30G(−)). The micelles were prepared and purified in the same manor as described previously. The blending of the targeted variant into the micelles did not change the size of the micelles as measured by DLS (Figure S4A) or the stability of the particles over time (Figure S4B). The surface charge of the particles remains negative but slightly increased to −10.7 ± 0.8 mV.
Targeted and non-targeted micelles were incubated with Her2/neu- (NIH/3T3) and Her2/neu+ (T6-17) cells at a concentration of 100 μg/ml for 45 minutes. The T2 relaxation time for the NIH/3T3 cells showed no difference between negative control particles, targeted particles, or cells incubated without particles indicating little to no nonspecific binding (Figure 3B). For the Her2/neu+ cell line, the cells incubated with the targeted particles show a significantly lower T2 relaxation time, consistent with the presence of SPIO, compared to cells with the negative control particles or cells incubated without particles. A competitive binding study was completed by adding excess Her2/neu affibody to the T6-17 cells before and during the incubation with the targeted particles. The presence of the free competing soluble affibody led to a significant increase in the T2 relaxation time. Therefore, these results provide clear evidence that Her2/neu oleosin micelles specifically bind to cells bearing Her2/neu.
In summary, our work demonstrates the ability to engineer the naturally occurring surfactant protein oleosin to stabilize and target SPIO-loaded micelles to Her2/neu+ cells. The functionalization of these particles is trivial due to the ease of incorporating biologically relevant motifs into the coat protein through molecular biology. These particles are extremely stable and display high relaxivity. We envision that oleosin stabilized nanoparticle micelles will represent a promising platform for targeted imaging applications. In addition, future work will focus on varying the surface charge and appending specific stealth ligands[14] to the particles to engineer modulate the toxicity of particles and maintain long circulation times.
Experimental Section
Gene synthesis
Genes were created using standard molecular biology techniques. Detailed cloning methods are included in supplemental information. All variants were confirmed through DNA sequencing.
Protein production and purification
Variants were expressed under the control of the lac promoter in E. Coli (BL21 DE3, Stratagene). The protein variants were solubilized according to the B-PER protocol and purified using IMAC following the Hispur Ni-NTA resin protocol.
FeO synthesis
Superparamagnetic iron oxide nanoparticles were synthesized according to a protocol adapted from Cheon et al.[15]
Nanoparticle assembly and purification
FeO-oleosin micelles were synthesized using an oil in water emulsion and purified using sequential centrifugation as previously reported[13].
Cell Viability Assay
The viability and proliferation of cells in the presence of Fe-Oleosin nanoparticles were evaluated by 3-[4,5-dimethylthialzol-2-yl]-2,5-diphenyltetrazolium bromide (MTT, Sigma) assay.
Cell lines
NIH/3T3 and T6-17 cells (i.e., NIH/3T3 cells engineered to stably express the Her2/neu/neu receptor, kindly provided by Dr. Mark Greene, University of Pennsylvania) were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin at 37 °C, and 5% CO2.
Her2/neu targeting
T6-17 and NIH/3T3 cells were incubated with 100 μg Fe/mL of Her2/neu-targeted SPIO micelles for 45 min in full media in triplicate. The media was removed and the cells were washed with PBS two times to remove any unbound micelles. Cells were trypsinized and counted. Cell suspensions were diluted to 0.4 × 106 cells/ml and T2 relaxation times were measured using a benchtop relaxometer (Bruker mq60).
Supplementary Material
Acknowledgments
K. Vargo and A. Al Zaki contributed equally to this work. We acknowledge funding for this work through NSF DMR – 1309556, NSF DMR-1120901, and NIH/NIBIB (R21EB013754). The project described was supported in part by Grant Number UL1RR024134 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. Supported in part by the Institute for Translational Medicine and Therapeutics’ (ITMAT) Transdisciplinary Program in Translational Medicine and Therapeutics.
Footnotes
Supporting Information is available from the Wiley Online Library or from the author.
References
- 1.a) Weissleder R, Hahn PF, Stark DD, Elizondo G, Saini S, Todd LE, Wittenberg J, Ferrucci JT. Radiology. 1988;169 doi: 10.1148/radiology.169.2.3174987. [DOI] [PubMed] [Google Scholar]; b) Weissleder R, Elizondo G, Wittenberg J, Rabito CA, Bengele HH, Josephson L. Radiology. 1990;175 doi: 10.1148/radiology.175.2.2326474. [DOI] [PubMed] [Google Scholar]; c) Shapiro EM, Skrtic S, Koretsky AP. Magnet Reson Med. 2005;53 doi: 10.1002/mrm.20342. [DOI] [PubMed] [Google Scholar]; d) Thorek DLJ, Tsourkas A. Biomaterials. 2008;29 doi: 10.1016/j.biomaterials.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.a) Wu SC, Chen YJ, Lin YJ, Wu TH, Wang YM. J Med Chem. 2013;56 doi: 10.1021/jm401060z. [DOI] [PubMed] [Google Scholar]; b) Petri-Fink A, Hofmann H. IEEE T Nanobiosci. 2007;6 doi: 10.1109/tnb.2007.908987. [DOI] [PubMed] [Google Scholar]; c) Matuszewski L, Persigehl T, Wall A, Schwindt W, Tombach B, Fobker M, Poremba C, Ebert W, Heindel W, Bremer C. Radiology. 2005;235 doi: 10.1148/radiol.2351040094. [DOI] [PubMed] [Google Scholar]; d) Hui JZ, Zaki AA, Cheng Z, Popik V, Zhang H, Luning Prak ET, Tsourkas A. Small. 2014 doi: 10.1002/smll.201303629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang AHC. Annu Rev Plant Physiol Plant Mol Biol. 1992;43 [Google Scholar]
- 4.Alexander LG, Sessions RB, Clarke AR, Tatham AS, Shewry PR, Napier JA. Planta. 2002;214 doi: 10.1007/s004250100655. [DOI] [PubMed] [Google Scholar]
- 5.Abell BM, Holbrook LA, Abenes M, Murphy DJ, Hills MJ, Moloney MM. Plant Cell. 1997;9 doi: 10.1105/tpc.9.8.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.a) Bhatla SC, Kaushik V, Yadav MK. Biotechnol Adv. 2010;28 doi: 10.1016/j.biotechadv.2010.01.001. [DOI] [PubMed] [Google Scholar]; b) Peng CC, Chen JCF, Shyu DJH, Chen MJ, Tzen JIC. J Biotechnol. 2004;111 doi: 10.1016/j.jbiotec.2004.03.013. [DOI] [PubMed] [Google Scholar]; c) Scott RW, Winichayakul S, Roldan M, Cookson R, Willingham M, Castle M, Pueschel R, Peng CC, Tzen JTC, Roberts NJ. Plant Biotechnol J. 2010;8 doi: 10.1111/j.1467-7652.2010.00522.x. [DOI] [PubMed] [Google Scholar]; d) Chang MT, Tsai TR, Lee CY, Wei YS, Chen YJ, Chen CR, Tzen JTC. J Agric Food Chem. 2013;61 doi: 10.1021/jf4019195. [DOI] [PubMed] [Google Scholar]; e) Chiang CJ, Lin CC, Lu TL, Wang HF. Nanotechnology. 2011;22 doi: 10.1088/0957-4484/22/41/415102. [DOI] [PubMed] [Google Scholar]; f) Chiang CJ, Lin LJ, Lin CC, Chang CH, Chao YP. Nanotechnology. 2011;22 doi: 10.1088/0957-4484/22/1/015102. [DOI] [PubMed] [Google Scholar]
- 7.Vargo KB, Parthasarathy R, Hammer DA. Proc Natl Acad Sci U S A. 2012;109 doi: 10.1073/pnas.1205426109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vargo KB, Sood N, Moeller TD, Heiney PA, Hammer DA. Submitted for Publication. 2014 doi: 10.1021/la502664e. [DOI] [PubMed] [Google Scholar]
- 9.Angilé FE, Vargo KB, Sehgal CM, Hammer DA, Lee D. Submitted for Publication. 2014 [Google Scholar]
- 10.a) Verma A, Stellacci F. Small. 2010;6 doi: 10.1002/smll.200901158. [DOI] [PubMed] [Google Scholar]; b) Cho EC, Au L, Zhang Q, Xia YN. Small. 2010;6 doi: 10.1002/smll.200901622. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Cho EC, Xie JW, Wurm PA, Xia YN. Nano Lett. 2009;9 doi: 10.1021/nl803487r. [DOI] [PubMed] [Google Scholar]
- 11.Orlova A, Magnusson M, Eriksson TLJ, Nilsson M, Larsson B, Hoiden-Guthenherg I, Widstrom C, Carlsson J, Tolmachev V, Stahl S, Nilsson FY. Cancer Res. 2006;66 doi: 10.1158/0008-5472.CAN-05-3521. [DOI] [PubMed] [Google Scholar]
- 12.a) Sreerama N, Venyaminov SY, Woody RW. Anal Biochem. 2000;287 doi: 10.1006/abio.2000.4879. [DOI] [PubMed] [Google Scholar]; b) Whitmore L, Wallace BA. Nucleic Acids Res. 2004;32 doi: 10.1093/nar/gkh077. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Sreerama N, Woody RW. Anal Biochem. 2000;287 doi: 10.1006/abio.2000.4880. [DOI] [PubMed] [Google Scholar]
- 13.Al Zaki A, Joh D, Cheng ZL, De Barros ALB, Kao G, Dorsey J, Tsourkas A. ACS Nano. 2014;8 doi: 10.1021/nn405701q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodriguez PL, Harada T, Christian DA, Pantano DA, Tsai RK, Discher DE. Science. 2013;339 doi: 10.1126/science.1229568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheon JW, Kang NJ, Lee SM, Lee JH, Yoon JH, Oh SJ. J Am Chem Soc. 2004;126 doi: 10.1021/ja038722o. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.