Abstract
T-lymphocyte migration is important for homing, cell trafficking, and immune surveillance. T-lymphocytes express lymphocyte function-associated antigen-1 (LFA-1; αLβ2) and very late antigen-4 (VLA-4; α4β1), which bind to their cognate ligands, intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1). These adhesive interactions provide T-lymphocytes with the ability to withstand hemodynamic shear forces to facilitate adhesion and migration along the blood endothelium. Recently, it has been shown that T-lymphocytes will crawl upstream against the direction of flow on surfaces functionalized with ICAM-1. Here, we have investigated whether the identity of the receptor and the magnitude of its engagement affects the direction of T-lymphocyte migration under flow. We used microcontact printed ICAM-1 and VCAM-1 PDMS surfaces on which density and type of adhesion molecule can be tightly controlled and non-specific adhesion adequately blocked. Using a laminar flow chamber, we demonstrate that T-lymphocytes migrate either upstream or downstream dependent upon ligand type, ligand concentration and shear rate. T-lymphocytes were found to migrate upstream on ICAM-1 but downstream on VCAM-1 surfaces – a behavior unique to T-lymphocytes. By varying concentrations of ICAM-1 and VCAM-1, directed migration under flow was observed to be dependent upon the type and concentration of ligand. As shear rates increase, T-lymphocytes favor upstream migration when any ICAM-1 is present, even in the presence of substantial amounts of VCAM-1. Furthermore, a loss of cytoskeletal polarity was observed upon introduction of fluid flow with reorganization that is dependent upon ligand presentation. These results indicate that T-lymphocytes exhibit two different modes of motility – upstream or downstream – under fluid flow that depends on ligand composition and the shear rate.
Introduction
For efficient homing, T-lymphocytes must withstand the hemodynamic forces caused by fluid flow to effectively adhere and migrate along the endothelium.1,2 T-lymphocytes express the integrins lymphocyte function-associated antigen-1 (LFA-1; αLβ2) and very late antigen-4 (VLA-4; α4β1), which bind to the ligands intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1), respectively. These integrins are known to be crucial for cell activation and to facilitate interactions with other leukocytes in order to elicit effector functions.3–5 LFA-1 and VLA-4 are also required for firm adhesion to the blood endothelium under shear flow to permit migration into lymph nodes or inflamed tissues.6–8
Previous work has shown the rather fascinating phenomenon that murine T-lymphocytes crawl efficiently against the direction of flow on immobilized ICAM-1 and ICAM-2 surfaces while undergoing recurrent downstream arrest on VCAM-1.9 Valignat et al. demonstrated that both freshly isolated and effector human T-lymphocytes increase their upstream migration against flow as shear rate increases on ICAM-1 surfaces.10 Furthermore, T-lymphocytes undergo adhesion strengthening on ICAM-1 surfaces upon spontaneous LFA-1-mediated adhesion under shear flow that is dependent upon calcium/calmodulin signaling and the assembly of the actin cytoskeleton.11
Often, multiple integrins are engaged, and a question that has not been fully addressed is how the engagement of multiple integrins controls T-lymphocyte motility under shear flow.12–15 It is known that chemokine engagement leads to increased integrin activation through heterologous modulation (inside-out signaling);16–18 on the other hand, homologous modulation, when one integrin binds its specific ligand activating a signaling pathway that then activates a different integrin, can also occur. For example, VCAM-1 engagement of VLA-4 is known to regulate β2-dependent adhesion under flow on ICAM-1 surfaces.5,19,20 Previous studies have also shown a synergistic response in adhesion strengthening and resistance to shear upon plating human T-lymphocytes on surfaces that have been co-immobilized with ICAM-1 and VCAM-1.19 However, it is not well understood how the simultaneous engagement of LFA-1 and VLA-4 with their cognate ligands controls the directional migration of T-lymphocytes under fluid flow, which is especially interesting given the dichotomous response of T-lymphocytes on each ligand alone.
We previously demonstrated that human primary T-lymphocytes are capable of spontaneous and robust migration under static conditions on microcontact printed ICAM-1 and VCAM-1 PDMS surfaces, but it is not well understood how the application of shear modulates this behavior.21 Here, we used the same surfaces, presenting ICAM-1 and VCAM-1 in a controllable ratio, to measure how ligand presentation affects directional migration under fluid flow. We quantified T-lymphocyte migration under shear flow using a parallel plate laminar flow assay.10,11,22–24 We show that under conditions of shear flow, T-lymphocytes can crawl either upstream or downstream, and the motility is dependent upon ligand concentration, type, and shear rate, over a range of physiological shear rates mimicking conditions encountered in the postcapillary venules where leukocyte extravasation predominantly takes place.25,26 Furthermore, we show that presentation of both ICAM-1 and VCAM-1 at different densities orients T-lymphocyte migration either upstream or downstream of fluid flow at low shear rates while at high shear rates preferential migration is observed upstream provided there is any ICAM-1 present. These results suggest that β2 integrins play a dominant role in dictating the direction of migration under fluid flow. Our current observations correspond well to the previous studies showing that VCAM-1 mediated motility exhibits lower migration speeds and, overall, less motility than on ICAM-1 surfaces and provides new insight into T-lymphocyte behavior on surfaces containing both ligands under shear flow.21
Results
Microcontact printing of protein A/G and surface quantification
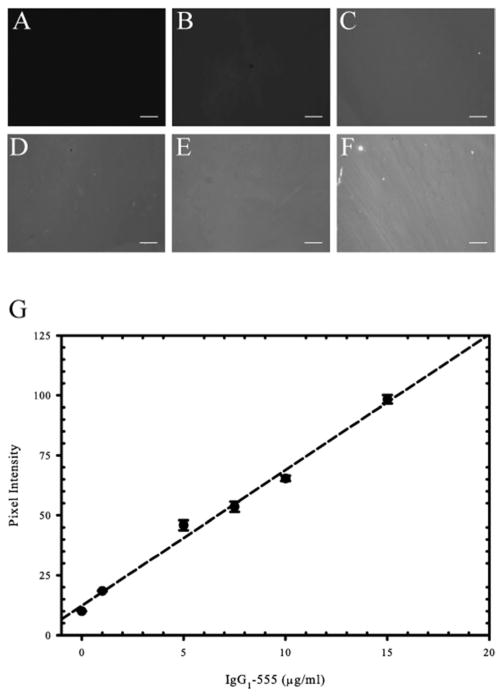
The relative composition of our surfaces were verified using fluorescently tagged human IgG1 molecules that bind to microcontact printed protein A/G. Briefly, Alexa Fluor-555 tagged IgG1 (IgG1-555) was mixed with unlabeled human IgG1 in different ratios to maintain a total protein solution concentration of 20 μg ml−1. Fig. 1A–F shows the fluorescence intensities of surfaces with increasing concentrations of IgG1-555. By quantifying the pixel intensities for each condition, we found a linear correlation (R2 = 0.991) between IgG1-555 concentration and pixel intensity (Fig. 1G). These data allows us to conclude that as ligand concentration is increased within solution (up to a concentration of 20 μg ml−1 IgG1) the amount of ligand is proportionately increased on our surfaces. The range of concentrations tested cover what was used in our study.
Fig. 1.
Increasing solution concentrations of Fc-containing ligand correlates to increasing surface ligand concentrations. Fluorescent images of surfaces prepared with increasing concentrations of Alexa Fluor-555 tagged human IgG1 (IgG1-555) mixed with unlabeled human IgG1 to maintain a total protein concentration of 20 μg ml−1. Solution concentrations of IgG1-555 depicted are as follows: (A) 0 μg ml−1, (B) 1 μg ml−1, (C) 5 μg ml−1, (D) 7.5 μg ml−1, (E) 10 μg ml−1, and (F) 15 μg ml−1. (G) The pixel intensities were calculated for each concentration and plotted. Data reveals a linear correlation between soluble IgG1-555 concentration and fluorescent signal present on the surface (R2 = 0.991). Scale bar = 50 μm.
ICAM-1, but not VCAM-1, supports T-lymphocyte migration against the direction of flow as a function of shear rate
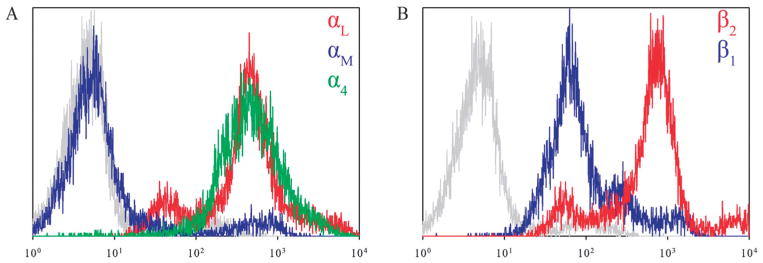
T-lymphocytes express the integrin heterodimers VLA-4 and LFA-1, and flow cytometry was employed to confirm the expression β1 and β2 on cultured primary human T-lymphocytes (Fig. 2). It was also verified that the cells do not express the MAC-1 integrin heterodimer (αMβ2) which is capable of binding to ICAM-1 (Fig. 2A), allowing us to conclude that the interactions observed on ICAM-1 are solely due to LFA-1 engagement in our experiments.
Fig. 2.
Expression of VLA-4 (α4β1) and LFA-1 (αLβ2) on primary human T-lymphocytes. (A) Expression of integrins αL (red colored line), αM (blue colored line), and the integrin α4 (green colored line). (B) Expression of integrins β2 (red colored line) and β1 (blue colored line). All negative controls are depicted in gray.
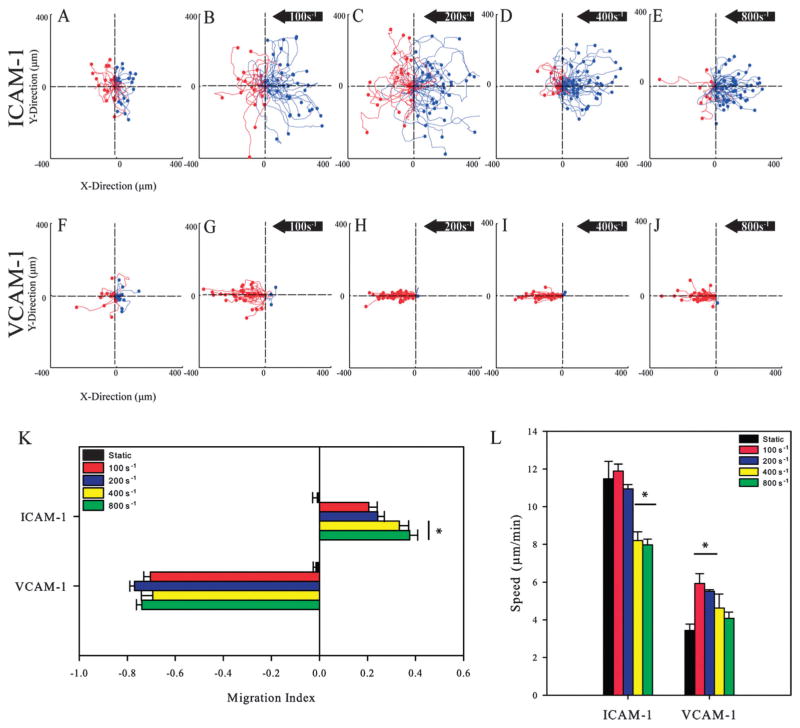
The motility of cells at different shear rates was measured on surfaces prepared by incubating either 5 μg ml−1 of ICAM-1 or VCAM-1 onto surfaces stamped with protein A/G. After injection and a resting time of 15 minutes to allow for adhesion, cells were exposed to shear rates of 100, 200, 400, or 800 s−1 for a time period of 30 minutes. As illustrated by representative cell traces (Fig. 3A and F), T-lymphocytes migrated substantial distances under static conditions on ICAM-1 or VCAM-1 surfaces, and upon application of flow, directional responses were observed (Movies S1–S4 in the ESI†). Blue traces indicate cells that traveled upstream in fluid flow while red traces indicate cells that traveled downstream. At a low shear rate of 100 s−1, cells on ICAM-1 surfaces showed a slight preference for migrating upstream, and with increasing shear rates, cells on ICAM-1 surfaces showed an increasing preference to migrate upstream (Fig. 3B–E). In contrast, cells on VCAM-1 surfaces preferred to migrate downstream with little to no migration upstream at any shear rate (Fig. 3G–J). To quantify the directional motion, a migration index (MI) was calculated for all cells; this is defined as the ratio of total x-displacement to total overall distance traveled, with positive values indicating motion upstream and negative values indicating motion downstream. Under static conditions, the MIs for cells on ICAM-1 and VCAM-1 were near 0, indicating random migration with no preference in direction. Upon exposure to flow, cells on ICAM-1 migrated upstream with increasing MIs as a function of increasing shear rate with values ranging from 0.21 to 0.38 (Fig. 3K). On VCAM-1 substrates, migration was downstream with MIs having no dependence on shear rate with values between −0.69 and −0.77 (Fig. 3K). Furthermore, T-lymphocytes exposed to surfaces of protein A/G alone did not adhere allowing us to assume that the observed directional migration on ICAM-1 and VCAM-1 surfaces is specifically due to the ligation of adhesion receptors, and not due to non-specific interactions.
Fig. 3.
T-lymphocytes crawl against the direction of flow on immobilized ICAM-1 but not on VCAM-1 to an extent that depends on shear rate. (A–E) Cell traces of T-lymphocytes under shear flow (right to left) on ICAM-1 (5.0 μg ml−1) and (F–J) VCAM-1 (5.0 μg ml−1) coated PDMS surfaces under varying shear rates. The traces depicted under flow are for one representative experiment in each group. Blue traces indicate cells that traveled upstream of flow while red traces indicate cells that traveled downstream. Black arrow indicates direction of shear flow for all conditions. Traces are in μm. (K) The direction of T-lymphocytes under shear flow as expressed as the migration index (MI). A positive MI indicates migration against the flow (upstream) while a negative MI indicates migration in the direction of flow (downstream). T-lymphocytes on ICAM-1 migrate upstream to an increasing extent with increasing shear rate while cells on VCAM-1 migrate downstream. The MI was calculated from T-lymphocyte tracks from three independent experiments. *p < 0.05. (L) T-lymphocytes on ICAM-1 migrate faster than cells on VCAM-1 and is decreased at higher shear rates. Migration speeds were calculated from three independent experiments each. *p < 0.05.
As shear rates increased, a decrease in migration speed was observed on both ligands (Fig. 3L). Migration speeds were higher on ICAM-1 surfaces than VCAM-1 under flow, which corresponds to previous observations under static conditions.21 On ICAM-1 surfaces, a significant decrease in speed was seen at larger shear rates (400 s−1 and 800 s−1) compared to under static conditions. The peak speed was observed at 100 s−1 (11.47 ± 0.93 μm min−1) with the lowest speed observed at 800 s−1 (7.97 ± 0.30 μm min−1). For VCAM-1 surfaces, application of 100 s−1 of fluid flow doubled the migration speed (5.93 ± 0.52 μm min−1) compared to what was observed under static conditions (3.44 ± 0.33 μm min−1). Furthermore, when shear rates were increased further on VCAM-1 substrates, the migration speeds decreased with increasing shear rate with the lowest speed being observed at 800 s−1 (4.08 ± 0.33 μm min−1).
Downstream migration of T-lymphocytes is dependent upon VCAM-1 concentration
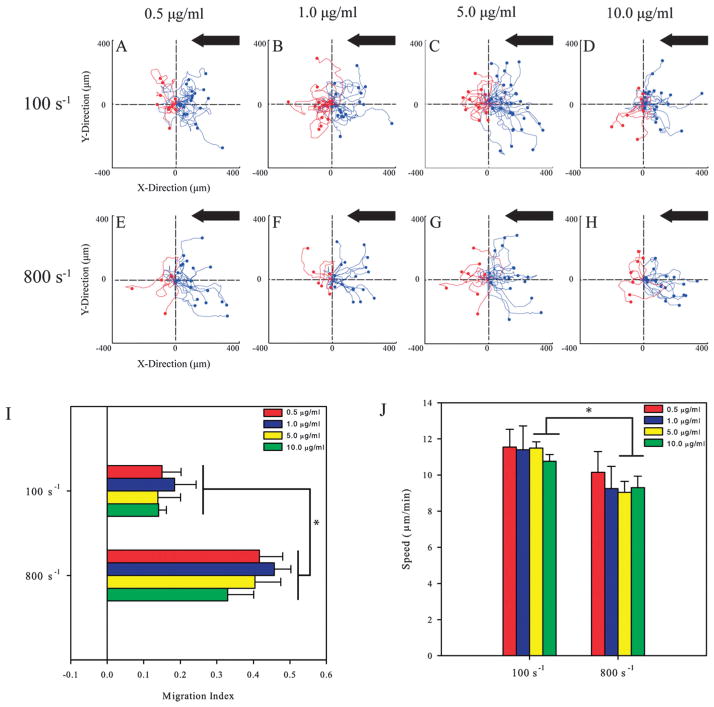
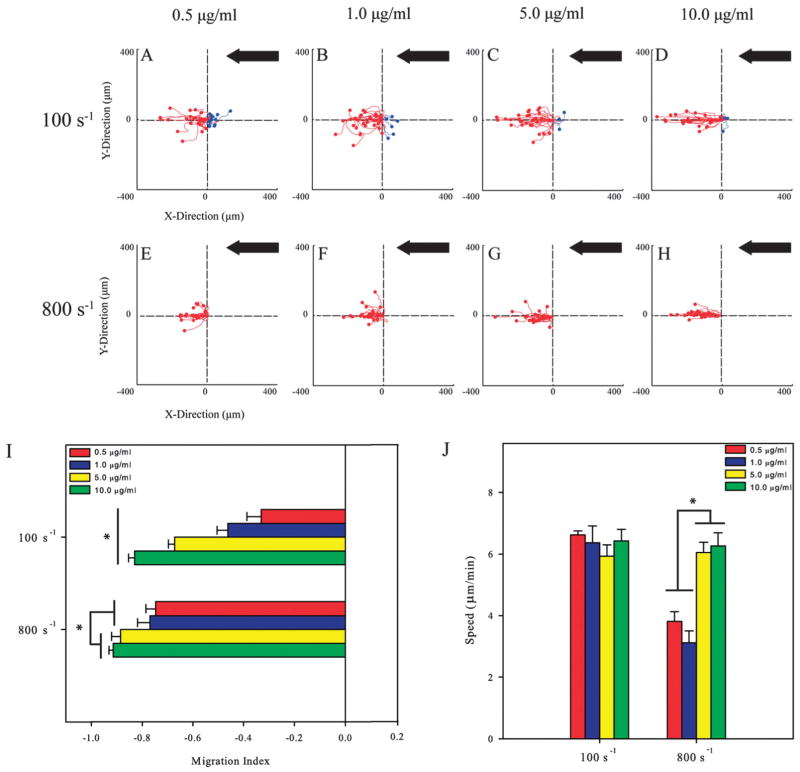
It has been previously demonstrated that the concentration of ICAM-1 and VCAM-1 controls cell speed and the random motility of primary human T-lymphocytes.21 This led us to investigate whether this is also true under shear flow. Cells were exposed to shear rates of 100 s−1 and 800 s−1 on ICAM-1 surfaces coated with 0.5, 1.0, 5.0, and 10.0 μg ml−1 of ICAM-1 Fc chimera. Representative cell traces shows that T-lymphocytes preferred to migrate upstream on ICAM-1 for both shear rates (Fig. 4A–D and E–H). The migration index was calculated for each condition to ascertain whether ligand concentration affects the directionality of migration along with shear rate. At a shear rate of 100 s−1, the MIs ranged between 0.14 to 0.21, and upon application of 800 s−1, these values increased significantly and ranged from 0.33 to 0.46 (Fig. 4I) with no dependence on ligand concentration for both shear conditions. For the lower concentrations of 0.5 and 1.0 μg ml−1 of ICAM-1, there were no differences in the speed of T-lymphocytes between shear rates of 100 s−1 and 800 s−1 (Fig. 4J). On surfaces prepared with concentrations of 5.0 and 10.0 μg ml−1 of ligand, speeds significantly decreased when shear rate increased from 100 s−1 to 800 s−1.
Fig. 4.
T-lymphocytes crawl upstream on different concentrations of immobilized ICAM-1. (A–D). Cell traces of T-lymphocytes crawling at a shear rate of 100 s−1 on varying concentrations of ICAM-1 PDMS surfaces. (E–H) Cell traces of T-lymphocytes under 800 s−1 of shear flow on varying concentrations of ICAM-1 PDMS surfaces. Black arrow indicates direction of shear flow for all conditions. The traces depicted under flow are for one representative experiment under the stated conditions. (I) The direction of T-lymphocyte migration under shear flow expressed as the migration index (MI). T-lymphocytes on ICAM-1 surfaces migrate upstream and directionality depends on shear rate. The MI was calculated from T-lymphocyte tracks from three independent experiments. *p <0.05. (J) T-lymphocytes on varying concentrations of ICAM-1 migrate with the same speed at each shear rate tested. Migrations speeds were calculated from three independent experiments each. *p < 0.05.
The motility of T-lymphocytes was also measured on VCAM-1 surfaces prepared at 0.5, 1.0, 5.0, and 10.0 μg ml−1 of the VCAM-1 Fc chimera. The representative cell traces show that at shear rates of 100 s−1 (Fig. 5A–D) and 800 s−1 (Fig. 5E–H) cells crawl downstream for all VCAM-1 concentrations. Unlike ICAM-1 surfaces, the MIs for cells on VCAM-1 surfaces strongly depend upon ligand concentration. Under 100 s−1 of flow, the MIs became more negative as VCAM-1 concentration increased, ranging from −0.33 (at 0.5 μg ml−1) to −0.83 (at 10 μg ml−1) (Fig. 5I). At a shear rate of 800 s−1, the effect of ligand density is more moderate with the two lower concentrations having similar MIs (−0.75 and −0.77) and the two larger concentrations having significantly larger values (−0.88 and −0.91). On VCAM-1 surfaces exposed to a shear rate of 100 s−1, crawling speeds ranged between 5.93 to 6.43 μm min−1 and were largely independent of ligand concentrations. However, at a shear rate of 800 s−1, cells exhibited lower migration speeds at lower ligand concentrations that doubled on surfaces made with higher ligand concentrations (3.12 versus 6.26 μm min−1; Fig. 5J).
Fig. 5.
The migration index of T-lymphocytes crawling in the direction of flow increases with concentration of immobilized VCAM-1. (A–D) Cell traces of T-lymphocytes at a shear rate of 100 s−1 on different concentrations of ICAM-1 on PDMS surfaces. (E–H) Cell traces of T-lymphocytes at a shear rate of 800 s−1 on varying concentrations of ICAM-1 PDMS surfaces. The traces depicted under flow are for one representative experiment in each group. Black arrow indicates direction of shear flow for all conditions. (I) The direction of T-lymphocytes under shear flow expressed as the migration index (MI). T-lymphocytes on VCAM-1 migrate downstream and the migration index is dependent upon ligand concentration. The MI was calculated from T-lymphocyte tracks from three independent experiments. *p <0.05. (J) T-lymphocytes on varying concentrations of VCAM-1 migrate with the same speed at a shear rate of 100 s−1 but increases speed on higher concentrations of VCAM-1 at a shear rate of 800 s−1. Migration speeds were calculated from three independent experiments each. *p < 0.05.
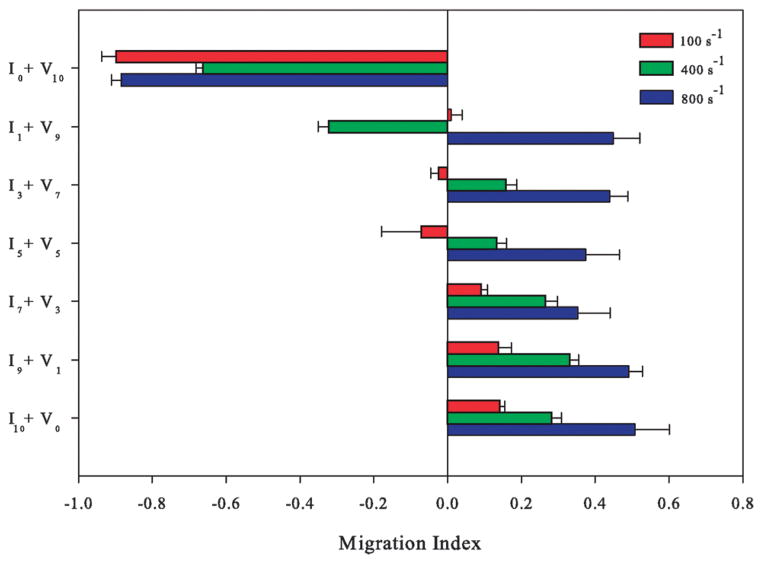
Directional migration of T-lymphocytes under fluid flow can be controlled through combinations of ICAM-1 and VCAM-1 under shear flow
T-lymphocytes were plated on surfaces where the ratio of ICAM-1 and VCAM-1 was varied by changing the ratios of ICAM-1 and VCAM-1 within solution while maintaining a constant total protein concentration of 10 μg ml−1. For example, by mixing 3 μg ml−1 of ICAM-1 and 7 μg ml−1 of VCAM-1, equaling to a total protein concentration of 10 μg ml−1, a surface called I3 + V7 was created; this surface was 30% ICAM-1 and 70% VCAM-1. On all surfaces, we measured the direction of migration either upstream or downstream as a function of the ratios of ICAM-1 and VCAM-1 present on the surface (Fig. 6; Movie S5 in the ESI†). As the ratio of ICAM-1 to VCAM-1 increased, cells experiencing a shear rate of 100 s−1 increased their orientation of migration against the direction of flow (increased their migration upstream). Upon exposure to 400 s−1, a preference in upstream migration was observed on most surfaces containing any amount of ICAM-1. Upstream migration was increased further upon exposure to 800 s−1 with the presence of any ICAM-1 on the surface even when large amounts of VCAM-1 were present (Movie S6 in the ESI†). At this shear rate, only surfaces made purely of VCAM-1 (V10) favored downstream migration.
Fig. 6.
T-lymphocytes elicit different responses on surfaces made with both immobilized ICAM-1 + VCAM-1 that are dependent upon shear rate. (Abbreviations: I – ICAM-1; V – VCAM-1; 10 – 10 μg ml−1; 7 – 7 μg ml−1; 5 – 5 μg ml−1; 3 – 3 μg ml−1; 1 – 1 μg ml−1) The direction of T-lymphocytes under shear flow expressed as the migration index (MI) under 100 s−1 (red bars), 400 s−1 (green bars) and 800 s−1 (blue bars). The total protein concentration was fixed at 10 μg ml−1 and the ratios of ICAM-1 to VCAM-1 were varied. Directional migration is dictated by ligand concentration at a shear rate of 100 s−1, with a substantial amount of ICAM-1 necessary for upstream migration. However, the presence of any ICAM-1 supports upstream migration at a shear rate of 400 s−1 (intermediate effect) and 800 s−1. The MI was calculated from T-lymphocyte tracks from three independent experiments.
T-lymphocyte resistance to shear flow is mediated through LFA-1–ICAM-1 interactions
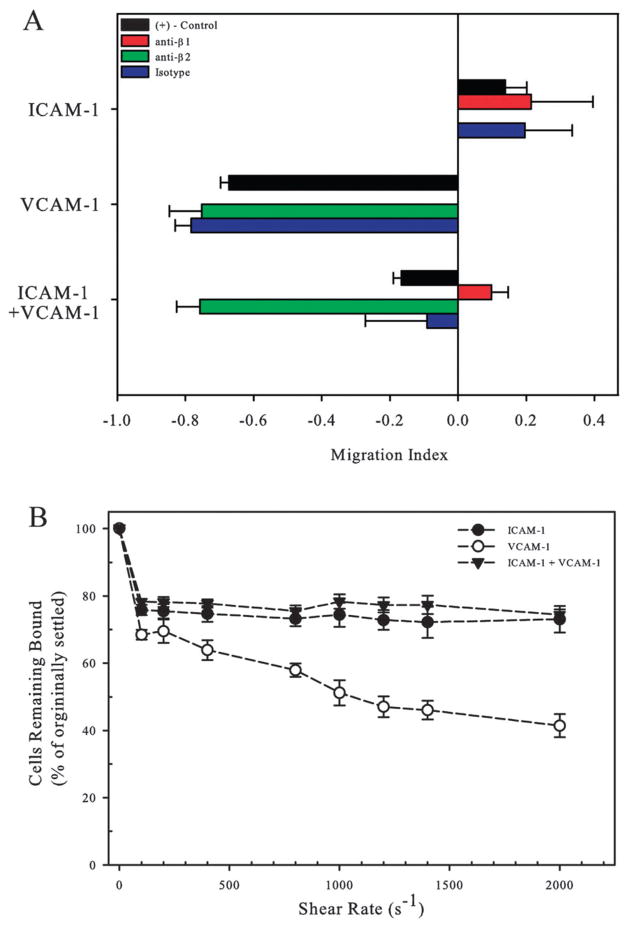
Antibodies were used to block either the β2 or β1 integrin subunits and cells were plated on surfaces of 5 μg ml−1 ICAM-1, 5 μg ml−1 VCAM-1, and 2.5 μg ml−1 ICAM-1 + 2.5 μg ml−1 VCAM-1. Upon blocking against the β2 integrin subunit on surfaces comprised of ICAM-1 alone, no cell adhesion was observed under flow meaning that the MI could not be calculated (Fig. 7A). The same was observed on surfaces made from VCAM-1 surfaces when the β1 integrin subunit was blocked. When T-lymphocytes were blocked against the β2 integrin and exposed to a surface of combined ICAM-1 and VCAM-1 ligand, we observed downstream migration of cells with fluid flow mimicking the behavior of cells on surfaces of VCAM-1 alone. Conversely, upon blocking against the β1 integrin, the MI was positive, indicating upstream migration – a result that was similar to what was observed on surfaces containing ICAM-1 alone. These results show that the motility observed on ICAM-1 and VCAM-1 surfaces under fluid flow are due to the specific interactions of the ligand-integrin pairs.
Fig. 7.
T-lymphocytes resist shear detachment through the β2 integrin. (A) T-lymphocytes pretreated with blocking antibodies against the β1 (red bar) or β2 (green bar) integrin subunits and were exposed to ICAM-1, VCAM-1, or ICAM-1 + VCAM-1 surfaces under 100 s−1 of fluid flow and the MI was determined. Data are mean ± SE (B) T-lymphocytes were exposed to increasing shear rates and the percent of cells remaining bound after 5 minutes of exposure was calculated. Shear rate was increased every 5 minutes starting at 100 s−1 and ending at 2000 s−1.
Next, the adhesion strength of T-lymphocytes was qualitatively assessed on printed surfaces of ICAM-1, VCAM-1, or ICAM-1 + VCAM-1 by exposing cells to increasing shear rates which were increased every 5 minutes for a period of 40 minutes. Cells were initially injected and allowed to adhere under static conditions for 15 minutes on surfaces of 5 μg ml−1 of ICAM-1, VCAM-1, or a combined surface of the two before exposure to shear. Fluid flow was then increased incrementally from 100 s−1 to 2000 s−1 and the number of cells that remained bound from those that originally settled on the surface was determined. Upon initial application of fluid flow at a shear rate of 100 s−1, approximately 20–30% of the cells were detached, presumably due to little or no adhesion to any of the three ligand conditions (Fig. 7B). For cells on ICAM-1, increasing the shear rate further did not decrease the number of remaining bound cells significantly (76 versus 73%). However, for cells on VCAM-1 surfaces, the number of bound cells significantly decreased as a function increasing shear rate from 68% to 41% bound. For surfaces on which both ICAM-1 and VCAM-1 were combined, there was no significant decrease in number of cells bound with increasing shear rates, similar to what was found on ICAM-1 surfaces.
Migration on ICAM-1 or VCAM-1 is robust and reversible but not on combined ligand surfaces
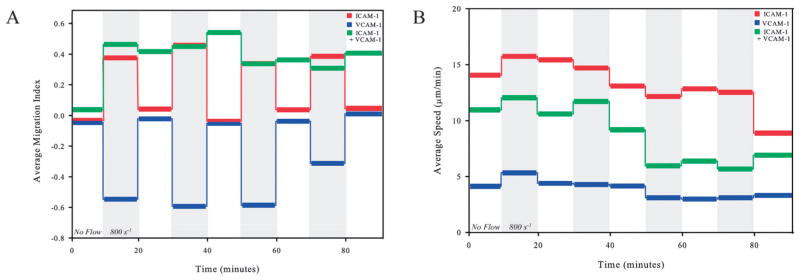
Next, the directional responses of T-lymphocytes on ICAM-1, VCAM-1, and ICAM-1 + VCAM-1 surfaces under high shear rates were reversible. Cells were allowed to migrate in periodic square waves of shear rate, alternating between no flow and a shear rate of 800 s−1, in increments of 10 minutes for a total time of 90 minutes. This technique is similar to that employed recently by Valignat et al.10 At shear rates of 800 s−1, cells on ICAM-1 migrate upstream (MI ~ 0.4) while cells on VCAM-1 migrate downstream (MI ~ −0.6) (Fig. 8A). Furthermore, upon application of alternating periods of no flow and shear rates of 800 s−1, the direction of migration is reversible with a return to a MI to nearly zero (random migration) demonstrating no memory of being exposed to shear flow. However, for cells on the combined ligand surface (I5 + V5), this behavior was not reversible; the MI remained positive during all periods of flow and even when the flow was removed. The exception was the absence of flow in the initial 10 minutes (Fig. 8A), when the cell had not been previously exposed to flow. During these periods of alternating shear flow, we found no change in cell speed in the presence or absence of flow (Fig. 8B). However, the average cell speeds on ICAM-1 alone during the time course of the experiment were always greater than those observed on VCAM-1 alone. Furthermore, cell speeds on surfaces with the combination of VCAM-1 and ICAM-1 remained between the speeds observed for ICAM-1 and VCAM-1 independently.
Fig. 8.
T-lymphocytes exhibit reversible directional responses to periodic shear flow on ICAM-1 and VCAM-1 but not on combined surfaces. (A) and (B) T-lymphocytes were exposed to periodic square waves of duration of 10 min and between shear rates of 0 s−1 and 800 s−1 (shaded region) on 5 μg ml−1 ICAM-1, 5 μg ml−1 VCAM-1, and 2.5 μg ml−1 each of both ICAM-1 and VCAM-1. The average MI and average speed over the 10 minute time interval were measured.
Shear flow leads to rearrangement of the T-lymphocyte cytoskeleton
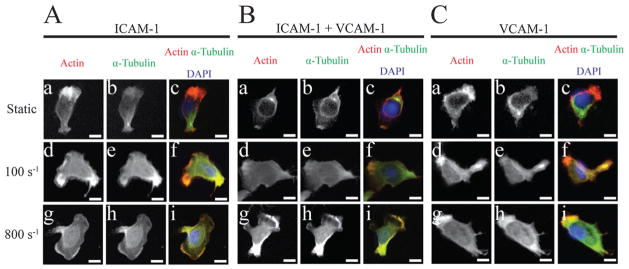
To further investigate the mechanisms of motility of T-lymphocytes, we exposed cells to shear flow on surfaces of 5 μg ml−1 of ICAM-1, VCAM-1, or combinations of the two followed by cell fixation, permeabilization, and fluorescent labeling of the actin and microtubule cytoskeletons. It has been previously shown that T-lymphocytes polarize on ICAM-1 and VCAM-1 surfaces alone in the absence of chemokine with an actin-rich lamellipod and an uropod containing the microtubule organizing center (MTOC).21 In the absence of flow for all three substrates, we observe distinct cytoskeletal polarity with actin predominantly located in the lamellipod and microtubules originating from the (MTOC) behind the nucleus (Fig. 9A(a–c), B(a–c), C(a–c)). Upon the introduction of shear flow, a loss of cytoskeletal polarity is observed with increasing co-localization of actin and tubulin on all three substrates; at both 100 s−1 and 800 s−1 shear rates, this rearrangement of cytoskeletal proteins is observed (Fig. 9A(d–i), B(d–i), C(d–i)). Cells migrating on either ICAM-1 or VCAM-1 alone under both shear rates display leading edges that contain brighter regions of actin which align in the direction of migration. On the other hand, cells migrating on combined surfaces of ICAM-1 + VCAM-1 under 100 s−1 of fluid flow display multiple regions along the edge of the cell that are rich in actin due to their dependence on ligand concentrations to control migration direction. Once the cells are exposed to 800 s−1 of fluid flow, the cells are observed to have increased actin at the leading edge in the direction of fluid flow similar to what is seen on ICAM-1 alone.
Fig. 9.
T-lymphocytes undergo cytoskeletal rearrangement upon application of shear flow during migration. (A) Fluorescent images of migrating T-lymphocytes on 5 μg ml−1 ICAM-1 under static (a–c), 100 s−1 (d–f), and 800 s−1 (g–i) shear rates. (B) Fluorescent images of migrating T-lymphocytes on 2.5 μg ml−1 ICAM-1 and 2.5 μg ml−1 VCAM-1 under static (a–c), 100 s−1 (d–f), and 800 s−1 (g–i) shear rates. (C) Fluorescent images of migrating T-lymphocytes on 5 μg ml−1 VCAM-1 under static (a–c), 100 s−1 (d–f), and 800 s−1 (g–i) shear rates. The direction of flow is from right to left. Scale bar = 5 μm.
Discussion
Intraluminal crawling under shear flow is required for T-lymphocyte extravasation and is dependent upon engagement of integrins to support adhesion and migration.27 Recently, Valignat et al. showed that primary human T-lymphocytes crawl against fluid flow in a shear dependent manner on ICAM-1 surfaces.10 In this study, we extend upon this recent work and report upon the effect of substrate chemistry on the directionality of primary human T-lymphocyte migration under fluid flow. Through the use of a parallel flow chamber, we quantified cell motility under fluid flow on substrates with different adhesive ligands in the absence of chemokine. Upon introduction of shear flow to cells exposed to either ICAM-1 or VCAM-1, we observed directional migration upstream or downstream of fluid flow, respectively. These results are dependent not only upon shear rate but also ligand concentration. Furthermore, to our knowledge, no one has investigated the effects of simultaneous engagement of LFA-1 and VLA-4 on the direction of T-lymphocyte motility under shear flow. Herein we report for the first time that T-lymphocytes favor LFA-1–ICAM-1 interactions to support migration upstream of fluid flow on surfaces presenting the adhesive ligands ICAM-1 and VCAM-1 together under high shear rates.
The integrins LFA-1 and VLA-4 are known to be mechano-responsive to force, leading to increased activation and adhesion stabilization.2,11,22,24,28 We expect that as shear rate is increased the integrin–ligand bonds strengthen to promote adhesion and migration. Overall, increasing shear rates led to an increase in the orientation of migration upstream of fluid flow on ICAM-1 surfaces but not VCAM-1 surfaces. These unique responses are likely caused by the inherent differences between these two integrins and their roles physiologically. The integrin LFA-1 binds to ICAM-1 and is required for firm adhesion and extravasation under fluid flow after transient adhesion events and chemokine exposure. Therefore, it appears that LFA-1–ICAM-1 interactions secure adhesion and promote lamellipodial extension upstream. VLA-4, on the other hand, is capable of supporting both tethering and rolling (in the absence of other adhesion ligands) through engagements with VCAM-1 and is required for capture of T-lymphocytes from fluid flow. Furthermore, after chemokine exposure, VLA-4 increases its affinity to promote firm adhesion and, along with LFA-1, mediates migration along the blood endothelium supporting diapedesis through the endothelium.4,27,29,30 Although VLA-4 participates in transient as well as firm adhesion events, we hypothesize that this integrin facilitates weaker adhesions compared to LFA-1 and cannot stabilize upstream interactions. Under flow, VLA-4 most likely supports a passive form of adhesion that can support downstream migration and prevent T-lymphocytes from being swept away. This has been proposed in other cell types, such as neutrophils, and is hypothesized to be due to lack of a mechanosensing mechanism through this receptor.10,31 Furthermore, unlike previous studies, we observed decreased speeds with increasing shear rate on both ICAM-1 and VCAM-1 surfaces. A possible explanation for the increased speed of upstream migration is the increase in the modulation of integrin–ligand affinity that leads to greater adhesive interactions.32,33 Interestingly, upon varying ligand concentration, increased upstream migration on ICAM-1 surfaces was observed to be dependent upon shear rate and not ligand density. On the other hand, cells migrating on increasing VCAM-1 concentrations showed increased downstream migration as ligand density increased but only during exposure to 100 s−1 of fluid flow.
Under homeostatic conditions, endothelial cells express basal levels of ICAM-1 and VCAM-1, and upon activation by inflammatory stimuli, expression of these molecules is upregulated to facilitate increased cell arrest and migration.34 Furthermore, it is known that physiologically relevant levels of shear stress due to pathologies can alter the expression of these adhesion molecules with decreasing shear stresses leading to decreased expression of ICAM-1 and increased expression of VCAM-1 while increasing shear stresses lead to increased expression of ICAM-1 and decreased expression of VCAM-1.35–37 Surfaces were created that present both ICAM-1 and VCAM-1 together at various densities to elucidate how simultaneous ligation of LFA-1 and VLA-4 controls migration under fluid flow at different shear stresses. Upon presentation of both adhesion molecules, we expected to observe motility that was intermediate between the behaviors of each ligand alone. Because VCAM-1 engagement can regulate β2-dependent adhesion on ICAM-1 surfaces, crosstalk between the two signaling pathways downstream of receptor engagement presents an additional layer of complexity in the regulation of T-lymphocyte motility.5,19,20,38 At a shear rate of 100 s−1, we observed directional responses that were dependent upon the concentrations of both ligands with preferred upstream migration on surfaces with higher concentrations of ICAM-1 and preferred downstream migration on surfaces with higher concentrations of VCAM-1. This behavior is consistent with the hypothesis that the phenotype of migration is consistent with the concentration of ligand. The magnitude of the migration index for each condition on combined surfaces is less than what is observed for each ligand individually, again consistent with an intermediate response.
On the other hand, upon application of high shear rates, such as 400 s−1 and 800 s−1 of fluid flow, we observe a shift in preferential migration upstream of flow that is dependent upon the presence of ICAM-1 on the surface. This effect was the most dramatic for the 800 s−1 condition with upstream migration occurring on all surfaces (except VCAM-1 alone) independent of the concentration of VCAM-1 present on the surface. It is known that adhesion strengthening occurs upon plating human T-lymphocytes on co-immobilized ICAM-1 and VCAM-1 surfaces.19 Together, these observations lead us to believe that under conditions of high shear, T-lymphocytes utilize LFA-1-ICAM-1 engagements over VLA-4-VCAM-1 engagements to resist detachment due to shear stress. These interactions stabilize adhesions supporting upstream migration when presented with both adhesion ligands. This shear resistance is hypothesized to occur as a result of outside-in integrin signaling triggered upon application of shear stress across the body of the cell that is translated into applied tension across the ligated integrin bonds thus increasing integrin activation. Furthermore, flow cytometry revealed that there are larger quantities of the β2 integrin than β1 present on the cell surface; by having more LFA-1 available to bind, smaller quantities of ICAM-1 may be capable of supporting upstream motion on the combined surfaces even in the presence of much larger quantities of VCAM-1. Also, if there is a larger number of activated LFA-1 versus VLA-4, this could explain why we observe more upstream motion on combined surfaces of the two ligands especially at high shear rates. Motility observed on surfaces of ICAM-1, VCAM-1, and ICAM-1 + VCAM-1 was shown to be due to specific ligand–integrin interactions as verified through functional integrin blocking. Through a shear flow cell detachment assay we demonstrated that cells on surfaces of ICAM-1 or combined ICAM-1 and VCAM-1 have greater resistance to shear stress than on surfaces of VCAM-1 alone. This further supports our claim that LFA-1-ICAM-1-mediated interactions are necessary for robust upstream migration and dominate under high shear on surfaces that present both adhesive ligands. Furthermore, utilization of the LFA-1 over VLA-4 under high shear rates may prove more advantageous to support migration since it is known that ICAM-1 expression is upregulated while VCAM-1 expression is downregulated on endothelial cells.37
T-lymphocyte upstream migration on ICAM-1 surfaces has been previously shown to be robust and reversible with cells migrating upstream upon application of flow and returning to random motion once it is removed.10 Here, the robustness of the response on surfaces of mixed chemistry was measured. We showed that motility is reversible on surfaces of VCAM-1 with downstream migration seen under fluid flow and random migration upon removal. On surfaces with ICAM-1 and VCAM-1 together, the directionality of motility is not reversible with T-lymphocytes maintaining upstream motion during periods of no flow. This suggests upstream motion is driven by signaling, not physical effects such as forces from shear stresses. This implies that memory occurs upon the removal of flow and this memory of signaling requires simultaneous ligation of both LFA-1 and VLA-4 since it is not observed upon ligation of each integrin receptor individually.
It has been speculated that shear stress may trigger signals or changes within the cytoskeletal machinery in T-lymphocytes. T-lymphocyte polarity and motility is highly dependent upon the arrangement of the actin and microtubule cytoskeletons through signaling pathways involving the Rho family GTPases.39–41 It has been previously shown that T-lymphocytes polarize on ICAM-1 and VCAM-1 surfaces alone in the absence of chemokine with an actin-rich lamellipod and microtubule dense uropod.21 Upon fluorescent labeling after exposing cells to shear flow, there was increased co-localization of these cytoskeletal proteins, and the absence of a clearly defined lamellipod. Even with this co-localization, dense regions of actin along the migrating leading edge are still observed matching to the preferred direction of migration under fluid flow. On the other hand, cells on the combined surface under low shear contain multiple dense regions of actin along the edge demonstrating their dependence on ligand concentration. We hypothesize that this increased co-localization of actin and tubulin occurs in order to withstand extracellular forces required to resist deformation caused by the shear stress. The cellular cytoskeleton is known to be involved with several signaling pathways thus possibly providing the signals required for adaptation to a new mechanical environment.42 This has been well established in other cells types, such as endothelial cells, which are capable of rearranging their cytoskeletons in response to fluid shear forces.43,44 This provides new insight into the role of integrins in mediating T-lymphocyte motility and understanding the critical role ligand composition can play in controlling the physiological response of T-lymphocytes to shear flow.
Supplementary Material
Insight, innovation, integration.
T-lymphocytes must attach and migrate along the blood endothelium to elicit their effector functions. Here, we demonstrate how ligand composition and varying shear rates modulate the orientation of human primary T-lymphocytes. Surfaces were created that allowed for dual presentation of two different adhesive ligands at fixed ratios. We demonstrate that the directionality of T-lymphocyte migration under shear flow is dependent upon the presentation of the ligand. Our work elucidates how T-lymphocytes respond to physiologic shear flow and the importance of integrin–ligand interactions to support motility.
Acknowledgments
Funding for this work was provided by National Institutes of Health (AI082292 and HL18208).
Footnotes
Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ib00201f
References
- 1.Luster AD, Alon R, von Andrian UH. Nat Immunol. 2005;6:1182–1190. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- 2.Alon R, Dustin ML. Immunity. 2007;26:17–27. doi: 10.1016/j.immuni.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Springer TA. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 4.von Andrian UH, Mackay CR. N Engl J Med. 2000;343:1020–1034. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- 5.Rose DM, Grabovsky V, Alon R, Ginsberg MH. J Immunol. 2001;167:2824–2830. doi: 10.4049/jimmunol.167.5.2824. [DOI] [PubMed] [Google Scholar]
- 6.Jones DA, McIntire LV, Smith CW, Picker LJ. J Clin Invest. 1994;94:2443–2450. doi: 10.1172/JCI117612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butcher EC, Picker LJ. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 8.Shulman Z, Cohen SJ, Roediger B, Kalchenko V, Jain R, Grabovsky V, Klein E, Shinder V, Stoler-Barak L, Feigelson SW, Meshel T, Nurmi SM, Goldstein I, Hartley O, Gahmberg CG, Etzioni A, Weninger W, Ben-Baruch A, Alon R. Nat Immunol. 2012;13:67–76. doi: 10.1038/ni.2173. [DOI] [PubMed] [Google Scholar]
- 9.Steiner O, Coisne C, Cecchelli R, Boscacci R, Deutsch U, Engelhardt B, Lyck R. J Immunol. 2010;185:4846–4855. doi: 10.4049/jimmunol.0903732. [DOI] [PubMed] [Google Scholar]
- 10.Valignat MP, Theodoly O, Gucciardi A, Hogg N, Lellouch AC. Biophys J. 2013;104:322–331. doi: 10.1016/j.bpj.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lek HS, Morrison VL, Conneely M, Campbell PA, McGloin D, Kliche S, Watts C, Prescott A, Fagerholm SC. J Biol Chem. 2013;288:14698–14708. doi: 10.1074/jbc.M112.430918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alon R, Kassner PD, Carr MW, Finger EB, Hemler ME, Springer TA. J Cell Biol. 1995;128:1243–1253. doi: 10.1083/jcb.128.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berlin C, Bargatze RF, Campbell JJ, von Andrian UH, Szabo MC, Hasslen SR, Nelson RD, Berg EL, Erlandsen SL, Butcher EC. Cell. 1995;80:413–422. doi: 10.1016/0092-8674(95)90491-3. [DOI] [PubMed] [Google Scholar]
- 14.Konstantopoulos K, Kukreti S, Smith CW, McIntire LV. J Leukocyte Biol. 1997;61:179–187. doi: 10.1002/jlb.61.2.179. [DOI] [PubMed] [Google Scholar]
- 15.Hyun YM, Chung HL, McGrath JL, Waugh RE, Kim M. J Immunol. 2009;183:359–369. doi: 10.4049/jimmunol.0803388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shimonaka M, Katagiri K, Nakayama T, Fujita N, Tsuruo T, Yoshie O, Kinashi T. J Cell Biol. 2003;161:417–427. doi: 10.1083/jcb.200301133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stachowiak AN, Wang Y, Huang YC, Irvine DJ. J Immunol. 2006;177:2340–2348. doi: 10.4049/jimmunol.177.4.2340. [DOI] [PubMed] [Google Scholar]
- 18.Hyun YM, Lefort C, Kim M. Immunol Res. 2009;45:195–208. doi: 10.1007/s12026-009-8101-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan JR, Hyduk SJ, Cybulsky MI. J Immunol. 2000;164:746–753. doi: 10.4049/jimmunol.164.2.746. [DOI] [PubMed] [Google Scholar]
- 20.May AE, Neumann FJ, Schömig A, Preissner KT. Blood. 2000;96:506–513. [PubMed] [Google Scholar]
- 21.Dominguez GA, Hammer DA. Integr Biol. 2014;6:862–873. doi: 10.1039/c4ib00094c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sigal A, Bleijs DA, Grabovsky V, van Vliet SJ, Dwir O, Figdor CG, van Kooyk Y, Alon R. J Immunol. 2000;165:442–452. doi: 10.4049/jimmunol.165.1.442. [DOI] [PubMed] [Google Scholar]
- 23.Chan JR, Hyduk SJ, Cybulsky MI. J Exp Med. 2001;193:1149–1158. doi: 10.1084/jem.193.10.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woolf E, Grigorova I, Sagiv A, Grabovsky V, Feigelson SW, Shulman Z, Hartmann T, Sixt M, Cyster JG, Alon R. Nat Immunol. 2007;8:1076–1085. doi: 10.1038/ni1499. [DOI] [PubMed] [Google Scholar]
- 25.Granger DN, Kubes P. J Leukocyte Biol. 1994;55:662–675. [PubMed] [Google Scholar]
- 26.Koutsiaris AG, Tachmitzi SV, Batis N, Kotoula MG, Karabatsas CH, Tsironi E, Chatzoulis DZ. Biorheology. 2007;44:375–386. [PubMed] [Google Scholar]
- 27.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 28.Chen C, Mobley JL, Dwir O, Shimron F, Grabovsky V, Lobb RR, Shimizu Y, Alon R. J Immunol. 1999;162:1084–1095. [PubMed] [Google Scholar]
- 29.Kinashi T. Nat Rev Immunol. 2005;5:546–559. doi: 10.1038/nri1646. [DOI] [PubMed] [Google Scholar]
- 30.Muller WA. Annu Rev Pathol: Mech Dis. 2011;6:323–344. doi: 10.1146/annurev-pathol-011110-130224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith LA, Aranda-Espinoza H, Haun JB, Hammer DA. Biophys J. 2007;92:632–640. doi: 10.1529/biophysj.105.079418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palecek SP, Loftus JC, Ginsberg MH, Lauffenburger DA, Horwitz AF. Nature. 1997;385:537–540. doi: 10.1038/385537a0. [DOI] [PubMed] [Google Scholar]
- 33.Zhu J, Luo BH, Xiao T, Zhang C, Nishida N, Springer TA. Mol Cell. 2008;32:849–861. doi: 10.1016/j.molcel.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pober JS, Cotran RS. Physiol Rev. 1990;70:427–451. doi: 10.1152/physrev.1990.70.2.427. [DOI] [PubMed] [Google Scholar]
- 35.Nagel T, Resnick N, Atkinson WJ, Dewey CF, Jr, Gimbrone MA., Jr J Clin Invest. 1994;94:885–891. doi: 10.1172/JCI117410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walpola PL, Gotlieb AI, Cybulsky MI, Langille BL. Arterioscler Thromb Vasc Biol. 1995;15:2–10. doi: 10.1161/01.atv.15.1.2. [DOI] [PubMed] [Google Scholar]
- 37.Ishibazawa A, Nagaoka T, Yokota H, Ono S, Yoshida A. Exp Eye Res. 2013;116:308–311. doi: 10.1016/j.exer.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 38.Porter JC, Hogg N. J Cell Biol. 1997;138:1437–1447. doi: 10.1083/jcb.138.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wittmann T, Waterman-Storer CM. J Cell Sci. 2001;114:3795–3803. doi: 10.1242/jcs.114.21.3795. [DOI] [PubMed] [Google Scholar]
- 40.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 41.Krummel MF, Macara I. Nat Immunol. 2006;7:1143–1149. doi: 10.1038/ni1404. [DOI] [PubMed] [Google Scholar]
- 42.Yu Y, Smoligovets AA, Groves JT. J Cell Sci. 2013;126:1049–1058. doi: 10.1242/jcs.098210. [DOI] [PubMed] [Google Scholar]
- 43.Dewey CF, Jr, Bussolari SR, Gimbrone MA, Jr, Davies PF. J Biomech Eng. 1981;103:177–185. doi: 10.1115/1.3138276. [DOI] [PubMed] [Google Scholar]
- 44.Nerem RM, Levesque MJ, Cornhill JF. J Biomech Eng. 1981;103:172–176. doi: 10.1115/1.3138275. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.