Cell therapy may transform the treatment of acute and chronic heart disease, with an anticipated impact rivaling the results of revascularization and reperfusion therapies developed in the last 50 years. Through controversies over the mechanisms of cell therapy, engraftment, and differentiation, the biological and translational (1) studies performed over the past decade shape the future of this very exciting new field of cardiovascular medicine (2). The lessons learned in the repair of ischemic and other myocardial diseases will also yield biological insights for the treatment of other organ systems.
Cell Therapy Programs of the 2000s
The basic notion of cell therapy for an acute or chronic injury was that the correct cell type—presumably a stem or precursor cell with the ability to engraft into an area of cardiac injury, and then to differentiate into lost cellular elements—can be sought and used for cardiac therapeutics. The idea of repopulating the bare patches of a lawn with new grass seed seems to have been a vast oversimplification, as we now understand the variety of pathways by which exogenous cell administration affect cardiac healing and remodeling (1,2). The translational application of candidate cell types has been criticized by some as being applied too rapidly before the cell biology is fully understood (3,4). Nonetheless, programs testing various cells in small- and large-animal models, followed by proof of principle human studies or early phase clinical trials, have moved the field forward substantially. As is the case in most early fields, translational studies have enabled further controversies as to whether appropriate strategies are being employed (5), and have focused the questions asked by pre-clinical investigators. Importantly however, translational research has provided an early vision of the future for this field, showing clearly what we know and what we don’t, and providing information on cell product preparation, delivery strategies (6), phenotypes of response to cell therapy (7), the measurement of clinically relevant and surrogate end points, and the identification of the clinical determinants of patient responsiveness (8).
Selecting Among Cell Types
The characteristics of the ideal cell type have been articulated by many experts: a cell type should be both quantitatively and temporally available, safe to administer, effective at engraftment, differentiation, and (most importantly) cardiac repair. Some argue that a source of autologous therapy is ideal so as to avoid any possibility of rejection, although it should be acknowledged that allogeneic cell therapy is also emerging as a strong possibility (8). Practical considerations, including the cost of therapy, will ultimately bear importantly on the accessibility of a new therapy.
Over the past decade, the translational pipeline has tested or is in the process of testing: 1) autologous whole bone marrow (AWBM); 2) skeletal myoblasts; 3) bone-marrow–derived mesenchymal stem cells (MSCs); 4) MSCs from other tissues, and MSC precursors; and 5) a variety of cardiac stem cell (CSC) preparations.
Pluripotent cells derived from embryonic stem cells (9,10) or induced pluripotent stem (11) cells are under vigorous study but are several years away from being tested in humans (12). Thus far, AWBM and skeletal myoblasts have yielded mixed results of surrogate efficacy. AWBM produces small but significant increases in ejection fraction, decreases in infarct size, and prevents remodeling (13); this cell therapy may have clinical benefits out of proportion to the degree of cardiac functional recovery (14). Bone-marrow–derived MSCs are the most rigorously studied stem cell population (Fig. 1), and this cell type is presently undergoing phase II testing for myocardial infarction and for heart failure (15).
Figure 1. MSC Research Publications.
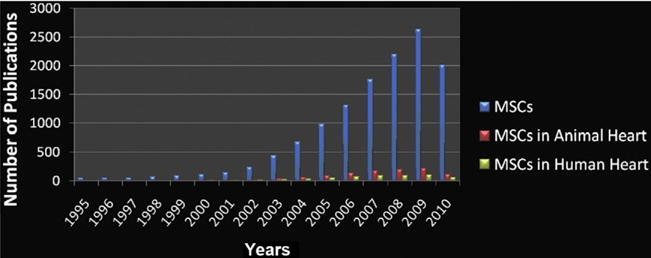
Publications on mesenchymal stem cell (MSC) research (blue bars) show substantial increases in this millennium, with parallel increases in cardiac experimental studies (red bars) and human studies (green bars) (2010 data through June 2010).
Cardiac stem cells, discovered in the early part of the millennium (16), are an exciting and promising potential class for cell-based therapies. The best understood is the cardiac c-kit cell, and it is definitively identified in the human heart (17); but other cardiac precursors are described (18,19). Enthusiasm for cardiac stem cells is very high as they fulfill most of the criteria anticipated for a cell therapeutic—they represent an autologous source of cardiopoietic and vasculogenic precursors that may be readily available. The 2 strategies for preparing cardiac stem cells farthest along the translational pipeline are to amplify c-kit CSCs from surgical biopsies (usually from the atrial appendage), and to prepare cardiospheres, the topic of the paper by Lee et al. (20) in this issue of the Journal. Cardiospheres are a collection of cells that can be coaxed to amplify from small pieces of heart tissue (21,22). Cardiospheres are composed of a mix of cell types, including 15% to 20% cardiac precursor cells, with the remainder being supporting cellular elements with mesenchymal features. The contributions from the Marbãn group represented by the current paper (20) and by that of Johnston et al. (23) are commendable for their use of rigorous translational large-animal models, the employment of delivery systems adaptable to human use, and sophisticated end point phenotyping. Porcine models can be designed closely to replicate the clinical scenario of reperfused infarction in either acute or chronic settings (24) and have led to Food and Drug Administration approval of a phase I trial of the cardiosphere strategy (25).
The heterogeneity of cellular constituents of the cardiosphere may prove to be advantageous, as the cell mixture may enable the recreation of the cardiopoietic niche (26). Indeed, this notion is a major conceptual advancement in the field of cell therapeutics and supports the idea that the stem cell niche may represent the functional unit of effective cardiac regeneration (1,26). In this regard, we have recently shown that bone marrow MSCs work in large part by cell-cell interactions with endogenous CPCs (1). Optimizing the process of therapeutic myogenesis may require a tailored combination of support cells and cardiopoietic precursors; although comparisons among cell types are at a very early stage of investigation, there is a large variety of therapeutic constructs from which to choose, and the insights gleaned from translational models offer major opportunities to refine and optimize cell therapeutics in the future.
Where Do We Go From Here?
The optimism for the field of cardiac cell therapy comes in large measure from the clear demonstrations of efficacy in large-animal models, which have also defined safety parameters and delivery strategies. If clinical trials replicate even a fraction of the benefit witnessed in the porcine model, the medical impact could be substantial. The results of animal studies coupled with ongoing early phase clinical trials suggest entry into the clinic in the next decade. For the next wave of trials to succeed, investigators must turn their attention to comparative studies seeking to define the best cell, cell combination, or therapeutic schedule. To the extent that various cell types produce similar outcomes (a seemingly likely eventuality for clinical outcomes), then consideration of cost and practicality will rise in importance.
There are critically important societal issues to consider as this field advances. Although cell therapy offers so much promise and fulfills a major unmet need for patients with chronic left ventricular dysfunction, public funding for clinical trials in the area is minimal. In addition, intellectual property concerns have almost entirely limited the entry of big pharma into the area. Treatments being offered to paying patients outside the United States are of uncertain safety and efficacy, yet are being widely sought by patients. Thus, the field finds itself in an awkward, paradoxical position—with an enormously promising transformative therapy looming on the horizon yet with progress being hindered by a relative lack of funding support for translational research on the one hand and scientific debate on the other.
Acknowledgments
This work was supported by National Heart, Lung, and Blood Institute grants U54-HL081028 (Specialized Center for Cell Based Therapy), R01-HL084275, and P20 HL101443. Dr. Hare is supported by RO1s AG025017, HL065455, and HL094849; is on the Steering Committee for Osiris; and is a consultant for Kardia. All other authors have reported they have no relationships to disclose.
Footnotes
Editorials published in the Journal of the American College of Cardiology reflect the views of the authors and do not necessarily represent the views of JACC or the American College of Cardiology.
References
- 1.Hatzistergos KE, Quevedo H, Oskouei BN, et al. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107:913–22. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anversa P, Kajstura J, Leri A, Bolli R. Life and death of cardiac stem cells: a paradigm shift in cardiac biology. Circulation. 2006;113:1451–63. doi: 10.1161/CIRCULATIONAHA.105.595181. [DOI] [PubMed] [Google Scholar]
- 3.Chien KR. Stem cells: lost in translation. Nature. 2004;428:607–8. doi: 10.1038/nature02500. [DOI] [PubMed] [Google Scholar]
- 4.Chien KR. Regenerative medicine and human models of human disease. Nature. 2008;453:302–5. doi: 10.1038/nature07037. [DOI] [PubMed] [Google Scholar]
- 5.Boyle AJ, Schulman SP, Hare JM, Oettgen P. Is stem cell therapy ready for patients? Stem cell therapy for cardiac repair. Ready for the next step. Circulation. 2006;114:339–52. doi: 10.1161/CIRCULATIONAHA.105.590653. [DOI] [PubMed] [Google Scholar]
- 6.Heldman AW, Hare JM. Cell therapy for myocardial infarction: special delivery. J Mol Cell Cardiol. 2007;44:473–6. doi: 10.1016/j.yjmcc.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quevedo HC, Hatzistergos KE, Oskouei BN, et al. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc Natl Acad Sci USA. 2009;106:14022–7. doi: 10.1073/pnas.0903201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hare JM, Traverse JH, Henry TD, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–86. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christoforou N, Oskouei BN, Esteso P, et al. Implantation of mouse embryonic stem cell-derived cardiac progenitor cells preserves function of infarcted murine hearts. PLoS ONE. 2010;5:e11536. doi: 10.1371/journal.pone.0011536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang L, Soonpaa MH, Adler ED, et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453:524–8. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- 11.van Laake LW, Qian L, Cheng P, et al. Reporter-based isolation of induced pluripotent stem cell- and embryonic stem cell-derived cardiac progenitors reveals limited gene expression variance. Circ Res. 2010;107:340–7. doi: 10.1161/CIRCRESAHA.109.215434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ieda M, Fu JD, Delgado-Olguin P, et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–86. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdel-Latif A, Bolli R, Tleyjeh IM, et al. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med. 2007;167:989–97. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- 14.Assmus B, Rolf A, Erbs S, et al. Clinical outcome 2 years after intracoronary administration of bone marrow-derived progenitor cells in acute myocardial infarction. Circ Heart Fail. 2010;3:89–96. doi: 10.1161/CIRCHEARTFAILURE.108.843243. [DOI] [PubMed] [Google Scholar]
- 15.Hare JM. Translational development of mesenchymal stem cell therapy for cardiovascular diseases. Tex Heart Inst J. 2009;36:145–7. [PMC free article] [PubMed] [Google Scholar]
- 16.Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–76. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 17.Bearzi C, Rota M, Hosoda T, et al. Human cardiac stem cells. Proc Natl Acad Sci USA. 2007;104:14068–73. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oh H, Bradfute SB, Gallardo TD, et al. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci USA. 2003;100:12313–8. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laugwitz KL, Moretti A, Lam J, et al. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–53. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee S-T, White AJ, Matsuhita S, et al. Intramyocardial injection of autologous cardiosphere or cardiosphere-derived cells preserves function and minimizes adverse ventricular remodeling in pigs with heart failure post-myocardial infarction. J Am Coll Cardiol. 2011;57:455–65. doi: 10.1016/j.jacc.2010.07.049. [DOI] [PubMed] [Google Scholar]
- 21.Messina E, De AL, Frati G, et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911–21. doi: 10.1161/01.RES.0000147315.71699.51. [DOI] [PubMed] [Google Scholar]
- 22.Smith RR, Barile L, Cho HC, et al. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 23.Johnston PV, Sasano T, Mills K, et al. Engraftment, differentiation, and functional benefits of autologous cardiosphere-derived cells in porcine ischemic cardiomyopathy. Circulation. 2009;120:1075–83. 7. doi: 10.1161/CIRCULATIONAHA.108.816058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schuleri KH, Boyle AJ, Centola M, et al. The adult Gottingen minipig as a model for chronic heart failure after myocardial infarction: focus on cardiovascular imaging and regenerative therapies. Comp Med. 2008;58:568–79. [PMC free article] [PubMed] [Google Scholar]
- 25.Marban E, Cheng K. Heart to heart: the elusive mechanism of cell therapy. Circulation. 2010;121:1981–4. doi: 10.1161/CIRCULATIONAHA.110.952580. [DOI] [PubMed] [Google Scholar]
- 26.Mazhari R, Hare JM. Mechanisms of action of mesenchymal stem cells in cardiac repair: potential influences on the cardiac stem cell niche. Nat Clin Pract Cardiovasc Med. 2007;4(Suppl 1):21–6. doi: 10.1038/ncpcardio0770. [DOI] [PubMed] [Google Scholar]


