Abstract
Background
Signs of cardiac transthyretin (TTR) amyloidosis (ATTR) in patients with echocardiographic increase in interventricular septal thickness (IVST) are lacking.
Objectives
To identify clinical and ECG/echocardiographic signs associated with increased IVST in ATTR.
Methods
Analysis of patients with baseline echocardiography in the Transthyretin Amyloidosis Outcomes Survey (THAOS) registry (N=1682). Patients were categorised into IVST classes according to the American Society of Echocardiography classification adapted to gender (ie, normal, mild, moderate, severe); then into two combined IVST classes (normal-mild and moderate-severe).
Results
425 patients were included: 336 with a TTR mutation (m-TTR) and 89 with wild-type TTR (WT-TTR). 72% were men. Median (25th, 75th centile) age was 62 (45, 72) years. Non-Val30Met and WT-TTR were frequent in moderate (41% and 35%) and severe (50% and 33%) IVST classes. Median IVST was 15 mm (14, 16) (moderate) and 20 mm (18, 22) (severe). In the combined moderate-severe class, 85% of patients were ≥55 years of age; 81% were men; 86% had blood pressure <140 mm Hg; and 77% had increased right ventricle thickness (≥7 mm). Up to 66% of patients had cardiac sparkling. Systolic dysfunction (left ventricular ejection fraction <50%), restrictive pattern and low voltage were less frequent, and observed in 49%, 18% and 33% of patients, respectively.
Conclusions
Increased IVST, especially in men ≥55 years with normal systolic blood pressure, increase in right ventricle free wall and valve thicknesses, and sparkling, should alert practitioners to the possibility of ATTR. Absence of restrictive pattern and low voltage should not rule out the suspicion.
Trial registration number:
NCT00628745 (clinicaltrials.gov).
Key questions.
What is already known about this subject?
Amyloidosis is a result of continuous accumulation of insoluble fibril proteins in the extracellular matrix in various organs including the heart. The two major proteins involved in this process are immunoglobulin light chains (AL) and transthyretin (TTR). Cardiac amyloid infiltration is the classic form of infiltrative hypertrophic cardiomyopathy (HCM) and is associated with abnormal increased interventricular septal thickening.
What does this study add?
We investigated and identified the clinical and echocardiographic signs that should alert practitioners to the possibility of TTR cardiac amyloidosis in patients with abnormal increased IVST. In future, additional research using new sensitive imaging techniques and including the other major type of cardiac amyloidosis, that is, light chain amyloidosis, and other types of HCM, should be undertaken to identify and validate specific diagnostic markers of the different types of cardiac amyloidosis.
How might this impact on clinical practice?
Cardiac amyloidosis is of poor prognosis and should be considered in the differential diagnosis of patients with abnormal increased IVST. When suspected, patients should be referred for genetic testing and/or other imaging evaluation (MRI and bone scintigraphy), and/or biopsy analysis should be identified.
Introduction
Recently, the European Society of Cardiology (ESC) published a position statement concerning hypertrophic cardiomyopathy, to raise clinicians’ awareness of the possible spectrum of abnormalities in patients, and eventually their families, with heart muscle diseases.1 Cardiac hypertrophy is generally identified by echocardiography and defined by an increase in interventricular septal thickness (IVST) and/or the left ventricular (LV) posterior wall thickness (PWT), with different cut-off values according to gender. This definition has been endorsed in the 2014 ESC guidelines.2–4 However, IVST increase alone cannot provide accurate information concerning the aetiology of heart disease. Hence, there is a need to develop appropriate diagnostic strategies based on clues from medical and family histories, physical examination, and non-invasive investigations such as ECG and echocardiography. Cardiac hypertrophy is now defined as a morphological increase in thickness of the LV wall that results from different conditions: cardiomyocyte hypertrophy or infiltration of either cardiomyocytes or the extracellular matrix.1 Cardiac transthyretin amyloidosis (ATTR) is a classic form of an infiltrative cardiomyopathy. It is a progressive lethal disease caused by continuous accumulation of insoluble TTR fibrils in the extracellular matrix.5
The hereditary form of ATTR expresses different phenotypes involving neuropathy and/or cardiomyopathy.6 More than 100 mutations have been reported.7 The Val30Met mutation is by far the most frequent mutation reported to cause ATTR, with two distinguishable forms depending on the age of onset: early (<50 years) and late (≥50 years).6 Early-onset Val30Met exhibits neuropathy as the major presenting feature and is endemic in Portugal, Japan and Brazil.6 Late-onset Val30Met patients exhibit neurological as well as cardiac abnormalities.6 Patients with non-Val30Met mutations were also reported to have cardiomyopathy and/or neuropathy.8 Wild-type (WT) ATTR, also known as senile systemic amyloidosis, usually affects the heart and primarily affects older male patients, with a prevalence of 25–36% observed in an autopsy series of elderly patients.9
Regardless of the genotype, identifying signs that may alert clinicians to the presence of ATTR cardiomyopathy are vital, as typical therapies for heart failure are not well tolerated; emerging treatments are designed to prevent progression of amyloid infiltration and prognosis is directly related to cardiac dysfunction, and is poor for later-stage disease.10
Accordingly, the aim of this study was to identify clinical, echocardiographic and ECG parameters associated with IVST increase in cardiac ATTR using the largest available international cohort (the Transthyretin Amyloidosis Outcomes Survey (THAOS)) on this disease to raise clinician's awareness of the diagnosis of cardiac ATTR.
Materials and methods
Patient population and data collection
The THAOS registry began enrolling patients in 2007. Details of this registry have been previously published.11 12
Data were obtained from the information given by the reporting centres, using a standardised dedicated website. Data entered into the database included, but were not limited to, demographics, clinical status, medical history, type of neuropathy when present, ECG and echocardiographic data, and TTR genotype.
All the participating clinical sites had received authorisation from their local ethical committees for use of the registry. Patients entered in the registry were required to be ≥18 years of age. Privacy and confidentiality of participating individuals were assured and each individual gave written consent.
Data extraction
By 30 June 2013, THAOS included data on 1682 patients, registered from 17 countries. This study reports the data of the 425 patients included in THAOS with a baseline echocardiography and IVST measurement in the range (3–40 mm), prespecified in the registry. Three patients with baseline echocardiography data were excluded due to out-of-range IVST values.
Definition of the interventricular septal thickness and genotype classifications
Patients were divided according to the IVST classification set by the American Society of Echocardiography.2 3 As stated, gender was also taken into consideration to limit misclassification.2 3 Classification was as follows: normal IVST group if thickness ≤9 mm in female subjects or ≤10 mm in male subjects; mildly abnormal if ≥10 and ≤12 mm in females, or ≥11 and ≤13 mm in males; moderately abnormal if ≥13 and ≤15 mm in females, or ≥14 and ≤16 mm in males;2 3 and severely abnormal if ≥16 mm in females or ≥17 mm in males.2 In a subsequent analysis, the normal/mild groups and moderate/severe groups were pooled to calculate clinical, ECG and echocardiographic prevalence signs associated with marked IVST increase.
Patients were also divided into four categories depending on ATTR genotype and onset: Val30Met early onset (<50 years), Val30Met late onset (≥50 years), non-Val30Met and WT-ATTR.
Definition of ECG and echocardiographic variables recorded
ECG analysis and measurements included heart rate, PR interval, QRS duration, QT interval, atrial fibrillation, pathological Q waves and low voltage. The latter was defined as QRS voltage amplitude <0.5 mV in all limb leads or <1 mV in all precordial leads.
Echocardiographic measurements were obtained in accordance with the American Society of Echocardiography recommendations. Wall and valve thickness (IVST; PWT; RV free-wall thickness; mitral, aortic and tricuspid valvular thickening) as well as LV and LA size (LV end-diastolic diameter (LVEDD) and left atrial diameter (LAD)) were measured on transthoracic echocardiograms. LV ejection fraction (LVEF) was measured using Simpson's biplane method. Peak E and A wave velocities were measured on Doppler mitral flow. Systolic pulmonary artery pressure (syst PAP) was estimated from the modified Bernoulli equation.13 Peak Ea velocity was measured on tissue Doppler images recorded at the annular mitral valve. Mitral regurgitation (MR) was quantified by measuring the MR area of the colour Doppler signal divided by the left atrial area measured in the four-chamber view.14 MR was considered ‘mild or less’ if MR area/LA area was <10%, ‘severe’ if MR area/LA area was >40% and ‘moderate’ if between the two.
Definition of selected clinical, ECG and echocardiographic classes
Patients were divided according to baseline symptoms. Symptoms were defined as ‘any symptom classified as possibly or definitely related to TTR amyloidosis as they were recorded in medical history or general examination in the THAOS registry’. To study the prevalence of potential signs associated with cardiac ATTR, clinical, ECG and echocardiographic characteristics were defined as follows: advanced age if ≥55 years, high systolic blood pressure (SBP) if ≥140 mm Hg,15 abnormal LVEF if <50%,16 hypertrophied posterior wall if PWT >13 mm for women and ≥14 mm for men,4 asymmetric hypertrophy if IVST/PWT ratio >1.3,17 restrictive filling pattern if E/A transmitral flow velocities >218 and increased RV free-wall if RV thickness ≥7 mm.19
Statistical analysis
Continuous variables are reported as median, and 25th and 75th centiles. For nominal qualitative data, n and percentage were reported. Statistical differences between patient groups were calculated using χ2 test for categorical variables. For continuous variables, the Mann-Whitney test was used when two groups were compared and the Kruskal-Wallis test when more than two groups were compared. Statistical analysis was performed using SAS software; p<0.05 was considered significant.
Results
Baseline characteristics of the echocardiographic THAOS subpopulation
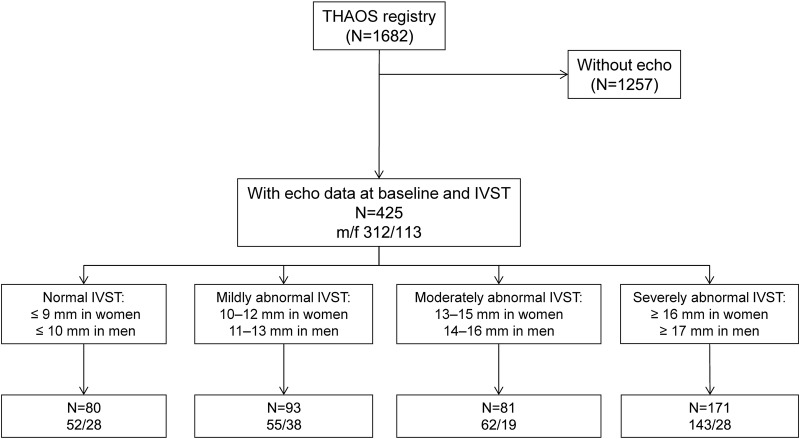
The median (25th and 75th centile) age of the study population of 425 patients was 62 (45, 72) years and 73% were men. Figure 1 presents the flow chart of the study, and prevalence of men and women, according to the IVST classification. A total of 336 patients had a diagnosis of m-ATTR and 89 of WT-ATTR (table 1). Val30Met mutation alone accounted for 50% of the m-ATTR subpopulation, of whom 104 were in early-onset and 63 were in late-onset groups (table 1).
Figure 1.
Study flow chart and IVST classification according to gender . *Prespecified IVST range 3–40 mm (IVST, interventricular septal thickness; THAOS, Transthyretin Amyloidosis Outcomes Survey).
Table 1.
Comparison of baseline clinical, biological and ECG variables among patients with normal, mild, moderate and severe IVST
| IVST | ||||||
|---|---|---|---|---|---|---|
| Variables | N | Normal | Mild | Moderate | Severe | p Value |
| N | 425 | 80 | 93 | 81 | 171 | |
| Genetic | ||||||
| ATTR FAP early Val30Met, n (%) | 104 | 50 (63) | 44 (47) | 8 (10) | 2 (1) | <0.0001* |
| ATTR FAP late Val30Met, n (%) | 63 | 7 (9) | 17 (18) | 12 (15) | 27 (16) | |
| ATTR FAP non-Val30Met, n (%) | 169 | 22 (28) | 29 (31) | 33 (41) | 85 (50) | |
| Wild type, n (%) | 89 | 1 (1) | 3 (3) | 28 (35) | 57 (33) | |
| Demographic and clinical | ||||||
| Age, years | 425 | 35 (31,50) | 49 (39,64) | 67 (58, 76) | 69 (61, 75) | <0.0001 |
| Male, n (%) | 425 | 52 (65) | 55 (59) | 62 (77) | 143 (84) | <0.0001 |
| Pacemaker, n (%) | 221 | 2 (12) | 6 (21) | 12 (24) | 35 (28) | 0.4524 |
| Myocardial infarction, n (%) | 421 | 0 (0) | 0 (0) | 2 (3) | 3 (2) | 0.3007 |
| Heart failure, n (%) | 421 | 8 (10) | 16 (18) | 45 (56) | 124 (73) | <0.0001 |
| Palpitations, n (%) | 421 | 6 (8) | 5 (6) | 11 (14) | 32 (19) | 0.0081 |
| Syncope, n (%) | 421 | 5 (6) | 5 (6) | 12 (15) | 32 (19) | 0.0044 |
| BMI, kg/m2 | 376 | 24 (21,26) | 24 (22,27) | 25 (22,27) | 25 (23,27) | 0.4255 |
| Karnofsky index, % | 360 | 90 (80,90) | 90 (80,90) | 80 (70,90) | 75 (60,80) | <0.0001 |
| NYHA II–IV vs I, n (%) | 190 | 6 (75)† | 12 (75) | 40 (89) | 112 (93) | 0.0866 |
| SBP, mm Hg | 374 | 121 (110,130) | 122 (114,132) | 120 (105,130) | 112 (104,130) | 0.0025 |
| DBP, mm Hg | 374 | 77 (70,84) | 78 (71,83) | 70 (66,80) | 70 (65,80) | 0.0002 |
| Age at onset, years | 423 | 31 (27,44) | 43 (33,57) | 60 (49,70) | 62 (53,70) | <0.0001 |
| Liver transplant, n (%) | 425 | 16 (20) | 11 (12) | 11 (14) | 12 (7) | 0.0268 |
| Age at liver transplant, years | 50 | 40 (35,52) | 44 (32,54) | 55 (48,61) | 50 (39,60) | 0.0675 |
| Motor neuropathy, n (%) | 425 | 31 (39) | 33 (36) | 33 (41) | 69 (40) | 0.8688 |
| Sensory neuropathy, n (%) | 425 | 69 (86) | 79 (85) | 54 (67) | 92 (54) | <0.0001 |
| Autonomic neuropathy, n (%) | 425 | 59 (74) | 59 (63) | 45 (56) | 98 (57) | 0.0529 |
| Biological | ||||||
| BNP, pg/mL | 141 | 32 (14,83) | 72 (37,179) | 591 (206,1027) | 552 (216,939) | 0.0031 |
| NT-proBNP, pg/mL | 152 | 55 (27,108) | 100 (47,649) | 939 (330,3445) | 3507 (1658,8982) | 0.4320 |
| Troponin I, ng/mL | 37 | 0.06 (0.06,0.06) | 0.06 (0.02,0.1) | 0.06 (0.02,0.09) | 0.12 (0.07,0.15) | 0.1329 |
| Troponin T, ng/mL | 122 | 0.01 (0.01,5) | 0.01 (0.01,0.04) | 0.04 (0.02,0.06) | 0.05 (0.03,0.08) | 0.1828 |
| ECG | ||||||
| Heart rate, bpm | 349 | 74 (68, 85) | 74 (65, 78) | 72 (64, 82) | 74 (65,85) | 0.6633 |
| PR interval, ms | 236 | 166 (148,188) | 161 (149,200) | 178 (152,208) | 193 (176,220) | 0.0011 |
| QRS interval, ms | 294 | 94 (86,100) | 98 (88,106) | 110 (88,126) | 116 (100,148) | <0.0001 |
| QT interval, ms | 280 | 378 (360,397) | 394 (361,413) | 424 (390,461) | 438 (398,470) | <0.0001 |
| Low voltage, n (%) | 265 | 2 (4) | 5 (9) | 20 (39) | 34 (30) | <0.0001 |
| Pathologic Q waves, n (%) | 229 | 3 (17) | 2 (7) | 17 (31) | 51 (41) | 0.0013 |
*Only TTR FAP was included in the analysis (wild type excluded).
†Data for this parameter were not reported in 90% of the patients of this group. Continuous variables are presented as median (25th, 75th centile). Percentage indicates proportion of patients with the variable within each IVST category.
ATTR, transthyretin amyloidosis; BMI, body mass index; BNP, brain natriuretic peptide; DBP, diastolic blood pressure; FAP, familial amyloid polyneuropathy; IVST, interventricular septal thickness; NT-proBNP, N-terminal proBNP; NYHA, New York Heart Association; SBP, systolic blood pressure.
Clinical, biological and ECG characteristics of the THAOS echo-subgroup according to the IVST classes
Baseline demographic, genetic, echocardiographic, ECG and biological parameters with respect to the four IVST classes, are shown in table 1. Briefly, moderate or severe IVST was observed more frequently in non-Val30Met and in WT-TTR amyloidosis. Of the early Val30Met patient group, 90% had normal or mild increased IVST.
Older age, male gender, cardiac symptoms, history of heart failure, higher brain natriuretic peptide (BNP) values, conduction abnormalities, low voltage and pathological Q waves, were more frequent in the moderate and severe IVST classes. No differences were observed in body mass index (BMI), modified BMI (mBMI), heart rate, troponin I and T values, and there was equal prevalence of pacemaker implant between the different IVST classes.
Echocardiographic characteristics of the THAOS echo-subgroup according to the IVST classes
Baseline echocardiographic data according to the IVST classification are summarised in table 2. Increased IVST severity was associated with higher sparkling, decreased LVEF, prevalence of moderate to severe MR, valvular thickening, increased LAD and increased syst PAP. E/A was significantly different in the four IVST classes, and was more elevated in patients with normal and severe IVST. In all groups, the median of E/A was <1.5.
Table 2.
Comparison of baseline echocardiographic characteristics among patients according to IVST classification
| IVST | ||||||
|---|---|---|---|---|---|---|
| N | Normal | Mild | Moderate | Severe | p Value | |
| IVST, mm | 425 | 9 (8, 10) | 11 (10, 12) | 15 (14, 16) | 20 (18, 22) | – |
| PWT, mm | 403 | 9 (8, 9) | 10 (9, 11) | 14 (12, 15) | 18 (16, 20) | <0.0001 |
| LVEDD, mm | 379 | 45 (42, 48) | 46 (42, 49) | 45 (41, 48) | 43 (39, 47) | 0.0129 |
| Sparkling, n (%) | 299 | 17 (33) | 23 (38) | 30 (53) | 93 (72) | <0.0001 |
| LVEF, % | 327 | 62 (56, 70) | 60 (55, 67) | 55 (45, 60) | 43 (35, 60) | <0.0001 |
| RV wall thickness, mm | 87 | 6 (5, 7) | 6 (5, 7) | 8 (6, 9) | 9 (7, 10) | 0.0086 |
| Mitral thickening, n (%) | 228 | 5 (14) | 4 (9) | 14 (33) | 51 (48) | <0.0001 |
| Aortic thickening, n (%) | 225 | 6 (16) | 9 (21) | 12 (29) | 42 (40) | 0.0184 |
| Tricuspid thickening, n (%) | 221 | 0 (0) | 2 (5) | 2 (5) | 18 (18) | 0.0031 |
| LAD, mm | 359 | 33 (30, 35) | 36 (33, 40) | 42 (39, 46) | 45 (42, 49) | <0.0001 |
| Syst PAP, mm Hg | 140 | 24 (19, 25) | 25 (20,28) | 30 (26, 40) | 40 (30,45) | <0.0001 |
| E, cm/s | 139 | 74 (62, 85) | 70 (63, 81) | 88 (65, 102) | 81 (67, 101) | 0.0107 |
| A, cm/s | 114 | 54 (48, 65) | 64 (56, 75) | 70 (56, 104) | 54 (40, 81) | 0.0048 |
| E/A | 109 | 1.3 (1, 1.7) | 1.1 (0.9, 1.2) | 0.9 (0.7, 1.6) | 1.3 (0.9, 2.4) | 0.0390 |
| E/Ea, mean±SD | 60 | 7.3±4.0 | 7.8±4.3 | 14.4±6.7 | 16.8±6.7 | 0.0001 |
Continuous variables are presented as median (25th, 75th percentile). Percentage indicates proportion of the patients with the variable within each IVST category.
IVST, interventricular septal thickness; LAD, left atrial diameter; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; PWT, posterior wall thickness; RV, right ventricle; syst PAP, systolic pulmonary arterial pressure.
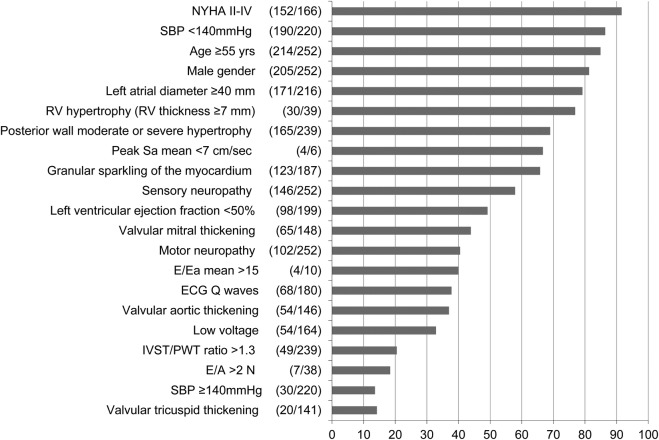
Prevalence of clinical, echocardiographic and ECG abnormal signs in patients with respect to IVST classes
The prevalence of clinical, echocardiographic and ECG abnormal signs in patients with moderate and severe IVST is shown in figure 2. The most frequent signs (>60%) are dyspnoea (New York Heart Association (NYHA) II–IV), age >55 years, male gender, LAD enlargement, increased RV free wall thickness, LV dysfunction measured by ejection fraction, and myocardial sparkling. IVST/PWT ratio >1.3, a sign of asymmetric septal hypertrophy encountered most commonly in sarcomeric cardiomyopathy, was reported in 21%; whereas low voltage, believed to be a strong sign of ATTR, was observed in only a third of these patients.
Figure 2.
Prevalence of the clinical, ECG and echocardiographic signs in the group with moderate and severe IVST increase in the THAOS population with echocardiography data available at baseline (N=252). *Women >13 mm, men >14 mm (IVST, interventricular septal thickness; n, number of patients with individual sign; N, number of patients with evaluations available for individual sign; NYHA, New York Heart Association; PWT, posterior wall thickness, RV, right ventricle; SBP, systolic blood pressure; THAOS, Transthyretin Amyloidosis Outcomes Survey).
Detailed comparisons of clinical, echocardiographic and ECG abnormal signs between the normal-mild IVST and moderate-severe IVST groups are shown in table 3. Briefly, patients with moderate to severe IVST were older, more symptomatic, male gender, and presented low voltage and Q waves, LV systolic dysfunction, increased filling pressure (Ea >15), and left atrial enlargement, and had more myocardial sparkling and valvular thickening, and increased RV wall and PWTs, than normal to mild IVST patients.
Table 3.
Prevalence of indicators in symptomatic subjects by IVST classification in patients classified in normal-mild and moderate-severe IVST classes
| IVST | N | Normal-mild (n=173) | Moderate-severe (n=252) | p Value |
|---|---|---|---|---|
| Clinical | ||||
| Age ≥55 years, n (%) | 425 | 56 (32) | 214 (85) | <0.0001 |
| NYHA II–IV, n (%) | 190 | 18 (75) | 152 (92) | 0.0134 |
| Male, n (%) | 425 | 107 (62) | 205 (81) | <0.0001 |
| SBP ≥140 mm Hg, n (%) | 374 | 19 (12) | 30 (14) | 0.7141 |
| Sensory neuropathy, n (%) | 425 | 148 (86) | 146 (58) | <0.0001 |
| Motor neuropathy, n (%) | 425 | 64 (37) | 102 (41) | 0.4698 |
| ECG | ||||
| Low voltage, n (%) | 265 | 7 (7) | 54 (33) | <0.0001 |
| Q waves, n (%) | 229 | 5 (10) | 68 (38) | 0.0002 |
| Echo | ||||
| LVEF <50%, n (%) | 327 | 18 (14) | 98 (49) | <0.0001 |
| Posterior wall moderate or severe hypertrophy (women ≥13 mm, men ≥14 mm), n (%) | 403 | 0 (0) | 165 (69) | <0.0001 |
| IVST/PWT ratio >1.3, n (%) | 403 | 11 (7) | 49 (21) | 0.0001 |
| E/A >2, n (%) | 109 | 5 (7) | 7 (18) | 0.0705 |
| E/Ea mean >15, n (%) | 60 | 4 (8) | 4 (40) | 0.0066 |
| RV free wall thickness ≥7 mm, n (%) | 87 | 20 (42) | 30 (77) | 0.0009 |
| LAD >40 mm, n (%) | 359 | 26 (18) | 171 (79) | <0.0001 |
| Sparkling, n (%) | 299 | 40 (36) | 123 (66) | <0.0001 |
| Aortic thickening, n (%) | 225 | 15 (19) | 54 (37) | 0.0052 |
| Mitral thickening, n (%) | 228 | 9 (11) | 65 (44) | <0.0001 |
| Tricuspid thickening, n (%) | 221 | 2 (3) | 20 (14) | 0.0053 |
IVST, interventricular septal thickness; LAD, left atrial diameter; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; PWT, posterior wall thickness; RV, right ventricle; SBP, systolic blood pressure.
Prevalence of clinical, echocardiographic and ECG abnormal signs in the moderate and severe group, according to genotype and disease onset
Of the overall early-onset and late-onset Val30Met, non-Val30Met and WT-ATTR, 10%, 62%, 70% and 96%, had moderate or severe IVST, respectively. Percentages of signs depending on genotype and/or disease onset for Val30Met in the moderate to severe IVST group are presented in table 4. Briefly, the prevalence of age ≥55 years and male gender were higher in the WT versus other ATTR groups (table 4). Neuropathy was more frequent in late Val30Met compared with non-Val30Met and WT patients. Hypertension or asymmetric ‘hypertrophy’ was more frequently observed in late Val30Met than in patients with non-Val30Met and WT-ATTR. Prevalence of dyspnoea, Q waves, left atrial dilation and sparkling was similar in the late Val30Met, non-Val30Met and WT-ATTR groups (table 4). However, it is important to note that no specific clinical, ECG or echocardiographic signs were exclusively associated with a given ATTR genotype.
Table 4.
Prevalence of indicators in symptomatic subjects with moderate to severe IVST depending on TTR mutation category
| IVST Moderate and severe |
Val30Met Early onset |
Val30Met Late onset |
non-Val30Met | WT |
|---|---|---|---|---|
| N | 10 | 39 | 118 | 85 |
| Clinical | ||||
| Age ≥55 years, n (%) | 5 (50) | 39 (100) | 85 (72) | 85 (100) |
| NYHA II–IV, n (%) | 2 (100) | 13 (87) | 65 (93) | 72 (91) |
| Gender, male, n (%) | 6 (60) | 31 (80) | 88 (75) | 80 (94) |
| SBP ≥140 mm Hg, n (%) | 3 (33) | 13 (37) | 8 (8) | 6 (8) |
| Sensory neuropathy, n (%) | 9 (90) | 37 (95) | 75 (64) | 25 (29) |
| Motor neuropathy, n (%) | 8 (80) | 30 (77) | 57 (48) | 7 (8) |
| ECG | ||||
| Low voltage, n (%) | 1 (17) | 2 (11) | 27 (38) | 24 (35) |
| Q waves, n (%) | 1 (17) | 8 (44) | 31 (38) | 28 (38) |
| Echocardiography | ||||
| LVEF <50%, n (%) | 0 (0) | 2 (15) | 53 (51) | 43 (55) |
| Posterior wall hypertrophy*, n (%) | 1 (10) | 17 (53) | 80 (71) | 67 (79) |
| IVST/PWT ratio> 1.3, n (%) | 3 (30) | 13 (41) | 23 (21) | 10 (12) |
| E/A >2, n (%) | 0 (0) | 1 (20) | 3 (14) | 3 (30) |
| E/Ea mean >15, n (%) | 0 (0) | NA | 3 (75) | 1 (25) |
| RV free wall thickness ≥7 mm, n (%) | 1 (33) | 7 (88) | 14 (74) | 8 (89) |
| LAD >40 mm, n (%) | 5 (56) | 28 (80) | 69 (73) | 69 (89) |
| Sparkling, n (%) | 4 (67) | 25 (74) | 56 (65) | 38 (62) |
| Aortic thickening, n (%) | 1 (33) | 2 (18) | 33 (46) | 18 (30) |
| Mitral thickening, n (%) | 0 (0) | 2 (18) | 37 (51) | 26 (43) |
| Tricuspid thickening, n (%) | NA | 1 (9) | 12 (17) | 7 (12) |
*Moderate or severe hypertrophy=women >13 mm and men >14 mm; NA, no data available for tricuspid valve thickening in early-onset Val30Met and for E/Ea in late onset for Val30Met.
IVST, interventricular septal thickness; LAD, left atrial diameter; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; PWT, posterior wall thickness; RV, right ventricle; SBP, systolic blood pressure.
Discussion
This study identified clinical, ECG and echocardiographic features that should alert cardiologists to suspect amyloidosis in patients with increased IVST, using the THAOS registry.
First, we demonstrated that advanced age, male gender, dyspnoea, increased RV free-wall thickness and echocardiographic myocardial sparkling, were frequently associated with moderate to severe IVST in patients with mostly normal SBP <140 mm Hg. Second, we showed that asymmetric LV ‘hypertrophy’ and absence of transmitral restrictive pattern or low voltage should not rule out a diagnosis of ATTR in these patients; and third, we pointed out that these methods could not discriminate the different TTR genotypes.
Interest of a large international registry
The THAOS registry represents the largest available database to document transversal ATTR characteristics and offers the unique opportunity to analyse data according to different genotypes and phenotypes. The international nature of the study allows for the generalisation of the findings, as it includes data from various countries and centres. A further advantage of such a large database is the use of standardised forms and the uniform manner in which data from a large number of patients with different genotypes are collected.
IVST measured by echocardiography as a useful tool to screen symptomatic patients for ATTR
Increased wall thickness of the heart is frequently diagnosed by echocardiography in patients; however, it could result from a number of different conditions. Progress in molecular biology has elucidated the underlying genetic abnormalities in many cases of cardiac hypertrophy; however, in daily practice, cardiologists have access first to the phenotype of patients rather than its genetic defects. As was pointed out in the recent ESC statement concerning hypertrophic cardiomyopathy, there is still a need to develop appropriate diagnostic strategies based on clues from medical and family histories, physical examination and non-invasive investigations such as echocardiography.1 Hence, identification of clinical signs associated with specific genetic disease, such as ATTR, is an important step before performing appropriate genetic testing.
Clinical and echocardiographic signs associated with increased IVST in ATTR
In the present study, we identified signs that may provide guidance in a possible diagnosis of ATTR. We demonstrated that age >55 years and male gender are frequently associated with increased IVST ≥13 mm in female, and ≥14 mm in male patients with ATTR. WT-ATTR, non-Val30Met and late-onset Val30Met patients showed higher IVST than early Val30Met patients. This may be explained by the relationship between increased IVST and age, and male gender, as shown in a previous study.16 Accordingly, in our study, WT-ATTR, non-Val30Met and late-onset Val30Met patients were older, and more frequently male, than early Val30Met patients. However, mechanisms other than age and gender might be involved in IVST increase, such as TTR fibril composition. Unfortunately, this information is lacking in the THAOS registry. Late-onset Val30Met and WT-ATTR showed fragmented TTR protein; conversely, whole TTR protein is observed in early-onset Val30Met.20 21
The striking difference in penetrance according to gender has been reported in several previous studies.22 23 Of note, 94% of patients with WT-ATTR were men; which is in accordance with reports in the literature.24–26 In Val30Met, as described previously,22 male gender dominated among late-onset patients compared to those with early onset. To the best of our knowledge, no pathophysiological explanation for this gender discrepancy has emerged.
In our study, most patients with increased IVST had normal blood pressure and dyspnoea. Hypertension is a ubiquitous cause of LV hypertrophy, with advanced age. Therefore, the combination of increased IVST and normal blood pressure should increase cardiologists’ awareness of the possibility of cardiac amyloidosis. Conversely, in daily practice, dyspnoea might not be useful to discriminate ATTR amyloidosis from other hypertrophic cardiomyopathies. Of course, these signs are also frequent in amyloid light-chain (AL) amyloidosis, and this diagnosis should be ruled out by identification of amyloidogenic monoclonal proteins (serum and urine immunofixation combined with free light-chain quantification) and demonstration of TTR amyloid deposition in a tissue specimen.
Increased RV wall thickness, valvular thickening and granular sparkling of the myocardium were frequently observed in patients with moderate to severe IVST, in accordance with previous findings.25 27 28 TTR infiltrates all the structures of the heart (left and right ventricle (RV) and valves) in contrast to hypertension, which only increases LV wall thickness.29 Increases in aortic valve and mitral valve thickening were frequently observed. In previous studies, atrioventricular thickening has been proposed as a marker for cardiac amyloidosis.28 Rapezzi et al30 showed recently that half of patients with ATTR exhibited abnormal valvular thickness whereas only 3% exhibited sarcomeric hypertrophic cardiomyopathies. Myocardial sparkling, a qualitative—but subjective and highly dependent on the echocardiographic technique (harmonic)—sign of amyloid infiltration, was observed in 65% of the patients with moderate to severe IVST in our study, which is in agreement with previous reports.31–33 Combination of increased RV wall thickness, valvular thickening and sparkling, are of particular relevance to the diagnosis of ATTR in patients with increased IVST. However, increased RV wall thickness can also be observed in cases of symmetric LV hypertrophy in Fabry disease or in mitochondrial cytopathy, and in cases of asymmetric LV hypertrophy in sarcomeric hypertrophic cardiomyopathy. Thus it is more the combination of signs rather than the sign alone that may suggest a diagnosis of TTR amyloidosis.
Absence of signs should not rule out the diagnosis
Cardiac amyloidosis is often described as a restrictive cardiomyopathy with symmetric hypertrophic pattern, preserved ejection fraction and ECG low voltage.5 34
In our study, the restrictive transmitral pattern was observed in only 18% of patients with ATTR moderate to severe IVST. Asymmetric LV hypertrophy pattern was not rare, as it was exhibited in 21% of patients with moderate and severe IVST. This asymmetric pattern was more frequently observed in late-onset Val30Met and in non-Val30Met than in WT-ATTR. Forty-nine per cent of patients with moderate to severe IVST had an ejection fraction <50%. Low voltage was observed in less than one-third of the patients. All of this suggests that absence of a restrictive pattern or low voltage, or presence of asymmetric hypertrophy pattern or LV systolic dysfunction, should not rule out the diagnosis of ATTR amyloidosis.
Study limitations
There are several limitations in investigations based on data collected in observational surveys such as THAOS. As data were collected during routine clinical practice from many centres/countries and at the discretion of the patient's physician, there are inevitably variations in the type of clinical investigations conducted. In particular, at the time when these data were reviewed (data cut-off date 30 June 2013), echocardiography was not considered as a routine clinical examination in many participating neurology centres. Thus, patients with primarily neurological manifestations of their disease are generally attended by neurologists and do not undergo heart examinations to a similar extent as those presenting with primarily heart problems and followed by cardiologists. Thus, there was a selection bias in investigations performed, with heart examinations rarely performed in patients with early-onset ATTR Val30Met disease. The high prevalence of heart failure (NYHA II–IV) noted in the group of patients with normal-mild increased IVST probably represents a selection bias, where patients with symptoms of dyspnoea are registered as having heart failure and undergo echocardiographic examination. Similarly, heart examinations are generally not carried out on asymptomatic carriers of an ATTR gene mutation, a group that otherwise would be interesting to analyse. Furthermore, the conclusion of this study may be applicable only for patients with the same characteristics as those included in this study.
Non-Val30Met includes many different mutations with phenotype heterogeneity that could not be addressed in this study due to the limited number of patients with echocardiography.
Echocardiographic measurements such as MR measurement by vena-contracta or proximal isovelocity surface area, or new echocardiographic techniques such as strain or strain rate, were not performed worldwide and are, therefore, not reported in the THAOS registry. Thus, although they are of interest in this disease,35 evaluation of MR severity and LV systolic function was limited. Not all echocardiography machines used were able to measure strain by Doppler tissue imaging or two-dimensional speckle-tracking, measurements may differ between brands of device. This makes it difficult to calculate a mean value.
Lastly, this study focused only on TTR amyloidosis, whereas most of the signs described are also present in AL cardiac amyloidosis. A diagnosis of AL cardiac amyloidosis should be ruled out by other means, such as demonstration of TTR amyloid deposition in a tissue specimen. Future studies are needed comparing patients with hypertensive heart disease and hypertrophic cardiomyopathy with patients having ATTR amyloidosis to determine sensitivity and specificity, and cut-off value of each sign, to predict ATTR amyloidosis.
Conclusion
TTR cardiac amyloidosis should be suspected in the presence of increased echocardiographic IVST, particularly in patients >55 years of age, of male gender, with normal SBP, increased thickness of RV free wall and valves, LAD enlargement and granular sparkling of the myocardium. Female gender or absence of restrictive pattern, or absence of low voltage or LV dysfunction, should not rule out the possibility of cardiac ATTR. ATTR genetic testing should be performed in patients with suspected TTR cardiomyopathy, as the criteria above are not specific and were present across the different ATTR genotypes and in WT-ATTR. Further studies, including control groups, are needed to determine the specificity, sensitivity and cut-off values of the criteria described in this study.
Acknowledgments
The authors would like to thank the physicians, patients and all persons who were involved in the THAOS registry, and Dr Aziz Guellich for advice and help in writing the manuscript. Editorial support was provided by Paul Hassan, PhD of Engage Scientific. The editorial support provided consisted solely of manuscript formatting and no contribution was made to editorial content.
Footnotes
Funding: This study was funded by Pfizer Inc.
Competing interests: TD has received research support from, and served on advisory boards for, Pfizer Inc. MSM has received support from FoldRx Pharmaceuticals—which was acquired by Pfizer Inc, in October 2010—as a clinical investigator and for scientific meeting expenses. His institution has received grant support from Pfizer Inc. CR has received research support from, and served on advisory boards for, Pfizer Inc, and serves on the scientific advisory board of the THAOS registry. VP-B has received support from FoldRx Pharmaceuticals, as a clinical investigator, and serves on the scientific advisory board of the THAOS registry. ONK and RM are employees of Pfizer Inc. OBS has served as an advisor for Alnylam Pharmaceuticals, Isis Pharmaceuticals and Pfizer Inc, and as an advisor and clinical investigator for FoldRx Pharmaceuticals. He currently serves on the scientific advisory board of the THAOS registry. AVK has received research support from, and served on advisory boards for, Pfizer Inc.
Ethics approval: Local institutional review boards approved the study.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Rapezzi C, Arbustini E, Caforio AL et al. Diagnostic work-up in cardiomyopathies: bridging the gap between clinical phenotypes and final diagnosis. A position statement from the ESC Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013;34:1448–58. 10.1093/eurheartj/ehs397 [DOI] [PubMed] [Google Scholar]
- 2.Lang RM, Bierig M, Devereux RB et al. , Chamber Quantification Writing Group; American Society of Echocardiography's Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005;18:1440–63. 10.1016/j.echo.2005.10.005 [DOI] [PubMed] [Google Scholar]
- 3.Lang RM, Badano LP, Mor-Avi V et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39.e14. 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 4.Elliott PM, Anastasakis A, Borger MA et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2733–79. 10.1093/eurheartj/ehu284 [DOI] [PubMed] [Google Scholar]
- 5.Falk RH, Dubrey SW. Amyloid heart disease. Prog Cardiovasc Dis 2010;52:347–61. 10.1016/j.pcad.2009.11.007 [DOI] [PubMed] [Google Scholar]
- 6.Planté-Bordeneuve V, Said G. Familial amyloid polyneuropathy. Lancet Neurol 2011;10:1086–97. 10.1016/S1474-4422(11)70246-0 [DOI] [PubMed] [Google Scholar]
- 7.Benson MD. Liver transplantation and transthyretin amyloidosis. Muscle Nerve 2013;47:157–62. 10.1002/mus.23521 [DOI] [PubMed] [Google Scholar]
- 8.Jacobson DR, Pastore RD, Yaghoubian R et al. Variant-sequence transthyretin (isoleucine 122) in late-onset cardiac amyloidosis in black Americans. N Engl J Med 1997;336:466–73. 10.1056/NEJM199702133360703 [DOI] [PubMed] [Google Scholar]
- 9.Cornwell GG III, Murdoch WL, Kyle RA et al. Frequency and distribution of senile cardiovascular amyloid. A clinicopathologic correlation. Am J Med 1983;75:618–23. 10.1016/0002-9343(83)90443-6 [DOI] [PubMed] [Google Scholar]
- 10.Hanna M. Novel drugs targeting transthyretin amyloidosis. Curr Heart Fail Rep 2014;11:50–7. 10.1007/s11897-013-0182-4 [DOI] [PubMed] [Google Scholar]
- 11.Planté-Bordeneuve V, Suhr OB, Maurer MS et al. The Transthyretin Amyloidosis Outcomes Survey (THAOS) registry: design and methodology. Curr Med Res Opin 2013;29:77–84. 10.1185/03007995.2012.754349 [DOI] [PubMed] [Google Scholar]
- 12.Coelho T, Maurer MS, Suhr OB. THAOS—The Transthyretin Amyloidosis Outcomes Survey: initial report on clinical manifestations in patients with hereditary and wild-type transthyretin amyloidosis. Curr Med Res Opin 2013;29:63–76. 10.1185/03007995.2012.754348 [DOI] [PubMed] [Google Scholar]
- 13.Yock PG, Popp RL. Noninvasive estimation of right ventricular systolic pressure by Doppler ultrasound in patients with tricuspid regurgitation. Circulation 1984;70:657–62. 10.1161/01.CIR.70.4.657 [DOI] [PubMed] [Google Scholar]
- 14.Helmcke F, Nanda NC, Hsiung MC et al. Color Doppler assessment of mitral regurgitation with orthogonal planes. Circulation 1987;75:175–83. 10.1161/01.CIR.75.1.175 [DOI] [PubMed] [Google Scholar]
- 15.Smith SC Jr, Collins A, Ferrari R et al. Our time: a call to save preventable death from cardiovascular disease (heart disease and stroke). Circulation 2012;126:2769–75. 10.1161/CIR.0b013e318267e99f [DOI] [PubMed] [Google Scholar]
- 16.Murtagh G, Dawkins IR, O'Connell R et al. Screening to prevent heart failure (STOP-HF): expanding the focus beyond asymptomatic left ventricular systolic dysfunction. Eur J Heart Fail 2012;14:480–6. 10.1093/eurjhf/hfs030 [DOI] [PubMed] [Google Scholar]
- 17.Fifer MA, Vlahakes GJ. Management of symptoms in hypertrophic cardiomyopathy. Circulation 2008;117:429–39. 10.1161/CIRCULATIONAHA.107.694158 [DOI] [PubMed] [Google Scholar]
- 18.Garcia MJ, Thomas JD, Klein AL. New Doppler echocardiographic applications for the study of diastolic function. J Am Coll Cardiol 1998;32:865–75. 10.1016/S0735-1097(98)00345-3 [DOI] [PubMed] [Google Scholar]
- 19.Rudski LG, Lai WW, Afilalo J et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010;23:685–713; quiz 86–8 10.1016/j.echo.2010.05.010 [DOI] [PubMed] [Google Scholar]
- 20.Ihse E, Ybo A, Suhr O et al. Amyloid fibril composition is related to the phenotype of hereditary transthyretin V30M amyloidosis. J Pathol 2008;216:253–61. 10.1002/path.2411 [DOI] [PubMed] [Google Scholar]
- 21.Ihse E, Suhr OB, Hellman U et al. Variation in amount of wild-type transthyretin in different fibril and tissue types in ATTR amyloidosis. J Mol Med (Berl) 2011;89:171–80. 10.1007/s00109-010-0695-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koike H, Misu K, Ikeda S et al. Type I (transthyretin Met30) familial amyloid polyneuropathy in Japan: early- vs late-onset form. Arch Neurol 2002;59:1771–6. 10.1001/archneur.59.11.1771 [DOI] [PubMed] [Google Scholar]
- 23.Hörnsten R, Pennlert J, Wiklund U et al. Heart complications in familial transthyretin amyloidosis: impact of age and gender. Amyloid 2010;17:63–8. 10.3109/13506129.2010.483114 [DOI] [PubMed] [Google Scholar]
- 24.Bhuiyan T, Helmke S, Patel AR et al. Pressure-volume relationships in patients with transthyretin (ATTR) cardiac amyloidosis secondary to V122I mutations and wild-type transthyretin: Transthyretin Cardiac Amyloid Study (TRACS). Circ Heart Fail 2011;4:121–8. 10.1161/CIRCHEARTFAILURE.109.910455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rapezzi C, Merlini G, Quarta CC et al. Systemic cardiac amyloidoses: disease profiles and clinical courses of the 3 main types. Circulation 2009;120:1203–12. 10.1161/CIRCULATIONAHA.108.843334 [DOI] [PubMed] [Google Scholar]
- 26.Pinney JH, Whelan CJ, Petrie A et al. Senile systemic amyloidosis: clinical features at presentation and outcome. J Am Heart Assoc 2013;2:e000098 10.1161/JAHA.113.000098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ng B, Connors LH, Davidoff R et al. Senile systemic amyloidosis presenting with heart failure: a comparison with light chain-associated amyloidosis. Arch Intern Med 2005;165:1425–9. 10.1001/archinte.165.12.1425 [DOI] [PubMed] [Google Scholar]
- 28.Simons M, Isner JM. Assessment of relative sensitivities of noninvasive tests for cardiac amyloidosis in documented cardiac amyloidosis. Am J Cardiol 1992;69:425–7. 10.1016/0002-9149(92)90250-3 [DOI] [PubMed] [Google Scholar]
- 29.Seward JB, Casaclang-Verzosa G. Infiltrative cardiovascular diseases: cardiomyopathies that look alike. J Am Coll Cardiol 2010;55:1769–79. 10.1016/j.jacc.2009.12.040 [DOI] [PubMed] [Google Scholar]
- 30.Rapezzi C, Quarta CC, Obici L et al. Disease profile and differential diagnosis of hereditary transthyretin-related amyloidosis with exclusively cardiac phenotype: an Italian perspective. Eur Heart J 2013;34:520–8. 10.1093/eurheartj/ehs123 [DOI] [PubMed] [Google Scholar]
- 31.Falk RH, Plehn JF, Deering T, et al Sensitivity and specificity of the echocardiographic features of cardiac amyloidosis. Am J Cardiol 1987;59:418–22. 10.1016/0002-9149(87)90948-9 [DOI] [PubMed] [Google Scholar]
- 32.Rahman JE, Helou EF, Gelzer-Bell R et al. Noninvasive diagnosis of biopsy-proven cardiac amyloidosis. J Am Coll Cardiol 2004;43:410–15. 10.1016/j.jacc.2003.08.043 [DOI] [PubMed] [Google Scholar]
- 33.Bhandari AK, Nanda NC. Myocardial texture characterization by two-dimensional echocardiography. Am J Cardiol 1983;51: 817–25. 10.1016/S0002-9149(83)80139-8 [DOI] [PubMed] [Google Scholar]
- 34.Dubrey SW, Hawkins PN, Falk RH. Amyloid diseases of the heart: assessment, diagnosis, and referral. Heart 2011;97:75–84. 10.1136/hrt.2009.190405 [DOI] [PubMed] [Google Scholar]
- 35.Damy T, Deux JF, Moutereau S et al. Role of natriuretic peptide to predict cardiac abnormalities in patients with hereditary transthyretin amyloidosis. Amyloid 2013;20:212–20. 10.3109/13506129.2013.825240 [DOI] [PubMed] [Google Scholar]