Abstract
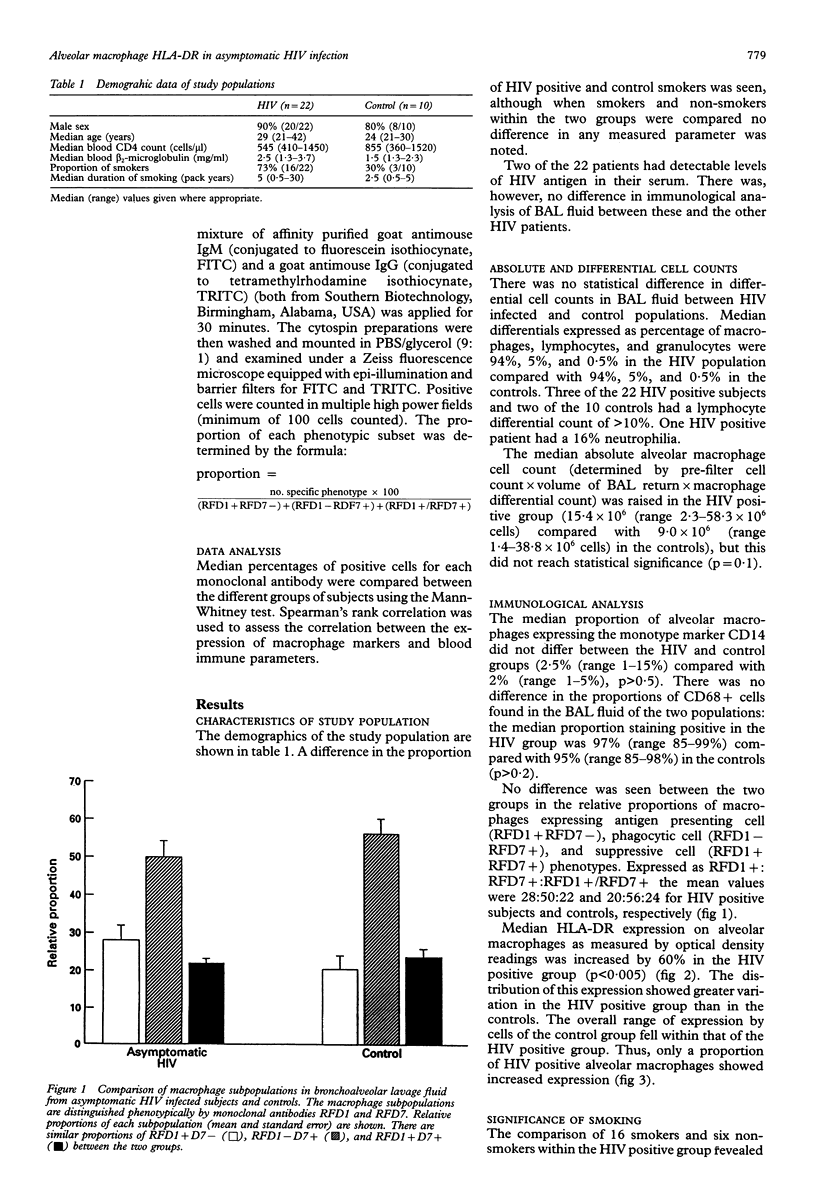
BACKGROUND--It has previously been shown that HIV infected individuals with pneumonitis have identifiable abnormalities in alveolar macrophages obtained by bronchoalveolar lavage (BAL). In particular, alterations in the expression of alveolar macrophage surface antigens associated with macrophage function have been reported. To determine whether these changes reflect HIV infection or the respiratory episode itself, a population of HIV infected patients with no respiratory disease was studied. METHODS--Twenty two HIV antibody positive individuals with a peripheral blood CD4 count of > 400/microliters and 10 healthy volunteer controls underwent bronchoscopy and BAL. Cytospin preparations from the recovered cells were stained using immunoperoxidase and double immunofluorescence techniques with monoclonal antibodies RFD1, RFD7, EBM11/CD68 (mature macrophages), UCHM1/CD14 (monocyte marker), and HLA-DR (RFDR1). Differential cell counts were also performed. RESULTS--There was an increase in overall alveolar macrophage HLA-DR expression in the HIV population. This was not reflected in a change in the percentage of cells staining CD14 (monocytes) or CD68 (mature macrophages) positive. The relative proportions of cells staining RFD1 + RFD7- (inducer cells), RFD1 - RFD7+ (effector cells), and RFD1 + RFD7+ (suppressive cells) were unchanged between HIV and control groups. CONCLUSIONS--In a population of HIV infected individuals with normal CD4 counts and no respiratory disease there was an increase in overall alveolar macrophage HLA-DR expression which occurred independently of any alteration in the relative proportions of alveolar macrophage subpopulations.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agostini C., Semenzato G. Does analysis of bronchoalveolar lavage fluid provide a tool to monitor disease progression or to predict survival in patients with HIV-1 infection? Thorax. 1994 Sep;49(9):848–851. doi: 10.1136/thx.49.9.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agostini C., Zambello R., Trentin L., Poletti V., Spiga L., Gritti F., Cipriani A., Salmaso L., Cadrobbi P., Semenzato G. Prognostic significance of the evaluation of bronchoalveolar lavage cell populations in patients with HIV-1 infection and pulmonary involvement. Chest. 1991 Dec;100(6):1601–1606. doi: 10.1378/chest.100.6.1601. [DOI] [PubMed] [Google Scholar]
- Belsito D. V., Sanchez M. R., Baer R. L., Valentine F., Thorbecke G. J. Reduced Langerhans' cell Ia antigen and ATPase activity in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1984 May 17;310(20):1279–1282. doi: 10.1056/NEJM198405173102002. [DOI] [PubMed] [Google Scholar]
- Bohnet S., Braun J., Dalhoff K. Intercellular adhesion molecule-1 (ICAM-1) is upregulated on alveolar macrophages from AIDS patients. Eur Respir J. 1994 Feb;7(2):229–234. doi: 10.1183/09031936.94.07020229. [DOI] [PubMed] [Google Scholar]
- Bray D. H., Squire S. B., Bagdades E., Mulvenna P. M., Johnson M. A., Poulter L. W. Alveolar macrophage populations are distorted in immunocompromised patients with pneumonitis. Eur Respir J. 1992 May;5(5):545–552. [PubMed] [Google Scholar]
- Bray D. H., Squire S. B., Kawana A., Johnson M. A., Poulter L. W. Antiretroviral treatment reverses HIV-induced reduction in the expression of surface antigens on alveolar macrophages in AIDS patients. Clin Exp Immunol. 1993 Jan;91(1):13–17. doi: 10.1111/j.1365-2249.1993.tb03346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhl R., Jaffe H. A., Holroyd K. J., Borok Z., Roum J. H., Mastrangeli A., Wells F. B., Kirby M., Saltini C., Crystal R. G. Activation of alveolar macrophages in asymptomatic HIV-infected individuals. J Immunol. 1993 Feb 1;150(3):1019–1028. [PubMed] [Google Scholar]
- Burney P., Chinn S. Developing a new questionnaire for measuring the prevalence and distribution of asthma. Chest. 1987 Jun;91(6 Suppl):79S–83S. doi: 10.1378/chest.91.6_supplement.79s. [DOI] [PubMed] [Google Scholar]
- Campbell D. A., Poulter L. W., du Bois R. M. Immunocompetent cells in bronchoalveolar lavage reflect the cell populations in transbronchial biopsies in pulmonary sarcoidosis. Am Rev Respir Dis. 1985 Dec;132(6):1300–1306. doi: 10.1164/arrd.1985.132.6.1300. [DOI] [PubMed] [Google Scholar]
- Ennen J., Seipp I., Norley S. G., Kurth R. Decreased accessory cell function of macrophages after infection with human immunodeficiency virus type 1 in vitro. Eur J Immunol. 1990 Nov;20(11):2451–2456. doi: 10.1002/eji.1830201114. [DOI] [PubMed] [Google Scholar]
- Haas J. G., Riethmüller G., Ziegler-Heitbrock H. W. Monocyte phenotype and function in patients with the acquired immunodeficiency syndrome (AIDS) and AIDS-related disorders. Scand J Immunol. 1987 Oct;26(4):371–379. doi: 10.1111/j.1365-3083.1987.tb02269.x. [DOI] [PubMed] [Google Scholar]
- Heagy W., Kelley V. E., Strom T. B., Mayer K., Shapiro H. M., Mandel R., Finberg R. Decreased expression of human class II antigens on monocytes from patients with acquired immune deficiency syndrome. Increased expression with interferon-gamma. J Clin Invest. 1984 Dec;74(6):2089–2096. doi: 10.1172/JCI111633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg N., MacDonald S., Slusarenko M., Beverley P. C. Monoclonal antibodies specific for human monocytes, granulocytes and endothelium. Immunology. 1984 Dec;53(4):753–767. [PMC free article] [PubMed] [Google Scholar]
- Janossy G., Bofill M., Poulter L. W., Rawlings E., Burford G. D., Navarrete C., Ziegler A., Kelemen E. Separate ontogeny of two macrophage-like accessory cell populations in the human fetus. J Immunol. 1986 Jun 15;136(12):4354–4361. [PubMed] [Google Scholar]
- Kelly P. M., Bliss E., Morton J. A., Burns J., McGee J. O. Monoclonal antibody EBM/11: high cellular specificity for human macrophages. J Clin Pathol. 1988 May;41(5):510–515. doi: 10.1136/jcp.41.5.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight S. C., Farrant J., Bryant A., Edwards A. J., Burman S., Lever A., Clarke J., Webster A. D. Non-adherent, low-density cells from human peripheral blood contain dendritic cells and monocytes, both with veiled morphology. Immunology. 1986 Apr;57(4):595–603. [PMC free article] [PubMed] [Google Scholar]
- Lim S. G., Condez A., Poulter L. W. Mucosal macrophage subsets of the gut in HIV: decrease in antigen-presenting cell phenotype. Clin Exp Immunol. 1993 Jun;92(3):442–447. doi: 10.1111/j.1365-2249.1993.tb03418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luján L., Begara I., Collie D. D., Watt N. J. Phenotypic analysis of cells in bronchoalveolar lavage fluid and peripheral blood of maedi visna-infected sheep. Clin Exp Immunol. 1993 Feb;91(2):272–276. doi: 10.1111/j.1365-2249.1993.tb05894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Maza O., Mitsuyasu R. T., Miles S. A., Giorgi J. V., Heitjan D. F., Sherwin S. A., Fahey J. L. Gamma-interferon-induced monocyte major histocompatibility complex class II antigen expression individuals with acquired immune deficiency syndrome. Cell Immunol. 1989 Oct 15;123(2):316–324. doi: 10.1016/0008-8749(89)90292-x. [DOI] [PubMed] [Google Scholar]
- Murray J. F., Mills J. Pulmonary infectious complications of human immunodeficiency virus infection. Part I. Am Rev Respir Dis. 1990 May;141(5 Pt 1):1356–1372. doi: 10.1164/ajrccm/141.5_Pt_1.1356. [DOI] [PubMed] [Google Scholar]
- Noble B., Du Bois R. M., Poulter L. W. The distribution of phenotypically distinct macrophage subsets in the lungs of patients with cryptogenic fibrosing alveolitis. Clin Exp Immunol. 1989 Apr;76(1):41–46. [PMC free article] [PubMed] [Google Scholar]
- Overland E. S., Nolan A. J., Hopewell P. C. Alteration of pulmonary function in intravenous drug abusers. Prevalence, severity, and characterization of gas exchange abnormalities. Am J Med. 1980 Feb;68(2):231–237. doi: 10.1016/0002-9343(80)90359-9. [DOI] [PubMed] [Google Scholar]
- Petit A. J., Terpstra F. G., Miedema F. Human immunodeficiency virus infection down-regulates HLA class II expression and induces differentiation in promonocytic U937 cells. J Clin Invest. 1987 Jun;79(6):1883–1889. doi: 10.1172/JCI113032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit A. J., Tersmette M., Terpstra F. G., de Goede R. E., van Lier R. A., Miedema F. Decreased accessory cell function by human monocytic cells after infection with HIV. J Immunol. 1988 Mar 1;140(5):1485–1489. [PubMed] [Google Scholar]
- Pittis M. G., Sternik G., Sen L., Diez R. A., Planes N., Pirola D., Estevez M. E. Impaired phagolysosomal fusion of peripheral blood monocytes from HIV-infected subjects. Scand J Immunol. 1993 Nov;38(5):423–427. doi: 10.1111/j.1365-3083.1993.tb02583.x. [DOI] [PubMed] [Google Scholar]
- Plata F., Autran B., Martins L. P., Wain-Hobson S., Raphaël M., Mayaud C., Denis M., Guillon J. M., Debré P. AIDS virus-specific cytotoxic T lymphocytes in lung disorders. Nature. 1987 Jul 23;328(6128):348–351. doi: 10.1038/328348a0. [DOI] [PubMed] [Google Scholar]
- Plaza V., Jiménez P., Xaubet A., Picado C., Torres A., Agustí C., Agustí-Vidal A. Bronchoalveolar lavage cell analysis in patients with human immunodeficiency virus related diseases. Thorax. 1989 Apr;44(4):289–291. doi: 10.1136/thx.44.4.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulter L. W., Campbell D. A., Munro C., Janossy G. Discrimination of human macrophages and dendritic cells by means of monoclonal antibodies. Scand J Immunol. 1986 Sep;24(3):351–357. doi: 10.1111/j.1365-3083.1986.tb02104.x. [DOI] [PubMed] [Google Scholar]
- Poulter L. W., Power C., Burke C. The relationship between bronchial immunopathology and hyperresponsiveness in asthma. Eur Respir J. 1990 Jul;3(7):792–799. [PubMed] [Google Scholar]
- Romani N., Lenz A., Glassel H., Stössel H., Stanzl U., Majdic O., Fritsch P., Schuler G. Cultured human Langerhans cells resemble lymphoid dendritic cells in phenotype and function. J Invest Dermatol. 1989 Nov;93(5):600–609. doi: 10.1111/1523-1747.ep12319727. [DOI] [PubMed] [Google Scholar]
- Roy G., Rojo N., Leyva-Cobián F. Phenotypic changes in monocytes and alveolar macrophages in patients with acquired immunodeficiency syndrome (AIDS) and AIDS-related complex (ARC). J Clin Lab Immunol. 1987 Jul;23(3):135–141. [PubMed] [Google Scholar]
- Schaberg T., Lauer C., Lode H., Fischer J., Haller H. Increased number of alveolar macrophages expressing adhesion molecules of the leukocyte adhesion molecule family in smoking subjects. Association with cell-binding ability and superoxide anion production. Am Rev Respir Dis. 1992 Nov;146(5 Pt 1):1287–1293. doi: 10.1164/ajrccm/146.5_Pt_1.1287. [DOI] [PubMed] [Google Scholar]
- Sei Y., Petrella R. J., Tsang P., Bekesi J. G., Yokoyama M. M. Monocytes in AIDS. N Engl J Med. 1986 Dec 18;315(25):1611–1612. doi: 10.1056/NEJM198612183152512. [DOI] [PubMed] [Google Scholar]
- Spiteri M. A., Clarke S. W., Poulter L. W. Phenotypic and functional changes in alveolar macrophages contribute to the pathogenesis of pulmonary sarcoidosis. Clin Exp Immunol. 1988 Dec;74(3):359–364. [PMC free article] [PubMed] [Google Scholar]
- Spiteri M. A., Poulter L. W. Characterization of immune inducer and suppressor macrophages from the normal human lung. Clin Exp Immunol. 1991 Jan;83(1):157–162. doi: 10.1111/j.1365-2249.1991.tb05607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twigg H. L., 3rd, Lipscomb M. F., Yoffe B., Barbaro D. J., Weissler J. C. Enhanced accessory cell function by alveolar macrophages from patients infected with the human immunodeficiency virus: potential role for depletion of CD4+ cells in the lung. Am J Respir Cell Mol Biol. 1989 Nov;1(5):391–400. doi: 10.1165/ajrcmb/1.5.391. [DOI] [PubMed] [Google Scholar]