Abstract
The authors present a case of a 28-year-old man with a known history of paroxysmal nocturnal haemoglobinuria (PNH), under platelet antiaggregation, admitted following recurrent transitory arterial ischaemic attacks. Concomitant thrombosis of the superior mesenteric artery and bilateral renal infarction was found. Cardioembolism, namely patent foramen ovale, was excluded and anticoagulation added, with no further events on 10-month follow-up. PNH is a rare acquired disorder of haematopoietic stem cells, characterised by haemolytic anaemia, pancytopenia and thrombotic events classically involving the venous system. Reports of cerebral artery stroke and accompanying intra-abdominal arterial thrombosis are especially rare. Complementary investigation and treatment options in these patients are discussed.
Background
Stroke in the young adult is uncommon and comprises a wide spectrum of aetiologies and risk factors, which include haematological conditions.1 Paroxysmal nocturnal haemoglobinuria (PNH) is a rare acquired disorder of haematopoietic stem cells, characterised by haemolytic anaemia, pancytopenia and thrombotic events.2 Reported incidence of clinically significant disease is in the range of 1–10 cases per million, although this may be an underestimate.3 It arises through a somatic mutation of the phosphatidylinositol glycan A gene, resulting in disruption of glycosylphosphatidylinositol (GPI) anchor biosynthesis in bone marrow stem cells and thereby a deficiency of all GPI-anchored proteins on the cell membrane, among which the complement regulatory proteins CD55 and CD59. Hence, the clonal expansion of these susceptible cells results in complement-mediated intravascular haemolysis, inflammation, platelet activation and thrombosis.4
Thrombotic events are the main cause of death in these patients and classically involve the venous system, with reports of less than 5% occurring in central nervous system arteries.5 Thrombophilia in PNH is thought to be multifactorial and a number of mechanisms have been hypothesised4 6—complement-mediated damage of GPI-deficient blood cells may result in the release of procoagulant microparticles into circulation; high levels of free haemoglobin promote scavenging of nitric oxide, which has been implicated in platelet activation and aggregation; complement C5a generates inflammatory cytokines; deficiency or absence of GPI-linked proteins such as urokinase-type plasminogen activator receptor, heparan sulfate and a coreceptor for tissue factor pathway inhibitor may contribute to defective fibrinolysis, among others.
Although, as mentioned, cerebrovascular complications mainly involve the venous territory, stroke caused by arterial occlusion is not negligible and may be the first manifestation of this condition.7
Case presentation
A 28-year-old man of African descent, with a known medical history of PNH diagnosed 7 years earlier and a left middle cerebral artery stroke the year before, with no neurological sequelae, presented to the emergency department (ED), with sudden onset and self-limited complaints of weakness and paraesthesias of the left hemibody, within a time span of approximately 15 min. He denied other neurological symptoms, but mentioned productive cough of mucopurulent sputum and unquantified fever in the previous days. His chronic medication consisted of prednisolone 10 mg, ranitidine, folic acid and weekly darbepoetin, with sporadic requirement of iron administration and red blood cell transfusion. Since the stroke, he was also prescribed secondary prophylaxis with aspirin and rosuvastatin. He denied other vascular risk factors. On neurological examination, no deficits were apparent and a CT of the brain showed no acute vascular lesions. His blood tests were remarkable for anaemia (haemoglobin 8.4 g/dL), raised lactate dehydrogenase (10.004 U/L) and C reactive protein (7.4 mg/dL); a chest radiograph suggested a discrete pulmonary infiltrate in the right lower lobe. He was empirically medicated with amoxicillin and clavulanate, and subsequently discharged. Two days later, he returned to the ED, following another self-limited episode of left hemibody weakness, with difficulty walking and mild dysarthria. On physical examination, besides pale mucous membranes, discrete left upper limb ataxia and dysdiadochokinaesia were noted. A diffusion-weighted brain MRI was performed (figure 1), which showed multiple acute ischaemic strokes on the left cerebellar hemisphere, and left frontal and right parietal convexities; additionally, a left insular sequelar infarction was evident.
Figure 1.
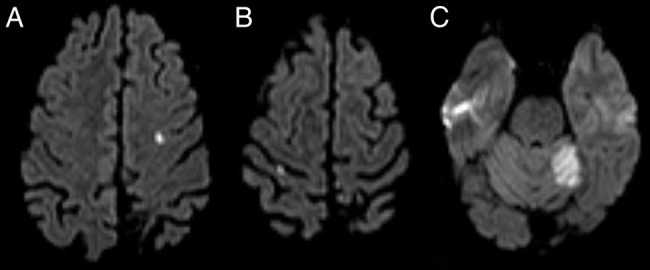
First diffusion-weighted brain MRI displays acute infarctions in multiple topographies. (A) Left frontal convexity cortex; (B) Right parietal convexity cortex; (C) Left cerebellar hemisphere.
On the first day of admission, the patient reported severe abdominal pain and an abdominopelvic CT angiography was conducted (figure 2). It revealed a fine hypodense mural thrombus at the periphery of the superior mesenteric artery and multiple wedge-shaped hypovascularised areas in both kidneys, indicative of infarction, in addition to non-specific early changes in hepatic vascularisation. Anticoagulation with enoxaparin was started (1 mg/kg two times a day) and platelet antiaggregation suspended, with improvement of the abdominal symptoms. In spite of treatment, during hospitalisation, the patient experienced another two transient ischaemic attacks, one with left-sided hemiparesis, and another with right-sided hemiparesis and non-fluent aphasia. A repeat brain MRI scan (figure 3) documented new small cortical acute infarctions on the left fronto-opercular and right parietal medium convexity, besides the expected progression to chronicity of the left cerebellar hemisphere ischaemic lesion. In light of these findings, platelet antiaggregation was reintroduced on the 10th day of admission. Complementary investigation up to that point had been otherwise normal: carotid and vertebral ultrasonography with colour Doppler and transcranial Doppler (TCD) ultrasonography were unremarkable; TCD monitoring for microembolic signals was negative, rendering right-to-left shunt unlikely; venous ultrasonography of the lower limbs showed no signs of thrombosis; electrocardiogram and 24 h Holter exhibited no dysrhythmia; besides pancytopenia and features of haemolytic anaemia, blood studies were negative for infectious agents, such as HIV and Treponema pallidum, autoimmune and hypercoagulability conditions other than PNH. A transoesophageal echocardiography, performed to exclude a cardiac source of embolism, described a patent foramen ovale (PFO), with suggestion of right to left contrast passage with Valsalva manoeuvre. However, this finding was subsequently not confirmed by cardiac catheterisation. A thoracic CT angiography also excluded pulmonary shunt.
Figure 2.
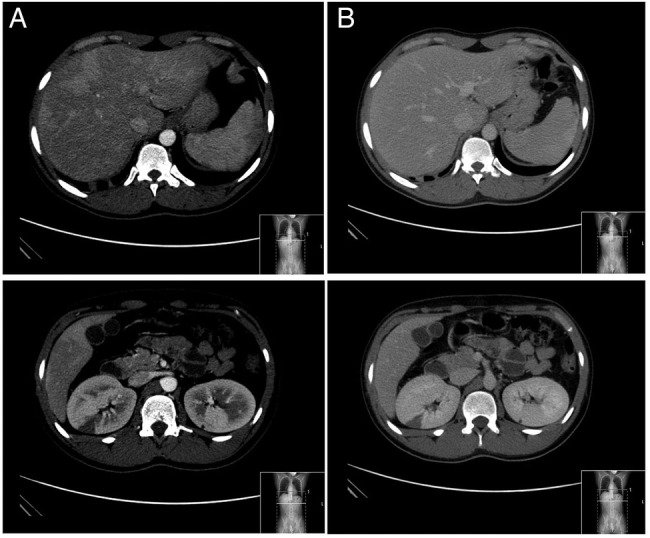
Abdominopelvic CT with contrast ((A) arterial phase; (B) portal venous phase). Heterogeneous contrast enhancement of the hepatic parenchyma in the arterial phase, which homogenise in the portal phase, suggestive of non-specific early changes in vascularisation; multiple wedge-shaped hypovascularised areas in both kidneys, observed in acquisition phases and indicative of infarction.
Figure 3.
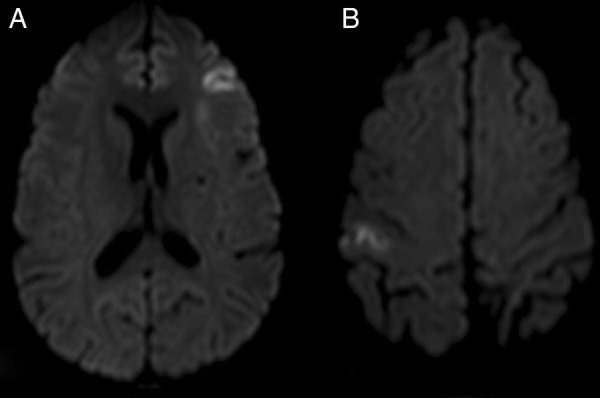
Second diffusion-weighted brain MRI. (A) Left fronto-opercular cortex acute infarction; (B) Right parietal medium convexity cortex acute infarction.
Outcome and follow-up
The patient was discharged on warfarin (target International Normalised Ratio 2–3) while maintaining platelet antiaggregation at a lower dose (aspirin 100 mg daily); darbepoetin was suspended on account of a potential prothrombotic effect. The patient was reassessed 10 months later, at a follow-up clinic, reporting no further episodes and his neurological examination was normal. He is currently awaiting bone marrow transplantation.
Discussion
The case reported illustrates cerebral artery thrombosis as a complication of PNH, with concomitant documentation of multiple intra-abdominal arterial thrombosis. To the best of our knowledge, this is an especially rare finding in a disease where thrombotic events classically involve the venous system. Extensive complementary investigation excluded right-to-left shunt and cardiac embolism in our patient, making the argument for a pathophysiological mechanism of in situ thrombosis in arteries without significant predisposing atherosclerotic disease. Nevertheless, it is mandatory to exclude PFO in patients with PNH and stroke, as its correction may reduce the risk of paradoxical embolism.
Episodes of haemolysis and thrombotic events may be triggered by various stimuli including surgery, strenuous physical activity, alcohol use or infections,8 as was the case in our patient, who was diagnosed with community-acquired pneumonia in the preceding days.
Thrombosis in PNH requires urgent intervention because of the high mortality and disability risk. The optimal management of acute thrombotic events involves immediate full anticoagulation.4 Vitamin K antagonists are generally recommended in the long term if there are no contraindications, as recurrent thromboses and extension of existing thromboses are frequent complications. Primary prophylactic anticoagulation is, however, an issue of debate due to lack of randomised and prospective studies. It should be considered in selected patients with large clones (>50%), stable platelet counts (>100 000/µL) and no known contraindication for anticoagulation.9 There is no published experience on the novel oral anticoagulants in this setting; information is also scarce on antiplatelet therapy, although its empirical use may be justified for refractory cases, as our patient illustrates.
In recent years, growing evidence supports the use of the humanised monoclonal antibody eculizumab.4 This antibody blocks terminal complement by binding to C5, inhibiting the formation of the membrane attack complex and, in doing so, compensating for the CD59 deficiency.10 Internationally accepted indications include transfusion-dependent haemolysis (4 or more transfusions in 12 months) and PNH-related complications (ie, thrombosis or renal failure), regardless of transfusion history.11 The first studies on its use have shown encouraging results with decreased need for blood transfusions, reduced rates of thrombotic events and marked improvement on quality of life.12 13 Nevertheless, prospective studies are lacking and a recent Cochrane systematic review concluded that its prescription can neither be supported nor rejected.14 Furthermore, it requires indefinite administration for a sustained response, making it a very costly treatment, besides not being readily available in most centres.
Allogeneic bone marrow transplantation (BMT) is the only potentially curative therapy available for PNH.2 It is usually reserved for patients who are severely hypoplastic, refractory to other forms of treatment, or if eculizumab is not available, as transplant-related morbidity and mortality are not negligible. Younger patients tend to be better candidates for BMT; however, the overwhelming lack of suitable donors entails a major limitation.
Learning points.
Paroxysmal nocturnal haemoglobinuria (PNH) is a rare acquired disorder of haematopoietic stem cells, characterised by haemolytic anaemia, pancytopenia and venous thrombotic events.
There are few reports of cerebral artery stroke in this setting, and concomitant intra-abdominal arterial thrombosis is especially rare.
Complementary investigation should exclude a right-to-left shunt.
As the leading cause of death in PNH, acute management of thrombosis requires immediate full anticoagulation; although its efficacy is not well-established, empirical antiplatelet therapy has been used as an adjuvant for refractory cases.
In recent years, growing evidence has supported use of the anticomplement monoclonal antibody, eculizumab, though it is not readily available in most centres; allogeneic bone marrow transplantation remains the only potentially curative therapy.
Footnotes
Contributors: LA wrote the manuscript. All the co-authors contributed to writing and revising the manuscript.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Ferro JM, Massaro AR, Mas JL. Aetiological diagnosis of ischaemic stroke in young adults. Lancet Neurol 2010;9:1085–96. 10.1016/S1474-4422(10)70251-9 [DOI] [PubMed] [Google Scholar]
- 2.Brodsky RA. Paroxysmal nocturnal hemoglobinuria. Blood 2014;124:2804–11. 10.1182/blood-2014-02-522128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gulbis B, Eleftheriou A, Angastiniotis M et al. Epidemiology of rare anaemias in Europe. Adv Exp Med Biol 2010;686:375–96. 10.1007/978-90-481-9485-8_22 [DOI] [PubMed] [Google Scholar]
- 4.Hill A, Kelly RJ, Hillmen P. Thrombosis in paroxysmal nocturnal hemoglobinuria. Blood 2013;121:4985–96; quiz 5105 10.1182/blood-2012-09-311381 [DOI] [PubMed] [Google Scholar]
- 5.Ziakas PD, Poulou LS, Rokas GI et al. Thrombosis in paroxysmal nocturnal hemoglobinuria: sites, risks, outcome. An overview. J Thromb Haemost 2007;5:642–5. 10.1111/j.1538-7836.2007.02379.x [DOI] [PubMed] [Google Scholar]
- 6.Van Bijnen ST, Van Heerde WL, Muus P. Mechanisms and clinical implications of thrombosis in paroxysmal nocturnal hemoglobinuria. J Thromb Haemost 2012;10:1–10. 10.1111/j.1538-7836.2011.04562.x [DOI] [PubMed] [Google Scholar]
- 7.Audebert HJ, Planck J, Eisenburg M et al. Cerebral ischemic infarction in paroxysmal nocturnal hemoglobinuria report of 2 cases and updated review of 7 previously published patients. J Neurol 2005;252:1379–86. 10.1007/s00415-005-0871-3 [DOI] [PubMed] [Google Scholar]
- 8.Pu JJ, Brodsky RA. Paroxysmal nocturnal hemoglobinuria from bench to bedside. Clin Transl Sci 2011;4:219–24. 10.1111/j.1752-8062.2011.00262.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall C, Richards S, Hillmen P. Primary prophylaxis with warfarin prevents thrombosis in paroxysmal nocturnal hemoglobinuria (PNH). Blood 2003;102:3587–91. 10.1182/blood-2003-01-0009 [DOI] [PubMed] [Google Scholar]
- 10.Rother RP, Rollins SA, Mojcik CF et al. Discovery and development of the complement inhibitor eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria. Nat Biotechnol 2007;25:1256–64. 10.1038/nbt1344 [DOI] [PubMed] [Google Scholar]
- 11.Rother RP, Bell L, Hillmen P et al. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA 2005;293:1653–62. 10.1001/jama.293.13.1653 [DOI] [PubMed] [Google Scholar]
- 12.Hill A, Hillmen P, Richards SJ et al. Sustained response and long-term safety of eculizumab in paroxysmal nocturnal hemoglobinuria. Blood 2005;106:2559–65. 10.1182/blood-2005-02-0564 [DOI] [PubMed] [Google Scholar]
- 13.Hillmen P, Muus P, Röth A et al. Long-term safety and efficacy of sustained eculizumab treatment in patients with paroxysmal nocturnal haemoglobinuria. Br J Haematol 2013;162:62–73. 10.1111/bjh.12347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martí-Carvajal AJ, Anand V, Cardona AF et al. Eculizumab for treating patients with paroxysmal nocturnal hemoglobinuria. Cochrane Database Syst Rev 2014;10:CD010340. [DOI] [PubMed] [Google Scholar]


