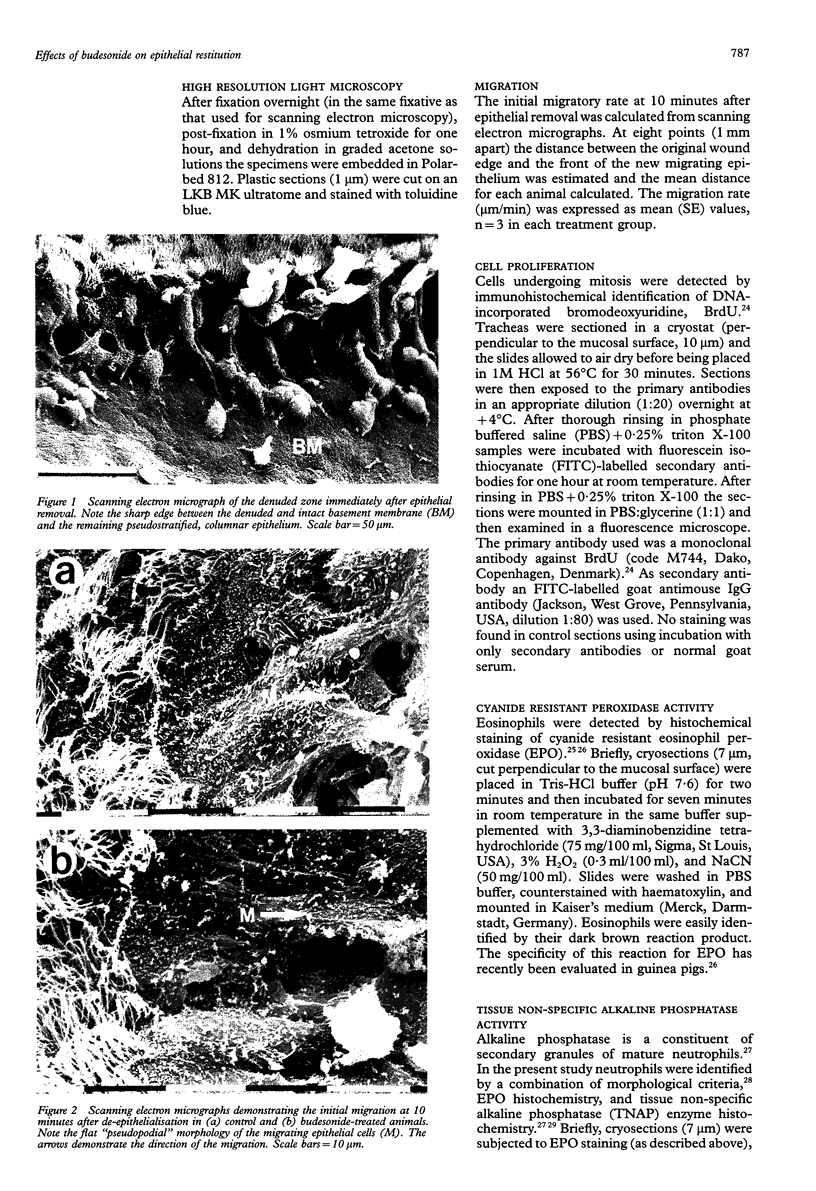
Abstract
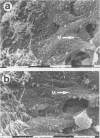
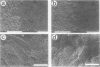
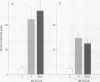
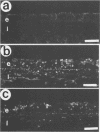
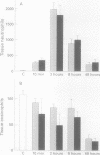
BACKGROUND--Continuous epithelial shedding and restitution processes may characterise the airways in diseases such as asthma. Epithelial restitution involves several humoral and cellular mechanisms that may potentially be affected by inhaled anti-asthma drugs. The present study examines the effect of a topical steroid on epithelial restitution in vivo in the guinea pig. METHODS--The airway epithelium was mechanically removed from well defined areas of guinea pig trachea without surgery and without damage to the basement membrane or bleeding. An anti-inflammatory dose of budesonide (1 mg) was administered repeatedly to the tracheal surface by local superfusion 24 hours before, at (0 hours), and 24 hours after the denudation. Migration of epithelial cells, formation of a plasma exudation-derived gel, and appearance of luminal leucocytes were recorded by scanning electron microscopy. Cell proliferation was visualised by bromodeoxyuridine immunohistochemistry and tissue neutrophils and eosinophils by enzyme histochemistry. RESULTS--Immediately after creation of the denuded zone ciliated and secretory cells on its border dedifferentiated, flattened out, and migrated speedily (mean (SE) 2.3 (0.3) micron/min) over the basement membrane. After 48 hours the entire denuded zone (800 microns wide) was covered by a tightly sealed epithelium; at this time increased proliferation was observed in new and old epithelium and subepithelial cells. Budesonide had no detectable effect on epithelial dedifferentiation, migration, sealing, or proliferation. Immediately after denudation and continuously during the migration phase plasma was extravasated creating a fibrinous gel rich in leucocytes, particularly neutrophils, over the denuded area. Budesonide had no effect on either the gel or the leucocyte density. CONCLUSIONS--These observations suggest that topical glucocorticoids may not interfere with a fast and efficient restitution of the epithelium in the airways.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes P. J., Pedersen S. Efficacy and safety of inhaled corticosteroids in asthma. Report of a workshop held in Eze, France, October 1992. Am Rev Respir Dis. 1993 Oct;148(4 Pt 2):S1–26. doi: 10.1164/ajrccm/148.4_Pt_2.S1. [DOI] [PubMed] [Google Scholar]
- Borish L., Mascali J. J., Dishuck J., Beam W. R., Martin R. J., Rosenwasser L. J. Detection of alveolar macrophage-derived IL-1 beta in asthma. Inhibition with corticosteroids. J Immunol. 1992 Nov 1;149(9):3078–3082. [PubMed] [Google Scholar]
- Boschetto P., Rogers D. F., Fabbri L. M., Barnes P. J. Corticosteroid inhibition of airway microvascular leakage. Am Rev Respir Dis. 1991 Mar;143(3):605–609. doi: 10.1164/ajrccm/143.3.605. [DOI] [PubMed] [Google Scholar]
- Bousquet J., Chanez P., Lacoste J. Y., White R., Vic P., Godard P., Michel F. B. Asthma: a disease remodeling the airways. Allergy. 1992 Feb;47(1):3–11. doi: 10.1111/j.1398-9995.1992.tb02242.x. [DOI] [PubMed] [Google Scholar]
- CORRELL N. O., Jr, BEATTIE E. J., Jr The characteristics of regeneration of respiratory epithelium. Surg Gynecol Obstet. 1956 Aug;103(2):209–211. [PubMed] [Google Scholar]
- DUNNILL M. S. The pathology of asthma, with special reference to changes in the bronchial mucosa. J Clin Pathol. 1960 Jan;13:27–33. doi: 10.1136/jcp.13.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djukanović R., Wilson J. W., Britten K. M., Wilson S. J., Walls A. F., Roche W. R., Howarth P. H., Holgate S. T. Effect of an inhaled corticosteroid on airway inflammation and symptoms in asthma. Am Rev Respir Dis. 1992 Mar;145(3):669–674. doi: 10.1164/ajrccm/145.3.669. [DOI] [PubMed] [Google Scholar]
- Erjefält I. A., Wagner Z. G., Strand S. E., Persson C. G. A method for studies of tracheobronchial microvascular permeability to macromolecules. J Pharmacol Methods. 1985 Dec;14(4):275–283. doi: 10.1016/0160-5402(85)90003-8. [DOI] [PubMed] [Google Scholar]
- Erjefält I., Greiff L., Alkner U., Persson C. G. Allergen-induced biphasic plasma exudation responses in guinea pig large airways. Am Rev Respir Dis. 1993 Sep;148(3):695–701. doi: 10.1164/ajrccm/148.3.695. [DOI] [PubMed] [Google Scholar]
- Erjefält I., Persson C. G. Increased sensitivity to toluene diisocyanate (TDI) in airways previously exposed to low doses of TDI. Clin Exp Allergy. 1992 Sep;22(9):854–862. doi: 10.1111/j.1365-2222.1992.tb02831.x. [DOI] [PubMed] [Google Scholar]
- Erjefält I., Persson C. G. Inflammatory passage of plasma macromolecules into airway wall and lumen. Pulm Pharmacol. 1989;2(2):93–102. doi: 10.1016/0952-0600(89)90030-6. [DOI] [PubMed] [Google Scholar]
- Erjefält J. S., Erjefält I., Sundler F., Persson C. G. Microcirculation-derived factors in airway epithelial repair in vivo. Microvasc Res. 1994 Sep;48(2):161–178. doi: 10.1006/mvre.1994.1047. [DOI] [PubMed] [Google Scholar]
- Erjefält J. S., Erjefält I., Sundler F., Persson C. G. Mucosal nitric oxide may tonically suppress airways plasma exudation. Am J Respir Crit Care Med. 1994 Jul;150(1):227–232. doi: 10.1164/ajrccm.150.1.8025753. [DOI] [PubMed] [Google Scholar]
- Fabbri L. M., Boschetto P., Zocca E., Milani G., Pivirotto F., Plebani M., Burlina A., Licata B., Mapp C. E. Bronchoalveolar neutrophilia during late asthmatic reactions induced by toluene diisocyanate. Am Rev Respir Dis. 1987 Jul;136(1):36–42. doi: 10.1164/ajrccm/136.1.36. [DOI] [PubMed] [Google Scholar]
- Frew A. J., Moqbel R., Azzawi M., Hartnell A., Barkans J., Jeffery P. K., Kay A. B., Scheper R. J., Varley J., Church M. K. T lymphocytes and eosinophils in allergen-induced late-phase asthmatic reactions in the guinea pig. Am Rev Respir Dis. 1990 Feb;141(2):407–413. doi: 10.1164/ajrccm/141.2.407. [DOI] [PubMed] [Google Scholar]
- Hutson P. A., Church M. K., Clay T. P., Miller P., Holgate S. T. Early and late-phase bronchoconstriction after allergen challenge of nonanesthetized guinea pigs. I. The association of disordered airway physiology to leukocyte infiltration. Am Rev Respir Dis. 1988 Mar;137(3):548–557. doi: 10.1164/ajrccm/137.3.548. [DOI] [PubMed] [Google Scholar]
- Jeffery P. K., Wardlaw A. J., Nelson F. C., Collins J. V., Kay A. B. Bronchial biopsies in asthma. An ultrastructural, quantitative study and correlation with hyperreactivity. Am Rev Respir Dis. 1989 Dec;140(6):1745–1753. doi: 10.1164/ajrccm/140.6.1745. [DOI] [PubMed] [Google Scholar]
- Jeffery P. K., Wardlaw A. J., Nelson F. C., Collins J. V., Kay A. B. Bronchial biopsies in asthma. An ultrastructural, quantitative study and correlation with hyperreactivity. Am Rev Respir Dis. 1989 Dec;140(6):1745–1753. doi: 10.1164/ajrccm/140.6.1745. [DOI] [PubMed] [Google Scholar]
- Jones H. B., Clarke N. A., Barrass N. C. Phenobarbital-induced hepatocellular proliferation: anti-bromodeoxyuridine and anti-proliferating cell nuclear antigen immunocytochemistry. J Histochem Cytochem. 1993 Jan;41(1):21–27. doi: 10.1177/41.1.8093255. [DOI] [PubMed] [Google Scholar]
- Keenan K. P., Combs J. W., McDowell E. M. Regeneration of hamster tracheal epithelium after mechanical injury. I. Focal lesions: quantitative morphologic study of cell proliferation. Virchows Arch B Cell Pathol Incl Mol Pathol. 1982;41(3):193–214. doi: 10.1007/BF02890281. [DOI] [PubMed] [Google Scholar]
- Laitinen L. A., Heino M., Laitinen A., Kava T., Haahtela T. Damage of the airway epithelium and bronchial reactivity in patients with asthma. Am Rev Respir Dis. 1985 Apr;131(4):599–606. doi: 10.1164/arrd.1985.131.4.599. [DOI] [PubMed] [Google Scholar]
- Laitinen L. A., Laitinen A., Haahtela T. A comparative study of the effects of an inhaled corticosteroid, budesonide, and a beta 2-agonist, terbutaline, on airway inflammation in newly diagnosed asthma: a randomized, double-blind, parallel-group controlled trial. J Allergy Clin Immunol. 1992 Jul;90(1):32–42. doi: 10.1016/s0091-6749(06)80008-4. [DOI] [PubMed] [Google Scholar]
- Lane B. P., Gordon R. Regeneration of rat tracheal epithelium after mechanical injury. I. The relationship between mitotic activity and cellular differentiation. Proc Soc Exp Biol Med. 1974 Apr;145(4):1139–1144. doi: 10.3181/00379727-145-37968. [DOI] [PubMed] [Google Scholar]
- Lapa e Silva J. R., Bachelet C. M., Pretolani M., Baker D., Scheper R. J., Vargaftig B. B. Immunopathologic alterations in the bronchi of immunized guinea pigs. Am J Respir Cell Mol Biol. 1993 Jul;9(1):44–53. doi: 10.1165/ajrcmb/9.1.44. [DOI] [PubMed] [Google Scholar]
- Lapa e Silva J. R., Bachelet C. M., Pretolani M., Baker D., Scheper R. J., Vargaftig B. B. Immunopathologic alterations in the bronchi of immunized guinea pigs. Am J Respir Cell Mol Biol. 1993 Jul;9(1):44–53. doi: 10.1165/ajrcmb/9.1.44. [DOI] [PubMed] [Google Scholar]
- Lundgren R., Söderberg M., Hörstedt P., Stenling R. Morphological studies of bronchial mucosal biopsies from asthmatics before and after ten years of treatment with inhaled steroids. Eur Respir J. 1988 Dec;1(10):883–889. [PubMed] [Google Scholar]
- Montefort S., Gratziou C., Goulding D., Polosa R., Haskard D. O., Howarth P. H., Holgate S. T., Carroll M. P. Bronchial biopsy evidence for leukocyte infiltration and upregulation of leukocyte-endothelial cell adhesion molecules 6 hours after local allergen challenge of sensitized asthmatic airways. J Clin Invest. 1994 Apr;93(4):1411–1421. doi: 10.1172/JCI117118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickard K. A., Taylor J., Rennard S. I., Spurzem J. R. Migration of bovine bronchial epithelial cells to extracellular matrix components. Am J Respir Cell Mol Biol. 1993 Jan;8(1):63–68. doi: 10.1165/ajrcmb/8.1.63. [DOI] [PubMed] [Google Scholar]
- Rosolia D. L., McKenna P. J., Gee M. H., Albertine K. H. Infusion of zymosan-activated plasma affects neutrophils in peripheral blood and bone marrow in sheep. J Leukoc Biol. 1992 Nov;52(5):501–515. doi: 10.1002/jlb.52.5.501. [DOI] [PubMed] [Google Scholar]
- Schleimer R. P. An overview of glucocorticoid anti-inflammatory actions. Eur J Clin Pharmacol. 1993;45 (Suppl 1):S3–S44. doi: 10.1007/BF01844196. [DOI] [PubMed] [Google Scholar]
- Sousa A. R., Poston R. N., Lane S. J., Nakhosteen J. A., Lee T. H. Detection of GM-CSF in asthmatic bronchial epithelium and decrease by inhaled corticosteroids. Am Rev Respir Dis. 1993 Jun;147(6 Pt 1):1557–1561. doi: 10.1164/ajrccm/147.6_Pt_1.1557. [DOI] [PubMed] [Google Scholar]
- Sur S., Crotty T. B., Kephart G. M., Hyma B. A., Colby T. V., Reed C. E., Hunt L. W., Gleich G. J. Sudden-onset fatal asthma. A distinct entity with few eosinophils and relatively more neutrophils in the airway submucosa? Am Rev Respir Dis. 1993 Sep;148(3):713–719. doi: 10.1164/ajrccm/148.3.713. [DOI] [PubMed] [Google Scholar]
- Ten R. M., Pease L. R., McKean D. J., Bell M. P., Gleich G. J. Molecular cloning of the human eosinophil peroxidase. Evidence for the existence of a peroxidase multigene family. J Exp Med. 1989 May 1;169(5):1757–1769. doi: 10.1084/jem.169.5.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILHELM D. L. Regeneration of tracheal epithelium. J Pathol Bacteriol. 1953 Apr;65(2):543–550. doi: 10.1002/path.1700650226. [DOI] [PubMed] [Google Scholar]
- Wallen N., Kita H., Weiler D., Gleich G. J. Glucocorticoids inhibit cytokine-mediated eosinophil survival. J Immunol. 1991 Nov 15;147(10):3490–3495. [PubMed] [Google Scholar]
- Wallen N., Kita H., Weiler D., Gleich G. J. Glucocorticoids inhibit cytokine-mediated eosinophil survival. J Immunol. 1991 Nov 15;147(10):3490–3495. [PubMed] [Google Scholar]
- Zahm J. M., Chevillard M., Puchelle E. Wound repair of human surface respiratory epithelium. Am J Respir Cell Mol Biol. 1991 Sep;5(3):242–248. doi: 10.1165/ajrcmb/5.3.242. [DOI] [PubMed] [Google Scholar]