Abstract
Incorporation of Zn atoms into a nanosize Cu lattice is known to alter the electronic properties of Cu, improving catalytic performance in a number of industrially important reactions. However the structural influence of Zn on the Cu phase is not well studied. Here, we show that Cu nano-clusters modified with increasing concentration of Zn, derived from ZnO support doped with Ga3+, can dramatically enhance their stability against metal sintering. As a result, the hydrogenation of dimethyl oxalate (DMO) to ethylene glycol, an important reaction well known for deactivation from copper nanoparticle sintering, can show greatly enhanced activity and stability with the CuZn alloy catalysts due to no noticeable sintering. HRTEM, nano-diffraction and EXAFS characterization reveal the presence of a small beta-brass CuZn alloy phase (body-centred cubic, bcc) which appears to greatly stabilise Cu atoms from aggregation in accelerated deactivation tests. DFT calculations also indicate that the small bcc CuZn phase is more stable against Cu adatom migration than the fcc CuZn phase with the ability to maintain a higher Cu dispersion on its surface.
Cu-based catalysts are widely used for many important reactions in the chemical industry, including methanol synthesis1,2,3, the water-gas shift reaction4,5,6, steam reforming7,8,9 and hydrogenation of dimethyl oxalate (DMO) to ethylene glycol (EG)10,11,12,13,14,15,16,17. The syngas (CO/H2) conversion to methanol over Cu/ZnO/Al2O3 is perhaps one of the most studied catalytic reactions over the past 40 years18. Electronic and structural modifications of the Cu phase, by varying support and additive(s), for enhanced catalytic performance have been investigated1,2,19. However a detailed understanding of this promotional effect is not yet reported due to the difficulties of detailed structural analysis of small alloy clusters on a multi-component support. Thanks to the rapid improvement of analytical surface science characterization techniques and theoretical methodologies, the potential effects induced by supported alloy catalysts are becoming clearer. Using advanced instruments researchers have recently shown that a very small number of Zn atoms are reduced from the ZnO support. These Zn atoms can decorate the Cu nanoparticle leading to a subtle change in electronic structure20,21,22. As a result, the intermediate formate ion has greater stabilization than that on the unmodified Cu surface20,21,22. Further reports have demonstrated that the decoration of stepped copper particles by Zn atoms in the industrial Cu/ZnO/Al2O3 catalyst is also identified, accounting for high activity and selectivity2. It has also recently been reported that addition of Ga3+ into Cu-ZnO precursors by co-precipitation gives a catalyst with exceptionally small but stable Cu crystallites (~0.5–2 nm) under methanol synthesis conditions23,24,25. One possibility is that the presence of Ga3+ facilitates the reduction of Zn atoms from ZnO, stabilizing the small Cu particles. It is long believed that Zn atoms are essential in catalysts for textural dispersion of the copper phase18,19,20,26,27,28,29, but the precise interaction between Zn and Cu still remains unclear. Moreover, the mechanism of stabilization from Zn and the configuration between Zn and Cu also need to be investigated.
In this work, we have systematically investigated Ga3+ doping into Cu-ZnO by controlling chemical composition and calcination temperature. We were particularly interested to develop these composite materials as effective catalysts for the hydrogenation of DMO to EG reaction due to the recent incentive to develop this non-oil based reaction route for chemicals synthesis. EG is an important raw material in the manufacture of polyester fibers and fabrics, and polyethylene terephthalate, widely used in our society10. It is produced almost exclusively from ethylene, derived from petroleum cracking, via hydrolysis of the intermediate ethylene oxide. However, the synthesis of ethylene glycol from natural gas, coal or biomass is becoming a strategic process due to the diversification in energy and chemical supplies for many nations. In fact, the insecure long-term prospect of petroleum supplies prompts the development of these non-oil based chemical processes. This involves the formation of syngas from coal or natural gas, followed by the coupling of CO with methyl nitrite to DMO catalysed by Pd and then hydrogenation to EG by Cu11,12,13,14,15. This synthesis route appears to be especially important for countries like China and the USA where coal is abundant. While the coupling reaction is efficiently taken place, one key issue of this new process is the latter catalytic hydrogenation reaction. This step has inherent problems with poor stability and short lifetime of the copper-based catalyst during the vapour phase hydrogenation of DMO to EG11,12,15,16,17. There is a strong tendency for copper nanoparticles to grow into larger crystallites through migration and coalescence, hence losing copper surface area during this reaction. Thus, finding a practical way to create and maintain highly dispersed copper on a catalyst surface has been proposed to be an important strategy to improve catalytic performance. Approaches to stabilize a high surface area of Cu comprise alloying Cu with a higher-melting point metal, encapsulation of the Cu nanoparticle in porous templates and the use of metal oxides with strong metal-support interaction to produce small Cu nanoparticles are included. In addition to stabilizing them against coalescence, the metal-support interaction of ZnO and Cr2O3 have been documented27,28,29,30,31.
Here, we report the progressive formation of Zn0 when Ga3+ is added to the Cu/ZnO precursors under H2, which gives rise to CuZn nanoclusters. A clear inverse correlation between Zn0 concentration and Cu particle size in the catalysts is, for the first time, revealed. From high resolution transmission electron microscopy (HRTEM), nano-diffraction, and extended X-ray absorption fine structure (EXAFS) analyses, a bcc CuZn phase can be identified. According to our density functional theory (DFT) calculations the bcc CuZn is more stable against metal sintering than the corresponding fcc Cu or fcc CuZn. Thus, this new type of catalyst shows remarkable activity and stability for the hydrogenation of DMO to EG. This conclusion, although demonstrated for the hydrogenation of DMO reaction, is expected to be applicable to other Cu catalyzed reactions that are susceptible to Cu sintering. It is thus believed that this finding can offer a new way to stabilize surface Cu clusters for high activity and stability.
Results
Progressive addition of Ga3+
Through incorporation of Ga3+ ions into the Cu and Zn containing precursor system a series of Zn modified Cu catalysts have been prepared by co-precipitation with control of precursor injection rate, pH value and precipitation temperature (refer to Methods and supplementary information (SI)). The calcination temperatures of the precursors and the chemical compositions were thoroughly investigated to correlate catalytic performance to various synthesis conditions of these Cu catalysts. Upon heating the solid mixed phases CuO, ZnO, ZnGa2O4 and (Cu,Zn)5(CO3)2(OH)16 were identified by powder XRD (Fig. S1). Under H2, the reduction of CuO to Cu occurred at lower temperature when doping with Ga3+ (see TPR in Fig. S2). As previously reported, XRD was unable to characterise the small Cu containing nano-clusters produced after reduction23,24,25. Thus, detailed XPS analysis was conducted which detected Cu0 (total reduction) and Ga3+ (no reduction) exclusively. XPS also indicated partial reduction of Zn2+ to form Zn0 after the H2 treatment of the co-precipitated precursors. Fig. S3a clearly confirms that the Zn 2p signal is composed of two peaks. They can be resolved to be 1023 eV and 1021 eV matching to the binding energies of Zn2+ and Zn0, respectively. Thus, the surface composition of catalysts analysed by XPS is presented in Table 1. It is apparent that the surface concentration of Zn0 varies with chemical composition and calcination temperature. The Zn0/(Zn0 + Zn2+) ratio increased as the content of Ga3+ increased to a maximum of 0.32 at a chemical composition of 43%Cu, 52%Zn, and 5%Ga. The ratio then decreased as the Ga3+ concentration was increased further. When using the same chemical composition but varying different calcination temperatures, the Zn0/(Zn0 + Zn2+) ratio was found to reach a maximum value when the sample was calcined at 330 °C. The CZG29 sample gave the highest Zn0/(Zn0 + Zn2+) ratio among all the studied catalysts, which means it has the highest ratio of Zn atoms decorated on Cu nanoparticles, and has the highest Zn0/Cu ratio than the other samples. Although XPS is a useful technique to obtain near surface composition, it cannot provide the topmost atomic layer analysis because the photoelectron escaping depth can reach to few nano-meters. Therefore, by using low energy ion, the penetration depth to the solid sample can be greatly restricted. As a result, apart from the XPS analysis, we also used the more surface-sensitive technique of high-sensitive low-energy ion scattering (HS-LEIS), which provided the elemental composition of the outermost surface (refer to SI for the details of the experiment of HS-LEIS), to characterise two selected samples, CZG28 and CZG29. As seen from Fig. S3b and insert table, an even higher degree of Zn decoration on the outmost surface of Cu of these two samples than that of XPS is depicted.
Table 1. XPS of CuZnGa samples with variations in composition and calcination temperature.
| Sample | Composition Cu:Zn:Ga | Zn/Ga | Zn/Cu | Zn0/ (Zn0 + Zn2+) | Zn0/Ga | Zn0/Cu |
|---|---|---|---|---|---|---|
| CZG34 | 43:56.5:0.5 | 87.68 | 3.66 | 0.19 | 16.66 | 0.70 |
| CZG33 | 43:54.5:2.5 | 18.03 | 3.47 | 0.22 | 3.97 | 0.76 |
| CZG29 | 43:52:5 | 7.1 | 4.14 | 0.32 | 2.72 | 1.32 |
| CZG28–280 | 43:47:10 | 3.05 | 1.93 | 0.29 | 0.92 | 0.58 |
| CZG28–330 | 43:47:10 | 5.97 | 3.39 | 0.30 | 1.79 | 1.02 |
| CZG28–380 | 43:47:10 | 4.71 | 3.61 | 0.20 | 0.94 | 0.72 |
| CZG28–450 | 43:47:10 | 4.72 | 2.99 | 0.18 | 0.85 | 0.54 |
| CZG28–500 | 43:47:10 | 4.06 | 2.04 | 0.17 | 0.69 | 0.35 |
| CZG30 | 43:37:20 | 2.03 | 4.01 | 0.2 | 0.53 | 0.8 |
| CZG31 | 43:28.5:28.5 | 0.97 | 2.1 | 0.14 | 0.14 | 0.29 |
| CZG32 | 43:19:38 | 0.43 | 2.4 | 0.1 | 0.08 | 0.24 |
Also, in the catalyst synthesis, an addition of Ga3+ into the Cu and Zn containing precursor system can clearly create a ZnGa2O4 phase, which depends on calcination temperature (>330 °C) and composition (Fig. S1). The apparent co-existence of ZnGa2O4 and ZnO phases (both identified in XRD) suggests the formation of a material interface between these two phases. It has been reported that the formation of heterojunction interface with two semiconducting solid phases (ZnGa2O4 and ZnO) with different band energies could lead to charge separation of the phases. In this case, the reducibility of the refractory ZnO support phase is greatly enhanced32. From the XPS result, the concentration of Zn0 indeed shows an optimum response to the presence of ZnGa2O4 and ZnO phases.
DMO hydrogenation
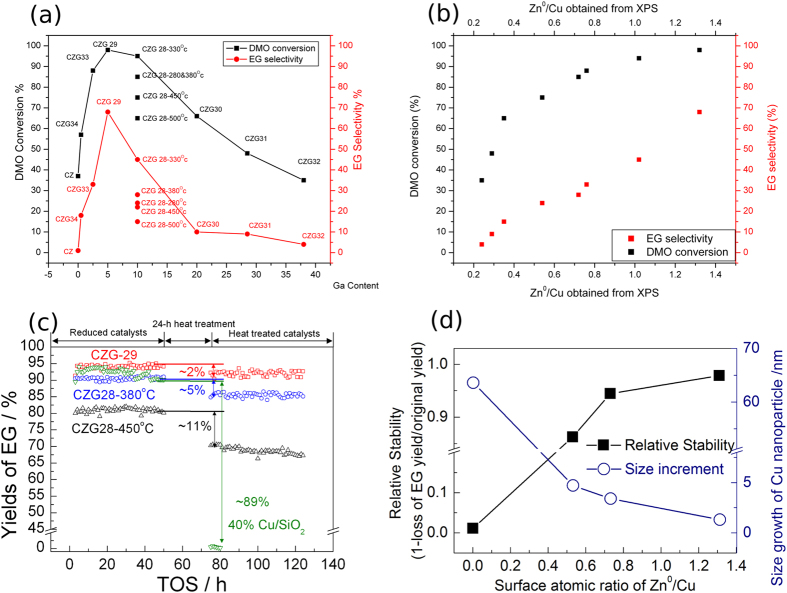
It is accepted that hydrogenation of DMO to EG can be effectively catalyzed by a Cu surface10,11,12,13,14,15,16,17. As a result, this reaction was studied over the series of CuZnGa catalysts. It can be seen from Fig. 1a that as the Ga3+ concentration increases, the activity increases, reaching a maximum value at the CZG29 sample. The activity then decreases as the Ga3+ concentration further increases. The EG selectivity has a similar trend to the activity. It can be seen that the calcination temperature of the CuZnGa precursors also affects the catalytic performance with a calcination temperature of 330 °C showing the best performance amongst the CZG28 series. Comparing the change in Ga3+ concentration with Zn0/(Zn0 + Zn2+) in Table 1 shows a trend that the activity and selectivity follow Zn0/(Zn0 + Zn2+) or Zn0/Cu ratio closely, see Fig. 1b. The catalytic performance of catalysts with an in-situ treatment (by raising the temperature to 400 °C for 24 h under N2) shows very different behaviours than that without the high temperature treatment (Fig. 1c). A highly active Cu/SiO2 catalyst containing no Zn was also introduced as a comparison. It can be seen that the EG yield of the Cu/SiO2 catalyst with an unmodified Cu surface, where the Cu particle size is 3.1 nm, reaches 95%, with both impressive activity and selectivity in this hydrogenation reaction. This differs from the methanol synthesis reaction where a pure Cu surface does not deliver an acceptable selectivity without Zn inclusion1,2,3,18,20,21,22. Thus, the electronic promotion from alloying Cu with Zn is perhaps unnecessary for this reaction, in addition, this catalyst is well-known for deactivation due to inherent problems of poor stability11,12,15,16,17. The EG yield of the Cu/SiO2 catalyst after heat treatment significantly decreased by 89%. In contrast, the CZG29 sample containing Zn0/Cu ratio of 1.32 only drops by 2% yield of EG after the 24 h accelerated heat treatment at 400 °C. Interestingly, samples CZG28–380 (Zn0/Cu ratio of 0.72) and CZG28-450 (Zn0/Cu ratio of 0.54) with intermediate Zn0/Cu ratios deactivated by 5 and 11% of EG yields accordingly. Thus, there is a clear correlation of stability with Zn0/Cu ratio, which suggests the active Cu particles might have severe sintering after heat treatment but the presence of Zn0 minimized the extent of sintering. Fig. S4 shows the TEM images before and after 24 h heat treatment of the samples. It can be seen that after 24 h heat treatment, Cu/SiO2 indeed suffered from serious particle aggregation (average diameter increased from 3.1 nm to 66.7 nm), whereas CZG29 maintained the small particle size (Figs S4 and S5). The results shown in Fig. 1d summarize the importance of Zn0/Cu which appears to stabilize the Cu nanoparticles from sintering at higher temperature, hence, minimizing the extent of deactivation. CZG29 is shown to be the most active and stable catalyst among those Cu-containing catalysts. Therefore, regarding the favorable Zn solubility into the Cu lattice, it is logical to assume that the Cu clusters from CuZnGa may be stabilized by Zn0 atoms through the formation of nano-CuZn alloy.
Figure 1.
(a) Catalytic performances of CZG samples prepared with various chemical compositions and calcination temperatures (all calcined at 330 °C unless otherwise indicated; 330 oC was found to be the optimal temperature for the establishment of ZnGa2O4/ZnO phases over other phases according to XRD) under reaction conditions of 220 °C, 3.0 MPa, H2/DMO = 80, WHSV = 0.6 g g−1 h −1 and mcatal. = 100 mg; (b) The correlation between Zn0/Cu obtained from XPS result with DMO hydrogenation catalytic performances of CZG samples; (c) Comparison of catalytic performance and stabilities of Cu-containing catalysts; reaction conditions: P(H2) = 3.0 MPa, H2/DMO = 80 (v/v), Temperature = 240 °C, WLSHV = 0.6 g·g−1·h−1 and mcatal. = 100 mg (20 mg Cu/SiO2 was diluted by 80 mg quartz sand); subjected to heat treatment at 400 °C for 24 h under N2 before resuming the testing; (d) the correlation of Zn0/Cu ratio with stability and particle size change.
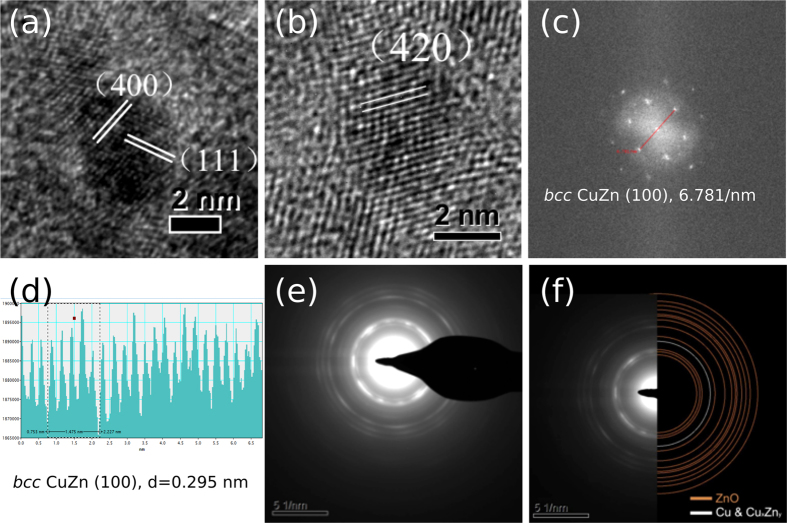
According to the phase diagram of Cu-Zn33, when the concentration of Zn is below 35% the CuZn alloy will stay as the fcc structure, as the same as Cu. However, when the Zn concentration is higher than 50%, as shown in most samples in our case, the structure tends to change to bcc. Then a change to complex cubic (Cu5Zn8) is seen when the Zn concentration is higher than 62%, and finally to hcp if Zn ratio is higher than 75%. These structural changes are thought to be attributed to the increasing electron contribution from Zn into anti-bonding bands of CuZn, hence making the primary structures unstable34. XRD could not be used to indicate the structure of these small crystallites of below 3–4 nm due to severe peak broadening. Thus, we carried out metal particle mapping and analysis by HRTEM and high resolution scanning transmission electron microscopy (HR-STEM). Close examination of the selected co-precipitated sample (CZG-29), containing the highest Zn0/Cu ratio, by microscopy clearly indicates a mixture of phases. The most prevalent phase in this reduced sample is the bulk ZnO (observable by XRD). There is also a clear evidence of the formation of the other phases: fcc Cu (may contain Zn as fcc CuZn with same lattice parameter), bcc CuZn and trace complex cubic Cu5Zn8 phases (at about 2 nm) (Fig. 2). Although the majority of nano-diffraction rings were generated from the substrate material, ZnO, Fig. 3 shows the statistical analysis of the Cu containing phases from the HR-STEM bright field images, when 140 sampling points were carried out. The coloured lines of Cu5Zn8 (Red), Cu (Green) and CuZn (Blue) represent the characteristic d-spacings of each structure: Cu (00–001–1241), CuZn (04–003–4270) and Cu5Zn8 (00–025–1128). The d-spacings of crystals were measured from the lattice fringes and the corresponding fast-Fourier Transform (FFT) images of selected HR-STEM images (Fig. S6). The three selected areas, (blue, green and red) in Fig. 3 indicate the frequent appearance of d-spacings, which is 0.148 nm from bcc CuZn, 0.181 nm from fcc Cu and 0.237 nm from complex cubic Cu5Zn8. Thus, nano-diffraction and microscopic techniques give a direct evidence of the presence of bcc CuZn and trace complex cubic Cu5Zn8 phases in the reduced CuZnGa catalyst (CZG29) when there is a large quantity of Zn0 in contact with Cu nanoparticles. This was predicted from the Cu-Zn phase diagram. On the other hand, the CZ catalyst without Ga (Cu/ZnO sample) contained exclusively the fcc Cu phase.
Figure 2.
Structural analysis of CZG29 sample: (a,b) HRTEM showing (400) and (420) bcc CuZn phase surrounding (111) fcc Cu nanoparticle core; (c) nano-diffraction of selected bcc CuZn phase; notice that the less well-defined diffraction spots could be arisen from the small sized and highly strained crystallite; (d) (100) phase of d = 0.295 nm; (e) selected area of diffraction rings; (f) diffraction rings matching well with simulated Braggs’ diffractions of Cu, ZnO and bcc CuZn phases.
Figure 3. Statistical d-spacing measurements from the lattice fringes and the corresponding FFT of selected HR-STEM images.
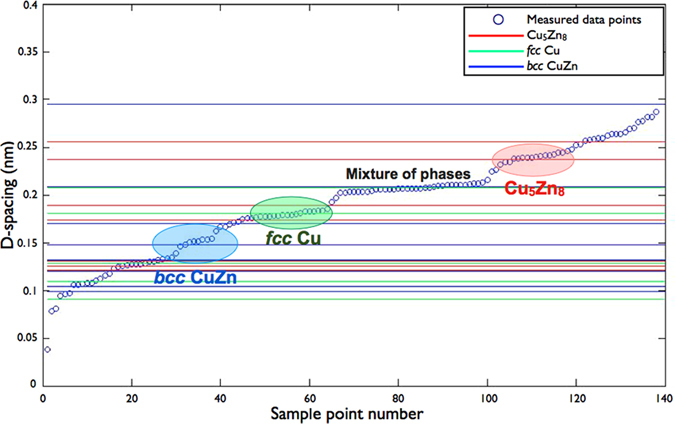
The coloured lines of Cu5Zn8 (Red), Cu (Green) and CuZn (Blue) are the d-spacing of each structures: Cu (00–001–1241); CuZn (04–003–4270) and Cu5Zn8 (00–025–1128).
To further confirm the incorporation of Zn0 into the nano Cu lattice, extended X-ray absorption fine structure (EXAFS) of Cu was studied. This technique explores the local structural information of the Cu atom. The experimental data for the reduced CuZnGa samples was recorded and satisfactory R fittings were achieved, as can be seen in Fig. S7, and Table 2. It was found that the spectra of most of the catalysts in present work could not be well modeled using only scattering parameters from metallic fcc Cu. Therefore, a mixed-structure model is introduced and it is found that the combination of both fcc metallic Cu and bcc CuZn alloy structures give significantly improved fits to the experimental spectra. We did not attempt to fit the complex cubic Cu5Zn8 since there was only a small quantity present in the sample. The EXAFS were fitted using scattering paths of Cu-Cu (2.56 Å) from metallic fcc Cu model, Cu-Zn (2.56 Å) and Cu-Cu (2.99 Å) from bcc Cu-Zn alloy model. Notice that the longer distance of Cu-Cu (2.99 Å) in the bcc Cu-Zn structure is distinctive from the shorter Cu-Cu (2.56 Å) of the fcc Cu model and Cu-Zn (2.56 Å) of the bcc Cu-Zn model. As shown in Table 2, all R-factors are below 0.8% with the coordination number of the Cu-Zn bond, derived from 2.56 Å scattering path, ranging from 6 to 9. As for the CN(Cu-Cu), derived from 2.99 Å scattering path, it can be seen that the CZG29 sample had the highest coordination number of bcc Cu-Cu bond, thus indicates the highest concentration of bcc Cu-Zn alloy. We also found the bcc CuZn concentration is consistent with the concentration of Zn0/Cu observed from the XPS result (Fig. S8). In addition, no Cu-Cu bond of 2.99 Å was observed in the 500 °C-calcined sample which suggests that no bcc CuZn alloy was formed during the reduction procedure if the CuZnGa precursor has been calcined at high temperature. The EXAFS confirmed the existence of bcc CuZn alloy in most of our samples, especially those with higher Zn0 concentration, i.e. CZG29 gives highest quantity of bcc CuZn alloy.
Table 2. EXAFS of CuZnGa samples of various calcination temperatures and chemical compositions.
| Sample | Cu:Zn:Ga | Enot* | CN (bcc Cu-Zn & fcc Cu-Cu) | (Cu-Zn & fcc Cu-Cu) | Bond length (Å) (Cu-Zn & fcc Cu-Cu) | CN (bcc Cu-Cu) | DW-factor (bcc Cu-Cu) | Bond length (Å) (bcc Cu-Zu) | R-factor |
|---|---|---|---|---|---|---|---|---|---|
| CZG28–280 | 43:47:10 | 4.2 | 6.9 (4) | 0.012 (1) | 2.55 (1) | 1.1 (4) | 0.013 (5) | 2.96 (3) | 0.8% |
| CZG28–330 | 43:47:10 | 4.8 | 6.4 (3) | 0.010 (1) | 2.53 (1) | 1.5 (5) | 0.014 (4) | 2.95 (3) | 0.6% |
| CZG28–380 | 43:47:10 | 4.7 | 7.6 (4) | 0.011 (1) | 2.54 (1) | 1.0 (5) | 0.014 (5) | 2.98 (4) | 0.8% |
| CZG28–450 | 43:47:10 | 4.2 | 6.4 (3) | 0.010 (1) | 2.54 (1) | 0.6 (3) | 0.011 (5) | 3.03 (4) | 0.7% |
| CZG28–500 | 43:47:10 | 4.5 | 9.3 (3) | 0.010 (1) | 2.54 (1) | None | None | None | 0.4% |
| CZG29 | 43:52:5 | 0.5 | 7.5 (4) | 0.010 (1) | 2.54 (1) | 1.8 (6) | 0.014 (3) | 2.94 (1) | 0.5% |
| CZG30 | 43:37:20 | 1.5 | 7.3 (2) | 0.008 (1) | 2.54 (1) | 1.0 (4) | 0.013 (4) | 3.00 (3) | 0.4% |
| CZG32 | 43:19:38 | 3.1 | 8.2 (2) | 0.009 (1) | 2.54 (1) | 0.6 (1) | 0.003 (2) | 3.01 (2) | 0.4% |
*Enot is the energy difference of absorption energy in experimental value and calculated value.
DFT Modeling
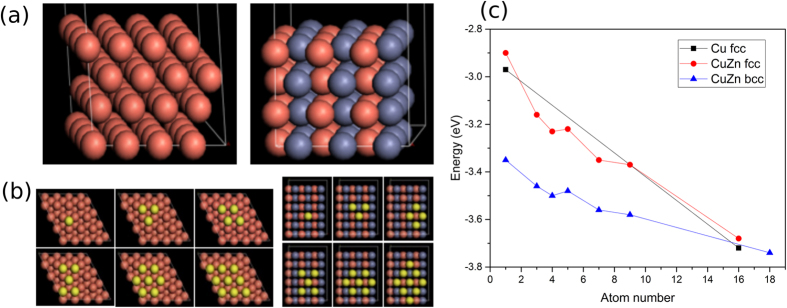
In the present work, fcc Cu (or fcc CuZn) and bcc CuZn can be clearly observed after the reduction procedure. It is intriguing to find that the sample containing a higher quantity of bcc CuZn shows better stability against particle sintering when high temperature treatment is applied. It has recently been reported that SiO2 supported Cu nanoparticles continue to grow, with an Ostwald ripening process, during DMO hydrogenation, causing deactivation of the Cu catalysts35. To further elucidate the Zn stabilization mechanism, DFT calculations were introduced. Fig. 4a illustrates the Cu fcc (111) 4 × 4 model (the similar surface fcc 50%Cu50%Zn (111), not shown) and 50%Cu50%Zn bcc (110) 3 × 3 model we adopted for the calculations. Additional Cu atoms (yellow) are placed on their surfaces progressively, as shown in Fig. 4b. As shown in Fig. 4c, the energy changes with increasing numbers of Cu atoms added to the surfaces containing exclusively topmost copper atoms (6 layered- fcc (111) Cu, fcc 50%Cu50%Zn (111) alloy and bcc 50%Cu50%Zn (110), Fig. S9). This simple calculation was used to model the reverse Ostwald ripening process; the higher stabilization energy for Cu atoms over this bcc CuZn surface reduces the Cu atoms migration, hence decreases the sintering extent. It is interesting to note, from Fig. 4c, that both fcc Cu and fcc CuZn show similar energy stabilizations upon addition of Cu atoms despite their difference in chemical composition. It can be clearly seen that the bcc CuZn surface, with the same Zn content as fcc CuZn, gives the highest energy stabilization to accommodate additional Cu atoms as compared to the other two surfaces. This clearly suggests that Cu atoms on bcc CuZn surface are more stable than the corresponding fcc Cu or fcc CuZn surfaces, which reduces the extent of sintering of Cu atoms due to the stronger geometric effects holding these atoms in position. Previous theoretical studies suggested that the phase change from fcc to bcc structure in CuZn when Zn ≥ 50% is attributed to the electronic energy stabilization of the system34. It is anticipated that this bcc arrangement can also exert stronger binding to Cu adatoms than that of fcc. This information also matches with known stronger binding properties of beta brass (bcc CuZn) in metallurgy, which can only be worked with hot, are harder, stronger and more suitable for casting. In contrast, alpha brass (fcc CuZn) is more malleable with weaker binding. It can be worked with cold and is used in pressing, forging or similar applications36. In this case, the dispersion of Cu atoms in nanosize bcc CuZn structure is shown to be more stable and can be used in Cu catalysts maintaining small CuZn clusters so as to keep their superior catalytic performance.
Figure 4.
(a) Cu fcc (111) 4 × 4 model and 50%Cu50%Zn bcc (110) 3 × 3 model; (b) additional 1, 3, 4, 5, 7 and 9 Cu atoms (yellow) on Cu fcc and 50%Cu50%Zn bcc surfaces; (c) plot of energy change of fcc Cu, fcc CuZn and bcc CuZn surfaces when adding increased number of Cu atoms onto those surfaces.
Concluding remarks
Nano alloys play a crucial role in many heterogeneous catalytic processes and their applications are expected to rise rapidly. This is due to the vast number of configurations and type of surface sites that multi-component materials can present. However, characterization of small alloy clusters of different compositions and temperature pre-treatments with respect to catalytic performance is technically challenging. In this case, using a range of surface sensitive, diffraction and microscopic techniques, we report for the first time that a clear structural role of Zn inclusion into the Cu nano-clusters by forming stable nano-beta phase CuZn. The structure can offer a good dispersion of active surface Cu atoms for catalysis and more importantly, stabilize them from aggregation. Regarding the hydrogenation of DMO the key challenge is to identify a new method to stabilize the Cu surface. A method to maintain higher Cu dispersion and thermal stability against sintering would provide an excellent direction to improve catalytic performance. We show that using highly dispersed bcc Cu-Zn catalysts may provide a good starting point for improving this reaction.
Methods
Synthesis of CuZnGa catalysts
CuZnGa catalysts were synthesized using a pH-controlled co-precipitation method. The metal precursors were hydrated metal nitrate salts: Cu(NO3)2·3 H2O (Aldrich), Zn(NO3)2·6 H2O (Aldrich), and Ga(NO3)3·9 H2O (Aldrich). For a typical preparation, the metal nitrates [3.77 g Cu(NO3)2·3 H2O; 5.53 g Zn(NO3)2·6 H2O; 0.75 g Ga(NO3)3·9 H2O] were dissolved completely in 100 mL deionized water. A Na2CO3 aqueous solution was prepared by dissolving 3.50 g of Na2CO3 in 100 mL of DI water. The solutions were added simultaneously into a plastic reactor containing 250 mL of preheated DI water. A delivery pump with two 50 mL syringes was used to inject the precursor metal nitrate solution at a constant rate of 0.42 mL/min in an automatic and reproducible manner. An HPLC pump was used to deliver the Na2CO3 solution at a rate of 0.35–0.70 mL/min. The mixture was stirred at 1000 rpm, with pH of the precipitating solution carefully maintained at 6.5. The precipitation process took place at around 80 °C. The pH of the liquid was measured using a temperature-dependent pH meter and was controlled at pH 6.5, with an error range of ± 0.1. Once the addition of the precursor metal nitrate solution was completed, the first aging was carried out under atmospheric conditions to let the pH value to become stable. After 30 min, the pH was measured again to ensure that the target pH had been reached before putting the lid onto the reactor. The resulting precipitate was continued to the second aging process in solution at 100 °C for 15 h. After aging, the precipitate was extracted by centrifugation at 5000 rpm. The centrifuged precipitate was washed with DI water five times at 5000 rpm to remove residual Na+ ions. The resulting wet solid was dried in air at 80 °C overnight and then calcined in static air, at a ramp of 5 °C/min up to desired temperature (330 °C, if not indicated) for 3 h to produce the final catalyst. For the Cu/SiO2 catalyst, the synthesis details could be found in the literature12.
DMO hydrogenation reaction
Catalytic reactions were conducted using continuous flow in a stainless steel tubular reactor equipped with a computer-controlled auto-sampling system. 100 mg of as-calcined catalyst with the particle diameter of 0.25 mm to 0.42 mm (40–60 meshes) was placed in the center of the reactor, and both sides of the catalyst bed were packed with quartz powders (40–60 meshes). Prior to the evaluation of the catalytic performance, the catalyst precursor was pre-reduced in a 5%H2-95%N2 atmosphere at 623 K for 4 h at a ramping rate of 2 °C/min. The catalyst bed was then cooled to the reaction temperature. Pure H2 was fed into the reactor and the system pressure was held at 3.0 MPa with the aid of a back-pressure regulator. For the hydrogenation of DMO, a 12 wt.% DMO-methanol solution was pumped into the catalyst bed with varying weight liquid hourly space velocity (WLHSVDMO) using a Series III digital HPLC pump (Scientific Systems, Inc.). Under a given condition, the outlet stream was sampled using an automatic Valco six-port sampling valve at 30 min intervals after the reaction reached a steady state. The products were analyzed using an online gas chromatograph (Shimadzu GC-2010) equipped with a DB-Wax capillary column (30 m × 0.32 mm × 0.25 μm) and a flame ionization detector with a relative standard deviation (RSD) of less than 2%. The RSD of analysis data for each sampling was less than 3%. The products were also collected and confirmed using a 7890GC–5975 MS system.
Additional Information
How to cite this article: Li, M. M.-J. et al. The remarkable activity and stability of a highly dispersive beta-brass Cu-Zn catalyst for the production of ethylene glycol. Sci. Rep. 6, 20527; doi: 10.1038/srep20527 (2016).
Supplementary Material
Acknowledgments
The financial support of this work from the EPSRC research council of UK is acknowledged. The authors wish to thank Shanghai Synchrotron Radiation Facility and Dr Xinlin Hong and his group of Wuhan University, China for the EXAFS measurement. MMJL acknowledges the Swire Scholarship of Oxford for her DPhil study. JWZ and YYZ are grateful to the financial support from the National Natural Science Foundation of China (No. 21473145).
Footnotes
Author Contributions M.M.M.L. carried out synthesis, X.P.S. and E.X.A.F.S. analysis; J.Z. performed catalyst testing and T.E.M.; J.Q. for D.F.T. calculations; W.C.H.K., S.S.S. and PP for nano-diffraction and H.R.T.E.M., F.L. and E.R. contributed for discussion in heterojunctions; J.Z. was supervised by Y.Y., M.M.M.L. and S.C.E.T. wrote the main manuscript text. All authors discussed and reviewed this paper.
References
- Liao F. et al. Electronic modulation of a copper/zinc oxide catalyst by a heterojunction for selective hydrogenation of carbon dioxide to methanol. Angew. Chem. Int. Ed. 51, 5832–5836 (2012). [DOI] [PubMed] [Google Scholar]
- Behrens M. et al. The active site of methanol synthesis over Cu/ZnO/Al2O3 industrial catalysts. Science 336, 893–897 (2012). [DOI] [PubMed] [Google Scholar]
- Kurtz M., Wilmer H., Genger T., Hinrichsen O. & Muhler M. Deactivation of supported copper catalysts for methanol synthesis. Catal. Lett. 86, 77–80 (2003). [Google Scholar]
- Li Y., Fu Q. & Flytzani-Stephanopoulos M. Low-temperature water-gas shift reaction over Cu- and Ni-loaded cerium oxide catalysts. Appl. Catal. B: Environ. 27, 179–191 (2000). [Google Scholar]
- Yahiro H., Murawaki K., Saiki K., Yamamoto T. & Yamaura H. Study on the supported Cu-based catalysts for the low-temperature water-gas shift reaction. Catal. Today 126, 436–440 (2007). [Google Scholar]
- Tanaka Y., Utaka T., Kikuchi R., Sasaki K. & Eguchi K. Water gas shift reaction over Cu- based mixed oxides for CO removal from the reformed fuels. Appl. Catal. A: Gen. 242, 287–295 (2003). [Google Scholar]
- Jeong H. et al. Hydrogen production by steam reforming of methanol in a micro-channel reactor coated with Cu/ZnO/ZrO2/Al2O3 catalyst. J. Power Sources 159, 1296–1299 (2006). [Google Scholar]
- Li Y. F., Dong X. F. & Lin W. M. Effects of ZrO2-promoter on catalytic performance of CuZnAlO catalysts for production of hydrogen by steam reforming of methanol. Int. J. Hydrogen Energy 29, 1617–1621 (2004). [Google Scholar]
- Ma L., Gong B., Tran T. & Wainwright M. S. Cr2O3 promoted skeletal Cu catalysts for the reactions of methanol steam reforming and water gas shift. Catal. Today 63, 499–505 (2000). [Google Scholar]
- Yue H., Zhao Y., Ma X. & Gong J. Ethylene glycol: properties, synthesis, and applications. Chem. Soc. Rev. 41, 4218–4244 (2012). [DOI] [PubMed] [Google Scholar]
- Chen L. F. et al. Cu/SiO2 catalysts prepared by the ammonia-evaporation method: Texture, structure, and catalytic performance in hydrogenation of dimethyl oxalate to ethylene glycol. J. Catal. 257, 172–180 (2008). [Google Scholar]
- He Z., Lin H., He P. & Yuan Y. Effect of boric oxide doping on the stability and activity of a Cu-SiO2 catalyst for vapor-phase hydrogenation of dimethyl oxalate to ethylene glycol. J. Catal. 277, 54–63 (2011). [Google Scholar]
- Yin A., Guo X., Dai W. L. & Fan K. The nature of active copper species in Cu-HMS catalyst for hydrogenation of dimethyl oxalate to ethylene glycol: New insights on the synergetic effect between Cu0 and Cu+. J. Phys. Chem. C 113, 11003–11013 (2009). [Google Scholar]
- Yin A., Guo X., Dai W. L., Li H. & Fan K. Highly active and selective copper-containing HMS catalyst in the hydrogenation of dimethyl oxalate to ethylene glycol. Appl. Catal. A: Gen. 349, 91–99 (2003). [Google Scholar]
- Zhu Y. Y. et al. The influence of copper particle dispersion in Cu/SiO2 catalysts on the hydrogenation synthesis of ethylene glycol. Catal. Lett. 135, 275–281 (2010). [Google Scholar]
- Yin A. Y., Guo X. Y., Fan K. N. & Dai W. L. Ion-exchange temperature effect on Cu/HMS catalysts for the hydrogenation of dimethyl oxalate to ethylene glycol. ChemCatChem 2, 206–213 (2010). [Google Scholar]
- Zhao S. et al. Chemoselective synthesis of ethanol via hydrogenation of dimethyl oxalate on Cu/SiO2: Enhanced stability with boron dopant. J. Catal. 297, 142–150 (2013). [Google Scholar]
- Olah G. A., Goeppert A. & Prakash G. K. S. Chemical recycling of carbon dioxide to methanol and dimethyl ether: from greenhouse gas to renewable, environmentally carbon neutral fuels and synthetic hydrocarbons. J. Org. Chem. 74, 487–498 (2009). [DOI] [PubMed] [Google Scholar]
- Fujitani T. & Nakamura J. The chemical modification seen in the Cu/ZnO methanol synthesis catalysts. Appl. Catal. A: Gen. 191, 111–129 (2000). [Google Scholar]
- Hambrock J., Schröter M. K., Birkner A., Wöll C. & Fischer. R. A. Nano-brass: Bimetallic copper/zinc colloids by a nonaqueous organometallic route using [Cu(OCH(Me)CH2NMe2)2] and Et2Zn as Precursors. Chem. Mater. 15, 4217–4222 (2003). [Google Scholar]
- Sanches S. G., Huertas F. J., de Avillez R. R. & Pais da Silva M. I. Influence of preparation methods and Zr and Y promoters on Cu/ZnO catalysts used for methanol steam reforming. Int. J. Hydrogen Energy 37, 6572–6579 (2012). [Google Scholar]
- Derrouiche S., Lauron-Pernot H. & Louis. C. Synthesis and treatment parameters for controlling metal particle size and composition in Cu/ZnO materials-first evidence of Cu3Zn alloy formation. Chem. Mater. 24, 2282–2291 (2012). [Google Scholar]
- Yu K. M. et al. Non-syngas direct steam reforming of methanol to hydrogen and carbon dioxide at low temperature. Nat. Commun. 3, 1230 (2012). [DOI] [PubMed] [Google Scholar]
- Tong W., Cheung K., West A., Yu K. M. & Tsang S. C. E. Direct methanol steam reforming to hydrogen over CuZnGaOx catalyst without CO post-treatment: Mechanistic considerations. Phys. Chem. Chem. Phys. 15, 7240–7248 (2013). [DOI] [PubMed] [Google Scholar]
- Tong W., West A., Cheung K., Yu K. M. & Tsang S. C. E. Dramatic effects of gallium promotion to methanol steam reforming Cu-ZnO catalyst for hydrogen production: Formation of 5Å copper clusters from CuZnGaOx. ACS Catal. 3, 1231–1244 (2013). [Google Scholar]
- Palo D. R., Dagle R. A. & Holladay J. D. Methanol steam reforming for hydrogen production. Chem. Rev. 107, 3992–4021 (2007). [DOI] [PubMed] [Google Scholar]
- Zander S. et al. The role of the oxide component in the development of copper composite catalysts for methanol synthesis. Angew. Chem. Int. Ed. 52, 6536–6540 (2013). [DOI] [PubMed] [Google Scholar]
- Spencer M. S. The role of zinc oxide in Cu/ZnO catalysts for methanol synthesis and the water-gas shift reaction. Top. Catal. 8, 259–266 (1999). [Google Scholar]
- Fujitani T. & Nakamura J. The effect of ZnO in methanol synthesis catalysts on Cu dispersion and the specific activity. Catal. Lett. 56, 119–124 (1998). [Google Scholar]
- Cheng. W. H. Reaction and XRD studies on Cu based methanol decomposition catalysts: Role of constituents and development of high-activity multicomponent catalysts. Appl. Catal. A: Gen. 130, 13–30 (1995). [Google Scholar]
- Kameoka S., Okada M. & Tsai A. P. Preparation of a novel copper catalyst in terms of the immiscible interaction between copper and chromium. Catal. Lett. 120, 252–256 (2008). [Google Scholar]
- Liao F. L. et al. A new class of tunable heterojunction by using two support materials for the synthesis of supported bimetallic catalysts. ChemCatChem 7, 230–235 (2015). [Google Scholar]
- Ahlers M. Martensite and equilibrium phases in Cu-Zn and Cu-Zn-Al alloys. Prog. Mater. Sci. 30, 135–186 (1986). [Google Scholar]
- Lee S. & Hoffmann R. Bcc and Fcc Transition Metals and Alloys: A Central Role for the Jahn-Teller Effect in Explaining Their Ideal and Distorted Structures. J. Am. Chem. Soc. 124, 4811–4823 (2002). [DOI] [PubMed] [Google Scholar]
- Zheng J. W. et al. CO-Mediated Deactivation Mechanism of SiO2-Supported Copper Catalysts during Dimethyl Oxalate Hydrogenation to Ethylene Glycol. J. Phys. Chem. C 119, 13758–13766 (2015). [Google Scholar]
- Müller S. & Zunger A. Structure of ordered and disordered α-brass. Phys. Rev. B 63, 094204 (2001). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.