Abstract
Telomere dysfunction participates in malignant transformation and tumorigenesis. Previous studies have explored the associations between telomere length (TL) and cancer susceptibility; however, the findings are inconclusive. The associations between genetic variants and TL have been verified by quite a few genome-wide association studies (GWAS). Yet, to date, there was no published study on the relationship between TL, related genetic variants and susceptibility to squamous cell carcinoma of the head and neck (SCCHN) in Chinese. Hence, we detected relative telomere length (RTL) by using quantitative PCR and genotyped seven selected single nucleotide polymorphisms by TaqMan allelic discrimination assay in 510 SCCHN cases and 913 controls in southeast Chinese. The results showed that RTL was significantly associated with SCCHN risk [(adjusted odds ratio (OR) = 1.19, 95% confidence interval (CI) = 1.08–1.32, P = 0.001]. Furthermore, among seven selected SNPs, only G allele of rs2736100 related to RTL in Caucasians was significantly associated with both the decreased RTL (P = 0.002) and the increased susceptibility to SCCHN in Chinese (additive model: adjusted OR = 1.17, 95%CI = 1.00–1.38, P = 0.049). These findings provide evidence that shortened TL is a risk factor for SCCHN, and genetic variants can contribute to both TL and the susceptibility to SCCHN in southeast Chinese population.
Telomeres consist of tandem (TTAGGG in humans)n of nucleotide repeats at the ends of chromosome1, which have an important effect on protecting the end of chromosomes from atypical recombination, breakage, degradation, loss of nucleotides, and end-to-end fusion2,3,4. Human telomeres are 10-15 kb long and are approximately shorten by ~30 to 200 bp in each cycle of mitotic division5. Therefore, TL has been supposed to define the cellular life span and number of cell divisions6. While the telomeres turn into a critical threshold, they may lead to double-strand break and cell senescence or apoptosis7. It has been reported that telomere dysfunction plays a complex role in oncogenesis, and both very short and very long telomeres in peripheral blood lymphocytes (PBL) promote carcinogenesis8,9. Specially, an increasing number of epidemiologic studies have identified the associations between TL in PBL and risk of several cancer types, such as lung10,11,12,13, ovarian14, bladder15,16, breast17, and colon or rectum cancer12,18,19. Thus, TL in PBL may serve as a common marker of risk in human cancers.
Previous studies have indicated that telomere length is affected by plenty of factors, such as cigarette smoking20, oxidative stress21 and chronic inflammation22. Additionally, the variation of telomere length was also attributed to heritable factors23. For example, GWASs have verified that genetic variants in multiple genome regions and genes (3q26-TERC, 2p16.2-ACYP2, 4q32.2-NAF1, 19p12-ZNF208, 10q24.33-OBFC1, and 20q13.3-RTEL1) were associated with telomere length in European populations24,25,26,27. Meanwhile, researchers have identified that the genetic variants related to telomeres were associated with the risk of multiple cancers11,24,25.
The incidence of squamous cell carcinoma of the head and neck (SCCHN) ranks about the fifth among cancers26. An estimated 348,300 new cases and 179,600 deaths from oral and pharynx cancer in 2008 worldwide27. A few studies have evaluated the associations among telomere length, SNPs related to TL and SCCHN risk; however, all of them were conducted in European population and the results have been inconsistent. For example, a recent study showed that TERT-CLPTM1L variants were associated with both of the mean relative telomere length (RTL) and SCCHN risk in Icelandic and European populations19, but another study reported that telomere length and functional polymorphisms of TERT were not associated with risk of SCCHN in American population28. Up to date, no study has investigated the correlation between SNPs, telomere length, and SCCHN risk in Chinese population.
Therefore, in the present study, we designed a case-control study including 510 SCCHN cases and 913 cancer free controls in southeast Chinese to comprehensively investigate the associations between telomere length, SNPs related to RTL, and SCCHN risk in Chinese.
Results
Primary information
The distributions of age, gender and smoking status (P = 0.440, 0.056 and 0.890, respectively) showed no significant difference between 510 SCCHN cases and 913 controls (Table 1). However, there were more drinkers in cases than that in controls (49.90% vs 37.13%, P < 0.001). Among 510 cases, 403(79.02%) were with the oral cavity cancer, and 107 (20.98%) with others (Table 1). RTL was measured on DNA from blood samples, and t test showed that older people (age ≥ 60) had shorter telomere length than younger people (age < 60) (P = 3.21 × 10−15). Moreover, the liner regression analysis identified a significant correlation between TL and age (r = −0.118, P = 0.001) (Table 2 and Supplementary Figure 1). Besides, we found that RTL in male were significantly shorted than that in female (P = 0.01) (Table 2 and Supplementary Figure 2A), these results are consistent with the previous studies29,30,31. But no difference was found in subgroups with different drinking or smoking status (P > 0.05) (Table 2, Supplementary Figures 1 and 2A).
Table 1. Characteristics of the subjects in this study.
| Variable | N (%) |
Pa | |
|---|---|---|---|
| Controls(n = 913) | Cases(n = 510) | ||
| Age at diagnosis (years) | |||
| Mean ± SD, y | 59.78 ± 9.37 | 61.28 ± 10.72 | 0.440 |
| <60 | 433(47.43) | 231(45.29) | |
| ≥60 | 480(52.57) | 279(54.71) | |
| Sex | |||
| Male | 682(74.70) | 357(70.00) | 0.056 |
| Female | 231(25.30) | 153(30.00) | |
| Smoking statusb | |||
| Never | 451(49.40) | 249(49.02) | 0.890 |
| Ever | 462(50.60) | 259(50.98) | |
| Drinking statusb | |||
| Never | 574(62.87) | 255(50.10) | 0.001 |
| Ever | 339(37.13) | 254(49.90) | |
| Tumor site | |||
| Oral | − | 403(79.02) | |
| Others | − | 107(20.98) | |
aTwo-sided Chi-square test.
bThe smoking and drinking status were unavailable for two and one controls, respectively.
Table 2. Distributions of relative telomere length (RTL) among controls.
| Selected variables | N | RTL, mean(95%CI) | Pa |
|---|---|---|---|
| Age at diagnosis (years) | |||
| <60 | 433 | 1.61(1.58–1.66) | 3.21 × 10−15 |
| ≥60 | 480 | 1.40(1.37–1.44) | |
| Sex | |||
| Male | 682 | 1.48(1.45–1.51) | 0.010 |
| Female | 231 | 1.56(1.51–1.62) | |
| Smoking status | |||
| Never | 451 | 1.51(1.48–1.55) | 0.569 |
| Ever | 462 | 1.50(1.46–1.53) | |
| Drinking status | |||
| Never | 574 | 1.52(1.48–1.55) | 0.232 |
| Ever | 339 | 1.49(1.44–1.53) | |
aTwo-sided t test.
The association between RTL and SCCHN risk
Then, we analyzed the associations between RTL and SCCHN risk and found that RTL in cases was significantly shorter than that in controls (OR = 1.19, 95%CI = 1.08–1.32, P = 0.001). In comparison with subjects with the first quarter (RTL ≥ 1.81), subjects with the third and fourth quarters had significantly increased risks of SCCHN with ORs (95%CIs) of 1.58(1.15–2.17) and 1.56(1.13–2.15), respectively (Table 3, Supplementary Figure 2B). When SCCHN cases were divided into two groups by tumor sites (oral cancer and other tumor sites), significant effect was only found for oral cancer (adjusted OR = 1.28, 95%CI = 1.14–1.43, P < 0.001), but not for other tumor sites (adjusted OR = 0.96, 95%CI = 0.80–1.15, P = 0.636), and the P value for heterogeneity test was 0.008 (Supplementary Table 1). Furthermore, the stratification analyses for the associations of RTL with SCCHN risk were conducted by age, gender, smoking and drinking status, and the results showed that the shorter RTL was associated with the increased risk of SCCHN in all subgroups except older (≥60). However, no significant heterogeneity was found among different strata (Supplementary Table 1).
Table 3. Association between relative telomere length (RTL) and SCCHN risk.
| Quartiles | RTL | Controls | Cases | Adjusted OR(95%CI)a | P |
|---|---|---|---|---|---|
| 75%~ | ≥1.81 | 228(24.97) | 98(19.22) | 1 | |
| 50% ~ 75% | 1.51–1.81 | 228(24.97) | 98(19.22) | 1.03(0.73–1.45) | 0.864 |
| 25% ~ 50% | 1.22–1.51 | 228(24.97) | 157(30.78) | 1.58(1.15–2.17) | 0.005 |
| ~25% | <1.22 | 229(25.09) | 157(30.78) | 1.56(1.13–2.15) | 0.006 |
| Trend | − | − | − | 1.19(1.08–1.32) | 0.001 |
aDerived from logistic regression with an adjustment for age at blood collection, sex, smoking and drinking status.
The effect of genetic variants on RTL
To evaluate the effect of genetic variants on RTL, we also analyzed the relevance between the seven SNPs reported in previous studies and RTL among 913 controls. Overall, we found that only the G allele of rs2736100 in TERT was significantly associated with the decreased RTL (P = 0.002), consistent with the findings in Caucasians32. But, we did not find any significant association for other six SNPs in controls (Table 4).
Table 4. Associations of the reported loci with relative telomere length (RTL) among controls.
| Loci | Chr. | Gene | Allelesa | Call rate (%) | MAFb | βc | Pc |
|---|---|---|---|---|---|---|---|
| rs10936599 | 3q26.2 | TERC | T/C | 98.1 | 0.465 | −0.022 | 0.370 |
| rs11125529 | 2p16.2 | ACYP2 | C/A | 98.6 | 0.214 | 0 | 0.991 |
| rs2736100 | 5p15.33 | TERT | T/G | 98.2 | 0.419 | −0.060 | 0.002 |
| rs4387287 | 10q24.33 | OBFC1 | A/C | 96.1 | 0.160 | −0.002 | 0.956 |
| rs755017 | 20q13.33 | RTEL1 | A/G | 93.8 | 0.443 | −0.002 | 0.924 |
| rs7675998 | 4q32.2 | NAF1 | A/G | 98.4 | 0.187 | −0.029 | 0.356 |
| rs8105767 | 19p12 | ZNF208 | A/G | 98.5 | 0.289 | −0.046 | 0.088 |
aEffect allele/alternative allele; effect allele is one associated with short telomeres, corresponding to the negative value of β estimates.
bMinor allele frequency among controls in this study.
cDerived from generalized linear models with an adjustment for age at blood collection and sex.
The associations between genetic variants and SCCHN risk
Next, we evaluated the effect of promising SNP (rs2736100) on the susceptibility to SCCHN. Interestingly, we identified that G allele of rs2736100 was significantly associated with an increased risk of SCCHN (dominant model: adjusted OR = 1.11, 95%CI = 1.02–1.20, P = 0.013; additive model: adjusted OR = 1.17, 95%CI = 1.00–1.38, P = 0.049) (Table 5). In addition, analyses in different subgroup stratified by sex, gender, drinking and smoking status and tumor sites demonstrated the risk effect of rs2736100-G allele on SCCHN remained significant in subjects with old age (≥60), female, no-smoking, no-drinking status and oral cancers (P < 0.05). However, heterogeneity test demonstrated no significant difference among different strata (Supplementary Table 2).
Table 5. Associations between rs2736100 and SCCHN risk.
| Gene | SNP | Controls | Casesa | Adjusted OR (95%CI)b | Pb |
|---|---|---|---|---|---|
| N (%) | N (%) | ||||
| TERT | rs2736100 | 897 | 495 | ||
| TT | 317(35.3) | 144(29.1) | 1 | ||
| TG | 419(46.7) | 255(51.5) | 1.37(1.06–1.78) | 0.015 | |
| GG | 161(18.0) | 96(19.4) | 1.32(0.95–1.83) | 0.099 | |
| TG + GG | 580(64.7) | 351(70.9) | 1.11(1.02–1.20) | 0.013 | |
| Additive model | 1.17(1.00–1.38) | 0.049 |
aGenotypes were available from 495 cases and 897 controls
bDerived from logistic regression with an adjustment for age, sex, smoking and drinking status.
Discussion
It is the first case-control study to investigate the relationship between telomere length, genetic variants related to RTL and SCCHN risk in Chinese. We discovered that SCCHN cases, especially oral cancer cases, had shorter telomere length than controls. Meanwhile, our study identified that rs2736100 (TERT SNP) related to RTL in European was associated with both telomere length and SCCHN risk in this southeast Chinese population. Taking account of these findings, we speculate that genetic variants in TERT may affect the telomere length and consequently induce the altered risk of SCCHN.
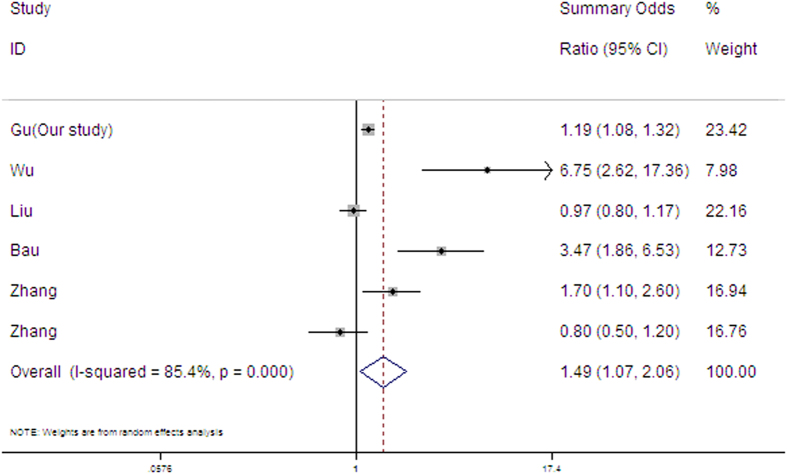
A few results have reported the relationship between RTL and SCCHN risk33,34,35,36, but the results are inconclusive because of different designs and sample size. We then performed a mini meta-analysis including our study and four available published studies on the association between RTL and SCCHN risk. The results of published studies were inconsistent with ORs of 6.75(2.62–17.36)37, 0.97(0.80–1.17)28, 3.47(1.86–6.53)36, 1.70(1.10–2.60)33 and 0.80(0.50–1.20)33. However, the pooled data showed that short RTL would significantly increase the risk of SCCHN (adjusted OR = 1.49, 95%CI = 1.07–2.06), supporting the findings in our study (Fig. 1 and Supplementary Table 3). Interestingly, our study also revealed a significant association between RTL and SCCHN in oral cancer, but not in other cancer sites, suggesting that telomere dysfunction might have different effects on the development of oral cancer and other HNSCC sites. However, these findings need the validation of other studies with larger samples and functional investigations.
Figure 1. Meta-analysis of associations between telomere length and risk of SCCHN.
Up to date, three GWAS studies have verified several SNPs related to RTL in European populations, including rs755017 at 20q13.33-RTEL1, rs10936599 at 3q26.2-TERC, rs4387287 at 10q24.33-OBFC1, rs7675998 at 4q32.2-NAF1, rs11125529 at 2p16.2-ACYP2, rs2736100 at 5p15.33-TERT, and rs8105767 at 19p12 -ZNF20838,39,40. In our study, we also discussed the association between above reported SNPs and RTL, and found that only rs2736100 in TERT was associated with RLT. TERT gene mapped to chromosome 5p15.33 encodes the telomerase enzyme and plays a key role in maintaining chromosomal stability and telomere DNA length41. Overexpression of TERT is likely to be involved in the tumorigenesis of multiple cancers including SCCHN34,35. The rs2736100 is located in intron 2 of TERT and the function of this SNP was still not clear. Although it is possible that this SNP has high linkage disequilibrium (LD) with other biologically plausible and cancer-causing mutations, a bioinformatics study indicated that rs2736100 may be localized in a regulatory region of the hTERT gene42. Thus, further functional investigations will be essential to deeply investigate the mechanism underlying these observations. Besides, we found no evidences of associations between other 6 SNPs and RTL, mostly because of genetic heterogeneity or small samples included in the published studies.
Recently, the relationship between genetic variants in TERT and the etiology of cancers has drawn increasing attention. Among those, rs2736100 is one of the most irrefutable identified SNP associated with cancer risk42,43,44. A recent meta-analysis including 16 published studies summarized the associations of rs2736100 with cancer risk and demonstrated that this SNP indeed affected the susceptibility to overall cancer45. However, no study has reported the role of rs2736100 in the development of SCCHN until now. In the present study, our results first identified that rs2736100 was associated with RTL and the altered risk of SCCHN in southeast Chinese population, providing more evidence that common genetic variants in TERT contribute to RTL and the carcinogenesis of SCCHN. Taking together, it may be plausible that rs2736100 can affect the hTERT expression, and subsequently generate a higher telomerase activity and an increased risk of SCCHN.
Additionally, it needs to be mentioned that RTL was significantly shorter both in the older (age ≥ 60) and in male, consistent with the findings in previous studies. For example, a lot of researches have confirmed that telomere length can act as a candidate biomarker of aging46,47,48,49. In additionally, several studies and a meta-analysis have identified that women have longer telomeres than men and such association would become stronger with increasing age43, which might result from a slower rate of telomere attrition in women50. Our study has several strengths. Up to now, it is the first study to systematically explore the associations among RTL, genetic variants related to TL and SCCHN risk in Chinese. Additionally, we recruited newly diagnosed cases and collected all blood samples prior to treatment, which may partly adjust the potential bias because of timing of blood draw for the case-control study. However, some insufficient points in our study also need to be improved. Firstly, we recruited SCCHN cases from hospitals and selected controls from communities, which might lead to potential selection bias. Second, a relative small sample size in this study might result in a lower statistical power. Third, we evaluated the RTL in peripheral blood leukocytes, but not in oral and neck tissue. However, some studies have investigated the consistence of TL between blood and tissues and showed significant linear correlation between leukocyte and some tissues51,52,53. Specially, Gadalla et al., observed a significant correlation between blood and buccal cells measurements of RTL (r = 0.74, p < 0.0001)52. Finally, it is a retrospective case-control study and the blood samples were collected after cancer diagnosis, this limitation could potentially cause a reverse causation bias. Therefore, the findings of associations between RTL, rs2736100 and SCCHN risk in this study still require further large prospective studies which would carefully address potential bias and functional studies to elucidate the mechanisms underlying such associations.
Materials and Methods
Study subjects
This study was approved by the institutional review board of Nanjing Medical University and informed consent has obtained from all participants. SCCHN cases were constantly recruited from hospitals including Jiangsu Stomatological Hospital, Nanjing, China and the First Affiliated Hospital of Nanjing Medical University, since May 2009 to October 2013. All of the SCCHN cases were positive histology, and those undergoing radiotherapy or chemotherapy or having any history of tumor were removed from this study. Age (±5 years) and sex matched controls were randomly selected from a cohort including 30,000~ participants in a screening program based on community for non-infective diseases in Jiangsu, China. After knowing-agreeing, the interview was performed with a structured questionnaire to acquire general information and environmental exposure history, such as age, sex, ethnicity, smoking and alcohol drinking. After interview, ~5 ml venous blood sample were collected. Finally, 510 incident SCCHN cases and 913 frequency-matched controls with enough qualified DNA were included in this study. Participants who drank more than twice per week for longer than 1 year were defined as drinkers and those who smoked at least per day for more than 1 year were considered smokers.
Measurement of relative telomere length
All methods and experimental protocols were approved by Nanjing Medical University, and carried out in accordance with the approved guidelines. GDNA was extracted from leukocyte of venous blood by phenol chloroform extraction. Each DNA sample was quantified and qualified by spectrophotometer and electrophoresis before genotyping. RTL was detected in genomic DNA using a modified RT-PCR protocol on an 7900HT(ABI PRISM) Sequence Detection System as we performed in previous study54. Telomere length was quantified by normalized the copy number of telomere repeats (T) to a single cope number (36B4, S). The reference pool contains equal amounts of genomic DNA from 5 healthy donors, to form a concentration related standard curve. The correlation coefficient (r2) of standard curve must be higher than 0.99. If the threshold cycle (Ct) value was out of the demarcation of the standard curves, the sample was repeated at a different concentration.
The q-PCR primers were shown in supplementary Table 4. Each well was poured into 50ng DNA pooled and 10μL SYBR® Green (Applied Biosystems) PCR Master Mix. All samples for both the telomere and 36B4 reactions were carried out in duplicate. Laboratory technicians were blinded to the case-control status and equivalent controls and cases were tested in each well of 384-plate. Cawthon’s formula 2−(ΔCt1−ΔCt2) = 2−ΔΔCt was used to calculated RTL.
SNP selection and genotyping
Up to date, three GWAS studies have discovered several genetic variants related to TL in European populations, such as rs398652, rs1317082 and so on38,39,40. However, no study has investigated the associations between these SNPs and TL or SCCHN risk in Chinese. According to above publications, we selected the reported SNPs with P ≤ 5 × 10−8 (genome-wide association significance level) and MAF ≥ 5% in Chinese population. After linkage disequilibrium (LD) analysis at r2 of 0.3, seven genetic variants related to telomere length were selected for genotyping, including rs755017 at 20q13.33-RTEL1, rs10936599 at 3q26.2-TERC, rs2736100 at 5p15.33-TERT, rs4387287 at 10q24.33-OBFC1, rs7675998 at 4q32.2-NAF1, rs11125529 at 2p16.2-ACYP2, and rs8105767 at 19p12-ZNF208. MAF and LD information were calculated by the 1000 Genomes Project (CHB&JPT subjects Phase I interim release).
The above SNPs were genotyped by means of the TaqMan allelic discrimination assay on the 7900HT (ABI PRISM Sequence Detection System). Supplementary Table 4 has shown the sequence of probes and primers. When genotyped, the lab technicians were blind with the subjects’ case or control status. Negative controls were added in every 384-plate and 10% of the samples were randomly selected to generate a 100% accordance. The yield rates of genotyping for the selected SNPs all reached 93% (Table 4).
Statistical analysis
Data of genotyping and demographic characteristics of subjects between the cases and the controls were calculated by chi-square test. T-test was used to evaluate the distributions of relative telomere length in different status among controls. The goodness-of-fit chi-square test was used to test the HWE of all SNPs among the controls. Linear regression model was used to explore the relationship of single variant and telomere length among controls. Logistic regression model was used to evaluate the association between RTL or genetic variants and cancer risk. The heterogeneity of ORs and 95% CIs derived from relevant subgroups were tested by the meta-analysis. All statistical analyses were calculated with Stata 12.0.
Additionally, a mini-meta analysis was conducted by Stata version 12.0 using the “metan” code. Six case-control studies (peripheral blood leukocytes as DNA source) were included in this meta-analysis. ORs and 95%CI s of risk of RTL were collected to calculate the summary OR.
Additional Information
How to cite this article: Gu, Y. et al. Telomere length, genetic variants and risk of squamous cell carcinoma of the head and neck in Southeast Chinese. Sci. Rep. 6, 20675; doi: 10.1038/srep20675 (2016).
Supplementary Material
Acknowledgments
This work was supported in part by National Natural Science Foundation of China (81473048 and 81302361), Innovative Practice Training Project for Jiangsu Higher Education Institutions Undergraduate (201310312003Z) and Priority Academic Program Development of Jiangsu Higher Education Institutions (Public Health and Preventive Medicine).
Footnotes
Author Contributions Y. G. and C.Y. designed the study and directed its implementation, including quality assurance and control and written this article. L. M., L.W., C.X. and W.X. helped constantly collect blood samples from hospitals including Jiangsu Stomatological Hospital, Nanjing, China and the First Affiliated Hospital of Nanjing Medical University. J.D., H.Y., J.D., G.J. and Z.H. helped conduct the literature review and prepare the methods. H.M. and H.S. helped supervise the field activities and designed the study’s analytic strategy and amend this article.
References
- Mao L. et al. Telomerase activity in head and neck squamous cell carcinoma and adjacent tissues. Cancer Res 56, 5600–5604 (1996). [PubMed] [Google Scholar]
- Li Z. et al. Shorter telomere length in peripheral blood leukocytes is associated with childhood autism. Sci Rep 4, 7073 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodelon C., Savage S. A. & Gadalla S. M. Telomeres in molecular epidemiology studies. Prog Mol Biol Transl Sci 125, 113–131 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Domenico E. G., Romano E., Del Porto P. & Ascenzioni F. Multifunctional role of ATM/Tel1 kinase in genome stability: from the DNA damage response to telomere maintenance. Biomed Res Int 2014, 787404 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley C. B. Human ageing and telomeres. Ciba Found Symp 211, 129–139 (1997). [DOI] [PubMed] [Google Scholar]
- Blackburn E. H. Switching and signaling at the telomere. Cell 106, 661–673 (2001). [DOI] [PubMed] [Google Scholar]
- Mathon N. F. & Lloyd A. C. Cell senescence and cancer. Nat Rev Cancer 1, 203–213 (2001). [DOI] [PubMed] [Google Scholar]
- Wu X. et al. Telomere dysfunction: a potential cancer predisposition factor. J Natl Cancer Inst 95, 1211–1218 (2003). [DOI] [PubMed] [Google Scholar]
- Gramatges M. M., Telli M. L., Balise R. & Ford J. M. Longer relative telomere length in blood from women with sporadic and familial breast cancer compared with healthy controls. Cancer Epidemiol Biomarkers Prev 19, 605–613 (2010). [DOI] [PubMed] [Google Scholar]
- Walcott F. et al. Telomere length and risk of glioma. Cancer Epidemiol 37, 935–938 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E. S. et al. Telomere length and recurrence risk after curative resection in patients with early-stage non-small-cell lung cancer: a prospective cohort study. J Thorac Oncol 10, 302–308 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosgood H. D. 3rd, Cawthon R., He X., Chanock S. & Lan Q. Genetic variation in telomere maintenance genes, telomere length, and lung cancer susceptibility. Lung cancer 66, 157–161 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan Q. et al. Longer telomere length in peripheral white blood cells is associated with risk of lung cancer and the rs2736100 (CLPTM1L-TERT) polymorphism in a prospective cohort study among women in China. PloS one 8, e59230 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirabello L. et al. Leukocyte telomere length in a population-based case-control study of ovarian cancer: a pilot study. Cancer causes control 21, 77–82 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broberg K., Bjork J., Paulsson K., Hoglund M. & Albin M. Constitutional short telomeres are strong genetic susceptibility markers for bladder cancer. Carcinogenesis 26, 1263–1271 (2005). [DOI] [PubMed] [Google Scholar]
- McGrath M., Wong J. Y., Michaud D., Hunter D. J. & De Vivo I. Telomere length, cigarette smoking, and bladder cancer risk in men and women. Cancer Epidemiol Biomarkers Prev 16, 815–819 (2007). [DOI] [PubMed] [Google Scholar]
- Shen J. et al. Short telomere length and breast cancer risk: a study in sister sets. Cancer Res 67, 5538–5544 (2007). [DOI] [PubMed] [Google Scholar]
- Pooley K. A. et al. Telomere length in prospective and retrospective cancer case-control studies. Cancer Res 70, 3170–3176 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafnar T. et al. Sequence variants at the TERT-CLPTM1L locus associate with many cancer types. Nat Genet 41, 221–227 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes A. M. et al. Obesity, cigarette smoking, and telomere length in women. Lancet 366, 662–664 (2005). [DOI] [PubMed] [Google Scholar]
- von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci 27, 339–344 (2002). [DOI] [PubMed] [Google Scholar]
- Schonland S. O. et al. Premature telomeric loss in rheumatoid arthritis is genetically determined and involves both myeloid and lymphoid cell lineages. Proc Natl Acad Sci USA 100, 13471–13476 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slagboom P. E., Droog S. & Boomsma D. I. Genetic determination of telomere size in humans: a twin study of three age groups. Am J Hum Genet 55, 876–882 (1994). [PMC free article] [PubMed] [Google Scholar]
- Jones A. M. et al. TERC polymorphisms are associated both with susceptibility to colorectal cancer and with longer telomeres. Gut 61, 248–254 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo A. et al. Shorter leukocyte telomere length is independently associated with poor survival in patients with bladder cancer. Cancer Epidemiol Biomarkers Prev 23, 2439–2446 (2014). [DOI] [PubMed] [Google Scholar]
- Marcu L. G. & Yeoh E. A review of risk factors and genetic alterations in head and neck carcinogenesis and implications for current and future approaches to treatment. J Cancer Res Clin Oncol 135, 1303–1314 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A. et al. Global cancer statistics. CA Cancer J Clin 61, 69–90 (2011). [DOI] [PubMed] [Google Scholar]
- Liu Z. et al. Telomere length and TERT functional polymorphisms are not associated with risk of squamous cell carcinoma of the head and neck. Cancer Epidemiol Biomarkers Prev 20, 2642–2645 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschenberger J. et al. Association of relative telomere length with progression of chronic kidney disease in two cohorts: effect modification by smoking and diabetes. Sci Rep 5, 11887 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H. et al. Long telomeres in peripheral blood leukocytes are associated with an increased risk of soft tissue sarcoma. Cancer 119, 1885–1891 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschenberger J. et al. Association of relative telomere length with cardiovascular disease in a large chronic kidney disease cohort: The GCKD study. Atherosclerosis 242, 529–534, (2015). [DOI] [PubMed] [Google Scholar]
- Nie W., Zang Y., Chen J. & Xiu Q. TERT rs2736100 polymorphism contributes to lung cancer risk: a meta-analysis including 49,869 cases and 73,464 controls. Tumour Biol 35, 5569–5574 (2014). [DOI] [PubMed] [Google Scholar]
- Zhang Y. et al. Telomere length in peripheral blood lymphocytes contributes to the development of HPV-associated oropharyngeal carcinoma. Cancer Res 73, 5996–6003 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unryn B. M., Hao D., Gluck S. & Riabowol K. T. Acceleration of telomere loss by chemotherapy is greater in older patients with locally advanced head and neck cancer. Clin Cancer Res 12, 6345–6350 (2006). [DOI] [PubMed] [Google Scholar]
- Patel M. M. et al. Clinical usefulness of telomerase activation and telomere length in head and neck cancer. Head Neck 24, 1060–1067 (2002). [DOI] [PubMed] [Google Scholar]
- Bau D. T. et al. Short telomere lengths in peripheral blood leukocytes are associated with an increased risk of oral premalignant lesion and oral squamous cell carcinoma. Cancer 119, 4277–4283 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentzensen I. M., Mirabello L., Pfeiffer R. M. & Savage S. A. The association of telomere length and cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev 20, 1238–1250 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codd V. et al. Identification of seven loci affecting mean telomere length and their association with disease. Nat Genet 45, 422–427, 427e421-422 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D. et al. Genome-wide association identifies OBFC1 as a locus involved in human leukocyte telomere biology. Proc Natl Acad Sci USA 107, 9293–9298 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojesen S. E. et al. Multiple independent variants at the TERT locus are associated with telomere length and risks of breast and ovarian cancer. Nat Genet 45, 371–384 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong Y. S., Wen J. & Bacchetti S. The human telomerase catalytic subunit hTERT: organization of the gene and characterization of the promoter. Hum Mol Genet 8, 137–142 (1999). [DOI] [PubMed] [Google Scholar]
- Machiela M. J. et al. Genetic variants associated with longer telomere length are associated with increased lung cancer risk among never-smoking women in Asia: a report from the female lung cancer consortium in Asia. Int J Cancer 137, 311–319 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M. et al. Gender and telomere length: systematic review and meta-analysis. Exp Gerontol 51, 15–27 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z. et al. Genetic polymorphisms of TERT and CLPTM1L, cooking oil fume exposure, and risk of lung cancer: a case-control study in a Chinese non-smoking female population. Med Oncol 31, 114 (2014). [DOI] [PubMed] [Google Scholar]
- Li C., Yin Z., Wu W., Li X. & Zhou B. Genetic variants in TERT-CLPTM1L genetic region associated with several types of cancer: a meta-analysis. Gene 526, 390–399 (2013). [DOI] [PubMed] [Google Scholar]
- Simm A. et al. Potential biomarkers of ageing. Biol Chem 389, 257–265 (2008). [DOI] [PubMed] [Google Scholar]
- Bekaert S., De Meyer T. & Van Oostveldt P. Telomere attrition as ageing biomarker. Anticancer Res 25, 3011–3021 (2005). [PubMed] [Google Scholar]
- von Zglinicki T. & Martin-Ruiz C. M. Telomeres as biomarkers for ageing and age-related diseases. Curr Mol Med 5, 197–203 (2005). [DOI] [PubMed] [Google Scholar]
- Butler R. N. et al. Biomarkers of aging: from primitive organisms to humans. J Gerontol A Biol Sci Med Sci 59, B560–567 (2004). [DOI] [PubMed] [Google Scholar]
- Okuda K. et al. Telomere length in the newborn. Pediatr Res 52, 377–381 (2002). [DOI] [PubMed] [Google Scholar]
- Gramatges M. M. & Bertuch A. A. Measuring relative telomere length: is tissue an issue? Aging 2, 756–757 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dlouha D., Maluskova J., Kralova Lesna I., Lanska V. & Hubacek J. A. Comparison of the relative telomere length measured in leukocytes and eleven different human tissues. Physiological research / Academia Scientiarum Bohemoslovaca 63 Suppl 3, S343–350 (2014). [DOI] [PubMed] [Google Scholar]
- Gadalla S. M., Cawthon R., Giri N., Alter B. P. & Savage S. A. Telomere length in blood, buccal cells, and fibroblasts from patients with inherited bone marrow failure syndromes. Aging 2, 867–874 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon R. M. Telomere measurement by quantitative PCR. Nucleic Acids Res 30, e47 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.