Abstract
The biosynthesis of long-chain polyunsaturated fatty acids (LC-PUFA) provides an intriguing example on how multi-enzymatic cascades evolve. Essential LC-PUFA, such as arachidonic, eicosapentaenoic, and docosahexaenoic acids (DHA), can be acquired from the diet but are also endogenously retailored from C18 precursors through consecutive elongations and desaturations catalyzed, respectively, by fatty acyl elongase and desaturase enzymes. The molecular wiring of this enzymatic pathway defines the ability of a species to biosynthesize LC-PUFA. Exactly when and how in animal evolution a functional LC-PUFA pathway emerged is still elusive. Here we examine key components of the LC-PUFA cascade, the Elovl2/Elovl5 elongases, from amphioxus, an invertebrate chordate, the sea lamprey, a representative of agnathans, and the elephant shark, a basal jawed vertebrate. We show that Elovl2 and Elovl5 emerged from genome duplications in vertebrate ancestry. The single Elovl2/5 from amphioxus efficiently elongates C18 and C20 and, to a marked lesser extent, C22 LC-PUFA. Lamprey is incapable of elongating C22 substrates. The elephant shark Elovl2 showed that the ability to efficiently elongate C22 PUFA and thus to synthesize DHA through the Sprecher pathway, emerged in the jawed vertebrate ancestor. Our findings illustrate how non-integrated “metabolic islands” evolve into fully wired pathways upon duplication and neofunctionalization.
The origin of complexity in living systems is a central question in evolution1,2. Pairwise interactions between molecules (e.g. ligand and receptors; enzymes and their substrates) and the impact of gene duplication on protein function have provided crucial insight into the understanding of physiological diversity3. Additionally, the association of different enzymes into single pathways and how these are affected by evolutionary processes is fundamental to reconstruct the history of metabolic gene networks4,5. The biosynthesis of long-chain (C ≥ 20) polyunsaturated fatty acids (LC-PUFA) in animals represents a fascinating example, where phylogenetically unrelated enzymes participate in a metabolic cascade to synthesize vital molecules such as arachidonic acid (ARA, 20:4n-6), eicosapentaenoic acid (EPA, 20:5n-3) and docosahexaenoic acid (DHA, 22:6n-3)6,7 (Fig. 1A). In addition to dietary input, LC-PUFA are synthesized endogenously from essential C18 polyunsaturated fatty acid (PUFA) precursors including linoleic acid (LOA, 18:2n-6) and α-linolenic acid (ALA, 18:3n-3) in mammals and teleosts, through a series of consecutive desaturation and elongation reactions8 (Fig. 1A). How and when this gene pathway has emerged and functionally diversified over time is still obscure. Typically in mammals, the metabolic cascade converting C18 PUFA into bioactive C20-22 LC-PUFA, such as ARA, EPA and DHA requires the concerted action of distinct Δ5 and Δ6 fatty acyl desaturase (FADS) enzymes, as well as that of elongation of long-chain fatty acids (ELOVL) proteins including ELOVL2 and ELOVL5 at specific steps in the pathway8 (Fig. 1A). Recently, the ability for direct ∆4 desaturation of 22:5n-3 to 22:6n-3 has been also shown in human FADS29. The mechanisms of LC-PUFA biosynthesis in teleost fish, particularly farmed species, have been extensively investigated in the past decades, and many aspects of these metabolic pathways are better understood in fish compared to mammals. For example, the specific ability to convert C18 PUFA into LC-PUFA is directly dependent on the exact Fads and Elovl gene repertoire as well as their substrate specificities10,11,12,13. It has been shown that the inability of most teleosts to utilize Δ5 desaturase substrates is linked to the specific loss of Fads111,12. Surprisingly, the small number of teleost species able to perform Δ5 conversions have a fads2 gene with Δ5 activity10,14,15,16.
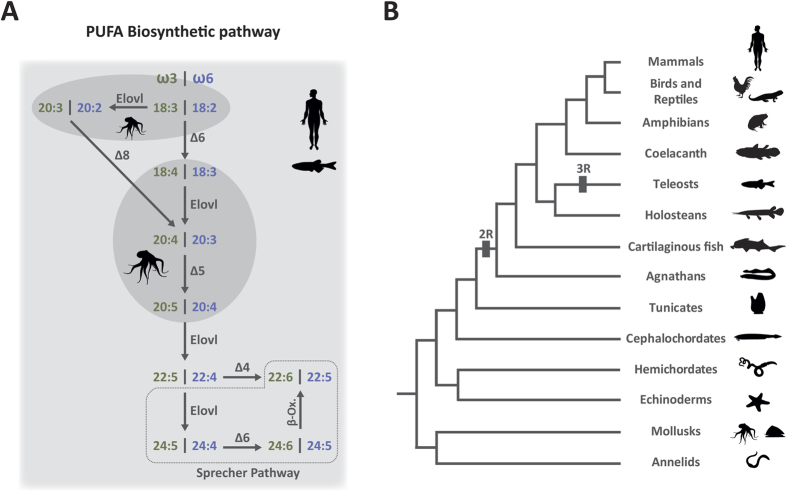
Figure 1. Biosynthetic pathway of LC-PUFA as determined in mammals and teleosts (all reactions shown), and octopus (confined to reactions in the two ellipses).
(A). Elongation (Elovl), desaturation (Δ4, Δ5 and Δ6) and β-oxidation (β-oxi) reactions are indicated. The omega-3 (ω3) and omega-6 (ω6) PUFA synthesis cascades are shown in parallel. Each composite number (e.g. 18:3) refers to a specific PUFA, with the first number indicating the number of carbon atoms and the second referring to the ethylenic bonds (details on each PUFA in supplementary Table 1). Phylogenetic tree of the major Bilaterian animal groups considered in this study (B). Genome duplications are indicated (2R and 3R).
Genes encoding ELOVL proteins have received comparatively less attention, although their action is critical for a complete and functional LC-PUFA pathway17 (Fig. 1A). Generally, mammalian ELOVL5 is involved in the elongation of C18 and C20 PUFA, whilst ELOVL2 is predominantly active towards C20 and C22 PUFA18,19 (Fig. 1A). In contrast, the bird ELOVL5 is, to some extent, able to convert docosapentaenoic acid (DPA, 22:5n-3) to C24 LC-PUFA, though with considerable less efficiency than ELOVL2, which displays a similar substrate preference to mammals20. The elovl gene repertoire in teleosts is also distinctive from that of tetrapods. Most species studied so far have a single elovl5 gene with the ability to elongate C18 and C20 PUFA substrates, with marginal activity towards C2221,22,23,24,25, with Atlantic salmon appearing as the sole fish species where two copies of elovl5 have been characterized11,26. In contrast, an elovl2 orthologue has been identified only in Atlantic salmon10 (Salmo salar), zebrafish27 (Danio rerio) and rainbow trout28 (Oncorhynchus mykiss), and with ray-finned fishes (including most marine species) appearing to lack elovl2 in their genomes11. Similar to their tetrapod counterparts, teleost elovl2 demonstrated the capacity to elongate DPA and thus contribute to DHA production through the so-called “Sprecher pathway”29 (Fig. 1A). From the above, Elovl5 appears to be unique in its capability to elongate C18 PUFA substrates and, similarly, Elovl2 towards C22 PUFA, while there is an overlap between both enzymes in their capacity to metabolize C20 substrates. However, when exactly Elovl2 and Elovl5 genes diverged and their respective functional fatty acid preferences emerged in metazoan evolution is presently unknown. Interestingly, various mollusk species, including the common octopus (Octopus vulgaris), the noble scallop (Chlamys nobilis) and cuttlefish (Sepia officinalis), have been shown to possess an Elovl gene, phylogenetically basal to the vertebrate Elovl2 and Elovl530,31,32. Curiously, the mollusk Elovl enzyme is only capable of metabolizing C18 PUFA and to lesser extent C2030,31,32 (Fig. 1A). The desaturase abilities in mollusks are also markedly different when compared to mammals and teleosts, since only Δ5 desaturases have been described so far33,34,35 (Fig. 1A). These results suggest a complex scenario regarding the evolutionary emergence of a complete LC-PUFA biosynthetic pathway.
Despite the significant effort made to clarify the LC-PUFA biosynthetic capabilities in some vertebrate lineages, the presently known complement of Fads and Elovl genes and their biosynthetic abilities in key evolutionary lineages hampers the precise evolutionary profiling of this pathway. Here we investigate the Elovl2/Elovl5 gene repertoire at a key evolutionary moment: the invertebrate/vertebrate transition (Fig. 1B). By examining three species, including the European amphioxus (Branchiostoma lanceolatum, cephalochordate), the sea lamprey (Petromyzon marinus, agnathan) and the elephant shark (Callorhinchus milii, basal gnathostome), we provide an insightful snapshot into the evolution of critical enzymes dictating the LC-PUFA biosynthetic pathways in chordates.
Results
Elovl2 and Elovl5 originated in the ancestor of vertebrates
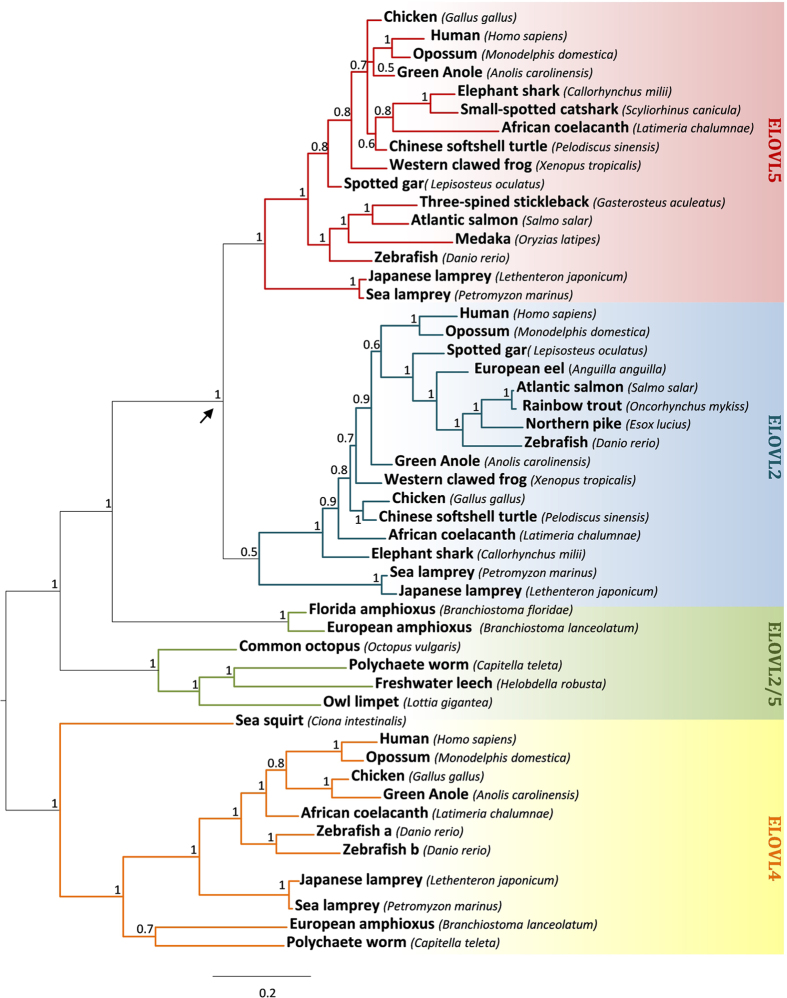
We analyzed the repertoire of Elovl2 and Elovl5 like genes in a total of 19 species representing all major vertebrate lineages (Sarcopterigii, Actinopterigii, Chondrichthyans and Agnathans) (Fig. 1B). In addition, we also investigated invertebrate species, representing four phyla from invertebrate protostomes and deuterostomes (Fig. 1B). The retrieved sequence dataset was used for phylogenetic reconstruction employing two methods, Bayesian analysis (BA) and Maximum likelihood (ML) (supplementary Fig. 1 for the ML phylogeny). We found two well-supported monophyletic groups, one containing all Elovl4 sequences, and another containing invertebrate single copy Elovl2/5 from cephalochordates and various protostome species and all vertebrate Elovl2 and Elovl5 sequences (Fig. 2). Within the latter group, gnathostome sequences formed two sister clades Elovl2 and Elovl5, respectively. Each of the lamprey sequences branched together with gnathostome Elovl2 and Elovl5, although with low statistical support in the case of Elovl2 (Fig. 2). Therefore, the overall tree topology is indicative of the timing of Elovl2/5 gene expansion, coincident with the evolution of the vertebrate lineage approximately 500 million years ago. No tunicate sequences were used in our analyses since no orthologues of Elovl2/5 were found in genomes from sea squirts (Ciona intestinatis and C. savignyi) and the star ascidian (Botryllus schlosseri), despite the former having an Elovl4-like gene with the ability to elongate C18 and C20 PUFA36. Additionally, while some studies in teleosts have suggested that Elovl4 can partly contribute to the LC-PUFA biosynthesis37 these enzymes are generally related to the biosynthesis of very long-chain (C > 24) fatty acids38, and thus were not considered in this study.
Figure 2. Bayesian molecular phylogenetic analysis of the Elovl2, Elovl5 and Elovl4 genes.
Numbers at nodes indicate posterior probabilities. Arrow denotes duplication timing of Elovl2/5. Rooted on the Elovl4 clade. Accession numbers for all sequences are provided in the supplementary Table 2.
Genome duplications generated Elovl2 and Elovl5 paralogues in vertebrates
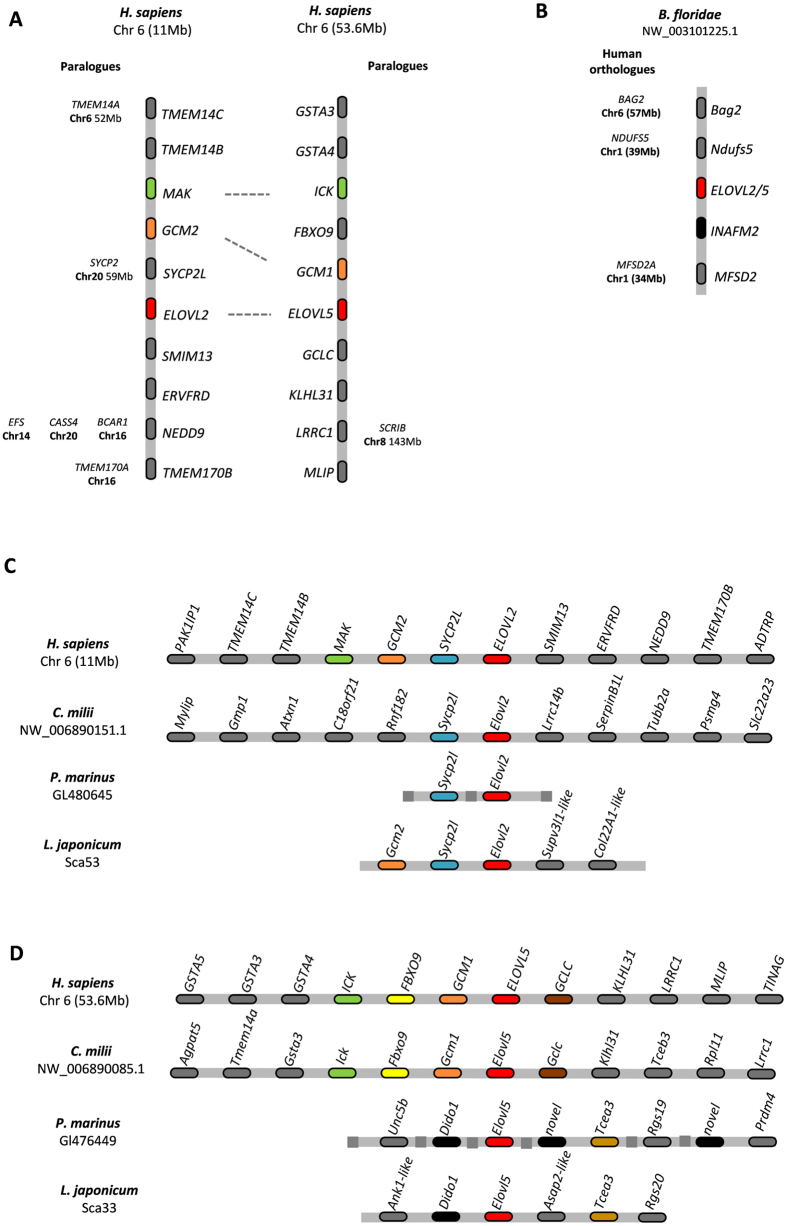
The phylogenetic analysis supported the timing of Elovl2 and Elovl5 origin to the ancestor of vertebrates. Thus, we hypothesize that genome duplications were involved in the diversification of Elovl2/5 genes. Human Elovl2 and Elovl5 localize to the same human chromosome (Hsa6) though at separate regions (Fig. 3A). These two genomic sections were linked to a four-fold paralogy originating from genome duplications38 (linkage group 4) involving a quartet of regions: paralogy A at Hsa20.5, paralogy B at Hsa2.1/6.6/6.8, paralogy C at Hsa6.2/8.2/8.4, and paralogy D at Hsa1.2 (supplementary Fig. 2). In effect, neighboring Elovl2 and Elovl5 gene families with a duplication history coincident with genome duplications, have, in most cases, a gene-by-gene paralogy in the expected regions (Fig. 3A). For example, GCM1 and ICK (neighbors of Elovl5) have a vertebrate specific paralog mapping to the Elovl2 locus, GCM2 and MAK, respectively. Also Sycp2l, localizing close to Elovl2, has a paralogue at Hsa20.5 as expected (paralogy group A) (Fig. 3A). Additionally, we also examined the Elovl2/5 genomic locus of the pre-duplicated genome of the Florida amphioxus (B. floridae) (Fig. 3B). Coherently, we found that the neighboring genes Bag2, Ndufs5 and Mfsd2 have their human orthologues localizing to Hsa6 (close to Elovl5) and Hsa1, part of linkage group C and D, respectively (Fig. 3B, supplementary Fig. 2). Thus, we can conclude that Elovl2 and Elovl5 have appeared as part of whole-genome duplications.
Figure 3. Comparative genomic maps of Elovl gene loci.
(A) Paralogy analysis of ELOVL2 and ELOVL5 human orthologues; (B) the amphioxus elovl2/5 gene locus; (C) synteny analysis of the Elovl2 genes in lampreys, human and elephant shark; (D) synteny analysis of the Elovl2 genes in lampreys, human and elephant shark.
Are Agnathan Elovl genes exact Elovl2 and Elovl5 orthologues?
To further clarify the orthology of the identified Elovl2/5 sequences, we examined the syntenic relationships of Elovl2/5 genes in key species. Gnathostome Elovl2 and Elovl5 gene loci were conserved, though with different degrees (Fig. 3C, D; supplementary Fig. 3). For example, Sycp2l flanks Elovl2 in humans and the elephant shark, indicative of a common origin (Fig. 3C). A strongly conserved syntenic pattern was also observed in the Elovl5 locus, with Gcm1 and Gclc outflanking this gene in all gnathostome species except the former in zebrafish (Fig. 3D; supplementary Fig. 3). The exact orthology of agnathan gene sequences poses some challenges, namely when evolutionary processes such as whole genome duplications and gene loss are involved40,41,42. Given that the putative lamprey Elovl2 was statistically weakly supported in the phylogenetic tree, we examined also the flanking gene families of the putative Elovl genes in both the sea lamprey and the Japanese lamprey (Lethenteron japonicum). In both species, the putative Elovl2 locus includes orthologues of Sycp2l and Gcm2 gene, denoting a strong conservation with the human locus (Fig. 3C). In contrast, the “Elovl5” locus of lampreys displays no synteny conservation with other vertebrates (Fig. 3D). Although we cannot exclude that this represents a different paralogue retained uniquely in lampreys, we suggest that this is a bona fide Elovl5 gene, in a highly rearranged locus.
Functional characterization of amphioxus, sea lamprey and elephant shark ELOVL enzymes
We next analyzed the substrate specificities of ELOVL enzymes from three chordate species, namely amphioxus, sea lamprey and elephant shark (Table 1). Transgenic yeast expressing the amphioxus Elovl2/5 ORF were able to elongate C18, C20 and, to a lesser extent, C22 PUFA substrates (Table 1). The sea lamprey Elovl5 showed relatively high activity towards C18 PUFA (18:4n-3 and 18:3n-6), and lower activity toward the C20 PUFA (20:5n-3 and 20:4n-6). Compared to the sea lamprey Elovl5, the Elovl2 was very efficient in the elongation of C20 to C22, with C18 PUFA being elongated to a lesser extent (Table 1). Interestingly, neither of the sea lamprey Elovl enzymes displayed the capacity to elongate C22 to C24 (Table 1). In order to investigate when the Elovl2 acquired the ability to elongate C22 PUFA, we tested the function of the elephant shark Elovl2. Consistent with the activities exhibited by fish and mammalian orthologues16,43 the elephant shark Elovl2 had marginal activity towards C18 PUFA and high elongation capability on C20 and C22 PUFA that were converted into the corresponding C22 and C24 elongation products, respectively (Table 1). Moreover, the functional characterization of the elephant shark Elovl5 confirmed its ability to elongate preferably C18 and C20 to C20 and C22 PUFA (Table 1), respectively, as typically observed in other vertebrate lineages16,17.
Table 1. Functional characterization of the amphioxus Elovl2/5, the sea lamprey Elovl5, Elovl2 and mutated Elovl2, and the elephant shark Elovl5 and Elovl2 in Saccharomyces cerevisiae.
| FA substrate | FA product | Amphioxus Elovl2/5 | Sea lamprey Elovl5 | Sea lamprey Elovl2 | Sea lampreymutated Elovl2 | Elephant shark Elovl5 | Elephant shark Elovl2 |
|---|---|---|---|---|---|---|---|
| 18:4n-3 | 20:4n-3 | 21 | 56 | 9 | 0 | 69 | 7 |
| 18:3n-6 | 20:3n-6 | 55 | 40 | 0 | 0 | 74 | 3 |
| 20:5n-3 | 22:5n-3 | 87 | 12 | 88 | 57 | 65 | 85 |
| 20:4n-6 | 22:4n-6 | 88 | 8 | 25 | 8 | 56 | 82 |
| 22:5n-3 | 24:5n-3 | 14 | 0 | 0 | 2 | 5 | 43 |
| 22:4n-6 | 24:4n-6 | 4 | 0 | 0 | 0 | 2 | 37 |
Conversions were calculated according to the formula (all product areas/(all products areas + substrate area)) ×10.
W231C substitution confers C22 to C24 elongation capacity to sea lamprey Elovl2
Functional characterization of the sea lamprey Elovl2 showed no ability to elongate C22 PUFA to C24 products contrary to those of gnathostome Elovl2. On the other hand, elephant shark Elovl2, whose sequence contains the specific cysteine (C) residue regarded as critical for elongation of C22 by Elovl241 (supplementary Fig. 4), showed ability to elongate C22 PUFA as in gnathostome lineages. Coherently, the sea lamprey Elovl2 exhibits a tryptophan (W) typical of Elovl5 sequences (supplementary Fig. 4). Thus, we next tested whether site-directed mutagenesis of W231C would drift the enzymatic activity towards C22 PUFA elongation as observed in the gnathostome orthologue. Our mutagenesis analysis showed that the W231C substitution conferred the sea lamprey Elovl2 the ability to elongate 22:5n-3 to 24:5n-3, although the conversion obtained in the yeast expression system (2%) was notably lower when compared to other Elovl2 proteins characterized in the present study and previously reported using similar systems11,27 (Table 1). Interestingly, the mutant retained its ability to elongate C20 PUFA such as 20:5n-3 and 20:4n-6 to the corresponding C22 PUFA, 22:5n-3 and 22:4n-6, but lost its ability to elongate C18 PUFA (Table 1). Overall, the functional characterization of sea lamprey Elovl2 mutant confirms that the cysteine (C) residue indicated above is key for the C22 to C24 elongation ability42, but the relatively low conversion observed in the yeast system suggests that other amino acids are also critical for an efficient conversion of C22 into C24 PUFA.
Discussion
Vertebrate radiation encompassed the acquisition of key physiological and anatomical innovations, as a consequence of gene and genome duplications44,45,46,47. Among others, these might have facilitated the challenge of colonizing new ecological niches with diverse nutrient composition such as, for example, LC-PUFA. ELOVL are key enzymes involved in the rate-limiting step of fatty acid elongation pathway by which β-ketoacyl-CoA is produced after the condensation of acyl-CoA molecule and malonyl-CoA17. Although these enzymes have been extensively studied in a number of metazoans including invertebrates and vertebrates, their evolution has yet to be deciphered. Here we focused on a subset of Elovl genes, namely Elovl2 and Elovl5, critical in the biosynthetic pathways of LC-PUFA17. Combining phylogenetics, comparative genomics and functional data, we have been able to deduce the early evolution of functional Elovl specificities in chordates.
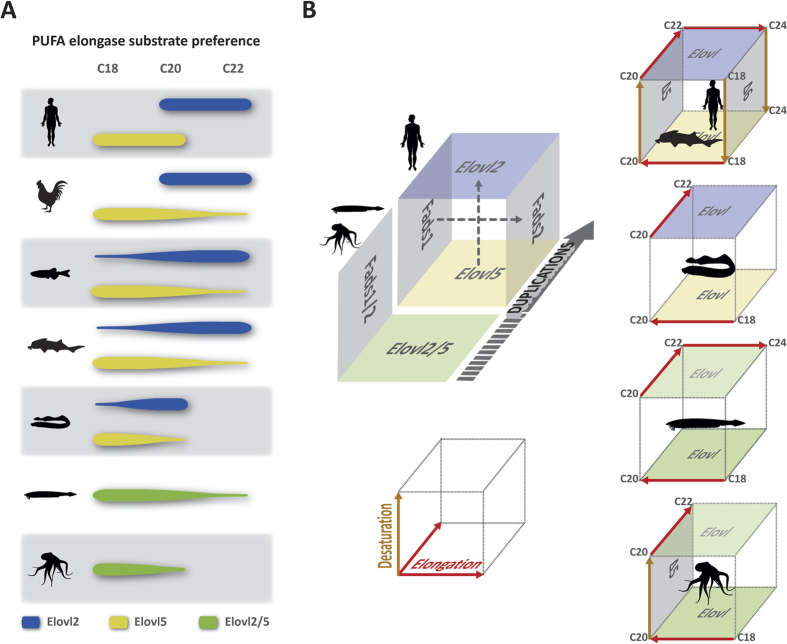
Phylogenetics and synteny revealed that orthologs of Elovl2 and Elovl5 occur only in vertebrate species. Thus, our data support that both Elovl5 and Elovl2 have evolved in agnathans, chondrichthyans, holosteans (spotted gar) and teleosts such as zebrafish and Atlantic salmon. Contrarily to Elovl5, Elovl2 is absent in most of the ray-finned fish branch due to a gene loss event as previously hypothesized11. Moreover, the finding of a single Elovl2/5 sequence in invertebrate deuterostomes and protostomes, and its basal position in the tree, defines the transition from invertebrate chordates to vertebrates as the exact timing at which diversification of Elovl2/5 gene family occurred. Typically in mammals, ELOVL2 are enzymes with high elongation efficiency towards C20 and C22 PUFA, and marginal (if any) activity towards C18 substrates8,17 (Fig. 4A). In contrast, ELOVL5 have C18 and C20 PUFA as preferred substrates, but have little or no capability to elongate C22 PUFA8,17. The former elongation specificity is largely exhibited by protostomes such as the octopus Elovl2/530 (Fig. 4A). In agreement, the here reported amphioxus ELOVL2/5 enzyme showed the same elongation pattern with C18 and C20 PUFA appearing as preferred substrates for elongation, although some ability to elongate C22 PUFA was also observed (Fig. 4A). The substrate preferences of the sea lamprey Elovl2 and the Elovl5 enzymes showed a complete inability to elongate C22 PUFA, whereas the elephant shark Elovl2 was able to effectively elongate C22 PUFA, 22:5n-3 and 22:4n-6, to their corresponding C24 products as shown in teleosts and mammalian ELOVL2 proteins11,17,27,28 (Fig. 4A). The amino acid alignment of the various Elovl2/5 sequences allowed us to identify that the elephant shark Elovl2, similar to orthologues from other gnathostome lineages including mammals, birds, amphibians and teleosts, contained within its sequence the cysteine (C) regarded as critical for C22 PUFA elongation by Elovl243, while this residue was substituted by a tryptophan (W) in the sea lamprey Elovl2. Using a site-directed mutagenesis approach, we showed that the mutated lamprey Elovl2 protein lost the ability to elongate C18 PUFA exhibited by the native protein and, more importantly, gained the ability to elongate C22 PUFA. However, the minute capacity to elongate C22 exhibited by the mutated lamprey Elovl2 suggests that other unidentified amino acids are also critical for this function.
Figure 4. Evolutionary scenario of LC-PUFA biosynthesis functional diversification in Bilateria.
(A) PUFA elongase substrate preference in octopus, amphioxus, lamprey, elephant shark, zebrafish, chicken and human; (B) schematic view of gene duplication events in the elongation/desaturation network along the invertebrate/vertebrate transition (top left) and known enzymatic activities of FADS and ELOVL (right; from top to bottom: octopus, amphioxus, lamprey and the gnathostomes elephant shark and human); Δ5 and Δ6 denote desaturation activities.
Apart from elongase activity, the complexity of the LC-PUFA biosynthetic network cannot be dissociated from LC-PUFA desaturation profiles. The combined analysis of Fads and Elovl gene repertoire and function in various species allows us to propose that a fragmented LC-PUFA pathway existed early in evolution (Fig. 4B). Data derived from mollusks strongly suggests that the ancestral bilaterian LC-PUFA biosynthetic pathway was composed of Fads and Elovl genes encoding, respectively, proteins with single desaturation (Δ5) and elongation (C18 to C22) enzymatic abilities30,31,32,33,34,35 (Fig. 4B), although the presence of additional uncharacterized desaturases in mollusks impedes a final conclusion48. An incomplete pathway also appears to exist in cephalochordates. Despite the functionalities of Elovl2/5 showing its ability to elongate PUFA ranging from C18 to C22, a full complement of desaturase abilities is likely absent as suggested by in silico searches, with a single Fads-like gene described so far in their genome12 (Fig. 4B). However, relevant levels of DHA were found in the digestive tract of amphioxus49. While they could be exclusively diet-derived, an endogenous DHA production cannot be excluded. Thus, the characterization of the single amphioxus FADS should be addressed in the future. In agnathans, on the other hand, the restricted elongation profiles demonstrated by the lack of elongation activity by both Elovl-like enzymes towards C22 may limit the LC-PUFA biosynthetic pathways regardless of the possible number of genes or desaturase activities existing in lampreys (Fig. 4B). Importantly, the combined activities of the elephant shark Elovl5 and Elovl2 enabling elongation up to C24 LC-PUFA and thus DHA biosynthesis29, as well as the existence of Δ6 and Δ5 Fads in chondrichthyans12, strongly suggest that a fully developed LC-PUFA biosynthetic pathway dependent on the sequential action of Elovl and Fads was first operational in gnathostomes (Fig. 4B). The overall pathway has been conserved throughout this lineage with localized episodes of gene loss, gene duplication and functional plasticity as demonstrated by the Δ5 capacity of some teleost Fads212.
However, it is difficult to foresee the exact evolutionary drivers accounting for the acquisition of a full biosynthetic pathway for LC- PUFA in organisms that have a likely supply in the diet. Clearly though, endogenous production of DHA, the final LC-PUFA in the cascade, is physiologically advantageous since it represents an additional source to cope with potential dietary scarcity, as well as satisfy particularly high requirements in early development50. Additionally, DHA levels are known to be especially high in tissues such as brain and retina, in mammals, teleosts and chondrichthyans27,50,51. Thus, it is conceivable to hypothesize that the elaboration of brain and eye function in vertebrate ancestry52, was paralleled by the capacity to endogenously regulate and synthesize DHA independently of exogenous sources.
In conclusion, the observed lineage-specific LC-PUFA biosynthetic profiles in chordate species were tailored by gene duplication events followed by enzymatic neofunctionalizations. We propose that the biosynthesis of the essential fatty acid DHA through the Sprecher pathway from C18 precursors was not fully resolved until gnathostomes emerged.
Methods
Sequence collection
ELOVL amino acid (aa) sequences were retrieved from Ensembl, GenBank, JGI (Joint Genome Institute), elephant shark genome project (http://esharkgenome.imcb.a-star.edu.sg/) and Japanese lamprey genome project (http://jlampreygenome.imcb.a-star.edu.sg/), databases through Blastp searches using as reference the annotated human ELOVL2, ELOVL5 and ELOVL4 aa sequences. Accession numbers are available in supplementary Table 2.
Phylogenetic analysis
A total of 50 ELOVL aa sequences were aligned with MAFFT53 (L-INS-i method). The sequence alignment was stripped from all columns containing gaps leaving 200 gap free sites for phylogenetic analysis. Bayesian phylogenetic analysis was performed using MrBayes v3.2.3 available in CIPRES Science Gateway V3.354. MrBayes was run for 5 million generations with the following parameters: rate matrix for aa = mixed, nruns = 2, nchains = 4, temp = 0.2, sampling set to 500 and burin to 0.25. Maximum likelihood phylogenetic analysis was performed in PhyML v3.0 server55 protein evolutionary model was calculated in PhyML using smart model selection resulting in JTT +G6 +I +F and the number of bootstrap replicates was set to 1000. The resulting trees were visualized in Fig Tree V1.3.1 available at http://tree.bio.ed.ac.uk/software/figtree/ and rooted with ELOVL4 sequences.
Synteny and comparative genomics
Elovl2 and Elovl5 genes were mapped onto the respective species genomes, using the latest genome assemblies available in Ensembl release (Ensembl release 80, May 2015). The elephant shark genomic information was collected from Ensembl Pre assembly ESHARK1 (http://ensembl.fugu-sg.org/index.html) and for Japanese lamprey synteny maps were inferred using the draft assembly LetJap1.0 available at GenBank. When possible, we analyzed a 1Mb window centered on the corresponding Elovl gene, using the human locus as reference for comparison. Paralogy studies used the ancestral chordate genome reconstruction39. Ensembl paralog and ortholog prediction tools were used to infer evolutionary history of flanking Elovl genes in addition to phylogenetic analysis reconfirmation using ML methods.
Elovl full ORF genes in amphioxus, sea lamprey and elephant shark
Total RNA was isolated from amphioxus (whole animal) and sea lamprey (kidney, liver and brain) using an Illustra RNAspin Mini RNA Isolation Kit (GE Healthcare, UK). All steps were performed according to the manufacturer’s recommendations, including the on-column treatment of isolated RNA with RNase-free DNaseI. One μg RNA was used for cDNA synthesis using the iScript cDNA Synthesis Kit (Bio-Rad) and following the manufacturer’s specifications. Initial isolation of the Elovl-like gene in amphioxus was achieved by PCR with Phusion® Flash (high-fidelity PCR master mix) using degenerate primers (supplementary Table 3). Initial PCR product was confirmed by sequencing and used to design gene specific primers (GSP) used obtain the full-length cDNA sequences by RACE PCR (SMARTer™ RACE cDNA Amplification, Clontech). For the sea lamprey, one complete and one incomplete Elovl2/5-like sequences were identified in the available genome. To obtain the open reading frames (ORF) of the incomplete Elovl2/5-like sequence (ENSPMAG00000005149), we carried out a RACE PCR. The elephant shark Elovl2 sequence was identified in the transcriptome and genome sequence56, and was chemically synthesized (Integrated DNA Technologies, Inc., Glasgow, UK).
Cloning into pYES2 vector and functional assays in yeast
Functional characterization of the ELOVL gene products from amphioxus, sea lamprey and elephant shark were investigated by heterologous expression in yeast Saccharomyces cerevisiae (strain InvSc1, Invitrogen). Briefly, the ORF of the target genes were cloned into the yeast expression vector pYES2 (Invitrogen) following a two-step routine. First, PCRs with specific primers flanking the full ORF were designed in the 5′ and 3′ UTR of each gene (supplementary Table 3) were performed using Phusion® Flash (high-fidelity PCR master mix) under the following conditions: initial denaturation at 98 °C for 10 s, followed by 25 cycles at 98 °C for 1 s annealing for 5 s and 72 °C for the required amount of time according to the product size. The second step consisted in re-amplification of the initial PCR product (diluted 1/50) with a set of primers containing the start and stop codons and restriction enzyme sites for further cloning into pYES2 (supplementary Table 3). PCR conditions were the same with the exception of the number of cycles that was increased to 35. The resulting PCR product was purified, digested with appropriate restriction enzymes and ligated into a similarly restricted pYES2 vector to produce the constructs pYES2-BlELOVL for B. lanceolatum Elovl2/5, pYES2-PmELOVL2 and pYES2-PmELOVL5 for P. marinus Elovl2 and Elovl5, respectively, and pYES2-CmELOVL2 and pYES2-CmELOVL5 for C. milii Elovl2 and Elovl5, respectively. Lamprey Elovl2 W231C mutant was produced by site directed mutagenesis PCR using pYES2-PmELOVL2 as template, and the PCR product was subsequently purified, digested with the restriction enzymes and ligated into pYES2 to produce pYES2-PmELOVL2-W231C. Accuracy of the DNA sequences was confirmed in all constructs by sequencing. Transformation and culture of yeast were conducted as previously described10,21. In order to assess the substrate specificity of the ELOVL enzymes from amphioxus, sea lamprey and elephant shark, transgenic yeast expressing the Elovl ORF were grown in the presence of the following PUFA substrates: 18:4n-3, 18:3n-6, 20:5n-3, 20:4n-6, 22:5n-3 and 22:4n-6. After 48 h of incubation, yeast were harvested, washed and total lipid extracted by homogenization in chloroform/methanol (2:1, v/v) containing 0.01% BHT13.
Fatty acid analysis of yeast and elongation conversions
Fatty acyl methyl esters (FAME), prepared from total lipids extracted from harvested cells, were analyzed using a Thermo Gas Chromatograph (Thermo Trace GC Ultra, Thermo Electron Corporation, Waltham, MA, USA) fitted with an on-column injection system and a FID detector. Further confirmation of FAME was performed with an Agilent 6850 Gas Chromatograph system coupled to a 5975 series MSD (Agilent Technologies, Santa Clara, CA, USA). The elongation conversion efficiencies from exogenously added PUFA substrates were calculated by the proportion of substrate fatty acid converted to elongated products as (all product areas/(all product areas + substrate area)) x 100.
Additional Information
How to cite this article: Monroig, Óscar. et al. Evolutionary functional elaboration of the Elovl2/5 gene family in chordates. Sci. Rep. 6, 20510; doi: 10.1038/srep20510 (2016).
Supplementary Material
Acknowledgments
This work was supported by the Fundação para a Ciência e a Tecnologia (FCT) (Strategic Funding UID/Multi/04423/2013, SFRH/BD/84238/2012 to M.L.-M. and SFRH/BPD/72519/2010 to R.R.) and partly by MINECO (Spanish Government - AGL2013-40986R). The access to the Institute of Aquaculture laboratories was funded by the European Union’s Seventh Framework Programme (FP7/2007-2013) under grant agreement n° 262336 (AQUAEXCEL), Transnational Access Project Number 0095/06/03/13.
Footnotes
Author Contributions Ó.M. and L.F.C.C. designed research; Ó.M., M.L.-M., J.C.N., F.H., D.R.T. and L.F.C.C. performed research; Ó.M., M.L.-M., J.C.N., F.H., R.R., M.M.S., B.V., D.R.T. and L.F.C.C. analyzed data; and Ó.M. and L.F.C.C. wrote the paper.
References
- Lynch M. & Conery J. S. The Evolutionary Fate and Consequences of Duplicate Genes. Science 290, 1151–1155 (2000). [DOI] [PubMed] [Google Scholar]
- Dean A. M. & Thornton J. W. Mechanistic approaches to the study of evolution: the functional synthesis. Nat. Rev. Genet. 8, 675–688 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgham J. T., Carroll S. M. & Thornton J. W. Evolution of Hormone-Receptor Complexity by Molecular Exploitation. Science 312, 97–101 (2006). [DOI] [PubMed] [Google Scholar]
- Phillips P. C. Epistasis – the essential role of gene interactions in the structure and evolution of genetic systems. Nat. Rev. Genet. 9, 855–867 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z., Jiang W., Lages N., Borcherds W. & Wang D. Relationship between gene duplicability and diversifiability in the topology of biochemical networks. BMC Genomics 15, 577 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tocher D. R. Metabolism and Functions of Lipids and Fatty Acids in Teleost Fish. Rev. Fish. Sci. 11, 107–184 (2003). [Google Scholar]
- Schmitz G. & Ecker J. The opposing effects of n−3 and n−6 fatty acids. Prog. Lipid Res. 47, 147–155 (2008). [DOI] [PubMed] [Google Scholar]
- Guillou H., Zadravec D., Martin P. G. & Jacobsson A. The key roles of elongases and desaturases in mammalian fatty acid metabolism: Insights from transgenic mice. Prog. Lipid. Res. 49, 186–199 (2010). [DOI] [PubMed] [Google Scholar]
- Park H. G., Park W. J., Kothapalli K. S. & Brenna J. T. The fatty acid desaturase 2 (FADS2) gene product catalyzes Δ4 desaturation to yield n-3 docosahexaenoic acid and n-6 docosapentaenoic acid in human cells. FASEB J. 29, 3911–9 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings N. et al. A vertebrate fatty acid desaturase with Δ5 and Δ6 activities. Proc. Natl. Acad. Sci. USA 98, 14304–14309 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais S., Monroig O., Zheng X., Leaver M. J. & Tocher D. R. Highly unsaturated fatty acid synthesis in Atlantic salmon: characterization of ELOVL5- and ELOVL2-like elongases. Mar. Biotechnol. (NY) 11, 627–639 (2009). [DOI] [PubMed] [Google Scholar]
- Castro L. F. C. et al. Functional Desaturase Fads1 (Δ5) and Fads2 (Δ6) Orthologues Evolved before the Origin of Jawed Vertebrates. PLoS ONE 7, e31950 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroig Ó., Tocher D. R., Hontoria F. & Navarro J. C. Functional characterisation of a Fads2 fatty acyl desaturase with Δ6/Δ8 activity and an Elovl5 with C16, C18 and C20 elongase activity in the anadromous teleost meagre (Argyrosomus regius). Aquaculture. 412–413, 14–22 (2013). [Google Scholar]
- Li Y. et al. Vertebrate fatty acyl desaturase with Δ4 activity. Proc. Natl. Acad. Sci. USA 107, 16840–16845 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanomman S., Ketudat-Cairns M., Jangprai A. & Boonanuntanasarn S. Characterization of fatty acid delta-6 desaturase gene in Nile tilapia and heterogenous expression in Saccharomyces cerevisiae. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 166, 148–156 (2013). [DOI] [PubMed] [Google Scholar]
- Fonseca-Madrigal J. et al. Diversification of substrate specificities in teleostei Fads2: characterization of Δ4 and Δ6Δ5 desaturases of Chirostoma estor. J. Lipid Res. 55, 1408–1419 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson A., Westerberg R. & Jacobsson A. Fatty acid elongases in mammals: their regulation and roles in metabolism. Prog. Lipid Res. 45, 237–249 (2006). [DOI] [PubMed] [Google Scholar]
- Leonard A. E. et al. Identification and expression of mammalian long-chain PUFA elongation enzymes. Lipids 37,733–740 (2002). [DOI] [PubMed] [Google Scholar]
- Leonard A. E., Pereira S. L., Sprecher H. & Huang Y. S. Elongation of long-chain fatty acids. Prog. Lipid Res. 43, 36–54 (2004). [DOI] [PubMed] [Google Scholar]
- Gregory M. K., Geier M. S., Gibson R. A. & James M. J. Functional Characterization of the Chicken Fatty Acid Elongases. J. Nutr. 143, 12–16 (2013). [DOI] [PubMed] [Google Scholar]
- Agaba M., Tocher D. R., Dickson C. A., Dick J. R. & Teale A. J. Zebrafish cDNA encoding multifunctional Fatty Acid elongase involved in production of eicosapentaenoic (20:5n-3) and docosahexaenoic (22:6n-3) acids. Mar. Biotechnol. (NY) 6, 251–261 (2004). [DOI] [PubMed] [Google Scholar]
- Zheng X. et al. Physiological roles of fatty acyl desaturases and elongases in marine fish: Characterisation of cDNAs of fatty acyl Δ6 desaturase and elovl5 elongase of cobia (Rachycentron canadum). Aquaculture 290, 122–131 (2009). [Google Scholar]
- Gregory M. K., See V. H., Gibson R. A. & Schuller K. A. Cloning and functional characterisation of a fatty acyl elongase from southern bluefin tuna (Thunnus maccoyii). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 155, 178–185 (2010). [DOI] [PubMed] [Google Scholar]
- Mohd-Yusof N. Y., Monroig O., Mohd-Adnan A., Wan K. L. & Tocher D. R. Investigation of highly unsaturated fatty acid metabolism in the Asian sea bass, Lates calcarifer. Fish Physiol. Biochem. 36, 827–843 (2010). [DOI] [PubMed] [Google Scholar]
- Morais S., Mourente G., Ortega A., Tocher J. A. & Tocher D. R. Expression of fatty acyl desaturase and elongase genes, and evolution of DHA:EPA ratio during development of unfed larvae of Atlantic bluefin tuna (Thunnus thynnus L.). Aquaculture 313, 129–139 (2011). [Google Scholar]
- Carmona-Antonanzas G., Tocher D. R. & Taggart J. B. & Leaver, M. J. An evolutionary perspective on Elovl5 fatty acid elongase: comparison of Northern pike and duplicated paralogs from Atlantic salmon. BMC Evol. Biol. 13, 85 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroig Ó., Rotllant J., Sanchez E., Cerda-Reverter J. M. & Tocher D. R. Expression of long-chain polyunsaturated fatty acid (LC-PUFA) biosynthesis genes during zebrafish Danio rerio early embryogenesis. Biochim. Biophys. Acta 1791, 1093–1101 (2009). [DOI] [PubMed] [Google Scholar]
- Gregory M. K. & James M. J. Rainbow trout (Oncorhynchus mykiss) Elovl5 and Elovl2 differ in selectivity for elongation of omega-3 docosapentaenoic acid. Biochim. Biophys. Acta. 1841, 1656–1660 (2014). [DOI] [PubMed] [Google Scholar]
- Sprecher H. Metabolism of highly unsaturated n-3 and n-6 fatty acids. Biochim. Biophys. Acta 1486, 219–231 (2000). [DOI] [PubMed] [Google Scholar]
- Monroig Ó., Guinot D., Hontoria F., Tocher D. R. & Navarro J. C. Biosynthesis of essential fatty acids in Octopus vulgaris (Cuvier, 1797): Molecular cloning, functional characterisation and tissue distribution of a fatty acyl elongase. Aquaculture 360–361, 45–53 (2012). [Google Scholar]
- Liu H. et al. Cloning and functional characterization of a polyunsaturated fatty acid elongase in a marine bivalve noble scallop Chlamys nobilis Reeve. Aquaculture 416–417, 146–151 (2013). [Google Scholar]
- Monroig Ó., Hontoria F., Varó I., Tocher D. R. & Navarro J. C. Investigating the essential fatty acids in the common cuttlefish Sepia officinalis (Mollusca, Cephalopoda): Molecular cloning and functional characterisation of fatty acyl desaturase and elongase. Aquaculture 450, 38–47 (2016). [Google Scholar]
- Monroig Ó., Navarro J. C., Dick J. R., Alemany F. & Tocher D. R. Identification of a Delta5-like fatty acyl desaturase from the cephalopod Octopus vulgaris (Cuvier 1797) involved in the biosynthesis of essential fatty acids. Mar. Biotechnol. (NY) 14, 411–422 (2012). [DOI] [PubMed] [Google Scholar]
- Li M. et al. Characterization of two Δ5 fatty acyl desaturases in abalone (Haliotis discus hannai Ino). Aquaculture 416–417, 48–56 (2013). [Google Scholar]
- Liu H. et al. Functional characterization of a Δ5-like fatty acyl desaturase and its expression during early embryogenesis in the noble scallop Chlamys nobilis Reeve. Mol. Biol. Rep. 41, 7437–7445 (2014). [DOI] [PubMed] [Google Scholar]
- Meyer A. et al. Novel fatty acid elongases and their use for the reconstitution of docosahexaenoic acid biosynthesis. J. Lipid Res. 45, 1899–1909 (2004). [DOI] [PubMed] [Google Scholar]
- Monroig Ó. et al. Expression and role of Elovl4 elongases in biosynthesis of very long-chain fatty acids during zebrafish Danio rerio early embryonic development. Biochim. Biophys. Acta 1801, 1145-1154 (2010). [DOI] [PubMed] [Google Scholar]
- Agbaga M.-P. et al. Role of Stargardt-3 macular dystrophy protein (ELOVL4) in the biosynthesis of very long chain fatty acids. Proc. Natl. Acad. Sci. USA 105, 12843–12848 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam N. H. et al. The amphioxus genome and the evolution of the chordate karyotype. Nature 453, 1064–1071 (2008). [DOI] [PubMed] [Google Scholar]
- Kuraku S. Impact of asymmetric gene repertoire between cyclostomes and gnathostomes. Semin. Cell Dev. Biol. 24, 119–127 (2013). [DOI] [PubMed] [Google Scholar]
- Mehta T. K. et al. Evidence for at least six Hox clusters in the Japanese lamprey (Lethenteron japonicum). Proc. Natl. Acad. Sci. USA 110, 16044–16049 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. J. & Keinath M. C. The sea lamprey meiotic map improves resolution of ancient vertebrate genome duplications. Genome Res. 25, 1081–1090 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory M. K., Cleland L. G. & James M. J. Molecular basis for differential elongation of omega-3 docosapentaenoic acid by the rat Elovl5 and Elovl2. J. Lipid Res. 54, 2851–2857 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno S. In Evolution by Gene Duplication 1st edn,(eds Ohno S.) Ch. 16, 98–105 (Springer Verlag, 1970). [Google Scholar]
- Braasch I., Volff J.-N. & Schartl M. The Endothelin System: Evolution of Vertebrate-Specific Ligand–Receptor Interactions by Three Rounds of Genome Duplication. Mol. Biol. Evol. 26, 783–799 (2009). [DOI] [PubMed] [Google Scholar]
- Minguillon C., Gibson-Brown J. J. & Logan M. P. Tbx4/5 gene duplication and the origin of vertebrate paired appendages. Proc. Natl. Acad. Sci. USA 106, 21726–21730 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann F. G., Opazo J. C. & Storz J. F. Whole-Genome Duplications Spurred the Functional Diversification of the Globin Gene Superfamily in Vertebrates. Mol. Biol. Evol. 29, 303–312 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surm J. M., Prentis P. J. & Pavasovic A. Comparative Analysis and Distribution of Omega-3 lcPUFA Biosynthesis Genes in Marine Molluscs. PLoS One 10, e0136301 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan D. et al. Ancestral genetic complexity of arachidonic acid metabolism in Metazoa. Biochim. Biophys. Acta 1841, 1272–84 (2014). [DOI] [PubMed] [Google Scholar]
- Lauritzen L., Hansen H. S., Jørgensen M. H. & Michaelsen K. F. The essentiality of long chain n-3 fatty acids in relation to development and function of the brain and retina. Prog. Lipid Res. 40,1–94 (2001). [DOI] [PubMed] [Google Scholar]
- Stoknes I. S., Økland H. M. W., Falch E. & Synnes M. Fatty acid and lipid class composition in eyes and brain from teleosts and elasmobranchs. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 138, 183–191 (2004). [DOI] [PubMed] [Google Scholar]
- Shimeld S. M. & Holland P. W. H. Vertebrate innovations. Proc. Natl. Acad. Sci. USA 97, 4449–4452 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K. & Toh H. Recent developments in the MAFFT multiple sequence alignment program. Brief. Bioinform. 9, 286–298 (2008). [DOI] [PubMed] [Google Scholar]
- Miller M. A. et al. A RESTful API for Access to Phylogenetic Tools via the CIPRES Science Gateway. Evol. Bioinform. Online 11, 43–48 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon S. et al. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol. 59, 307–321 (2010). [DOI] [PubMed] [Google Scholar]
- Venkatesh B. et al. Elephant shark genome provides unique insights into gnathostome evolution. Nature 505, 174–179 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.