Biochemical, morphological, and transcriptomic characterization of an unusual surface wax produced by Bayberry fruits reveals a cutin-related biosynthetic pathway that synthesizes oilseed-like glycerolipids in plants.
Abstract
Bayberry (Myrica pensylvanica) fruits synthesize an extremely thick and unusual layer of crystalline surface wax that accumulates to 32% of fruit dry weight, the highest reported surface lipid accumulation in plants. The composition is also striking, consisting of completely saturated triacylglycerol, diacylglycerol, and monoacylglycerol with palmitate and myristate acyl chains. To gain insight into the unique properties of Bayberry wax synthesis, we examined the chemical and morphological development of the wax layer, monitored wax biosynthesis through [14C]-radiolabeling, and sequenced the transcriptome. Radiolabeling identified sn-2 monoacylglycerol as an initial glycerolipid intermediate. The kinetics of [14C]-DAG and [14C]-TAG accumulation and the regiospecificity of their [14C]-acyl chains indicated distinct pools of acyl donors and that final TAG assembly occurs outside of cells. The most highly expressed lipid-related genes were associated with production of cutin, whereas transcripts for conventional TAG synthesis were >50-fold less abundant. The biochemical and expression data together indicate that Bayberry surface glycerolipids are synthesized by a pathway for TAG synthesis that is related to cutin biosynthesis. The combination of a unique surface wax and massive accumulation may aid understanding of how plants produce and secrete non-membrane glycerolipids and also how to engineer alternative pathways for lipid production in non-seeds.
INTRODUCTION
The aerial surfaces of plants are covered with a variety of soluble and insoluble lipids that together constitute the cuticle. Waxes are a component of the plant cuticle and together with the glycerolipid polyester cutin provide plants with their primary interface with the external environment. The most common surface waxes found on plants are derived from fatty acids modified to form very-long-chain hydrocarbons, alcohols, aldehydes, ketones, and wax esters of fatty acids and alcohols (Samuels et al., 2008). However, the wax that accumulates on the surfaces of the fruits of some species of North American Bayberry plants (Myrica [or Morella] pensylvanica) is strikingly unusual in abundance and composition. Bayberry wax represents up to 29% of the fresh mass of the fruit (reported from Myrica carolinesis species) and contains the glycerolipids triacylglycerol (TAG) (Harlow et al., 1965), diacylglycerol (DAG), and monoacylglycerol (MAG) (Kraut, 1965; Hawthorne and Miller, 1987). Adding to its distinctiveness, the fatty acids in the surface glycerolipids are entirely saturated, primarily palmitic (C16:0) and myristic (C14:0). Despite these unique features, Bayberry is rarely cited as a massive accumulator of surface wax or as a plant tissue that produces large amounts of glycerolipids, and in the past 100 years, there have been very few studies examining the morphology of Bayberry fruits and the development and chemical composition of their surface wax (Youngken, 1915; McKay, 1948; Harlow et al., 1965; Kraut, 1965; Hawthorne and Miller, 1987). The thick unusual wax layer is attractive to and digestible by some species of birds (Fordham, 1983) and may have evolved as a seed dispersal mechanism (Place and Stiles, 1992). Owing to the striking abundance and exclusively saturated fatty acid content that confers a high melting point to the wax, a traditional use of Bayberry wax is to make holiday candles (Williams, 1958).
In plants, TAG is most abundantly produced in seeds, pollen, and the mesocarps of some fleshy fruits (i.e., avocado [Persea americana], oil palm, and olive). The simplest pathway to produce TAG in those tissues involves two acylations of glycerol-3-phosphate (G3P) with fatty acyl-CoA to produce phosphatidic acid. After phosphate removal, the resulting DAG is further acylated to yield TAG (Chen et al., 2015) that is then sequestered into oil bodies or lipid droplets within the cytoplasm (Chapman et al., 2012). Although Bayberry is exceptional in its extracellular accumulation of TAG and DAG, plants do accumulate extracellular lipids containing glycerol in the form of cutin and suberin. Extracellular or surface lipids are synthesized and secreted from the single layer of epidermal cells on the outer plane of aerial tissues. Aerial epidermal lipid metabolism is largely devoted to the production and secretion of lipids, with two-thirds or more of all acyl chains synthesized in this cell monolayer destined to become wax or cutin (Suh et al., 2005). However, in contrast to seeds and other TAG-accumulating plant tissues, surface lipids do not accumulate within epidermal cells, and unlike the fatty acids of membrane and storage lipids, most fatty acids in cutin and suberin are modified by omega and mid-chain oxygenations that allow interesterification reactions to form a lipophilic, insoluble polymer (Pollard et al., 2008; Samuels et al., 2008).
Epidermal cells clearly evolved mechanisms to separate membrane fatty acids from the unusual fatty acids that form the cuticle and the ability to secrete the lipids through the cell membrane, cell wall, and onto external surfaces. Because Bayberry accumulates very large quantities of glycerolipids on its fruit surface that are typically intracellular, it was not clear how these lipids are exported and whether TAG and DAG are synthesized by reactions similar to oilseeds or perhaps by a previously undescribed pathway. The objective of this study was to identify similarities or differences between Bayberry wax production and conventional surface lipid and intracellular glycerolipid production. Research on plants that produce such large amounts of surface lipids may provide insights into the molecular features and biochemical pathways for plant lipid secretion. In addition, studying Bayberry wax triacylglycerol production may help elucidate mechanisms for non-polar lipid production in non-seed tissues. We initiated this work by examining changes in fruit anatomy, details of the chemical structures secreted by Bayberry fruits and quantifying the accumulation of wax through an entire growing season. Biochemical pathway analysis by [14C]-labeling and transcript analysis by RNA-seq revealed features of Bayberry wax accumulation that are distinctly different from conventional TAG production. Together, these results indicate that the extracellular glycerolipids in Bayberry wax, including TAG, are synthesized by a pathway that differs from previously defined TAG biosynthesis pathways in plants. An increased understanding of this process may prove useful in engineering plants for secretion of high value lipids, particularly those that have toxic or negative consequences when accumulated inside cells.
RESULTS AND DISCUSSION
Bayberry Surface Wax Accumulates to the Highest Levels Reported for Plants and Is Composed Entirely of Saturated Glycerolipids
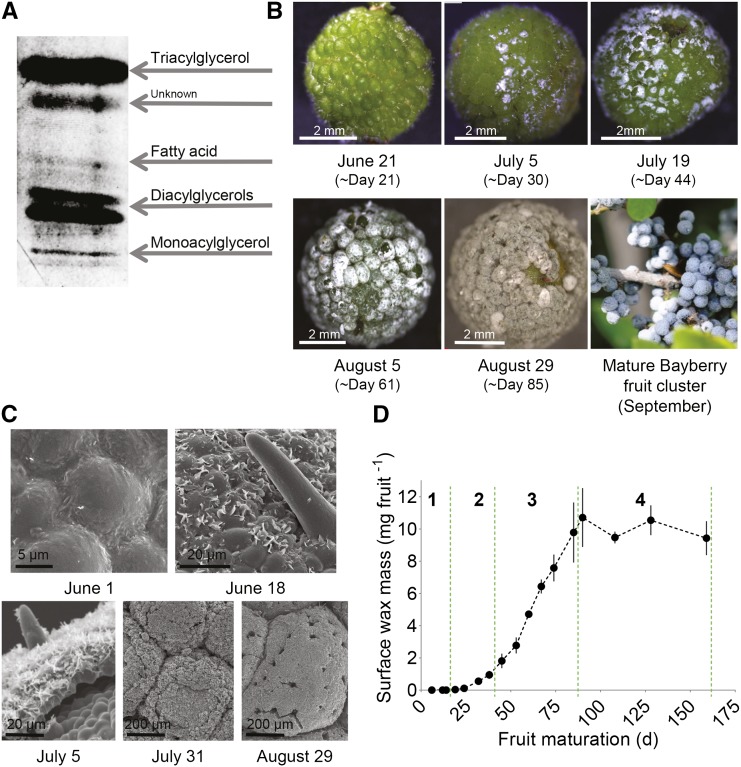
Mature Bayberry fruits are covered with an unusually thick layer of surface wax (Figure 1). As with most other soluble waxes on the surfaces of plants (Jetter et al., 2006), Bayberry wax can be removed by immersing the fruits for a short time (30 s) in chloroform, without removing typical internal membrane lipids (Supplemental Figure 1). Gravimetric determination of surface wax from mature Bayberry fruits yielded 9.8 (±1.2) mg fruit−1. This is 32% of the entire fruit dry weight (Table 1), comparable to 29% from an earlier report (Harlow et al., 1965), and when calculated based on fruit surface area is 8.7 mg cm−2. The amount of surface wax on mature Bayberry fruits is higher than any levels of surface lipid reported for the plant kingdom (including Carnauba [Copernicia prunifera] leaf wax) and possibly in nature (Kolattukudy, 1976; Baker, 1982; Jetter et al., 2006).
Figure 1.
Progression of Wax Accumulation on the Surfaces of Bayberry Fruits through Development.
(A) TLC separation of mature Bayberry wax. After development the plate was sprayed with primuline and viewed under UV light. The higher and lower diacylglycerol bands represent sn-1,3-diacylglycerol and sn-(1,2)(2,3)- diacylglycerol isoforms, respectively.
(B) Light microscopy images through the growth season.
(C) Scanning electron microscopy images of Bayberry fruits.
(D) Mass of glycerolipids in Bayberry wax through the season, determined by GC with flame ionization detection. Stages of wax accumulation are designated by the dotted green lines. Each point represents the mean of three to four replicates ± se.
Table 1. Wax Accumulation on Bayberry Fruits and Knobs Based on Different Fruit Measurements.
| In a Single Fruit | In a Single Knob | |
|---|---|---|
| Total surface wax | 9.8 mg | 0.043 mg |
| Wax per surface area | 8.7 mg cm−2 | – |
| Wax per fresh mass | 15% | 28% |
| Wax per dry mass | 32% | 56% |
Wax and fruit parts were measured gravimetrically.
The composition of chloroform-soluble surface wax of Bayberry fruits was analyzed by thin-layer chromatography (TLC) and by high-temperature gas chromatography (GC). In contrast to other plants, Bayberry contains almost exclusively glycerolipids in its surface wax. TLC identified the major components of the wax as TAG and DAG with minor bands aligning with MAG and free fatty acid (FFA) standards (Figure 1A). Analysis of intact lipids by GC confirmed TAG, DAG, and MAG as the major components of mature Bayberry surface wax and indicated a molar ratio of 30:62:8 between them (Supplemental Figure 2). Common plant surface waxes (i.e., alkanes, etc.) were not detected on the TLC plate or in the GC chromatogram and gravimetric quantification (9.8 mg fruit−1) of the surface wax was very similar to the GC quantification (10.7 ± 1.9 mg fruit−1). Also intriguing was that only saturated fatty acid species were detected in TAG, DAG, and MAG (C16:0 [>75%] C14:0 [< 25%], and trace amounts of C18:0 [<1%]). TAG or DAG containing such a high percentage of C16:0 fatty acids, with no unsaturated fatty acids, has also not been reported for plants (Badami and Patil, 1980; Banerji et al., 1984; Gunstone et al., 2007).
Bayberry Wax Accumulates Continuously through 8 Weeks of Fruit Maturation
Cuticular lipid production (i.e., wax and cutin) by epidermal cells involves the coordinated action of intracellular lipid synthesis, trafficking of lipids within the cells, and movement of the cuticular lipids through the cell membrane and the cell wall (Beisson et al., 2012). The cuticular lipid layer is established during the initial stages of tissue development and cuticular lipid synthesis is highest in young and expanding tissue (Suh et al., 2005). Because of the unique composition and abundance of Bayberry surface wax, we asked whether its mechanism of wax production is similar to conventional cuticular lipid production and secretion (Harlow et al., 1965; Samuels et al., 2008). Figure 1 presents images of the developmental progression of Bayberry fruits by light microscopy (Figure 1B) and scanning electron microscopy (Figure 1C). Surface wax became first visible to the naked eye ∼1 month after pollen production by the male flowers. At that time, the fruits were green and fully exposed from the flower bracts, and morphological features appeared to be fully established. The surface wax initially appeared in small separated clusters on the fruit surface, but gradually thickened and spread to cover the entire surface of the fruits. This process extended over ∼8 weeks, after which the wax layer persisted on the fruits through the fall and winter months. Scanning electron microscope images of the fruits prior to wax detection revealed a smooth surface devoid of any of the crystals that appear later in the season (Figure 1C). At the earliest stages of wax accumulation, sharp crystals were clustered in the junctions between the epidermal pavement cells and as the season progressed the ultrastructure of the layer became fissured and crust-like. In this regard, mature Bayberry wax appeared similar to many succulents that accumulate thick layers of wax (Barthlott et al., 1998). A time-lapse video of the growth of the wax layer also showed the emergence of local wax deposits, followed by the wax accumulating over the entire fruit surface and thickening (Supplemental Movie 1).
The quantity and composition of the surface wax was determined by GC throughout 159 d of development, beginning ∼2 weeks after pollen production (Figure 1D). In agreement with the images, surface waxes were detected after day 14, with the highest amounts observed 70 d after initial detection of the wax (day 90 of this analysis). Three distinct periods of accumulation were identified during this analysis: stage 1, days 1 to 19, when 2% of the wax was deposited; stage 2, days 19 to 45, when 15% of wax was deposited; and stage 3, days 45 to 90, when the remaining 83% of the wax was deposited. The mean daily rate of wax accumulation was 3 mg g fresh weight−1 or 200 µg fruit−1 during stage 3. Interestingly, the fruits approached their maximum fresh weights prior to the majority of the wax being deposited (Supplemental Figure 3). This suggested that most of the wax on Bayberry fruits was deposited on fully formed fruit tissues, rather than accumulating through the initial stages of fruit development like cutin and most conventional surface waxes (Suh et al., 2005; Jetter et al., 2006). During a fourth stage, from days 90 to 159, there was a 5 to 10% loss in surface wax, which may be attributable to some shedding of the wax layer, animal or insect feeding, or microorganism degradation.
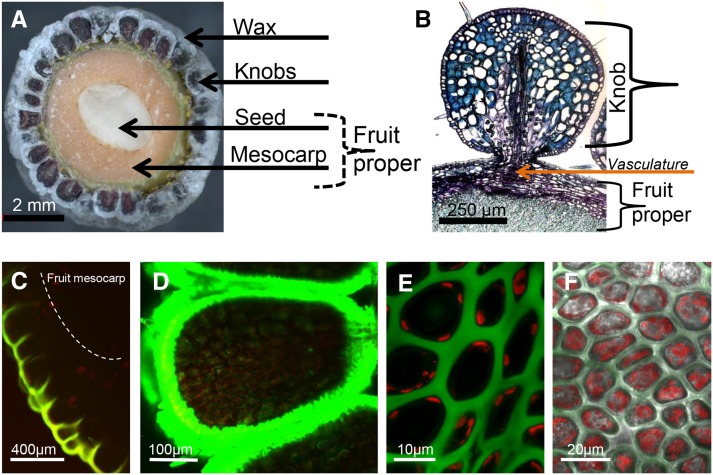
Bayberry Surface Wax Is Produced by “Knobs,” an Unusual Multicellular Tissue That Extends from the Fruit Exocarp
The wax that accumulates on the surface of Bayberry fruits appears on three sides of multicellular structures that extend from the surface of the fruit proper (exocarp) (Figure 2A). These structures are referred to as “knobs” (Harlow et al., 1965). The fruit (drupe) is 5 mm in diameter and its entire surface is covered with 200 to 250 knobs. Each knob is ∼500 µm in diameter, contains more than 10,000 cells, and is connected to the fruit proper by a vascular system that runs along the circumference of the fruit into each knob (Figure 2B). The large size and uniform distribution of the knobs on the fruit make them distinctly different from other surface protrusions in plants, such as trichomes. Fruits of other Myricaceae species that do not appear to produce the same abundant layer of surface wax as M. pensylvanica (i.e., Myrica rubra) also have knobs, suggesting these structures are a feature of the fruits of the Myricaceae family (Feng et al., 2012) rather than specific for a large accumulation of surface wax.
Figure 2.
Structure of Bayberry Knobs.
(A) Cross section of a mature Bayberry fruit.
(B) Paraffin-embedded cross section stained with toluidine blue shows the connection of a single Bayberry knob to the fruit proper.
(C) to (F) Confocal fluorescence images of Bayberry fruits stained for neutral lipids with Nile red ([C] and [E]) or BODIPY 493/503 ([D] and [F]). Green color in images is lipid and red color is chlorophyll florescence.
(C) Cross section of a young Bayberry knob and fruit (∼5% of final wax load).
(D) Cross section of a Bayberry knob in mid-development (∼50% of final wax load).
(E) Top-down view into knob epidermal cells.
(F) Similar view into knob epidermal cells, but after surface wax removed from the fruits. Note the reduction of interstitial fluorescence from the stained lipids in (F) compared with (E) and that both appear not to contain neutral lipids within cells.
To investigate cellular and subcellular sites associated with the production and secretion of surface wax, the neutral lipid fluorescent dyes Nile Red or BODIPY493/503 were applied to freshly harvested intact fruits and cross sections of the fruits were imaged by confocal microscopy (Figures 2C to 2F). At both early and mid-stages of development, staining by both dyes was strongest around the knobs and not within any structures of the fruit proper (Figures 2C and 2D). This is unlike oil palm, olive, or avocado, which accumulate lipids as a major component of their fruit mesocarp. There was also very little lipid staining within the inner interstitial layers of the knobs. Moreover, we did not detect any stained subcellular structures characteristic of lipid droplets or other defined structures indicative of accumulation of lipids within the knob cells. Staining fruits after removal of wax also did not expose intracellular or interstitial lipids that might not have been visible due to the strong signal from the lipids on the immediate surface (Figure 2F). Together, these results indicate that the wax, or neutral lipid precursors, does not accumulate to detectable levels within the cells or interstitial spaces.
Each knob produces and secretes ∼40 µg of wax, which is greater than 50% of its dry weight (Table 1). The crystallization temperature of extracted Bayberry wax, as determined by differential scanning calorimetry, was greater than 40°C (Supplemental Figure 4), which is much higher than other plant oils that accumulate within cells (Gunstone et al., 2007). Presumably, if these surface glycerolipids accumulated within the knob cells, they might exist as a solid, potentially restricting further movement to the surface, and also affecting cell function.
The gradual accumulation of surface wax and the apparent lack of significant lipid storage inside the fruit tissues indicate that Bayberry surface wax is actively secreted from the knobs through development, rather than released as a senescence-related disintegration of outer layers of the fruit. The lack of storage within knob cells is in contrast to other TAG-accumulating plant tissues. For example, extracellular triacylglycerol estolides on the surface of “wet” stigmas and lipids that coat pollen appear to initially accumulate inside cells and are deposited on the surface when cells rupture (Konar and Linskens, 1966; Murphy, 2001). Instead, the mechanism that Bayberry employs is similar to the deposition of conventional surface lipids, where lipids do not accumulate within cells (Pighin et al., 2004) and are exported to the surface immediately after synthesis.
The Distribution and Structure of Surface Glycerolipids throughout Development Provides an Initial Suggestion of a Pathway to Synthesize TAG for Bayberry Wax That Is Different from Oilseeds
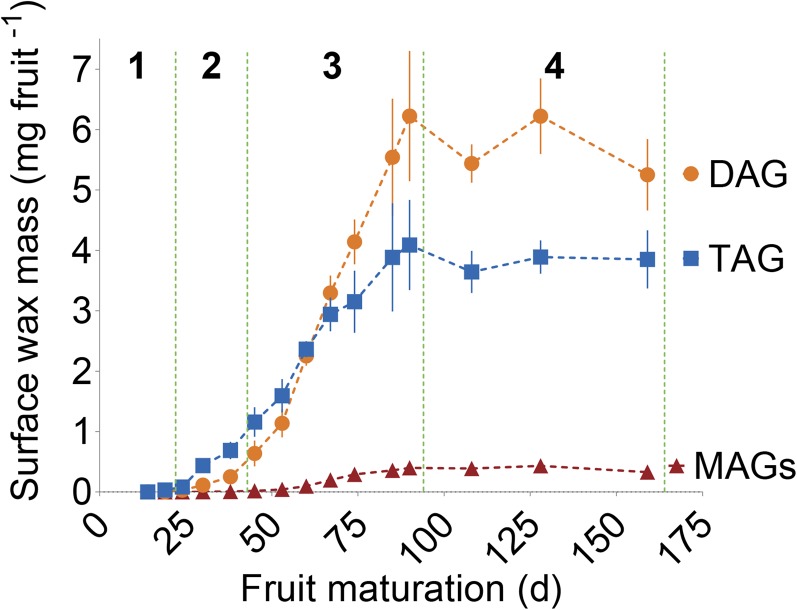
TAG was the only lipid detected in Bayberry surface wax at the earlier stages of development and was the most abundant surface glycerolipid throughout stages 1 and 2 (Figure 3; Supplemental Table 1). However, DAG accumulation gradually overtook TAG midway through wax production, and at maturity the mol % of DAG was 2-fold greater than TAG. DAG and TAG exhibited similar rates of accumulation in the wax for ∼40 to 60 d, with both peaking between days 53 and 60, but, from days 60 to 90, the rate of TAG accumulation declined (Supplemental Figure 5). The accumulation of DAG to greater levels than TAG is unusual for a tissue that accumulates large levels of TAG. For example, in oilseeds, TAG represents greater than 95% of total glycerolipids with DAG constituting less than 5% of the lipids at maturity (Slack et al., 1980).
Figure 3.
The Mass per Fruit of Individual Glycerolipids Found in Bayberry Wax during Fruit Maturation.
Stages, as defined in Figure 1D, are designated by the dotted green line. Each point in the graphs represents the mean of three to four replicates ± se.
The increasing levels of DAG through the season occurred without any reduction in total TAG levels, suggesting that DAG was derived from new synthesis and not due to degradation (lipolysis) of TAG already present in the surface wax. Consistent with this suggestion, FFA, the other product of lipolysis, was present only at trace levels in the wax. Instead, we tentatively concluded that DAG accumulated late in the season because its rate of production may have exceeded the capacity of the final acylation of DAG to TAG.
Also intriguing, and in sharp contrast to oilseeds, was that MAG accumulated to 4% of the mass of the surface wax (Figure 3). The kinetics of MAG accumulation was similar to DAG in that it was detected and produced at its highest rate late in the wax accumulation period (Supplemental Figure 5). The regiospecificity of the acyl chain of MAG, determined by GC, was primarily in the sn-2 position of the glycerol backbone at all stages of development (i.e., sn-2 MAG) (Supplemental Figure 6A). Additional analysis by mass spectrometry confirmed the identity of the isoforms and revealed a molar ratio of sn-2 MAG: sn-1/3 MAG of 7:1 for both C16:0-MAG and C14:0-MAG (Supplemental Figure 6B). (Methods used in this study do not distinguish between a fatty acid that is esterified to the sn-1 or sn-3 positions; therefore, they are denoted as sn-1/3 throughout the manuscript.) We were unable to find any reports on the accumulation of MAG in oilseeds (Slack et al., 1980) or other oil accumulating plant tissues. However, sn-2 MAG is produced as an intermediate for the biosynthesis of the surface glycerolipid polymer cutin and accumulates as a component of root waxes (Li et al., 2007; Pollard et al., 2008; Yang et al., 2010).
Together, the accumulation of MAG and DAG and the enrichment of acyl chains in the sn-2 position of MAG implied that Bayberry DAG and TAG synthesis differs from conventional TAG synthesis in plants. Furthermore, the kinetics of sn-2 MAG and DAG accumulation in the wax is consistent with a role as intermediates for TAG synthesis, as opposed to products of lipolysis.
[14C]-Glycerol Radiolabeling Supports a Pathway from sn-2 MAG → DAG → TAG
To investigate how Bayberry synthesizes its unusual surface wax, knob tissue was incubated with [14C]-acetate and [14C]-glycerol precursors, and the incorporation of the label into glycerolipids was monitored through time. [14C]-glycerol is converted into G3P, the initial molecule that is acylated to produce glycerolipids, while [14C]-acetate enters fatty acid synthesis and labels newly synthesized fatty acids (Slack et al., 1977). Both labels have been used extensively to study membrane and storage lipid synthesis in leaves and oilseeds and to infer metabolic precursor-product relationships, pool sizes, and fluxes (Harwood, 1988; Bates and Browse, 2012; Allen et al., 2015) but have not been used to identify pools of glycerolipid intermediates for cuticular lipid synthesis.
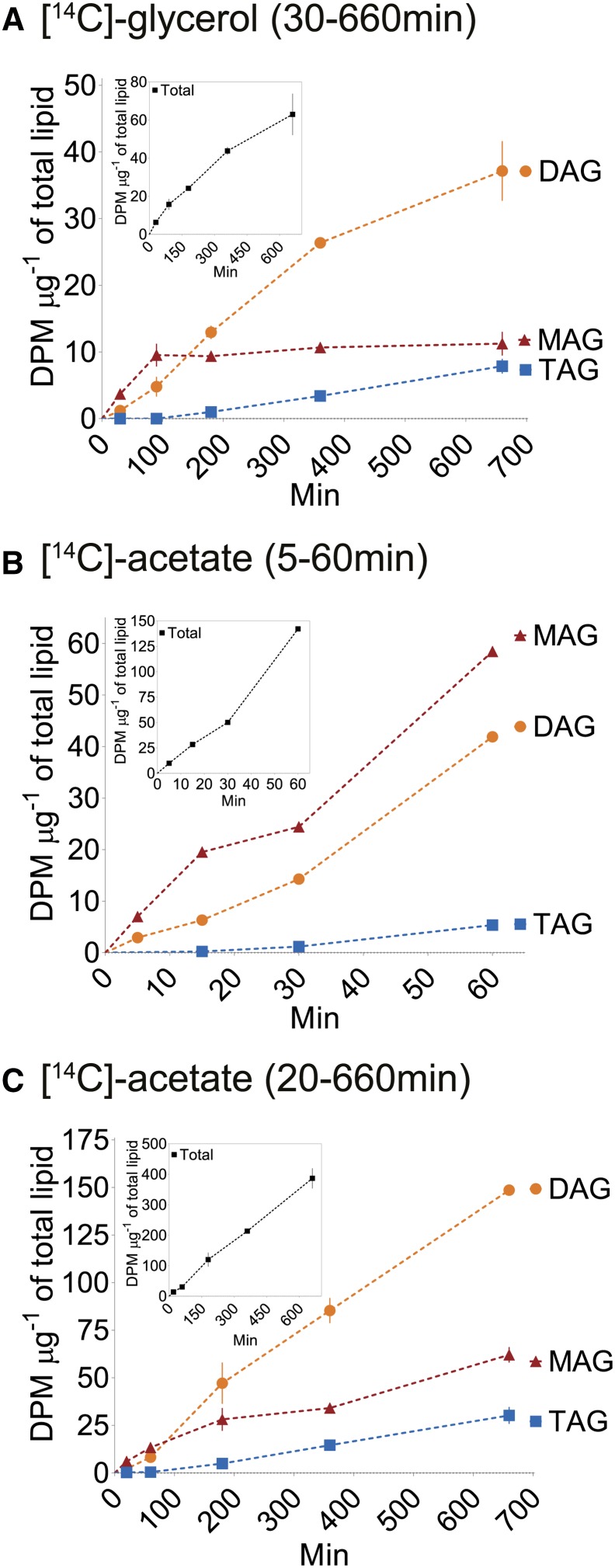
Knobs isolated from freshly harvested fruits were incubated for up to 11 h with [14C]-glycerol, and [14C]-incorporation into lipids was analyzed by TLC at five time points. In agreement with the composition of the surface wax, [14C]-MAG, -DAG, and -TAG were abundantly labeled, representing 80 to 90% of the [14C]-glycerol incorporated into lipids throughout the time course (Supplemental Table 2). To identify the position of the acyl group on glycerol, the sn-1/3 and sn-2 isoforms of [14C]-MAG were separated by borate-impregnated TLC (Thomas et al., 1965). Consistent with the identification of sn-2 MAG in Bayberry wax (Supplemental Figure 6), over 80% of the newly synthesized [14C]-MAG occurred as the sn-2 isoform (Supplemental Figure 7). These data point toward sn-2 MAG as an intermediate in the pathway to produce Bayberry surface DAG and TAG. Other [14C]-lipids, notably phosphatidylcholine (PC), which is extensively labeled in leaf or seed incubations (Slack et al., 1977, 1978) represented less than 5% of the total radioactivity (Supplemental Table 2).
We next asked whether sn-2 MAG is radiolabeled in a pattern consistent with its participation as an intermediate for Bayberry DAG and TAG synthesis. Because some [14C]-glycerol can enter glycolysis and subsequently label acyl chains (Slack et al., 1977, 1978), radiolabel specifically in the glycerol backbone of MAG, DAG, and TAG was determined (see Supplemental Table 3 for acyl chain versus glycerol labeling results). At the earliest time point (30 min), [14C]-MAG represented ∼85% of the [14C]-glycerol backbone of glycerolipids and [14C]-MAG increased through 90 min, after which it remained at a steady state (Figure 4A). [14C]-glycerol incorporation into DAG lagged behind MAG through the first 90 min of labeling, but afterwards [14C]-DAG became the most abundant radiolabeled lipid. Since MAG represented less than 1% of the glycerolipid mass of the tissue at this stage of development, these kinetics are consistent with the filling of a precursor pool of [14C]-MAG, followed by [14C]-MAG acylation to form [14C]-DAG. [14C]-TAG was labeled after [14C]-MAG and [14C]-DAG, with a substantially longer lag time, and [14C]-TAG continually increased through the remainder of the 11-h labeling period. The labeling of TAG is consistent with a model of glycerol backbone flux from [14C]-DAG into [14C]-TAG. Of note, after 11 h, radiolabel in [14C]-DAG was still 4- to 5-fold more abundant than [14C]-TAG, despite the fact that the rate of TAG accumulation in the wax at this stage of development was equal to DAG (Supplemental Figure 5 , days 38 to 45). This long lag in [14C]-TAG accumulation also suggested that there was a very large DAG intermediate pool used as the substrate for TAG synthesis and that this pool was not completely filled with radiolabeled DAG even after 11 h.
Figure 4.
Time Course of Incorporation of [14C]-Lipid Precursors into MAG, DAG, and TAG.
(A) [14C]-glycerol incorporation into the glycerol backbones (acyl chain labeling subtracted).
(B) [14C]-acetate incorporation into acyl chains through 60 min of labeling.
(C) [14C]-acetate incorporation into acyl chains through 11 h of labeling.
The inset in each graph presents the total radioactivity incorporated into glycerol backbone (A) or the organic fraction ([B] and [C]) at each time point. The data are expressed as the mean of two replicates ± range. Disintegrations per minute (DPM) was normalized to the total lipid content in the tissue at each time point. Labeling was performed in late July (days 38 to 45) with knobs that had a total lipid content (including wax) of 2000 to 3000 µg fruit−1.
Together, the kinetics of [14C]-glycerol incorporation demonstrated that sn-2 MAG is an initial glycerolipid intermediate in the production of DAG, and DAG is further acylated to form TAG. The low level of PC labeling, initial synthesis of sn-2 MAG, and the very long lag in TAG labeling is distinctly different from oilseed labeling experiments (Slack et al., 1977; Bates et al., 2007, 2009).
[14C]-Acetate Labeling Indicates Acyl Chains Enter sn-2-MAG Prior to Incorporation into DAG and TAG
The kinetics of [14C]-glycerol incorporation (Figure 4A) provided information on the flux of the glycerol backbone into MAG, DAG, and TAG. To understand the kinetics of acyl chain incorporation into glycerolipids, Bayberry knobs were incubated with [14C]-acetate for 5 min to 11 h. As with [14C]-glycerol labeling, [14C]-MAG, -DAG, and -TAG represented the majority of [14C]-acetate incorporated into lipids. [14C]-PC was never more abundant than [14C]-MAG, particularly at the earliest time points, and represented less than 10% of total [14C] incorporation (Supplemental Table 4). Notably, and unlike results from most oilseed labeling experiments (Bates et al., 2009; Allen et al., 2015), the initial kinetics of [14C]-acyl chain incorporation was very similar to the [14C]-glycerol incorporation. [14C]-sn-2 MAG was again the most abundantly labeled lipid at the initial time points (5 to 60 min), and there was a 60- to 90-min lag in radiolabel incorporation into [14C]-DAG and a much longer lag for [14C]-TAG synthesis (Figures 4B and 4C). The time-course experiments were repeated numerous times over two separate growing seasons with tissue of different ages with similar results.
The rapid initial incorporation of [14C]-acetate into the acyl chain of sn-2 MAG provided additional evidence that MAG is an early intermediate in Bayberry surface wax production. Because fatty acyl-CoA pools in plants are very small and would introduce a lag of less than 1 min (Larson and Graham, 2001; Tjellström et al., 2012), the labeling of [14C]-MAG with no discernable lag is consistent with acylation of G3P by acyl-CoA. However, the substantial lag in acyl chain incorporation into [14C]-DAG and the even longer lag in [14C]-TAG production is not observed with acyl chain labeling for plants which accumulate primarily saturated and monounsaturated fatty acids (e.g., Cacao, Cuphea, and avocado mesocarp) (Stobart and Stymne, 1985; Griffiths et al., 1988; Bafor et al., 1990; Griffiths and Harwood, 1991; Bates and Browse, 2012). In these tissues, rather than initial incorporation into PC, newly synthesized fatty acids apparently enter a common acyl-CoA pool where they have an equal chance of reacting with G3P, lysophosphatidic acid, or DAG via the Kennedy pathway and thus produce [14C]-DAG and [14C]-TAG at similar initial rates. In contrast, the significant lag time in acyl chain labeling of Bayberry [14C]-DAG and very long lag time for [14C]-TAG implied that the acyl donor pools for these acylation reactions are distinct from G3P acylation to synthesize MAG, and also from each other. One possibility is that acyl chains are incorporated into DAG and TAG via reactions that are acyl-CoA independent. Thus, the incorporation of acyl chains into Bayberry fruit DAG and TAG appears to differ from previously reported pathways for TAG synthesis in plants, including those for which PC is not an intermediate.
Evidence for DAG Biosynthesis by an Acyl-CoA-Independent Transacylase
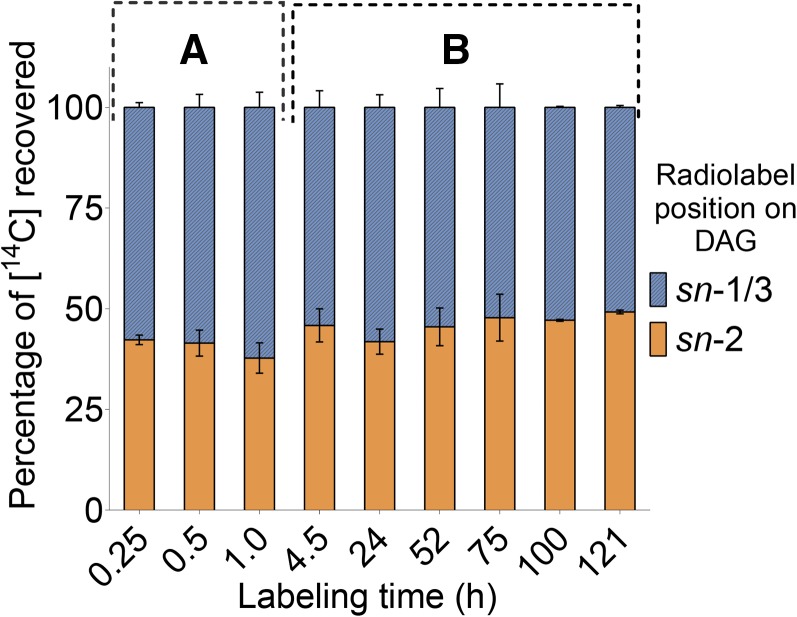
The substantial lag prior to linear accumulation of [14C]-acetate-labeled fatty acids into [14C]-DAG suggested that the pool of precursors or intermediates that provided acyl chains to MAG to form [14C]-DAG was larger than the pool of fatty acyl-CoAs that synthesized [14C]-MAG. To further examine possible reactions for DAG biosynthesis, we analyzed the distribution through time of [14C]-acyl chains on the sn-1/3 and sn-2 positions of DAG after incubations with [14C]-acetate. The regiospecific localization of [14C]-acyl chains was determined by digesting sn-1,2(2,3)-DAG with pancreatic lipase (Christie and Han, 2010) followed by TLC analysis of the resulting FFA (representing acyl chains from the sn-1/3 positions of DAG) and MAG (representing the sn-2 acyl chain of DAG).
Digestion of [14C]-DAG after both short (15 to 60 min) and long labeling incubations (up to 5 d) revealed that its [14C]-acyl chains were distributed almost equally between the sn-1/3 and sn-2 positions of its glycerol backbone (Figure 5). An equal distribution of [14C] in the sn-1/3 and sn-2 positions of DAG, as observed here, would not occur if the acyl donor had significantly lower or greater specific activity than the [14C]-sn-2 MAG acyl acceptor (Supplemental Figure 8). Instead, the equal distribution of [14C] on both acyl chains of DAG indicated that [14C]-DAG was synthesized by acylation of the [14C]-sn-2 MAG precursor at its sn-1 (or sn-3) position by a [14C]-acyl donor with a very similar specific activity.
Figure 5.
Distribution of [14C]-Acyl Chains on the sn-1/3 and sn-2 Positions of [14C]-DAG.
Two separate incubations with [14C]-acetate were analyzed: up to 1 h labeling of dissected knobs (A) and up to 5 d of labeling with whole fruits (B). After incubations with labeled substrates, [14C]-lipids were extracted and separated by TLC. [14C]-DAG was recovered, acetylated, and digested with pancreatic lipase to determine the locations of the [14C]-acyl chains on glycerol of DAG. Each bar is the mean from two independently collected labeled Bayberry tissues ± range.
The equal distribution of [14C]-acyl chains at both positions of DAG was established within 15 min of labeling, which was before the intermediate [14C]-MAG pool had filled (i.e., reached maximum specific activity or steady state) (Figure 4). While the identity of the acyl donor to [14C]-sn-2 MAG to produce [14C]-DAG was not directly determined by these experiments, some inferences can be made. Because MAG represented almost all of the [14C]-labeled compounds at the earliest time points and, as discussed earlier, the acyl donor to MAG (to form DAG) is likely not acyl-CoA, a simple interpretation consistent with these data is that the acyl donor was another molecule of [14C]-sn-2 MAG. In this scenario, based on the almost equal distribution of labeled acyl chains at sn-1/3 and sn-2 positions of DAG, the sn-2 MAG acyl donor may be from the same intermediate pool of sn-2 MAG that provided the glycerol backbone and sn-2 acyl chain of DAG. This reaction can be considered a MAG:MAG transacylation. We note that acyl-CoA-independent MAG:MAG transacylation (forming DAG) has been described in animals via a reaction catalyzed by phospholipase A2 (iPLA2) (Waite and Sisson, 1973; Jenkins et al., 2004; Gao and Simon, 2005). In addition, safflower microsomes incorporated both the acyl and glycerol moieties from [14C]-sn-2 MAG into DAG and TAG (Stobart et al., 1997).
Evidence for TAG Synthesis by an Acyl-CoA-Independent Mechanism
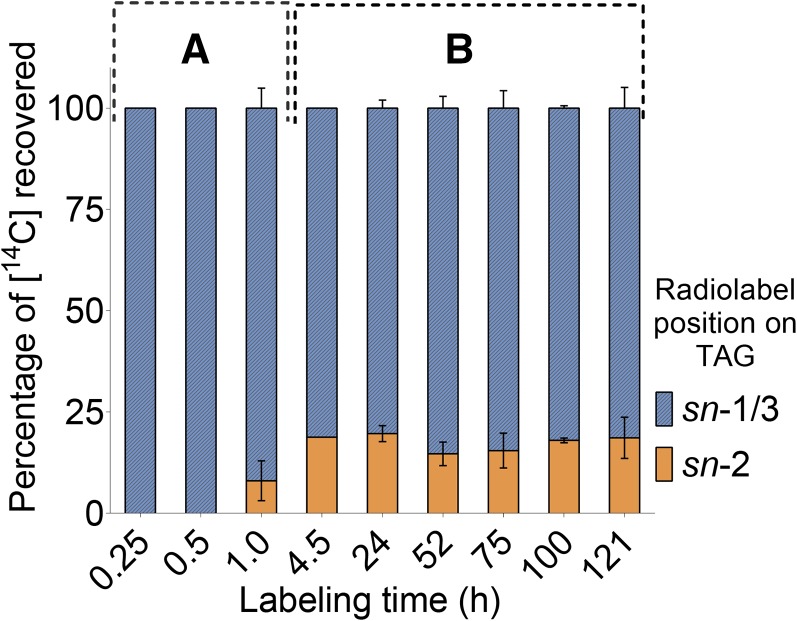
To evaluate the reaction mechanism and possible acyl donor for TAG synthesis, the positional distribution of [14C]-acyl chains on the glycerol backbone of TAG was examined by digestion with pancreatic lipase. The positional labeling of acyl chains of [14C]-TAG differed markedly from [14C]-DAG (Figure 6). After 30 min of [14C]-acetate labeling, radiolabel could only be detected in the sn-1/3 positions of TAG, and at 60 min, 90% of label was derived from the sn-1/3 positions. Furthermore, even after 5 d of continuous incubation with [14C]-acetate, acyl chains in the sn-1/3 positions were still at least 4-fold more highly labeled than [14C] at the sn-2 position of TAG. This indicated that the [14C]-acyl donor pool had a consistently higher [14C]-per acyl chain (i.e., specific activity) compared with the DAG backbone (i.e., acceptor) pool. One interpretation of the distribution of labeled acyl chains is that most of the [14C]-TAG synthesized was derived from an unlabeled DAG, rather than from the newly synthesized [14C]-DAG molecules. The fact that this result was observed throughout 5 d of labeling indicates that the biosynthetically active DAG pool involved in TAG synthesis is very large.
Figure 6.
Distribution of [14C]-Acyl Chains on the sn-1/3 and sn-2 Positions of [14C]-TAG.
Two separate incubations with [14C]-acetate were analyzed: up to 1 h labeling of dissected knobs (A) and up to 5 d of labeling with whole fruits (B). After incubations with labeled substrates, [14C]-lipids were extracted and separated by TLC. [14C]-TAG was recovered and digested with pancreatic lipase to determine the locations of the [14C]-acyl chains on sn-1/3 and sn-2 positions of glycerol. Each bar is the mean from two independently collected labeled Bayberry tissues ± range.
As discussed above (i.e., Figures 4B and 4C), the kinetics of [14C]-acetate incorporation into DAG and TAG was very different from other plant tissues with a high content of saturated and monounsaturated fatty acids. Specifically, in those examples, acyl-CoA provides the acyl chains for DAG and TAG and the reactions are catalyzed by acyl-CoA-dependent acyltransferase enzymes (i.e., LPAAT, DGAT1, and DGAT2). In contrast, labeling results for Bayberry wax synthesis point toward acyl-CoA-independent reactions for DAG and TAG synthesis. A reaction mechanism for TAG synthesis that is supported by the labeling kinetics of sn-2 MAG, by the regiospecific labeling of DAG, and by lack of label in other compounds is acylation of unlabeled or less labeled DAG with a [14C]-acyl chain from [14C]-sn-2 MAG (Supplemental Figure 9 schematically outlines the alternative scenarios for TAG labeling). This reaction would be considered a MAG:DAG transacylase.
The labeling data do not support other acyl-CoA-independent reactions for TAG synthesis. In plants, the enzyme phospholipid:diacylglycerol acyltransferase (PDAT) produces TAG via PC donating an acyl chain from its sn-2 position onto DAG (Dahlqvist et al., 2000). However, PDAT was not considered as a significant source of acyl chains for Bayberry TAG because PC was not abundantly labeled, and PDAT expression was barely detectable (see RNA-seq results below). The labeling data were also not consistent with TAG synthesis by a DAG:DAG transacylase, as occurs in animal cells (Lehner and Kuksis, 1993; Yamashita et al., 2014), yeast (Ghosal et al., 2007), and safflower microsomes (Stobart et al., 1997). Specifically, a DAG:DAG transacylase would catalyze an exchange of acyl chains between two molecules of [14C]-DAG. However, in Bayberry, because both acyl chains of [14C]-DAG were equally labeled, the DAG:DAG transacylase reaction would result in the retention of label in TAG from the sn-2 position of the acceptor DAG. In this case, both sn-1/3 and sn-2 positions would be labeled, resulting in a ratio close to 2:1 of [14C] in the sn-1/3 and sn-2 positions of [14C]-DAG. This distribution is in contrast to the consistently greater than 4:1 ratio observed in [14C]-TAG produced by Bayberry (Figure 6).
Evidence for Extracellular Accumulation of Newly Synthesized sn-2 MAG, DAG, and TAG
To evaluate the secretion of Bayberry wax, the distribution of newly synthesized [14C]-glycerolipids between the extracellular wax and the lipids that remained associated with the knob tissue after wax removal was determined. Intact Bayberry fruits were incubated for 1 h to 5 d with [14C]-acetate, and at each harvest time the surface lipids were separated from lipids that remained associated with knob tissue by dipping fruits in chloroform for 30 s. Within 1 h after addition of labeled acetate to the tissue, [14C]-MAG, -DAG, and -TAG were predominantly detected in the extracellular wax (Supplemental Figure 10A). At 1 h, label incorporation into DAG and TAG was still in the lag phase, indicating intermediate pools of MAG and DAG were not saturated with [14C]. When the proportion of [14C]-glycerolipids in the two fractions was averaged across multiple time points collected through two experiments (Supplement Figure 10B), [14C]-MAG accumulated to comparable levels in both the extracellular lipids and the lipids that remained in the knob tissue, while in contrast, [14C]-DAG and [14C]-TAG were found almost exclusively in the extracellular surface wax extract.
The rapid accumulation of [14C]-glycerolipids in the extracellular lipids, notably [14C]-DAG and -TAG, was consistent with the neutral lipid-specific staining (Figure 2), which illustrated a lack of accumulation of glycerolipids within knob cells. Furthermore, the detection of [14C]-MAG in the extracellular lipids suggests that MAG may be biosynthetically active outside of the cell. In cutin synthesis, sn-2 MAG is secreted from epidermal cells to the surface (Li et al., 2007) and sn-2 MAG is an acyl donor for cutin polyester synthesis via a GDSL-motif enzyme (Yeats et al., 2012). In Bayberry, MAG is only a minor (0.5 to 1% wax mass) component of the surface wax at developmental stages used for labeling, while [14C]-MAG represented up to 70% of the [14C]-glycerolipids in the extracellular wax extract. Thus, by analogy, these data suggest that extracellular sn-2 MAG may be an acyl donor for extracellular glycerolipid synthesis for Bayberry wax.
Exogenously Added sn-2 MAG Is Incorporated into DAG and TAG
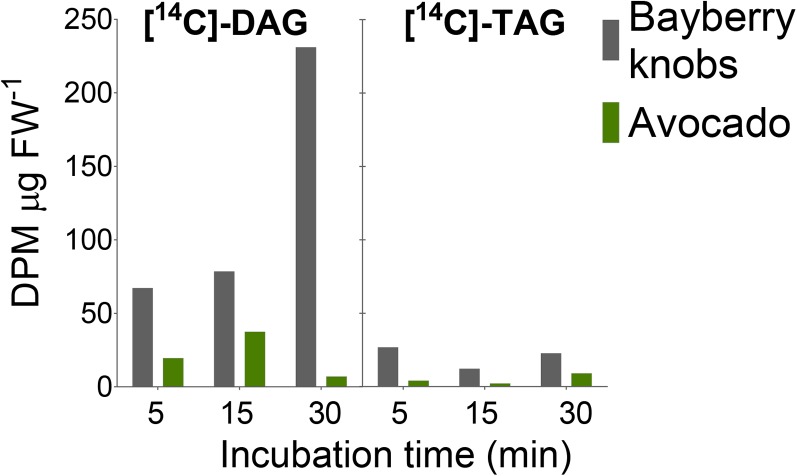
The kinetics of [14C]-acetate incorporation into labeled acyl chains together with the distribution of the acyl chains on the glycerol backbone suggested that acyl-CoA-independent reactions participate in DAG and TAG synthesis and that sn-2 MAG may act as an acyl donor. To directly test the capability of Bayberry knobs to incorporate radiolabel from sn-2 MAG into DAG and TAG, knobs were incubated with [14C]-sn-2 MAG for 5 to 30 min (Figure 7). Radiolabeled DAG and TAG were both detected within 5 min and [14C]-DAG increased through time. Reverse-phase TLC of the radiolabeled DAG and TAG isolated from the incubations confirmed their identities. [14C]-DAG and [14C]-TAG synthesis in these incubations was 3- to 4-fold higher than in parallel control incubations with avocado mesocarp. Thus, these results provide direct evidence that Bayberry knobs can synthesize [14C]-DAG and [14C]-TAG from [14C]-sn-2 MAG.
Figure 7.
Synthesis of [14C]-DAG and [14C]-TAG by Bayberry Knobs or Avocado Mesocarp Tissue Incubated with [14C]-sn-2 MAG.
Each bar represents the total disintegrations per minute (DPM) recovered from a lipid extract of the tissue and incubation media. Data were normalized to the tissue fresh weight. This experiment was repeated and incubation was extended to 3 h. Similar trends were observed at the initial time points (1 h), but avocado DAG and TAG production accelerated after 1 h. Gray, Bayberry knobs; green, avocado mesocarp tissue. FW, fresh weight.
Genes Related to Biosynthesis and Secretion of Surface Lipids Are Very Highly Expressed in Bayberry Knobs
To identify transcripts for enzymes and proteins expressed during Bayberry surface wax production, mRNA from seven developmental stages of Bayberry knobs was analyzed by RNA-seq. The knob transcriptome was strikingly dominated by very high expression of transcripts associated with acyl lipid and cutin synthesis. Overall, 13 out of the top 55 most highly expressed annotated genes from Bayberry knobs are associated with acyl lipid metabolism (Table 2). This abundance of acyl lipid-associated transcripts exceeds castor (60% oil) and even oil palm mesocarp (90% oil), where less than 10 of the 50 most highly expressed genes are acyl lipid associated (Bourgis et al., 2011; Troncoso-Ponce et al., 2011).
Table 2. Rank (Out of ∼10,000 Contigs Annotated to Arabidopsis Loci) and Expression (RPKM) of the Highest Expressed Acyl Lipid-Related Contigs in Bayberry Knobs.
| Expression Rank | RPKM Sum | Protein Encoded by Arabidopsis Homolog | Pathway/Reaction | ||
|---|---|---|---|---|---|
| Top lipid-related transcripts | |||||
| 1 | 81,312 | Lipid transfer protein (LTP) type 1 (AT2G38540) | Lipid secretion | ||
| 4 | 36,499 | DCR/PEL3/HXXXD acyltransferase | Cutin synthesis | ||
| 8 | 23,603 | sn-2 GPAT4/8 | Cutin synthesis | ||
| 11 | 12,112 | sn-2-GPAT6 | Cutin synthesis | ||
| 19 | 10,147 | GDSL1-motif lipase/transacylase (CuS1/SiCD1) | Cutin synthesis | ||
| 32 | 8,029 | GDSL2-motif lipase/transacylase (AT3G16370) | Cutin synthesis | ||
| 33 | 7,817 | LTP_2 (AT1G48750) | Lipid secretion | ||
| 35 | 7,061 | Keto-acyl CoA synthase (KCS) 10 | Fatty acid elongation | ||
| 36 | 6,926 | KCS19 | Fatty acid elongation | ||
| 37 | 6,705 | LTP_2 (AT3G18280) | Lipid secretion | ||
| 49 | 5,426 | ABCG Transporter 1 (WBC1) | Lipid secretion | ||
| 52 | 4,707 | Acyl carrier protein (ACP) 4 | Fatty acid synthesis | ||
| 54 | 4,705 | Acyl-ACP thioesterase B (FATB) | Fatty acid synthesis | ||
| Transcripts for conventional TAG synthesis | |||||
| 1538 | 418 | Putative diacylglycerol acyltransferase 3 (DGAT3) | |||
| 1613 | 401 | Lysophosphatidic acid acyltransferase 3 (LPAAT3) | |||
| 2372 | 268 | Phosphatidylcholine: diacylglycerol acyltransferase 1 (PDAT1) | |||
| 3960 | 151 | Diacylglycerol acyltransferase 2 (DGAT2) | |||
| 4495 | 126 | sn-1 glycerol-3-phosphate acyltransferase 9 (GPAT9) | |||
| *DGAT1 AND LPAAT 2, 4 were not detected | |||||
The ranks were determined from the RPKM summed across seven developmental stages. Each gene is annotated according to its Arabidopsis homolog. The complete list of genes and developmental profiles are presented in Supplemental Data Set 1.
The identities of these very highly expressed transcripts in Bayberry knobs are illustrative of its highly specialized lipid metabolism. First, the most abundantly expressed acyltransferases were a sn-2 glycerol-3-phosphate acyltransferase (sn-2 GPAT) (Zheng et al., 2003; Beisson et al., 2007); an HXXXD-motif acyltransferase closely related to Arabidopsis DEFECTIVE IN CUTICULAR RIDGES (DCR) (Panikashvili et al., 2009); and GDSL-motif lipase/transacylases related to CUTIN DEFICIENT1 (CD1) (Girard et al., 2012; Yeats et al., 2012). As described below, the putative or reported activities of these acyltransferases are consistent with the lipid structures produced for Bayberry surface wax and with the enzymatic reactions predicted by the labeling data and with the extracellular localization of the glycerolipids. Furthermore, transcripts for these three cutin-associated acyltransferases were expressed at levels 50-fold or higher than acyltransferases in known TAG assembly pathways (e.g., GPAT9, LPAAT, DGAT, and PDAT), and none of the “conventional” TAG-associated acyltransferases were ranked among the top 1400 most highly expressed transcripts of Bayberry knobs (Table 2). Second, Bayberry surface glycerolipids are exceptional in that over 99% of the fatty acids are saturated. In agreement, the most highly expressed transcript among the fatty acid synthesis pathway was acyl-ACP thioesterase B (FATB), the enzyme that preferentially releases saturated fatty acids from ACP in the fatty acid synthetase complex. Third, the secretion of waxes and cutin precursors to the plant surface requires ATP binding cassette transporters of the G subfamily (ABCG) and is believed to be coordinated with lipid transfer proteins (LTPs) (Samuels et al., 2008). Accordingly, transcripts assigned to one ABCG transporter and three LTPs were among the most highly expressed transcripts in Bayberry knobs.
The three extremely highly expressed cutin-associated acyltransferases, sn-2 GPAT, DCR, and GDSL-motif lipase/transacylase, represent enzymes that together with the labeling and biochemical data provide insights into biosynthetic mechanisms for Bayberry wax synthesis. First, unlike the GPAT reaction that acylates the sn-1 position of G3P to initiate intracellular TAG synthesis, the sn-2 GPATs are bifunctional enzymes that acylate the sn-2 position of G3P and remove the phosphate to produce sn-2 MAG. Among the three sn-2 GPAT clades previously characterized (Li et al., 2007; Li-Beisson et al., 2009; Yang et al., 2010), the highly expressed Bayberry GPAT transcripts were most closely related to genes required for cutin biosynthesis (Arabidopsis sn-2 GPAT 4/8 and 6 isoforms) and were less related to suberin associated or other members of the sn-2 GPAT family (Supplemental Figure 12A). Furthermore, their expression increased through the development of the wax layer (Supplemental Figure 11A).
Next, the most abundant transcript that encodes an enzyme in Bayberry knobs is a close homolog to the Arabidopsis HXXXD (BAHD) acyltransferase encoded by DCR (Supplemental Figures 11C and 12C). Arabidopsis DCR is a soluble, cytosolic enzyme that is essential for production of dihydroxy16:0 rich cutin (Panikashvili et al., 2009). In vivo substrates for DCR were not identified; however, it was proposed that DCR might be involved in intracellular concatenation of the cutin polyester (Panikashvili et al., 2009; Molina and Kosma, 2015). Although DCR has diacylglycerol acyltransferase activity in vitro (Rani et al., 2010), flax DCR could not complement the Arabidopsis dgat1 knockout mutant (Pan et al., 2013) and DAG or TAG has not been identified as an intermediate in cutin production. That DCR is the most highly expressed of all enzyme transcripts in Bayberry knobs and is temporally correlated with wax production implies that the enzyme has a key role in surface wax production in Bayberry.
Finally, transcripts encoding two highly expressed GDSL-motif enzymes (referred to here as GDSL1 and GDSL2) may also catalyze the assembly of Bayberry glycerolipids. Although often annotated as “lipase,” GDSL-motif enzymes can catalyze transacylase reactions, which are typically favored under acidic and low-water environments (Schmid and Verger, 1998; Akoh et al., 2004). Such conditions exist at aqueous-aliphatic interphases within the apoplast surrounding epidermal cells, where the final assembly of the cutin polyester is thought to be catalyzed by extracellular localized GDSL-motif or related lipase-like enzymes (Beisson et al., 2012; Girard et al., 2012 Yeats et al., 2012). Bayberry GDSL1 was most highly expressed at the first two stages of RNA analysis but decreased to very low levels during the remainder of the time course. GDSL2 was highly expressed at all stages of wax accumulation (Supplemental Figure 11B). GDSL1 is closely related to CD1 (74% identical; 87% similar), the “cutin synthase” identified in tomato (Solanum lycopersicum; Girard et al., 2012; Yeats et al., 2012) (Supplemental Figure 12B). Interestingly, a minor reaction in CD1 assays was the production of DAG and TAG (Yeats et al., 2012). The other highly expressed GDSL contig (GDSL2) is 53% similar to CD1 but falls into another clade for which no members have been characterized.
A Model for Bayberry Wax Biosynthesis
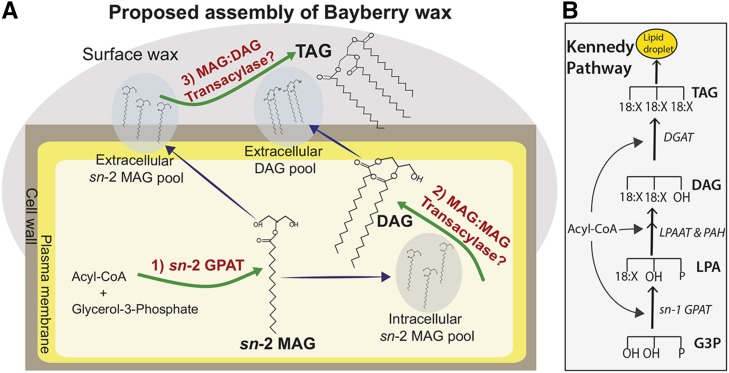
Based on a combination of molecular species analysis of glycerolipids, microscopy, radiolabeling by [14C]-acetate, [14C]-glycerol, and [14C]-MAG, and transcript analysis, we propose a sequence of intra- and extracellular reactions that result in the accumulation of glycerolipid surface wax on Bayberry fruits (Figure 8). Fluxes of glycerol and acyl chains are also presented in Supplemental Figure 13. Most aspects of the pathway are in sharp contrast to glycerolipid synthesis in oilseeds and other TAG-accumulating plant tissues and clearly indicate that Bayberry has evolved a novel pathway for soluble glycerolipid synthesis in plants. The reaction sequence proposed includes intracellular synthesis of DAG via a MAG:MAG transacylase and TAG synthesis outside of the cells via a MAG:DAG transacylase. However, we acknowledge that there may be other reactions, including some, or perhaps exclusive, extracellular DAG synthesis. The key observations that were used to formulate and support the model in Figure 8 are as follows: (1) The continuous 8-week accumulation of surface wax coupled with the light and microscopy images of the fruits indicate that components of the surface wax are not stored within the cells and instead point toward the knob cells actively secreting neutral lipids to their surface throughout development. (2) Bayberry wax contains a high proportion of DAG (∼65%) and up to 4% sn-2 MAG, which are not significant constituents of oilseeds. The fact that sn-2 MAG is an intermediate in the synthesis of cutin initially suggested that Bayberry surface wax may be synthesized by enzymatic reactions related to cutin and not conventional TAG synthesis reactions. (3) The kinetics of [14C]-glycerol labeling confirmed that sn-2 MAG is an initial intermediate and revealed that the glycerol backbone flux proceeds from sn-2 MAG →DAG→TAG. (4) [14C]-acetate labeling indicated that newly synthesized acyl chains also flux from sn-2 MAG to DAG and then to TAG with increasing lag times similar to [14C]-glycerol labeling. This indicated that the acylation of each position on the glycerol backbone of MAG, DAG, and TAG utilizes different pools of acyl-chain donors. (5) Analysis of the regiospecific distribution of [14C]-acyl chains on DAG and TAG supports a model where DAG and TAG are synthesized by acyl-CoA-independent mechanisms, and, as discussed, sn-2 MAG is a likely acyl donor for both reactions. (6) The observation that the majority of [14C]-acyl chains in [14C]-TAG are in the sn-1/3 positions (Figure 6) indicated that the DAG pool used for TAG synthesis was not completely filled with [14C]-DAG (even after 5 d of continuous labeling). Instead, these data indicate that the [14C]-DAG molecules in the active DAG pool were diluted substantially by a large amount of unlabeled DAG molecules. Because a large DAG pool inside cells was not detected by neutral lipid-specific staining (Figure 2), and major accumulation of DAG inside the cells would be expected to disrupt lipid bilayers (Goñi and Alonso, 1999), these data strongly suggest that TAG synthesis occurs outside of the cells. (7) In agreement with this hypothesis, our model also proposes a physical separation for DAG and TAG synthesis (i.e., DAG is synthesized intracellularly and TAG is synthesized extracellulary). This hypothesis is consistent with the very different lag times in DAG and TAG labeling indicating formation from independent acyl donor pools (Figure 4) but that the acyl donor for both reactions is likely sn-2 MAG. (8) The rate of DAG and MAG accumulation increased or remained high late in the season (stage 3), whereas the TAG accumulation rate decreased (Supplemental Figure 5). Possibly, increased levels of surface wax physically restrict the final extracellular acylation for TAG synthesis, whereas intracellular reactions to synthesize MAG and DAG are less affected. (9) [14C]-sn-2 -MAG, -DAG, and -TAG appeared rapidly in the extracellular wax is consistent with extracellular glycerolipid synthesis and [14C]-MAG as an acyl-donor. (10) RNA-seq of Bayberry knobs revealed that a striking proportion of highly expressed transcripts are associated with the production and secretion of cutin, while transcripts encoding enzymes of conventional TAG synthesis were low or undetectable.
Figure 8.
Initial Model for Bayberry Surface Wax Biosynthesis.
(A) The pathway proposed here is supported by multiple lines of evidence (see text), including lipid structural analysis, radiolabeling, and the identity of very highly expressed acyltransferase transcripts in Bayberry knobs (sn-2 GPAT, DCR, and GDSL-motif). The pathway begins with acylation of G3P by 16:0-CoA and synthesis of sn-2 MAG catalyzed by bifunctional sn-2 GPAT (1). The intracellular pool of sn-2 MAG is then used as a substrate for DAG synthesis by a putative MAG:MAG transacylase (2). The DAG product and a portion of the sn-2 MAG are transported outside of cells. TAG synthesis occurs when sn-2 MAG donates an acyl chain to DAG through a proposed extracellular MAG:DAG transacylase (3). The MAG:MAG transacylase and MAG:DAG transacylase reactions proposed are based on radiolabeling data and have not been demonstrated by direct assays with purified enzymes. Other enzyme reactions and/or an alternative scenario in which DAG synthesis occurs outside cells cannot be ruled out.
(B) The Kennedy pathway for TAG synthesis is also presented to illustrate the contrasting reactions of oilseed TAG synthesis to Bayberry surface wax synthesis.
Together, microscopy, biochemical analysis, radiolabeling, and expression data support a model of biosynthesis of the unique Bayberry surface wax via reactions and intermediates analogous to the synthesis of the insoluble surface glycerolipid cutin. Specifically, the highly expressed and bifunctional sn-2 GPATs would produce sn-2 MAG. Transcripts encoding a homolog of the intracellular HXXXD-motif DCR were the most highly expressed of any enzyme in Bayberry knobs, and we propose this enzyme contributes to intracellular DAG synthesis. Finally, secretion of both MAG and DAG would provide substrates for an extracellularly localized GDSL-motif enzyme to catalyze a transacylase reaction forming TAG, which is analogous to models for cutin assembly (Yeats et al., 2012, 2014).
While the data clearly identify a novel pathway to produce TAG in plants and suggest a combination of intracellular and extracellular reactions with sn-2 MAG as a primary acyl-donor, some aspects of the proposed model are uncertain and a number of questions remain. Specifically, despite the fact that most of the radioactivity applied to Bayberry fruits was recovered in MAG, DAG, and TAG, another intermediate or acyl donor, other than sn-2 MAG, for DAG or TAG acylation reactions cannot be ruled out. Although [14C]-sn-2 MAG added to knobs was a substrate for DAG and TAG synthesis, more direct evidence for the proposed reactions could be provided by enzyme assays after purification of the enzymes highly expressed in Bayberry knobs. However, we were unable to detect enzymatic activity when Bayberry DCR or GDSL-motif enzymes were heterologously expressed in Escherichia coli, yeast, or transiently in Nicotiana benthamiana. Thus, we could not directly demonstrate which enzymatic reactions are catalyzed by the DCR and GDSL-motif enzymes. GDSL-motif enzymes have broad substrate specificity (Akoh et al., 2004), and the GDSL-motif enzymes in Bayberry might be active in both intra- and extracellular synthesis of Bayberry DAG and TAG. Indeed, related lipase-like enzymes in animals can catalyze an acyl-CoA-independent formation of DAG and TAG (Yamashita et al., 2014). DCR transcripts were the highest of any enzyme in Bayberry, but a reaction mechanism by which it contributes to the assembly of Bayberry wax is not clear. Although Bayberry wax does not contain hydroxy-fatty acids as do the cutin monomers impacted in dcr, the structure of sn-2 MAG and DAG are chemically analogous in that their glycerol backbone possesses free hydroxyls. In a previous study, Arabidopsis DCR was expressed in E. coli and yeast and exhibited diacylglycerol acyltransferase activity (DGAT) and monoacylglycerol acyltransferase (MGAT) activity (Rani et al., 2010). However, radiolabeling kinetics together with the positional distribution of [14C]-acyl chains of Bayberry wax implied that DAG and TAG are not synthesized by acyl-CoA-dependent acyl transferases. Future work should explore the possibility of DCR catalyzing a CoA-independent transacylase reaction and/or identify other enzymatic partners or cofactors for alternative reactions catalyzed by DCR. Regardless of the absence of direct in vitro evidence for the proposed enzyme reactions, this study clearly indicates that plants can synthesize TAG by a novel pathway involving sn-2 MAG as an intermediate and acyltransferases related to cutin biosynthesis.
Conclusions and Significance to Understanding TAG and Surface Lipid Biosynthesis
Bayberry fruit accumulates the largest known quantity of surface wax among plants, is the only plant reported to contain almost entirely TAG and DAG in its surface wax, and may be the only example of plant TAG synthesis from exclusively saturated fatty acids with chain lengths greater than C12:0. We conclude that Bayberry wax glycerolipids are synthesized by a combination of intracellular and extracellular reactions via enzymes related to biosynthesis of the insoluble surface glycerolipid cutin. However, Bayberry wax is quite different from cutin in its structure and in the fact that the fatty acids are not modified or linked together or to the cell wall as an insoluble polyester. Why did Bayberry evolve its unique surface wax? Seed dispersal is a driving force for evolution of the very large diversity of fruit structures and composition (Lorts et al., 2008). By providing a high calorie and chemically stable food source for birds, the glycerolipids on the surface of Bayberry fruits could increase seed dispersal resulting in a selective advantage for evolution of the very abundant surface wax layer (Fordham, 1983; Place and Stiles, 1992). Bayberry knobs clearly upregulated the expression of a very specific subset of genes associated with cutin production and saturated fatty acids to achieve its extraordinarily specialized metabolism.
Bayberry could be a useful model to study surface lipid production and secretion in addition to TAG synthesis in non-seed tissues. The fact that Bayberry produces surface lipids at many fold higher levels than other plant species may allow for improved visualization and biochemical analysis of the mechanism for surface lipid secretion and a better understanding of how surface lipid synthesis interacts with other lipid metabolism in plant cells. Second, the genes and enzymatic reactions to synthesize and secrete soluble surface glycerolipids may provide new insights in understanding and potentially manipulating lipid synthesis in plants. Many efforts to produce higher value lipids and increase quantities of lipids in crop species have been stymied by deleterious effects on cell metabolism and pathway bottlenecks (e.g., Bates and Browse, 2011; Carlsson et al., 2011). Bayberry provides an example where modifications in surface lipid pathways, specifically in genes used to produce cutin, can enable the plant to synthesize extracellular TAGs. Future work should address what specific gene modifications may be required to secrete DAG and TAG and how it can be used to as a strategy to engineer the accumulation of valuable, but potentially deleterious, lipids in plants. Furthermore, the mechanism Bayberry employs to secrete lipids might also be useful to secrete other types of hydrocarbons.
METHODS
Plant Material and Collection
Myrica pensylvanica fruits were collected from three separate stands of plants on the campus of Michigan State University (42°43N, 84°23W). Experiments were done on tissue harvested from 2011 to 2015. Fruit development was comparable each year, although we noticed that fruits developed earlier (∼1 to 2 weeks) in 2012 when spring temperatures were warm. Fruits were used fresh (i.e., within minutes after collection from plants) or were immediately frozen in liquid nitrogen and stored at −80°C for later analysis. Intact knobs were either dissected from the fruits by gently scraping them with a syringe needle or when the fruits hardened the knobs were removed be gently grinding the whole fruit with a mortar and pestle or Polytron.
Stereospecific Numbering Nomenclature
In this manuscript, the sn (stereospecific numbering) nomenclature is used to denote the position of fatty acids on the glycerol backbone of MAGs, DAGs, and TAGs. sn-2 refers to fatty acids esterified to carbon 2 of glycerol (also called the beta carbon). sn-1 and sn-3 refer to the terminal carbons of the glycerol backbone (also called alpha carbons). Methods used in this study do not distinguish between a fatty acid that is esterified to the sn-1 or sn-3 positions; therefore, they are denoted as sn-1/3 throughout the manuscript.
Wax and Lipid Extraction
Surface wax was extracted by immersing whole intact Bayberry fruits in 5 to 20 mL (depending on the number of fruits used) of chloroform for 20 to 30 s. Testing of various extraction times indicated that 20 to 30 s releases greater than 90% of the available surface wax monomers. The chloroform fraction with the soluble waxes was separated from the fruits, and the solvent was removed under N2. In some experiments, after surface lipid extraction, the knob lipids or whole fruit lipids were recovered by quenching the tissue in 85°C isopropanol for 10 min, grinding with a Polytron followed by a 3:2 hexane:isopropanol extraction (Hara and Radin, 1978). After drying under N2, surface wax and internal lipid extractions were redissolved in 1 mL of toluene or hexanes or chloroform, capped under N2, and stored at −20°C until further analysis.
Lipid Quantification
The glycerolipids of Bayberry wax were analyzed by GC with flame ionization detection using a high-temperature DB-5 column (length 30 m × 0.25 mm i.d., 0.1-µm film thickness; Agilent). To facilitate better separation of hydroxyl-containing compounds (i.e., FFA, MAG, and DAG) in the wax, samples were derivatized overnight at 50°C with bis-N,N-(trimethylsilyl) trifluoroacetamide in pyridine and toluene as cosolvent. Derivatized samples were then dried under N2 and immediately and redissolved in 1:1 n-heptane:toluene (1:1 [v/v]). The GC temperature program was as follows: inlet temperature of 380°C, oven temperature of 250°C for 3 min, and then ramped to 370°C at 10°C min−1 and held for 15 min. Because the response factors for intact TAG decreases as the carbon number increases, correction factors were applied by creating standard curves using saturated TAG standards from Tri13:0 to Tri 20:0 (Buchgraber et al., 2004).
In addition to intact glycerolipid analysis, some lipid samples were quantified based on their transmethylated fatty acid content (i.e., as FAMEs). Samples were transmethylated in 1 mL of 5% H2SO4 (v/v) and supplemented with 300 μL of toluene at 85°C for 2 h. FAMEs were extracted once with 2 mL of hexane, and the solvent was evaporated under N2. FAMEs were then redissolved in n-heptane and analyzed by GC according to Li-Beisson et al. (2013).
For all lipid quantification, appropriate glycerolipid or FAME internal standards were added during the extraction and prior to analysis.
Microscopic Analysis of Bayberry Tissue
Visualization of Bayberry fruits was performed with standard light microscopy, scanning electron microscopy, and confocal fluorescence microscopy. To characterize the developmental progression of Bayberry fruits, fresh and paraffin-fixed tissue was photographed using a dissecting or compound light microscope with an attached digital camera. Fixation of Bayberry fruits was done essentially as described by Karlgren et al. (2009). Sections were cut to thickness of 10 to 20 µm, attached to a microscope slide, and stained with toluidine blue for 5 to 10 min (Brundrett et al., 1991).
For scanning electron microscopy, whole fruits were frozen in liquid nitrogen followed by freeze drying (Electron Microscopy Sciences model EMS750X). Dried samples were mounted on aluminum stubs using carbon suspension cement and high vacuum carbon tabs. Samples were then coated with gold (∼20 nm thickness) and osmium (∼10 nm thickness) and examined in a JEOL 6610LV scanning electron microscope (tungsten hairpin electron emitter).
For confocal microscopy, freshly harvested fruits were hand-sectioned in half and stained with 2 µg mL−1 BODIPY 493/503 (excitation wavelength 488 nm, detected between 505 and 545nm) or Nile red (excitation wavelength 559 nm, detected between a 570 and 640nm). Stained and unstained (control) samples were then mounted in 5% glycerol and viewed under an Olympus FluoView FV1000 confocal laser scanning microscope configured on an IX81 automated inverted microscope using a 100× UPlanSApo (NA 1.4) objective. Chlorophyll autofluorescence was also collected using an excitation wavelength or 488 nm and collected between 655 and 755nm. All images obtained were analyzed using FluoView FV1000 FV10-ASW advanced software version 4.2.
Radiolabeling of Bayberry Fruits with [14C]-Acetate or [14C]-Glycerol
Radiolabeling was conducted with both dissected knobs and intact whole fruits. Freshly harvested tissue was incubated in buffer containing 20 mM MES (pH 5.7), 0.1 M sorbitol, 0.1× Murashige and Skoog salts, 0.01% Tween, 1 g of sucrose, and either 200 µCi [1-14C] of acetate (specific activity 52 mCi mmol−1; Perkin-Elmer) or 16 µCi [14C-(U)] glycerol (specific activity 150 mCi mmol−1; Perkin-Elmer). The dissected knobs were incubated in 5 to 10 mL of buffer, while whole fruits were incubated in 20 to 25 mL of buffer. The tissue was gently shaken at room temperature under 50 to 100 µE of white light. At each harvesting time, a portion of the tissue was removed from the buffer, and lipids were extracted as described above. To account for different amounts of knob tissue harvested at different time points, radioactivity was normalized based on the total fatty acids content of respective lipid extracts, or when whole fruits were labeled, to the number of fruits harvested. Total radioactivity in lipids was quantified by scintillation counting (LS 6500; Beckman Coulter). In some incubations with whole fruits, surface wax was first extracted from the fruits with chloroform and then lipids were extracted from the knobs, as described above.
Radiolabeled lipids were separated on 20 × 20-cm K6 TLC plates by developing the TLC plate first to 12 to 14 cm in chloroform:methanol:acetic acid: water (85:15,10.3.5, v/v/v/v) to separate polar lipids, followed by neutral lipid separation by developing the TLC plate to its full length in hexane:diethyl ether:acetic acid (70:30:1, v/v/v). Labeled lipids were identified and quantified by autoradiography of TLC plates exposed in a Bio-Rad cassette for 3 to 24 h and using a PMI FX phosphor imager (Bio-Rad). Labeled lipid classes were positively identified by comigration with nonlabeled standards.
For [14C]-glycerol-labeled lipids, the amount of label in the acyl groups was compared with the backbone. Bands were eluted from TLC plates using chloroform:methanol:water (5:1:1, v/v/v) and then transmethylated followed by scintillation counting of the separated organic and aqueous phases.
Regiospecific Analysis of the Radiolabeled Acyl Chains
Regiochemistry of acyl chains in DAG and TAG was performed as previously described (Christie and Han, 2010). Briefly, 1,2(2,3)-DAG or TAG was purified by TLC. DAG was then acetylated with acetic anhydride and methanol at 50°C overnight, and the acetylated DAG was repurified by TLC (hexane:diethyl ether:acetic acid, 70:30:1, v/v/v). A dried mixture of acetylated Bayberry DAG or TAG containing 100 µg of unlabeled C18:1-methyl ester was incubated in 900 μL of 1 M Tris-HCl (pH 8.0), 125 μL of 2.2% CaCl3, 250 μL of 0.25% bile salts, and 25 μL of hexanes. The hexanes and FAME were added to aid in solubilization of the completely saturated Bayberry lipid mixtures (Brockerhoff, 1965; Barford et al., 1966). The reaction was preincubated in an orbital shaker at 400 rpm at 42°C for 15 min and then 0.5 mg of pancreatic lipase was added. After a 4-min incubation with the lipase, the reaction was immediately quenched with 1 mL of 6 M HCl and 1 mL of ethanol and then extracted three times with diethyl ether. Lipids were dried under N2 and separated by TLC and analyzed as described above.
Synthesis of [14C]-sn-2 MAG Substrate and Incubation with Bayberry Fruits
[14C]-myristoyl-sn-2 MAG was prepared by pancreatic lipase digestion of trimyristin [1-14C] (specific activity 55 mCi mmol−1; American Radiochemicals) as described above, except that the digestion time was extended to 15 min and the reaction quenched by adding 2 mL of 5% boric acid in 50% ethanol to minimize acyl migration in MAG (Thomas et al., 1965). Digested lipids were then separated by TLC impregnated with 5% boric acid, and [14C]-sn-2 MAG was eluted from the TLC and used in an assay within 1 d. Approximately 12% of the [14C]-TAG added was recovered in [14C]-sn-2 MAG.
A standard assay contained ∼0.7 µCi of [14C]-sn-2 MAG in 500 μL of labeling buffer added to knobs dissected from one Bayberry fruit. This equated to a concentration of 60 µM sn-2 MAG per assay. At each time point, the knobs and surrounding media were collected and lipids were extracted using 1:2 chloroform:methanol.
RNA-Seq of Bayberry Knob Tissue
RNA was extracted from Bayberry knobs through seven stages of wax development, from when surface wax was undetectable to ∼50% of its final production. Knobs were collected by gently scraping the fruit with a syringe needle. For comparison, RNA from young and mature Bayberry leaves was also extracted for sequencing. Triplicate biological replicates were collected at one stage of fruit development, three time points were collected in duplicate, and three time points were collected with one replicate. Total RNA was extracted from knobs and leaves using a method similar to Meisel et al. (2005). For each extraction, knobs or leaves were first ground to a fine powder in liquid N2, followed by adding hot (∼65°C) extraction buffer that was vigorously mixed with the tissue and then incubated at 65°C for 10 min. The extraction buffer contained 2% (w/v) CTAB, 2 M NaCl, 0.05% (w/v) spermidine, 100 mM Tris-HCl (pH 8.0), 25 mM EDTA, 3% (v/v) β-mercaptoethanol, and 3% (w/v) PVPP at a ratio (w/v) of tissue to buffer of 1:10. The mixture was allowed to cool to room temperature and insoluble material was removed by two extractions with 24:1 chloroform:isoamyl-alcohol (10,000g for 20 min at room temperature). The aqueous layer containing the nucleic acids was precipitated twice, once with ethanol containing 50 μg mL−1 glycogen (Ambion) at −20°C overnight followed by a second overnight precipitation with 2.5 M lithium chloride in 100 μL of RNase free water at 4°C. The concentrated RNA was treated with DNase (Ambion) and then cleaned using the RNeasy MinElute kit (Invitrogen). The purity of the RNA was assessed with an Agilent 2100 Bioanalyzer.
A total of 14 RNA samples from knobs and leaves were sequenced at the Joint Genome Institute. For each sample, stranded cDNA libraries were generated using Illumina mRNA sample preparation protocols. Briefly, mRNA was extracted from 10 µg of total RNA with magnetic beads containing poly(T) oligos. mRNA was then fragmented with divalent cations and high temperature and then reverse transcribed using random hexamers and SSII (Invitrogen) followed by second-strand synthesis. The cDNA was then treated with end-pair, A-tailing, adapter ligation, and 10 cycles of PCR. qPCR was then used to determine the concentration of the libraries.
The cDNA was subjected to paired-end sequencing using an Illumina HiSeq, obtaining a read length of 150 nucleotides. The data were computed using a combination of CLC workbench (version 7.5; www.clcbio.com) and Trinity software package (version r20140413). Briefly, reads were screened against the silva rRNA database (Quast et al., 2013) and trimmed on quality and adapter sequence using the Trim Sequences program from CLC Genomics Workbench. Reads were normalized with Trinity’s in silico normalization package and assembled in stranded orientation. De novo transcriptome assembly was done with a sample that contained a mixture of RNA from knobs from seven developmental stages and from two stages of leaves (Grabherr et al., 2011). Contigs were annotated against the Arabidopsis thaliana proteome (TAIR 10.0) with BLASTX. Arabidopsis was selected because annotations of its proteins are the most authoritative and well supported by data (Li-Beisson et al., 2013). Reads from each time point and leaves were mapped against the de novo assembly and quantified using the Genomics Workbench RNA-seq Analysis program (similarity fraction, 0.8; length fraction, 0.75). RPKM for each contig, top Arabidopsis homolog, and assembly statistics for each library can be found in Supplemental File 1.
Phylogenetic Tree Construction
Multiple alignments and the phylogenetic trees were constructed with bioinformatic tools available at www.phylogeny.fr using the default settings (MUSCLE 3.7 was used for multiple alignment, Gblocks 0.91b was used for alignment refinement, and PhyML 3.0 was used to generate the phylogenies), and trees were modified with MEGA6. Text files of the alignments can be found in Supplemental Data Set 2.
Accession Numbers
Bayberry sequences can be downloaded from the JGI genome portal (http://genome.jgi.doe.gov/) under project ID 1007578, and sequencing details can be found at NCBI under BioProject number PRJNA250987. The non-Bayberry sequences used for each tree and sequence identifiers are as follows: (A) GPATs (all from Arabidopsis [At]): GPAT1 (At1g06520), GPAT2 (At1g02390), GPAT3 (At4g01950), GPAT4 (At1g01610), GPAT5 (At3g11430), GPAT6 (At2g38110), GPAT7 (At5g06090), and GPAT8 (At4g00400); (B) GDSL-motif enzymes: tomato (Si) CD1/CuS1 (Solyc11g006250), CGT (E7AIM3); Agave americana (Aga) SGNH hydrolase (Q5J7N0); At-CuS1 (At3g04290), At-CDEF1 (At4g30140), At-GLIP2 (At1g53940), and At3g16370; Jacaranda mimosifolia JNP1 (EU350954); Physcomitrella patens (Pp) CuS1 (Pp1s34 96V6.1); (C) DCR and other HXXXD-motif acyl transferases: Linum usitatissimum (Lu) DCR1 (AHA57444), DCR2 (Lus10039256); At-DCR (At5g23940), At-CER26 (At4g13840), At-CER2 (AT4G24510), At-HCT (At5g48930), At-ASFT (At5g41040), At-FACT (At5g63560), and At3G62160; rice (Os) PMT (LOC_Os01g18744).
Supplemental Data
Supplemental Figure 1. Extraction of Bayberry surface wax by chloroform.
Supplemental Figure 2. A representative GC-FID separation of Bayberry surface wax.
Supplemental Figure 3. Mass of Bayberry fruit parts through development.
Supplemental Figure 4. Crystallization temperature of purified Bayberry wax determined by differential scanning calorimetry.
Supplemental Figure 5. Rates of accumulation of each glycerolipid class in the surface wax through development.
Supplemental Figure 6. Monoacylglycerols in Bayberry wax occur predominately as the sn-2 isomer.
Supplemental Figure 7. TLC separation of MAG isoforms after labeling Bayberry knobs.
Supplemental Figure 8. Alternative scenarios for DAG synthesis.
Supplemental Figure 9. Alternative scenarios for TAG synthesis.
Supplemental Figure 10. Distribution of [14C]-glycerolipids recovered in the extracellular wax lipids and the tissue after wax extraction (i.e., the cellular or knob lipids).
Supplemental Figure 11. Time course of expression of the abundant cutin-associated acyltransferases/transacylases in Bayberry knobs.
Supplemental Figure 12. Phylogenetic relationships between highly expressed Bayberry acyltransferases/transacylases and predicted or characterized genes in other plants.
Supplemental Figure 13. Flux of intact glycerolipids, glycerol backbone, and acyl chains for Bayberry surface wax synthesis.
Supplemental Table 1. Molar composition of acylglycerol species in surface wax through development.
Supplemental Table 2. Distribution of radioactivity in different lipid classes after incubation of knobs with [14C]-glycerol for the times indicated.
Supplemental Table 3. Percentage of radiolabel in the glycerol backbones of MAG, DAG, and TAG after incubation of knobs with [14C]-glycerol.
Supplemental Table 4. Percentage of [14C]-acetate incorporation into glycerolipids and unknown compounds through time.
Supplemental Movie 1. Time-lapse video of Bayberry wax secretion.
Supplemental Data Set 1. Annotations and expression levels (RPKM) of transcripts identified in Bayberry knobs and leaves.
Supplemental Data Set 2. Text file of the alignment corresponding to the phylogenetic analysis in Supplemental Figure 12.
Supplementary Material
Acknowledgments
We thank Mike Pollard and Henrik Tjellström for suggestions, advice, and help with labeling and lipid analysis. We also thank Patrick Horn and Anthony Schilmiller for critical reading of the manuscript (all of Michigan State University [MSU]). We thank Abby Vanderberg and Melinda Frame of the MSU Center for Advanced Microscopy for sample preparation and assistance with scanning electron and confocal microcopy, respectively. Mathias Schuetz (University of British Columbia) also helped with confocal microscopy, and Starla Zemelis (MSU) provided guidance for fixation of Bayberry tissue for microscopy. Adam Rice (MSU) performed the differential scanning calorimetry analysis of Bayberry wax. RNA-seq was performed by the DOE JGI, with special assistance from Kerrie Barry, Erika Lindquist, and Anna Lipzen. Transcriptome assembly and databases were provided by Nick Thrower (MSU) and Curtis Wilkerson (MSU). We also thank Kurt Stepnitz (MSU) for assistance with time-lapse photography and the MSU Grounds department for maintenance of the Bayberry shrubs. We thank three anonymous reviewers for very helpful suggestions on improving the manuscript. This work was supported by Department of Energy-Great Lakes Bioenergy Research Center Cooperative Agreement DE-FC02-07ER64494. J.P.S. received a National Science and Engineering Research Council of Canada postgraduate fellowship (PGS-D3).
AUTHOR CONTRIBUTIONS
J.P.S. and J.B.O. designed and conducted the research and wrote the manuscript.
Glossary
- TAG
triacylglycerol
- DAG
diacylglycerol
- MAG
monoacylglycerol
- G3P
glycerol-3-phosphate
- GC
gas chromatography
- TLC
thin-layer chromatography
- FFA
free fatty acid
- PC
phosphatidylcholine
- PDAT
phospholipid:diacylglycerol acyltransferase
Footnotes
Articles can be viewed online without a subscription.
References
- Akoh C.C., Lee G.C., Liaw Y.C., Huang T.H., Shaw J.F. (2004). GDSL family of serine esterases/lipases. Prog. Lipid Res. 43: 534–552. [DOI] [PubMed] [Google Scholar]
- Allen D.K., Bates P.D., Tjellström H. (2015). Tracking the metabolic pulse of plant lipid production with isotopic labeling and flux analyses: Past, present and future. Prog. Lipid Res. 58: 97–120. [DOI] [PubMed] [Google Scholar]
- Badami R.C., Patil K.B. (1980). Structure and occurrence of unusual fatty acids in minor seed oils. Prog. Lipid Res. 19: 119–153. [DOI] [PubMed] [Google Scholar]
- Bafor M., Jonsson L., Stobart A.K., Stymne S. (1990). Regulation of triacylglycerol biosynthesis in embryos and microsomal preparations from the developing seeds of Cuphea lanceolata. Biochem. J. 272: 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker E.A. (1982). Chemistry and morphology of plant epicuticular waxes. In The Plant Cuticle, Culter D.J., Alvin K.L., Price C.E., eds (London: Academic Press; ), pp. 139–165. [Google Scholar]
- Banerji R., Chowdhury A.R., Misra G., Nigam S.K. (1984). Butter from plants. Fett Wiss. Technol. 86: 279–284. [Google Scholar]
- Barford R.A., Luddy F.E., Magidman P. (1966). The hydrolysis of long chain trisaturated triglycerides by pancreatic lipase. Lipids 1: 287. [DOI] [PubMed] [Google Scholar]
- Barthlott W., Neinhuis C., Cutler D., Ditsch F., Meusel I., Theisen I., Wilhelmi H. (1998). Classification and terminology of plant epicuticular waxes. Bot. J. Linn. Soc. 126: 237–260. [Google Scholar]
- Bates P.D., Browse J. (2011). The pathway of triacylglycerol synthesis through phosphatidylcholine in Arabidopsis produces a bottleneck for the accumulation of unusual fatty acids in transgenic seeds. Plant J. 68: 387–399. [DOI] [PubMed] [Google Scholar]
- Bates P.D., Browse J. (2012). The significance of different diacylgycerol synthesis pathways on plant oil composition and bioengineering. Front. Plant Sci. 3: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates P.D., Durrett T.P., Ohlrogge J.B., Pollard M. (2009). Analysis of acyl fluxes through multiple pathways of triacylglycerol synthesis in developing soybean embryos. Plant Physiol. 150: 55–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates P.D., Ohlrogge J.B., Pollard M. (2007). Incorporation of newly synthesized fatty acids into cytosolic glycerolipids in pea leaves occurs via acyl editing. J. Biol. Chem. 282: 31206–31216. [DOI] [PubMed] [Google Scholar]
- Beisson F., Li Y., Bonaventure G., Pollard M., Ohlrogge J.B. (2007). The acyltransferase GPAT5 is required for the synthesis of suberin in seed coat and root of Arabidopsis. Plant Cell 19: 351–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisson F., Li-Beisson Y., Pollard M. (2012). Solving the puzzles of cutin and suberin polymer biosynthesis. Curr. Opin. Plant Biol. 15: 329–337. [DOI] [PubMed] [Google Scholar]
- Bourgis F., Kilaru A., Cao X., Ngando-Ebongue G.F., Drira N., Ohlrogge J.B., Arondel V. (2011). Comparative transcriptome and metabolite analysis of oil palm and date palm mesocarp that differ dramatically in carbon partitioning. Proc. Natl. Acad. Sci. USA 108: 12527–12532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockerhoff H. (1965). Stereospecific analysis of triglycerides: an analysis of human depot fat. Arch. Biochem. Biophys. 110: 586–592. [DOI] [PubMed] [Google Scholar]
- Brundrett M.C., Kendrick B., Peterson C.A. (1991). Efficient lipid staining in plant material with sudan red 7B or fluorol [correction of fluoral] yellow 088 in polyethylene glycol-glycerol. Biotech. Histochem. 91: 111–116. [DOI] [PubMed] [Google Scholar]
- Buchgraber M., Ulberth F., Anklam E. (2004). Interlaboratory evaluation of injection techniques for triglyceride analysis of cocoa butter by capillary gas chromatography. J. Chromatogr. A 1036: 197–203. [DOI] [PubMed] [Google Scholar]
- Carlsson A.S., Yilmaz J.L., Green A.G., Stymne S., Hofvander P. (2011). Replacing fossil oil with fresh oil - with what and for what? Eur. J. Lipid Sci. Technol. 113: 812–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman K.D., Dyer J.M., Mullen R.T. (2012). Biogenesis and functions of lipid droplets in plants: Thematic review series: Lipid droplet synthesis and metabolism from yeast to man. J. Lipid Res. 53: 215–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Woodfield H.K., Pan X., Harwood J.L., Weselake R.J. (2015). Acyl-trafficking during plant oil accumulation. Lipids 50: 1057–1068. [DOI] [PubMed] [Google Scholar]
- Christie W.W., Han X. (2010). Lipid Analysis: Isolation Separation and Lipodomic Analysis, 4th ed. (Bridgewater, UK: The Oily Press; ). [Google Scholar]
- Dahlqvist A., Stahl U., Lenman M., Banas A., Lee M., Sandager L., Ronne H., Stymne S. (2000). Phospholipid:diacylglycerol acyltransferase: an enzyme that catalyzes the acyl-CoA-independent formation of triacylglycerol in yeast and plants. Proc. Natl. Acad. Sci. USA 97: 6487–6492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng C., Chen M., Xu C.J., Bai L., Yin X.R., Li X., Allan A.C., Ferguson I.B., Chen K.S. (2012). Transcriptomic analysis of Chinese bayberry (Myrica rubra) fruit development and ripening using RNA-Seq. BMC Genomics 13: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fordham A.J. (1983). Of birds and bayberries: Seed dispersal and propagation of three Myrica species. Arnoldia 43: 20–23. [Google Scholar]
- Gao J., Simon M. (2005). Identification of a novel keratinocyte retinyl ester hydrolase as a transacylase and lipase. J. Invest. Dermatol. 124: 1259–1266. [DOI] [PubMed] [Google Scholar]
- Ghosal A., Banas A., Ståhl U., Dahlqvist A., Lindqvist Y., Stymne S. (2007). Saccharomyces cerevisiae phospholipid:diacylglycerol acyl transferase (PDAT) devoid of its membrane anchor region is a soluble and active enzyme retaining its substrate specificities. Biochim. Biophys. Acta 1771: 1457–1463. [DOI] [PubMed] [Google Scholar]
- Girard A.L., et al. (2012). Tomato GDSL1 is required for cutin deposition in the fruit cuticle. Plant Cell 24: 3119–3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goñi F.M., Alonso A. (1999). Structure and functional properties of diacylglycerols in membranes. Prog. Lipid Res. 38: 1–48. [DOI] [PubMed] [Google Scholar]
- Grabherr M.G., et al. (2011). Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 29: 644–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G., Harwood J.L. (1991). The regulation of triacylglycerol biosynthesis in cocoa (Theobroma cacao) L. Planta 184: 279–284. [DOI] [PubMed] [Google Scholar]
- Griffiths G., Stymne S., Stobart A.K. (1988). Phosphatidylcholine and its relationship to triacylglycerol biosynthesis in oil-tissues. Phytochemistry 27: 2089–2093. [Google Scholar]
- Gunstone F.D., Harwood J.L., Dijkstra A.J. (2007). The Lipid Handbook. (Boca Raton, FL: CRC Press; ). [Google Scholar]
- Hara A., Radin N.S. (1978). Lipid extraction of tissues with a low-toxicity solvent. Anal. Biochem. 90: 420–426. [DOI] [PubMed] [Google Scholar]
- Harlow R.D., Litchfie C., Fu H.C., Reiser R. (1965). Triglyceride composition of Myrica Carolinenis fruit coat fat (Bayberry Tallow). J. Am. Oil Chem. Soc. 42: 4. [Google Scholar]
- Harwood J.L. (1988). Fatty acid metabolism. Annu. Rev. Plant Physiol. 39: 37. [Google Scholar]
- Hawthorne S.B., Miller D.J. (1987). Analysis of commercial waxes using capillary supercritical fluid chromatography-mass spectrometry. J. Chromatogr. A 388: 397–409. [Google Scholar]
- Jenkins C.M., Mancuso D.J., Yan W., Sims H.F., Gibson B., Gross R.W. (2004). Identification, cloning, expression, and purification of three novel human calcium-independent phospholipase A2 family members possessing triacylglycerol lipase and acylglycerol transacylase activities. J. Biol. Chem. 279: 48968–48975. [DOI] [PubMed] [Google Scholar]
- Jetter R., Kunst L., Samuels L. (2006). Composition of plant cuticular waxes. In Biology of the Plant Cuticle, Riederer M., Muller C., eds (Blackwell Publishers; ), pp. 145–181. [Google Scholar]
- Karlgren A., Carlsson J., Gyllenstrand N., Lagercrantz U., Sundström J.F. (2009). Non-radioactive in situ hybridization protocol applicable for Norway spruce and a range of plant species. J. Vis. Exp. (26): 1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolattukudy P.E. (1976). Chemistry and Biochemistry of Natural Waxes. (New York: Elseiver; ). [Google Scholar]
- Konar R.N., Linskens H.F. (1966). The morphology and anatomy of the stigma of Petunia hybrida. Planta 71: 356–371. [DOI] [PubMed] [Google Scholar]
- Kraut A. (1965). The Chromatographic Resolution of Bayberry Wax. (Winnipeg, Canada: University of Manitoba; ). [Google Scholar]
- Larson T.R., Graham I.A. (2001). Technical Advance: a novel technique for the sensitive quantification of acyl CoA esters from plant tissues. Plant J. 25: 115–125. [DOI] [PubMed] [Google Scholar]
- Lehner R., Kuksis A. (1993). Triacylglycerol synthesis by an sn-1,2(2,3)-diacylglycerol transacylase from rat intestinal microsomes. J. Biol. Chem. 268: 8781–8786. [PubMed] [Google Scholar]
- Li Y., Beisson F., Ohlrogge J., Pollard M. (2007). Monoacylglycerols are components of root waxes and can be produced in the aerial cuticle by ectopic expression of a suberin-associated acyltransferase. Plant Physiol. 144: 1267–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li-Beisson Y., Pollard M., Sauveplane V., Pinot F., Ohlrogge J., Beisson F. (2009). Nanoridges that characterize the surface morphology of flowers require the synthesis of cutin polyester. Proc. Natl. Acad. Sci USA 106: 22008–22013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li-Beisson, Y., et al. (2013). Acyl-lipid metabolism. In The Arabidopsis Book 11: e0133, doi/10.1199/tab.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorts C.M., Briggeman T., Sang T. (2008). Evolution of fruit types and seed dispersal: A phylogenetic and ecological snapshot. J. Syst. Evol. 46: 396–404. [Google Scholar]
- McKay A.F. (1948). Occurrence of stearic acid in bayberry tallow (wax). J. Org. Chem. 13: 86–88. [DOI] [PubMed] [Google Scholar]
- Meisel L., Fonseca B., González S., Baeza-Yates R., Cambiazo V., Campos R., Gonźalez M., Orellana A., Retamales J., Silva H. (2005). A rapid and efficient method for purifying high quality total RNA from peaches (Prunus persica) for functional genomics analyses. Biol. Res. 38: 83–88. [DOI] [PubMed] [Google Scholar]
- Molina I., Kosma D. (2015). Role of HXXXD-motif/BAHD acyltransferases in the biosynthesis of extracellular lipids. Plant Cell Rep. 34: 587–601. [DOI] [PubMed] [Google Scholar]
- Murphy D.J. (2001). The biogenesis and functions of lipid bodies in animals, plants and microorganisms. Prog. Lipid Res. 40: 325–438. [DOI] [PubMed] [Google Scholar]
- Pan X., Siloto R.M., Wickramarathna A.D., Mietkiewska E., Weselake R.J. (2013). Identification of a pair of phospholipid:diacylglycerol acyltransferases from developing flax (Linum usitatissimum L.) seed catalyzing the selective production of trilinolenin. J. Biol. Chem. 288: 24173–24188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panikashvili D., Shi J.X., Schreiber L., Aharoni A. (2009). The Arabidopsis DCR encoding a soluble BAHD acyltransferase is required for cutin polyester formation and seed hydration properties. Plant Physiol. 151: 1773–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pighin J.A., Zheng H., Balakshin L.J., Goodman I.P., Western T.L., Jetter R., Kunst L., Samuels A.L. (2004). Plant cuticular lipid export requires an ABC transporter. Science 306: 702–704. [DOI] [PubMed] [Google Scholar]
- Place A.R., Stiles E.W. (1992). Living off the wax of the land - Bayberries and yellow-rumped warblers. Auk 109: 334–345. [Google Scholar]
- Pollard M., Beisson F., Li Y., Ohlrogge J.B. (2008). Building lipid barriers: biosynthesis of cutin and suberin. Trends Plant Sci. 13: 236–246. [DOI] [PubMed] [Google Scholar]
- Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P., Peplies J., Glöckner F.O. (2013). The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41: D590–D596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rani S.H., Krishna T.H., Saha S., Negi A.S., Rajasekharan R. (2010). Defective in cuticular ridges (DCR) of Arabidopsis thaliana, a gene associated with surface cutin formation, encodes a soluble diacylglycerol acyltransferase. J. Biol. Chem. 285: 38337–38347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughan P.G., Slack C.R. (1982). Cellular oranization of glycerolipid meatbolism. Annu. Rev. Plant Physiol. 33: 97–112. [Google Scholar]
- Samuels L., Kunst L., Jetter R. (2008). Sealing plant surfaces: cuticular wax formation by epidermal cells. Annu. Rev. Plant Biol. 59: 683–707. [DOI] [PubMed] [Google Scholar]
- Schmid R.D., Verger R. (1998). Lipases: Interfacial enzymes with attractive applications. Angew. Chem. Int. Ed. 37: 1608–1633. [DOI] [PubMed] [Google Scholar]
- Slack C.R., Bertaud W.S., Shaw B.D., Holland R., Browse J., Wright H. (1980). Some studies on the composition and surface properties of oil bodies from the seed cotyledons of safflower (Carthamus tinctorius) and linseed (Linum ustatissimum). Biochem. J. 190: 551–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack C.R., Roughan P.G., Balasingham N. (1977). Labelling studies in vivo on the metabolism of the acyl and glycerol moieties of the glycerolipids in the developing maize leaf. Biochem. J. 162: 289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack C.R., Roughan P.G., Balasingham N. (1978). Labelling of glycerolipids in the cotyledons of developing oilseeds by [1-14C] acetate and [2-3H] glycerol. Biochem. J. 170: 421–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobart A.K., Stymne S. (1985). The regulation of the fatty-acid composition of the triacylglycerols in microsomal preparations from avocado mesocarp and the developing cotyledons of safflower. Planta 163: 119–125. [DOI] [PubMed] [Google Scholar]
- Stobart K., Mancha M., Lenman M., Dahlqvist A., Stymne S. (1997). Triacylglycerols are synthesised and utilized by transacylation reactions in microsomal preparations of developing safflower (Carthamus tinctorius L) seeds. Planta 203: 58–66. [Google Scholar]
- Suh M.C., Samuels A.L., Jetter R., Kunst L., Pollard M., Ohlrogge J., Beisson F. (2005). Cuticular lipid composition, surface structure, and gene expression in Arabidopsis stem epidermis. Plant Physiol. 139: 1649–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A.E., Scharoun J.E., Ralston H. (1965). Quantitative estimation of isomeric monoglycerides by thin-layer chromatography. J. Am. Oil Chem. Soc. 42: 789. [Google Scholar]
- Tjellström H., Yang Z., Allen D.K., Ohlrogge J.B. (2012). Rapid kinetic labeling of Arabidopsis cell suspension cultures: implications for models of lipid export from plastids. Plant Physiol. 158: 601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troncoso-Ponce M.A., Kilaru A., Cao X., Durrett T.P., Fan J., Jensen J.K., Thrower N.A., Pauly M., Wilkerson C., Ohlrogge J.B. (2011). Comparative deep transcriptional profiling of four developing oilseeds. Plant J. 68: 1014–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waite M., Sisson P. (1973). Utilization of neutral glycerides and phosphatidylethanolamine by the phospholipase A-1 of the plasma membranes of rat liver. J. Biol. Chem. 248: 7985–7992. [PubMed] [Google Scholar]
- Williams L.O. (1958). Bayberry wax and bayberries. Econ. Bot. 12: 103–107. [Google Scholar]
- Yamashita A., Hayashi Y., Nemoto-Sasaki Y., Ito M., Oka S., Tanikawa T., Waku K., Sugiura T. (2014). Acyltransferases and transacylases that determine the fatty acid composition of glycerolipids and the metabolism of bioactive lipid mediators in mammalian cells and model organisms. Prog. Lipid Res. 53: 18–81. [DOI] [PubMed] [Google Scholar]
- Yang W., Pollard M., Li-Beisson Y., Beisson F., Feig M., Ohlrogge J. (2010). A distinct type of glycerol-3-phosphate acyltransferase with sn-2 preference and phosphatase activity producing 2-monoacylglycerol. Proc. Natl. Acad. Sci. USA 107: 12040–12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeats T.H., Huang W., Chatterjee S., Viart H.M., Clausen M.H., Stark R.E., Rose J.K. (2014). Tomato Cutin Deficient 1 (CD1) and putative orthologs comprise an ancient family of cutin synthase-like (CUS) proteins that are conserved among land plants. Plant J. 77: 667–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeats T.H., Martin L.B., Viart H.M., Isaacson T., He Y., Zhao L., Matas A.J., Buda G.J., Domozych D.S., Clausen M.H., Rose J.K. (2012). The identification of cutin synthase: formation of the plant polyester cutin. Nat. Chem. Biol. 8: 609–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngken H.W. (1915). The Comparative Morphology, Taxonomy and Distribution of the Myricaceae of the Eastern United States. PhD dissertation (Philadelphia: University of Pennsylvania; ). [Google Scholar]
- Zheng Z., Xia Q., Dauk M., Shen W., Selvaraj G., Zou J. (2003). Arabidopsis AtGPAT1, a member of the membrane-bound glycerol-3-phosphate acyltransferase gene family, is essential for tapetum differentiation and male fertility. Plant Cell 15: 1872–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.