Abstract
Transcranial direct current stimulation (tDCS) targeting the prefrontal cortex reduces the size and speed of standing postural sway in younger adults, particularly when performing a cognitive dual task. Here, we hypothesized that tDCS would alter the complex dynamics of postural sway as quantified by multiscale entropy (MSE). Twenty healthy older adults completed two study visits. Center-of-pressure (COP) fluctuations were recorded during single-task (i.e., quiet standing) and dual-task (i.e., standing while performing serial subtractions) conditions, both before and after a 20-min session of real or sham tDCS. MSE was used to estimate COP complexity within each condition. The percentage change in complexity from single- to dual-task conditions (i.e., dual-task cost) was also calculated. Before tDCS, COP complexity was lower (p = 0.04) in the dual-task condition as compared to the single-task condition. Neither real nor sham tDCS altered complexity in the single-task condition. As compared to sham tDCS, real tDCS increased complexity in the dual-task condition (p = 0.02) and induced a trend toward improved serial subtraction performance (p = 0.09). Moreover, those subjects with lower dual-task COP complexity at baseline exhibited greater percentage increases in complexity following real tDCS (R = −0.39, p = 0.05). Real tDCS also reduced the dual-task cost to complexity (p = 0.02), while sham stimulation had no effect. A single session of tDCS targeting the prefrontal cortex increased standing postural sway complexity with concurrent non-postural cognitive task. This form of noninvasive brain stimulation may be a safe strategy to acutely improve postural control by enhancing the system's capacity to adapt to stressors.
Keywords: Postural control, Dual task, tDCS, Elderly, Multiscale entropy
Introduction
Standing upright is a complex task that is often performed concurrently with non-postural cognitive tasks (i.e., talking, reading, remembering a grocery list) (Huxhold et al. 2006). This “dual tasking” disrupts postural control, and such “dual-task costs” are greater in aging (Rankin et al. 2000) and disease (Teasdale et al. 1993). Upright stance is enabled by a multifaceted system comprising peripheral sensorimotor elements and a host of spinal and supraspinal networks that interact nonlinearly to regulate the body's postural sway over time. As such, the dynamics of postural sway are exceedingly complex, that is, that they contain “meaningful structural richness” marked by a degree of non-random fluctuations over multiple temporal–spatial scales (Ivanov et al. 1998; Ashkenazy et al. 2002; Lipsitz 2009). This “complexity” is believed to reflect the capacity of the postural control system to adapt to stressors (e.g., environmental and/or task constraints, perturbations, aging, disease) (Goldberger et al. 2002; Lipsitz 2002). Age-related deterioration to numerous peripheral (Manor et al. 2010a) and central (Yang et al. 2013a) elements of the postural control system often results in loss of this complexity and consequentially diminished capacity of the system in question to adapt to the stressors of everyday life (Lipsitz 2009; Manor et al. 2010a, 2012a; Manor and Lipsitz 2013).
Transcranial direct current stimulation (tDCS) is a safe and noninvasive method of altering cortical excitability. A single, 20-min session of tDCS targeting the dorsolateral prefrontal cortices (administered at rest) has been shown to enhance a host of both cognitive and motor functions, including working memory (Fregni et al. 2005), problem solving (Metuki et al. 2012), decision making (Hecht et al. 2010; Ragert et al. 2008), and movement accuracy during reaching tasks (Reis and Fritsch 2011). Recently, Zhou et al. (2014b) provided evidence that real tDCS targeting this brain region, as compared to sham stimulation, effectively improves postural control during the performance of a non-postural cognitive dual task, as evidenced by a reduced dual-task costs to traditional parameters of postural sway, including average sway speed and fluctuation size over time.
The effects of tDCS on postural sway complexity, however, are currently unknown. Multiscale entropy (MSE) (Costa et al. 2007) is one method commonly used to estimate the complexity of postural sway, as reflected by center-of-pressure time series recorded with a force place, that is sensitive to aging and disease (Costa et al. 2002). MSE utilizes a “coarse-graining” technique to quantify sample entropy at multiple timescales, thereby estimating the re-occurrence of repetitive patterns over multiple scales of time. Relatively low MSE values thus indicate a more regular or deterministic pattern, whereas relatively high MSE values indicate a more irregular and information rich pattern. This metric has been widely used to quantify the complexity contained within the dynamics of numerous biological systems (Petersen et al. 1999; Chesnokov 2008; Trunkvalterova et al. 2008) and understand the mechanisms of cognitive and behavioral control (Breakspear and McIntosh 2011). As most biological systems, including the postural control system, operate over multiple spatial and temporal scales, MSE is often a more sensitive measure of system function (Costa et al. 2007; Gruber et al. 2011; Liang et al. 2014; Wayne et al. 2014) and predictor of task performance (Zhou et al. 2014a).
Here, we hypotheses that as compared with sham tDCS, real tDCS would increase the complexity of postural control and reduce the dual-task costs to this metric, in healthy older adults. We tested this hypothesis by conducting a double-blinded study in which postural control was assessed immediately before and after a single session of tDCS targeting the prefrontal cortices.
Materials and methods
Subjects
Twenty healthy older adults (11 men and nine women, age = 63 ± 3.6 years, height = 1.63 ± 0.05 m, body mass = 72 ± 8 kg) provided written informed consent as approved by the Institutional Review Board of Peking University First Hospital, Beijing. All subjects were right-handed as determined by the Edinburgh Handedness Inventory (Oldfield 1971). Exclusion criteria included any self-reported cardiovascular, neurological, or musculoskeletal disorder, current use of any centrally acting medication, recent hospitalization, an inability to stand or walk unassisted, and any other condition resulting in abnormal physical function.
Experimental protocol
Subjects completed two separate study visits separated by 1 week (Fig. 1). On each visit, postural control was assessed immediately before and after either real or sham tDCS. tDCS conditions were completed in random order. Subjects were unaware of the tDCS condition being administered, and the investigator who administered the stimulation was uninvolved in all other study procedures. At the end of each experimental visit, subjects completed a short questionnaire to record any possible side effects of tDCS (Poreisz et al. 2007).
Fig.
Experimental protocol. Subjects completed two study visits separated by 1 week. Each visit was completed at the same time of day. During each visit, single- and dual-task standing postural control was assessed immediately before and after either real or sham tDCS targeting the left prefrontal cortex. The order of tDCS condition was randomized, as was the testing order of single- and dual-task trials within each assessment period
tDCS settings
tDCS was delivered with a battery-driven electrical stimulator (Chattanooga Ionto® Iontophoresis System) connected to a pair of saline-soaked 35 cm2 synthetic surface sponges placed on the scalp. The anode (i.e., positive electrode) was placed over the left prefrontal cortex, relating to the F3 region of the 10/20 EEG electrode placement system. The cathode (i.e., negative electrode) was placed over the right supraorbital region (Boggio et al. 2008). This montage is believed to facilitate neuronal activity under the anode and has been shown to acutely enhance various cognitive functions (Javadi and Walsh 2011). The real tDCS condition comprised 20 min of stimulation at target intensity of 2.0 mA. This amount of stimulation is safe and has been shown to induce acute changes in cortical excitability (Herwig et al. 2003). At the beginning of stimulation, current was increased manually from 0.1 to 2.0 mA in 0.1-mA increments. Subjects were instructed to notify the investigator if and when they felt any uncomfortable sensations arising from the stimulation. The ramp-up procedure was stopped at this point, and for the remainder of the session, tDCS was delivered at an intensity of 0.1 mA below the highest level reached. At the end of the session, current was automatically ramped down to 0.0 mA over a 10-s period. The sham tDCS condition was administered using an inactive stimulation protocol, rather than the “off-target” active protocol, in order to minimize subject risk (Davis et al. 2013). The same electrode montage, session duration, and ramp-up procedures as the real tDCS condition were followed, except that current was only delivered for the first 60 s of the session before being automatically ramped back down to zero. This design is a reliable control as sensations arising from tDCS become negligible after the first minute of stimulation (Gandiga et al. 2006).
Assessments of postural control
Standing postural control was assessed by measuring center-of-pressure (COP) (i.e., postural sway) as subjects stood upright with bare feet and eyes open on a stationary force platform (Kistler Instrument Corp., Amherst, NY). Two 60-s trials were completed under two different experimental conditions: standing quietly (i.e., single task) and standing while performing a non-postural cognitive task (i.e., dual task) consisting of verbalized serial subtractions of three from a random three-digit number. The starting number was provided by the investigator immediately before the trial began. No instructions or cues were given regarding task prioritization within this dual-task condition.
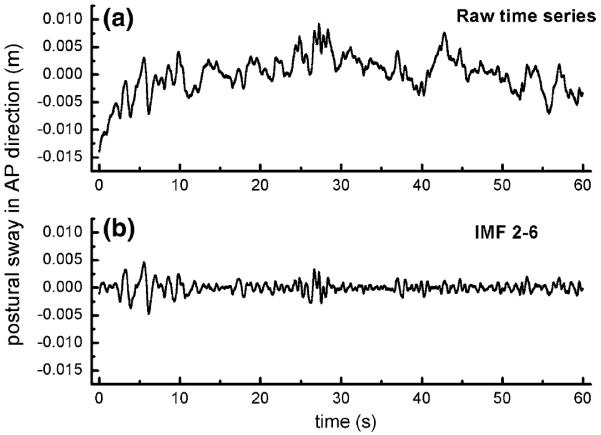
Trial order was randomized within each set of the four trials (i.e., two single tasking and two dual tasking) that were completed before and after real and sham tDCS. During each trial, COP fluctuations were recorded at a sampling frequency of 240 Hz (Fig. 2a).
Fig. 2.
Raw and empirical mode decomposition (EMD)-filtered postural sway (i.e., center-of-pressure, COP) time series in the anterior–posterior (AP) direction. The raw postural sway time series (a) was recorded at a sampling frequency of 240 Hz. As both high-frequency noise and low-frequency trends interfere with estimates of complexity, these fluctuations were removed using EMD to produce a new time series (b). Multiscale entropy (i.e., complexity) was derived from this “detrended” time series
Complexity analysis
This study focused on the acute effects of tDCS targeting the left prefrontal cortex on single- and dual-task postural sway complexity, as derived from MSE analysis (see “Appendix” for full description). We concentrated this analysis on anterior-posterior (AP) COP patterns, since fluctuations in this direction are often preferentially influenced by aging (Blaszczyk and Klonowski 2001) and have a relatively higher signal-to-noise ratio within the frequency range of interest as compared to medial–lateral sway fluctuations (Kang et al. 2009). Prior to MSE calculation, we detrended the original COP time series using ensemble empirical mode decomposition (EEMD) into 13 “intrinsic mode functions” that each contain fluctuations within a frequency range. We then excluded very high- and low-frequency fluctuations to ensure reliable estimates of complexity (Costa et al. 2005; Peng et al. 2009; Manor et al. 2010a). Specifically, we removed IMF 1 (frequencies above 20 Hz) as fluctuations in this range are unlikely to reflect balance-related biological processes (Wayne et al. 2014). We also removed IMFs 7–13 (frequencies below 0.2 Hz) to ensure a sufficient number of dynamic patterns within the COP time series (Manor et al. 2010a). The remaining IMFs (2–6) were then summed into a new time series used for MSE calculation (Fig. 2b).
A “coarse-graining” procedure was then used to derive six new time series by dividing the EMD-filtered time series into non-overlapping windows of length equaling a scale factor, τ, ranging from 3 to 8 data points. The coarse-grained series at the largest scale thus had 1800 points (i.e., 14400 points/8), which is of sufficient length to ensure reliable estimates of entropy (Richman and Moorman 2000; Costa et al. 2002). By using this coarse-graining procedure, we effectively examined COP fluctuations at sampling frequencies ranging from 12.5 to 33.3 ms. We then computed the sample entropy (m = 2 and r = 15 %) of each coarse-grained time series (Costa et al. 2005). COP complexity was finally determined by calculating a complexity index (Ci), as defined by the area under the entropy versus timescale curve, such that relatively larger areas are taken to reflect greater complexity.
In addition to calculating the complexity of single- and dual-task postural sway, the dual-task cost (i.e., the change of complexity from single- to dual tasking) (Beauchet et al. 2008) was calculated as shown in Eq. 1.
| (1) |
Meanwhile, cognitive task performance in each dual-task trial was recorded as the counting error rate, that is, the number of mistakes divided by the total number of responses.
Statistical analysis
Significance level was set to p < 0.05 for all analyses. Two-way repeated-measures ANOVAs were used to analyze the effects of tDCS on COP complexity. Model effects included tDCS condition (real, sham), time (pre-, post-tDCS), and their interaction. Tukey's post hoc testing was completed on significant models in order to identify differences between variable means within each tDCS condition and time combination. One-way ANOVA was used to determine the effects of real versus sham tDCS on the dual-task cost to COP complexity. Effect sizes were estimated using partial eta-squared (ηp).
Results
Subject characteristics
All 20 older adults completed all study procedures. The average intensity at which real tDCS was delivered was 1.4 ± 0.4 mA. Three subjects received the maximum intensity of 2 mA. Stimulation was well tolerated by all individuals, and no adverse events were reported.
Baseline COP complexity
Figure 3 illustrates the cohort MSE curves averaged across the two baseline assessments (i.e., prior to both real and sham tDCS) for single- and dual-task conditions. COP complexity (Ci), as defined by the area under the MSE curve, within the dual-task condition (mean ± SD 3.64 ± 0.76) was significantly lower than the single-task condition (mean ± SD 4.10 ± 0.80) (F1,78 = 4.58, p = 0.04). The observation is consistent with previous reports that “dual tasking” is associated with diminished complexity of COP (Kang et al. 2009).
Fig. 3.
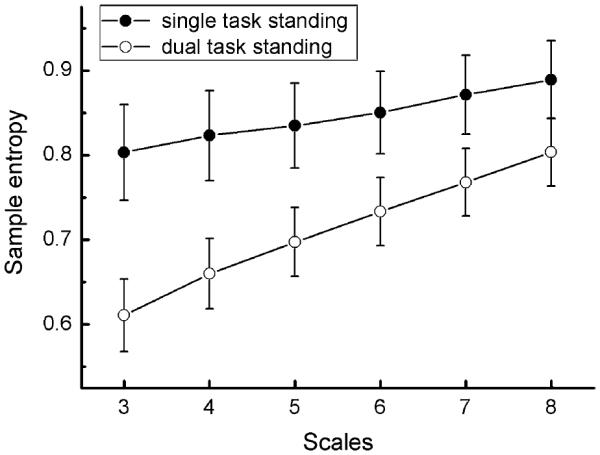
Multiscale entropy curves (mean ± SE) of postural sway under single- and dual-task conditions, prior to the administration of tDCS. Curves have been averaged across the two baseline assessments; that is, before sham and real stimulation. Error bars represent 1 SE from the mean at each scale within each condition. It can be observed that postural sway complexity (Ci), as indicated by the area under the MSE curve, was lower in dual-task conditions as compared to single-task conditions (F1,78 = 4.58, p = 0.04)
No associations were found between baseline COP complexity in single- or dual-task conditions and demographic characteristics, including age (single task: R = 0.18, p = 0.52; dual task: R = −0.22, p = 0.77), body mass (single task: R = −0.3, p = 0.11; dual task: R = −0.21, p = 0.67), and height (single task: R = −0.29, p = −0.11; dual task: R = −0.17, p = 0.51).
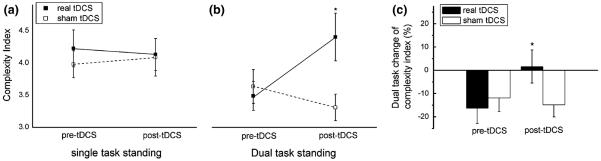
The effects of tDCS on COP complexity
The effects of tDCS on COP complexity are presented in Fig. 4. Neither real nor sham tDCS affected COP complexity in the single-task condition. However, within the dual-task condition, a significant interaction was observed between time (pre-, post-tDCS) and tDCS condition (real, sham) (F1,76 = 5.16, p = 0.02, ηp = 0.06). Post hoc analysis revealed that COP complexity was greater following real tDCS as compared to following sham tDCS and as compared to both pre-tDCS conditions.
Fig. 4.
Effects of noninvasive tDCS on standing postural sway complexity. Postural sway was recorded under single-task (i.e., standing quietly with eyes open) and dual-task (i.e., eye-open standing while completing a non-postural cognitive task) conditions, immediately before and after real or sham tDCS targeting the left prefrontal cortex. tDCS did not alter sway complexity in the single-task condition (a). When compared with sham tDCS, real tDCS resulted in a significant improvement in Ci when standing with performing a cognitive task (i.e., dual task) (b) and the percentage change of complexity from normal to dual task was reduced significantly as compared to sham condition (c). *Significant interaction (p < 0.05) between tDCS condition (real, sham) and time (pre-tDCS, post-tDCS). Error bars represent 1 SE from the mean
Real tDCS was also associated with a reduction in the dual-task cost to COP complexity (F1,76 = 6.28, p 0.02, ηp = 0.08). Post hoc testing revealed that the dual-task cost was smaller following real tDCS (mean ± SD 1.6 ± 31.8 %) as compared to following sham tDCS (mean ± SD −14.8 ± 23.3 %) and prior to both real (mean ± SD −16.3 ± 29.3 %) and sham (mean ± SD −11.8 ± 26.3 %) tDCS.
Correlation analyses indicated that within the real tDCS condition, those subjects who demonstrated greater dual-task COP complexity prior to the administration of tDCS experienced greater percentage increases in COP complexity as a result of brain stimulation (R = −0.39, p = 0.05) (Fig. 5). In other words, real tDCS tended to have a greater effect on COP complexity in those with lower complexity under baseline conditions.
Fig. 5.
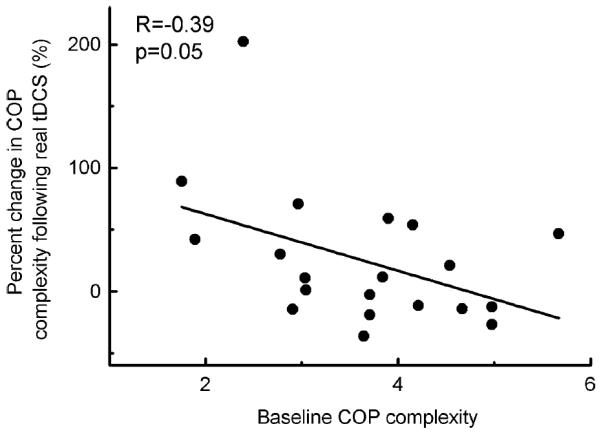
Relationship between the percentage change of COP complexity after real tDCS and complexity before tDCS in dual-task condition. It was observed that the percentage change of complexity after real tDCS was correlated with the complexity before tDCS in dual-task condition (R = −0.39, p = 0.05) and those demonstrated lower COP complexity before tDCS increased more after tDCS. This might indicate that tDCS had a greater effect on people with lower complexity
The effects of tDCS on non-postural task performance
Real tDCS also appeared to have a beneficial effect on serial subtraction performance within dual-task trials. Specifically, a trend toward a time X tDCS condition interaction was observed for serial subtraction error rate (F1,76 = 2.84, p = 0.09, ηp = 0.05). The error rate appeared to be lower following real tDCS (Mean ± SD 3.4 ± 0.7 %) as compared to baseline (Mean ± SD 6.2 ± 0.9 %), while no effects were observed in the sham tDCS condition (Mean ± SD 3.6 ± 0.6 % before and 3.4 ± 0.8 % after sham stimulation).
Discussion
Standing posture is maintained by multiple physiological systems interacting with one another over multiple scales of time and space. This highly complex procedure affords the postural control system the ability to adapt to the innumerable stressors of everyday life (Lipsitz 2002). Aging from adulthood into senescence often diminishes postural sway complexity (Zhou et al. 2013), and this loss has been linked to the capacity of the postural control system to produce successful adaptive responses to stressors (Lipsitz 2009; Marsh and Geel 2000). Recently, tDCS has been shown to improve the ability of the postural control system to adapt to non-postural cognitive tasks. Here, we demonstrate for the first time that a single session of tDCS increases the complexity of standing postural sway dynamics in older adults.
Mounting evidence indicates that age-related functional impairments are reversible with proper intervention (Lipsitz 2002) and that higher levels of function and system adaptability are associated with greater multiscale irregularity (i.e., complexity) within the dynamics of that system's (Manor and Lipsitz 2013; Wayne et al. 2014; Wang et al. 2014; Yang et al. 2013b). Liang and colleagues recently reported that a single session of tDCS targeting the prefrontal cortex enhances inhibitory cognitive control. Intriguingly, this cognitive improvement was correlated with an increase in the MSE-derived complexity of related EEG dynamics (Liang et al. 2014). Our observations suggest that real tDCS targeting this same region of the brain increases the complexity of standing postural sway dynamics under stressful (i.e., dual-task) conditions. Furthermore, real tDCS mitigated the “cost” of dual tasking on postural sway complexity (i.e., the percentage change from single-task conditions). This observation, taken together with the previous report that tDCS improves traditional parameters of postural control (Zhou et al. 2014b), suggests that facilitation of prefrontal cortical excitability via tDCS may improve the adaptive capacity of older adults specifically by counteracting the age-related loss of postural sway complexity.
Sample entropy is one metric that is often used to quantify the irregularity of non-stationary time series (Pincus 1991), including standing postural sway (Ramdani et al. 2009). MSE extends this technique by employing a “coarse-graining” technique to estimate the degree of irregularity within a time series over multiple scales of time (Costa et al. 2007). In the current study, we quantified the complexity of postural sway dynamics over relatively short timescales, ranging from 12.5 to 33.3 ms. These scales were chosen to ensure that results were uninfluenced by very high-frequency noise, as well as by insufficient repetitions of lower-frequency dynamical patterns (Costa et al. 2008). Both spinal and supraspinal feedback-mediated postural reflexes occur on millisecond timescales (Applegate et al. 1988). Although additional research is needed, tDCS-mediated increases in the complexity of fluctuations at these timescales may have thus stemmed in part from enhancement of cortical excitability to brain regions involved in these reflex pathways.
There are also several potential neurological mechanisms that may have led to tDCS-induced improvements specifically within dual-task condition. In the capacitysharing theory, it suggests that the cognitive resources are limited, and performing concurrent tasks that require shared resources will diminish performance in one or both tasks (Tombu and Jolicoeur 2003). Real tDCS may thus increase the availability of cognitive resources and optimize the allocation of available resources to one or both tasks (Filmer et al. 2013). On the other hand, the bottleneck theory of dual-task control concludes that a “bottleneck” occurs when two tasks are processed within the same neural networks, so that processing of one task will be delayed until the network or processor is free from the other task (Ruthruff et al. 2001). After real tDCS, increased processing speed and/or shortened time delay between two tasks results in those dual-task improvements (Redfern et al. 2001). Future work is thus needed to examine standing during concurrent performance of several cognitive tasks that vary in difficulty to enable further insight into the effects of tDCS on the interplay between cognitive and motor function.
We chose to target the prefrontal cortex with tDCS in this study because this region is closely involved in executive function (Kane and Engle 2002), attention (Knight et al. 1995), short-term memory (Fregni et al. 2005), verbal task performance (Javadi et al. 2012), and the ability to perform multiple tasks at the same time (Szameitat et al. 2002). This region is also involved in the control of motor tasks, including human standing (Goble et al. 2011). Still, the neural mechanisms of observed tDCS-related enhancement in postural dynamics are unclear. By targeting only one region, we were unable to determine whether changes resulted from specific neuronal changes within the left prefrontal cortex or from more general changes in brain excitability. Studies that examine the effects of the tDCS targeting one or more other brain regions (e.g., the sensory or motor cortices) are therefore worthy of investigation. Additionally, future work might utilize transcranial magnetic stimulation (TMS), functional imaging techniques, and/or electroencephalography (EEG) to link tDCS-induced changes in cortical neurophysiology with behavioral changes. It is also possible that multiple tDCS sessions may result in persistent changes in both sensorimotor (Zimerman et al. 2012) and cognitive function (Dockery et al. 2009), and as such, additional research into the longer-term effects of tDCS on dual-task postural control is warranted.
In the current study, real tDCS tended to increase dual-task COP complexity more in those subjects who exhibited lower COP complexity at baseline (see Fig. 5). This observation may have been caused in part by a ceiling effect of Ci, such that those with greater COP complexity prior to the administration of real tDCS had relatively less room for improvement. On the other hand, previous studies indicate that aging and disease not only diminish postural sway complexity, but increase the postural control system's dependence upon cognitive input and underlying brain networks (Manor et al. 2010b, 2012b). As such, those with impaired postural control (i.e., lower COP complexity) may stand to benefit more from tDCS-induced facilitation of cortical excitability. Future, larger-scale studies are therefore warranted to delineate the influence of baseline postural control characteristics on the effectiveness of tDCS as a therapeutic strategy.
We chose to utilize serial subtractions of three as the non-postural cognitive dual task. Although results indicated a trend toward a significant performance improvement in this task following real tDCS, subjects performed quite well on this task, and our results may have been confounded by a ceiling effect. Further studies using paradigms comprising multiple non-postural cognitive tasks of varying difficulty levels are encouraged to determine the extent to which tDCS improves dual-task performance under conditions of increasing cognitive demand. Finally, as we focused on postural sway within the AP direction, additional work is needed to elucidate the effects of tDCS on dynamics within the ML direction.
Conclusion
The present study indicates that a noninvasive intervention, tDCS, may increase the complexity of standing postural sway, specifically under dual-task conditions. tDCS might therefore serve as an effective strategy to help combat the age-related loss of complexity and associated reduction in the adaptive capacity of the postural control system.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (Grant Number 11372013) and the National Institute on Aging (1K01AG044543-01A1). We sincerely appreciate Dapeng Bao and the Beijing Sport University for providing the equipment needed to measure body postural sway.
Appendix
Multiscale entropy
MSE was calculated according to the procedure proposed by Costa (2007). For a given one-dimensional discrete time series of length N = {x1,…, xi,…, xN}, the set of consecutive coarse-grained time series {y(τ)} constructed was given by
| (2) |
where xi represents the original time series, τ is the scale factor, and 1 ≤ J ≤ N/τ. In other words, the coarse-grained series at different timescales are obtained by taking arithmetic averages of τ which neighbors original values without overlapping. Thus, the length of each coarse-grained series is given by N/τ, such that scale 1 reflects the original time series.
The sample entropy (SE) of each coarse-grained time series was then calculated. SE, which is related to approximate entropy (AE), calculates the irregularity of a given time series using the following steps:
- For the coarse-gained time series y(i), we could form the vectors Y(i) as:
where m is the pattern length parameter; - Define the distance between vector Y(i) and Y(j) as
(3) - For each i ≤ N − m, calculate the quality
the tolerance level r is set at a percentage of the SD of the time series.(4) Repeat steps (1)–(3) with embedding dimension m + 1;
Thus, SE reflects the conditional probability that a time series of length N/τ, repeating itself within given tolerance r for m points, will also repeat itself for m + 1 points without self-matches. Thus, both the tolerance level r and pattern length m need to be set in SE algorithm for the MSE calculation. Here, we choose a tolerance level of r = 0.15 × SD of the time series to avoid distortion due to the variability in signal magnitude (Costa et al. 2007). Additionally, we set m = 2 as traditionally recommended.
Footnotes
Conflict of interest All the authors declare that there is no further conflict of interest in this study.
Ethical standard All procedures performed in studies involving human subjects were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- Applegate C, Gandevia SC, Burke D. Changes in muscle and cutaneous cerebral potentials during standing. Exp Brain Res. 1988;71:183–188. doi: 10.1007/BF00247533. [DOI] [PubMed] [Google Scholar]
- Ashkenazy Y, Hausdorff JM, Ivanov PC, Stanley HE. A stochastic model of human gait dynamics. Phys A. 2002;316:662–670. [Google Scholar]
- Beauchet O, Annweiler C, Allali G, Berrut G, Herrmann FR, Dubost V. Recurrent falls and dual task-related decrease in walking speed: is there a relationship? J Am Geriatr Soc. 2008;56(7):1265–1269. doi: 10.1111/j.1532-5415.2008.01766.x. [DOI] [PubMed] [Google Scholar]
- Blaszczyk JW, Klonowski W. Postural stability and fractal dynamics. Acta Neurobiol Exp. 2001;61:105–112. doi: 10.55782/ane-2001-1390. [DOI] [PubMed] [Google Scholar]
- Boggio PS, Rigonatti SP, Ribeiro RB, et al. A randomized, double-blind clinical trial on the efficacy of cortical direct current stimulation for the treatment of major depression. Int J Neuropsychopharmacol. 2008;11:249–254. doi: 10.1017/S1461145707007833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breakspear M, McIntosh AR. Networks, noise and models: reconceptualizing the brain as a complex, distributed system. NeuroImage. 2011;58:293–295. doi: 10.1016/j.neuroimage.2011.03.056. [DOI] [PubMed] [Google Scholar]
- Chesnokov YV. Complexity and spectral analysis of the heart rate variability dynamics for distant prediction of paroxysmal atrial fibrillation with artificial intelligence methods. Artif Intell Med. 2008;43:151–165. doi: 10.1016/j.artmed.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Costa MD, Goldberger AL, Peng CK. Multiscale entropy analysis of complex physiologic time series. Phys Rev Lett. 2002;89:068102–068104. doi: 10.1103/PhysRevLett.89.068102. [DOI] [PubMed] [Google Scholar]
- Costa MD, Goldberger AL, Peng CK. Multiscale entropy analysis of biological signals. Phys Rev E. 2005;71:021906. doi: 10.1103/PhysRevE.71.021906. [DOI] [PubMed] [Google Scholar]
- Costa MD, Priplata AA, Lipsitz LA, et al. Noise and poise: enhancement of postural complexity in the elderly with a stochastic-resonance-based therapy. Europhys Lett. 2007;77:68008. doi: 10.1209/0295-5075/77/68008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa MD, Peng CK, Goldberger AL. Multiscale analysis of heart rate dynamics: entropy and time irreversibility measures. Cardiovasc Eng. 2008;8(2):88–93. doi: 10.1007/s10558-007-9049-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis NJ, Gold E, Pascual-Leone A, Bracewell RM. Challenges of proper placebo control for non-invasive brain stimulation in clinical and experimental applications. Eur J Neurosci. 2013;38:2973–2977. doi: 10.1111/ejn.12307. [DOI] [PubMed] [Google Scholar]
- Dockery CA, Hueckel-Weng R, Birbaumer N, Plewnia C. Enhancement of planning ability by transcranial direct current stimulation. J Neurosci. 2009;29(22):7271–7277. doi: 10.1523/JNEUROSCI.0065-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filmer HL, Mattingley JB, Dux PE. Improved multitasking following prefrontal tDCS. Cortex. 2013;49(10):2845–2852. doi: 10.1016/j.cortex.2013.08.015. [DOI] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Nitsche M, et al. Anodal transcranial direct current stimulation of prefrontal cortex enhances working memory. Exp Brain Res. 2005;166:23–30. doi: 10.1007/s00221-005-2334-6. [DOI] [PubMed] [Google Scholar]
- Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol. 2006;117:845–850. doi: 10.1016/j.clinph.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Goble DJ, Coxon JP, Van Impe A, et al. Brain activity during ankle proprioceptive stimulation predicts balance performance in young and older adults. J Neurosci. 2011;31(45):16344–16352. doi: 10.1523/JNEUROSCI.4159-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberger AL, Peng CK, Lipsitz LA. What is physiologic complexity and how does it change with aging and disease? Neurobiol Aging. 2002;23:23–26. doi: 10.1016/s0197-4580(01)00266-4. [DOI] [PubMed] [Google Scholar]
- Gruber AH, Busa MA, Gorton GE, III, Van Emmerik RE, Masso PD, Hamil Jl. Time-to-contact and multiscale entropy identify differences in postural control in adolescent idiopathic scoliosis. Gait Posture. 2011;34:13–18. doi: 10.1016/j.gaitpost.2011.02.015. [DOI] [PubMed] [Google Scholar]
- Hecht D, Walsh V, Lavidor M. Transcranial direct current stimulation facilitates decision making in a probabilistic guessing task. J Neurosci. 2010;30:4241–4245. doi: 10.1523/JNEUROSCI.2924-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herwig U, Satrapi P, Schonfeldt-Lecuona C. Using the international 10–20 EEG system for positioning of transcranial magnetic stimulation. Brain Topogr. 2003;16:95–99. doi: 10.1023/b:brat.0000006333.93597.9d. [DOI] [PubMed] [Google Scholar]
- Huxhold O, Li SC, Schmiedek F, Lindenberger U. Dual-tasking postural control: aging and the effects of cognitive demand in conjunction with focus of attention. Brain Res Bull. 2006;69:294–305. doi: 10.1016/j.brainresbull.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Ivanov PC, Amaral LN, Goldberger AL, Stanley HE. Stochastic feedback and the regulation of biological rhythms. Europhys Lett. 1998;43(4):363. doi: 10.1209/epl/i1998-00366-3. [DOI] [PubMed] [Google Scholar]
- Javadi AH, Walsh V. Transcranial direct current stimulation (tDCS) of the left dorsolateral prefrontal cortex modulates declarative memory. Brain Stimul. 2011;5:231–241. doi: 10.1016/j.brs.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Javadi AH, Cheng P, Walsh V. Short duration transcranial direct current stimulation (tDCS) modulates verbal memory. Brain Stimul. 2012;5(4):468–474. doi: 10.1016/j.brs.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Engle RW. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: an individual-differences perspective. Psychon Bull Rev. 2002;9(4):637–671. doi: 10.3758/bf03196323. [DOI] [PubMed] [Google Scholar]
- Kang HG, Costa MD, Priplata AA, et al. Frailty and the degradation of complex balance dynamics during a dual-task protocol. J Gerontol A Biol Sci Med Sci. 2009;64:1304–1311. doi: 10.1093/gerona/glp113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight RT, Grabowecky MF, Scabini D. Role of human prefrontal cortex in attention control. Adv Neurol. 1995;66:21–34. [PubMed] [Google Scholar]
- Liang WK, Lo MT, Yang AC, et al. Revealing the brain's adaptability and the transcranial direct current stimulation facilitating effect in inhibitory control by multiscale entropy. Neuroimage. 2014;90:218–234. doi: 10.1016/j.neuroimage.2013.12.048. [DOI] [PubMed] [Google Scholar]
- Lipsitz LA. Dynamics of stability: the physiologic basis of functional health and frailty. J Gerontol A Biol Sci Med Sci. 2002;57:B115–B125. doi: 10.1093/gerona/57.3.b115. [DOI] [PubMed] [Google Scholar]
- Lipsitz LA. Physiological complexity, aging, and the path to frailty. Sci Aging Knowledge Environ. 2009;16:e16. doi: 10.1126/sageke.2004.16.pe16. [DOI] [PubMed] [Google Scholar]
- Manor B, Lipsitz LA. Physiologic complexity and aging: implications for physical function and rehabilitation. Prog Neuropsychopharmacol Biol Psychiatry. 2013;45:287–293. doi: 10.1016/j.pnpbp.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manor B, Costa MD, Hu K, et al. Physiological complexity and system adaptability: evidence from postural control dynamics of older adults. J Appl Physiol. 2010a;109:1786–1791. doi: 10.1152/japplphysiol.00390.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manor B, Hu K, Zhao P, et al. Altered control of postural sway following cerebral infarction A cross-sectional analysis. Neurology. 2010b;74(6):458–464. doi: 10.1212/WNL.0b013e3181cef647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manor B, Hu K, Peng CK, Lipsitz LA, Novak V. Posturo-respiratory synchronization: effects of aging and stroke. Gait Posture. 2012a;36(2):254–259. doi: 10.1016/j.gaitpost.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manor B, Newton E, Abduljalil A, Novak V. The relationship between brain volume and walking outcomes in older adults with and without diabetic peripheral neuropathy. Diabetes Care. 2012b;35(9):1907–1912. doi: 10.2337/dc11-2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AP, Geel SE. The effect of age on the attentional demands of postural control. Gait Posture. 2000;12:105–113. doi: 10.1016/s0966-6362(00)00074-6. [DOI] [PubMed] [Google Scholar]
- Metuki N, Sela T, Lavidor M. Enhancing cognitive control components of insight problems solving by anodal tDCS of the left dorsolateral prefrontal cortex. Brain Stimul. 2012;5:110–115. doi: 10.1016/j.brs.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Peng CK, Costa MD, Goldberger AL. Adaptive data analysis of complex fluctuations in physiologic time series. Adv Adapt Data Anal. 2009;1(1):61–70. doi: 10.1142/S1793536909000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Pincus SM. Approximate entropy as a measure of system complexity. Proc Natl Acad Sci USA. 1991;88:2297–2301. doi: 10.1073/pnas.88.6.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poreisz C, Boros K, Antal A, Paulus W. Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Res Bull. 2007;72:208–214. doi: 10.1016/j.brainresbull.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Ragert P, Vandermeeren Y, Camus M, Cohen LG. Improvement of spatial tactile acuity by transcranial direct current stimulation. Clin Neurophysiol. 2008;119:805–811. doi: 10.1016/j.clinph.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramdani S, Seigle B, Lagarde J, Bouchara F, Bernard PL. On the use of sample entropy to analyze human postural sway data. Med Eng Phys. 2009;31(8):1023–1031. doi: 10.1016/j.medengphy.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Rankin JK, Woollacott MH, Cook AS, Brown LA. Cognitive influence on postural stability: a neuromuscular analysis in young and older adults. J Gerontol A Biol. 2000;55A(3):M112–M119. doi: 10.1093/gerona/55.3.m112. [DOI] [PubMed] [Google Scholar]
- Redfern MS, Jennings JR, Martin C, Furman JM. Attention influences sensory integration for postural control in older adults. Gait Posture. 2001;14(3):211–216. doi: 10.1016/s0966-6362(01)00144-8. [DOI] [PubMed] [Google Scholar]
- Reis J, Fritsch B. Modulation of motor performance and motor learning by transcranial direct current stimulation. Curr Opin Neurol. 2011;24:590–596. doi: 10.1097/WCO.0b013e32834c3db0. [DOI] [PubMed] [Google Scholar]
- Richman JS, Moorman JR. Physiological time-series analysis using approximate entropy and sample entropy. Am J Physiol Heart Circ Physiol. 2000;278:H2039–H2049. doi: 10.1152/ajpheart.2000.278.6.H2039. [DOI] [PubMed] [Google Scholar]
- Ruthruff E, Pashler HE, Klaassen A. Processing bottlenecks in dual-task performance: structural limitation or strategic postponement? Psychon Bull Rev. 2001;8:73–80. doi: 10.3758/bf03196141. [DOI] [PubMed] [Google Scholar]
- Szameitat AJ, Schubert T, Müller K, Von Cramon DY. Localization of executive functions in dual-task performance with fMRI. J Cogn Neurosci. 2002;14(8):1184–1199. doi: 10.1162/089892902760807195. [DOI] [PubMed] [Google Scholar]
- Teasdale N, Lajoie Y, Bard C, et al. Cognitive processes involved for maintaining postural stability while standing and walking. In: Stelmach GE, Homberg V (eds) Sensorimotor Impairment in the Elderly. Kluwer Academic Publishers, Boston. 1993:157–168. [Google Scholar]
- Tombu M, Jolicoeur P. A central capacity sharing model of dual-task performance. J Exp Psychol Hum Percept Perform. 2003;29:3–18. doi: 10.1037//0096-1523.29.1.3. [DOI] [PubMed] [Google Scholar]
- Trunkvalterova Z, Javorka M, Tonhajzerova I. Reduced short-term complexity of heart rate and blood pressure dynamics in patients with diabetes mellitus type 1: multiscale entropy analysis. Physiol Meas. 2008;29:817–828. doi: 10.1088/0967-3334/29/7/010. [DOI] [PubMed] [Google Scholar]
- Wang CH, Tsai CH, Tseng P, et al. The association of physical activity to neural adaptability during visuo-spatial processing in healthy elderly adults: a multiscale entropy analysis. Brain Cogn. 2014;92:73–83. doi: 10.1016/j.bandc.2014.10.006. [DOI] [PubMed] [Google Scholar]
- Wayne PM, Gow BJ, Costa MD, et al. Complexity-based measures inform effect of Tai Chi training on standing postural control: cross-sectional and randomized trial studies. PloS One. 2014;9(12):e114731. doi: 10.1371/journal.pone.0114731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang AC, Chu-Chung H, Heng-Liang Y, et al. Complexity of spontaneous BOLD activity in default mode network is correlated with cognitive function in normal male elderly: a multiscale entropy analysis. Neurobiol Aging. 2013a;34(2):428–438. doi: 10.1016/j.neurobiolaging.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Yang AC, Wang SJ, Lai KL, et al. Cognitive and neuropsychiatric correlates of EEG dynamic complexity in patients with Alzheimer's disease. Prog Neuropsychopharmacol Biol Psychiatry. 2013b;47:52–61. doi: 10.1016/j.pnpbp.2013.07.022. [DOI] [PubMed] [Google Scholar]
- Zhou J, Manor B, Liu D, Hu K, Zhang J, Fang J. The complexity of standing postural control in older adults: a modified detrended fluctuation analysis based upon the empirical mode decomposition algorithm. PLoS One. 2013;8:e62585. doi: 10.1371/journal.pone.0062585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Bao D, Zhang J, et al. Noise stimuli improve the accuracy of target aiming: possible involvement of noise-enhanced balance control. Exp Mech. 2014a;54(1):95–100. [Google Scholar]
- Zhou J, Hao Y, Wang Y, et al. Transcranial direct current stimulation (tDCS) reduces the cost of performing a cognitive task on gait and postural control. Eur J Neurosci. 2014b;39(8):1343–1348. doi: 10.1111/ejn.12492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimerman M, Heise KF, Hoppe J, et al. Modulation of training by single-session transcranial direct current stimulation to the intact motor cortex enhances motor skill acquisition of the paretic hand. Stroke. 2012;43(8):2185–2191. doi: 10.1161/STROKEAHA.111.645382. [DOI] [PMC free article] [PubMed] [Google Scholar]