Abstract
Studies of rare hereditary disorders are intended to find treatments, but they can also bring other discoveries. One such study links the dysfunction of a protein to that of the cell’s energy producers, the mitochondria.
In Greek, ‘ataxic’ (ατάξις) means ‘disordered’. In neurological diseases known as ataxias, the lack of order is mainly one of movement: patients cannot coordinate voluntary movements because of the loss of certain neural circuits, making it difficult for them to walk, reach for objects and speak. Increasingly, researchers are finding1, 2 that the underlying cause of some hereditary ataxias is another sort of disorder — that of mitochondria, the intracellular organelles that act as the cell’s powerhouses. However, we know very little about the mechanisms that trigger certain other hereditary ataxias, such as the condition known as autosomal recessive spastic ataxia of Charlevoix–Saguenay, or ARSACS. The disease is caused by mutations in the SACS gene3, but the link between the mutations and the neuronal loss has been unclear. Writing in Proceedings of the National Academy of Sciences, Girard et al.4 find that mitochondrial dysfunction is just such a link, and in the process provide clues about the cellular role of the SACS-encoded protein, sacsin*.
The ataxia in ARSACS is due to a progressive loss of neurons called Purkinje cells, which are found in the cerebellum5, a part of the brain involved in muscle coordination. Affected patients carry mutations in both chromosomal copies of SACS, so that their cells lack functional sacsin3, 6. The disorder was first described6 in 1978 in patients from the Charlevoix and Saguenay regions of Quebec, Canada, where it is much more common than in the rest of the world. One in 22 of the regions’ residents carries a mutation in one of their two copies of SACS — probably brought by French immigrants four centuries ago.
To understand sacsin’s functions, Girard et al.4 analysed the location of sacsin within various types of cells, including neurons, in culture and observed that the protein co-localizes with mitochondria. Moreover, mitochondria in mouse cells in which sacsin expression had been inhibited, and in cells from patients with ARSACS, had markedly abnormal shapes: they were larger and formed more clumps than mitochondria in normal cells. The authors then generated mice that lacked the protein. These animals progressively lost Purkinje cells as they aged, making them potential models for studying at least some aspects of ARSACS. Taken together, Girard and colleagues’ results suggest that sacsin deficiency results in unusually shaped, large mitochondria, and that this in turn leads to Purkinje-cell loss.
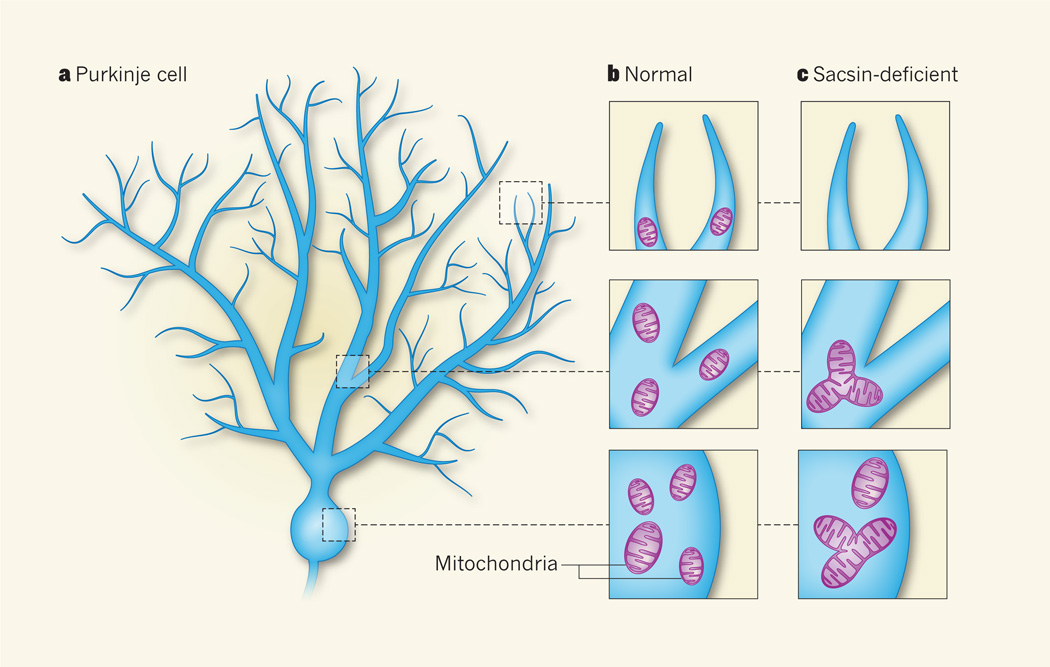
The shape of mitochondria is dynamically controlled by their fission and fusion. But why do unusually shaped mitochondria harm Purkinje cells more than other cells? Each Purkinje cell has many long, branching projections that form an extensive tree-like structure (Fig. 1a), establishing about 150,000 synaptic connections with other neurons5. The synapses are energy-demanding because of the need for continual pumping of ions through the cell membrane — to keep the ‘lines’ clear for neural transmission — and thus they require functional mitochondria to be located nearby. To enter the narrow neuronal projections, small mitochondria need to be pinched off by fission from large ones7 (Fig. 1b). Moreover, fission has a role in preserving mitochondrial health by eliminating organelles that become damaged by oxidative molecules produced during normal mitochondrial activity8. Purkinje cells seem to be highly dependent on the quality and efficient distribution of mitochondria throughout the cell, because their robust activity carries a high oxidative burden and they have a higher number of narrow prolongations than other cells. Indeed, Girard et al.4 find that the clumped, fused mitochondria in sacsin-deficient neurons fail to distribute into the cellular prolongations (Fig. 1c), and that damaged mitochondria seem to be more abundant than in normal cells.
Figure 1. Out-of-shape organelles lead to disease.
a, Neurons known as Purkinje cells have many long, branching prolongations and are found in the cerebellum, an area of the brain that controls movement. These cells progressively die off in patients with a neurological disease called autosomal recessive spastic ataxia of Charlevoix–Saguenay (ARSACS), which is caused by mutations in the gene encoding the protein sacsin. b, Mitochondria are usually found throughout the body of normal neurons, including in the narrow cellular prolongations. c, Girard et al.4 find that mitochondria in sacsin-deficient mouse cells and in cells from patients with ARSACS are larger than those in normal cells and accumulate in clumps. Such large mitochondria do not distribute into the cellular prolongations in sacsin-lacking neurons. These results, together with other data from sacsin-deficient mice, suggest that mitochondrial dysfunction is involved in the death of Purkinje cells in patients with ARSACS.
The authors’ findings4 raise several questions. For example, how does sacsin affect the size and distribution of mitochondria? Girard and colleagues propose that the protein may be needed for efficient fission of the organelle. In addition to the abnormal mitochondrial shapes in sacsin-deficient cells, the researchers4 report that sacsin binds to the Drp1 protein, which is essential for mitochondrial division. During fission, Drp1 polymerizes to form a spiral around the outside of the organelle, constricting it and facilitating its division9, 10.
The hypothesis that sacsin is required for full Drp1 activity is consistent with previous research11, 12 showing that mice with brain-specific Drp1 deficiency display features similar to those observed by Girard et al.4 in sacsin-deficient mice — a loss of Purkinje cells, together with the presence of oversized mitochondria that fail to spread into the neuronal projections. And it has been reported8 that damaged mitochondria are not efficiently degraded in cultured cells in which Drp1 activity is inhibited. Therefore, the dependence of Purkinje cells on the shape and health of mitochondria may explain why they are preferentially lost in Drp1- and sacsin-deficient mice, and in patients with ARSACS.
Additional questions remain, such as why sacsin binds to Drp1 and how this binding may affect mitochondrial shape. Some portions of the sacsin protein are similar to chaperones (proteins that help other proteins to fold efficiently), so it is tempting to speculate that sacsin may be needed for Drp1 or other proteins involved in mitochondrial division to assume their proper conformation. The link between sacsin and Drp1 also raises the possibility that sacsin regulates the division of peroxisomes, another type of intracellular organelle. Drp1 elimination in mice results in abnormally long peroxisomes11, and so it would be interesting to test whether the cells of patients with ARSACS contain longer peroxisomes.
Girard and colleagues’ study of ARSACS strikes a common theme in research into neurodegenerative disorders: insight from clinical studies reveals new perspectives for cell biology, and vice versa. For instance, the study of families affected by optic atrophy type 1 revealed the protein OPA1 as being essential for mitochondrial fusion12, 13. And research in the fruitfly revealed that the parkin protein — the dysfunction of which is the leading cause of recessive Parkinson’s disease — regulates mitochondrial shape14. These examples demonstrate the importance of fostering interaction between the clinic and the lab. Thus, we can continue to learn about neurological disease from model organisms such as the fruitfly, and about mitochondrial biology from people in Quebec who have ataxia. With further collaboration between clinicians and scientists, this understanding might translate into treatments for these devastating disorders in the not-too-distant future.
Footnotes
This News & Views article was published online on 7 March 2012.
References
- 1.Babcock M, et al. Science. 1997;276:1709–1712. doi: 10.1126/science.276.5319.1709. [DOI] [PubMed] [Google Scholar]
- 2.Van Goethem G, et al. Neurology. 2004;63:1251–1257. doi: 10.1212/01.wnl.0000140494.58732.83. [DOI] [PubMed] [Google Scholar]
- 3.Engert JC, et al. Nature Genet. 2000;24:120–125. doi: 10.1038/72769. [DOI] [PubMed] [Google Scholar]
- 4.Girard M, et al. Proc. Natl Acad. Sci. USA. 2012;109:1661–1666. doi: 10.1073/pnas.1113166109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kandel ER, Schwartz JH, Jessell TM. Principles of Neural Science. McGraw-Hill; 2000. [Google Scholar]
- 6.Bouchard JP, Barbeau A, Bouchard R, Bouchard RW. Can. J. Neurol. Sci. 1978;5:61–69. [PubMed] [Google Scholar]
- 7.Li Z, Okamoto K, Hayashi Y, Sheng M. Cell. 2004;119:873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Twig G, et al. EMBO J. 2008;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ingerman E, et al. J. Cell Biol. 2005;170:1021–1027. doi: 10.1083/jcb.200506078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Labrousse AM, Zappaterra MD, Rube DA, van der Bliek AM. Mol. Cell. 1999;4:815–826. doi: 10.1016/s1097-2765(00)80391-3. [DOI] [PubMed] [Google Scholar]
- 11.Ishihara N, et al. Nature Cell Biol. 2009;11:958–966. doi: 10.1038/ncb1907. [DOI] [PubMed] [Google Scholar]
- 12.Delettre C, et al. Nature Genet. 2000;26:207–210. doi: 10.1038/79936. [DOI] [PubMed] [Google Scholar]
- 13.Alexander C, et al. Nature Genet. 2000;26:211–215. doi: 10.1038/79944. [DOI] [PubMed] [Google Scholar]
- 14.Greene JC, et al. Proc. Natl Acad. Sci. USA. 2003;100:4078–4083. doi: 10.1073/pnas.0737556100. [DOI] [PMC free article] [PubMed] [Google Scholar]