Abstract
Tissue engineering aims to fabricate functional tissue for applications in regenerative medicine and drug testing. More recently, 3D printing has shown great promise in tissue fabrication with a structural control from micro- to macro-scale by using a layer-by-layer approach. Whether through scaffold-based or scaffold-free approaches, the standard for 3D printed tissue engineering constructs is to provide a biomimetic structural environment that facilitates tissue formation and promotes host tissue integration (e.g., cellular infiltration, vascularization, and active remodeling). This review will cover several approaches that have advanced the field of 3D printing through novel fabrication methods of tissue engineering constructs. It will also discuss the applications of synthetic and natural materials for 3D printing facilitated tissue fabrication.
1. Introduction
3D printing holds remarkable promise for tissue engineering as it can potentially provide a rapid and robust approach to assemble functional tissue in vitro.[1–5] A functional macro-tissue requires a specific set of micro-architecture that provides the structural and mechanical support, sufficient nutrient supply, the necessary cell types, and the ability to actively remodel once implanted.[6–10] 3D printing proposes an effective means to assemble all of these necessary components through the use of biomaterials, printing techniques, and cell delivery methods (Figure 1). Initial strategies included printing complex scaffolds followed by a cell seeding process,[11,12] whereas current strategies aim to minimize steps and deliver structure and cells simultaneously through either scaffold-based designs or scaffold-less designs.[2,3,13–16]
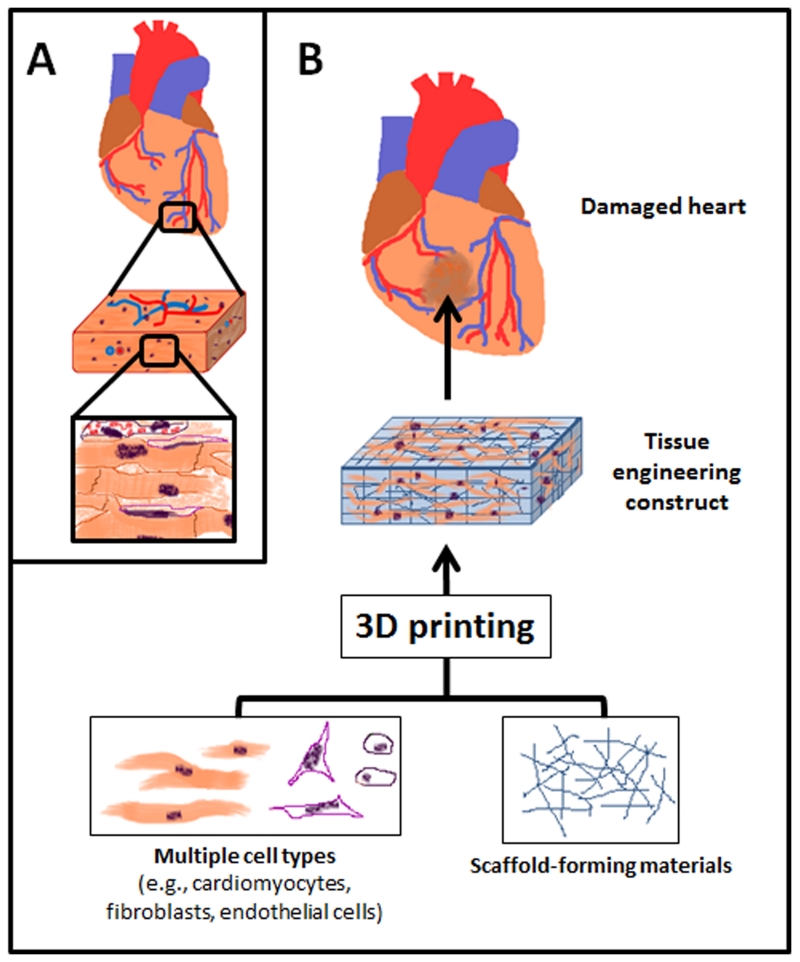
Figure 1.
Schematic representation of 3D printing for tissue engineering applications, such as for cardiac tissue engineering. (A) Tissues are composed of multiple types of cells assembled into hierarchal structures. (B) 3D printing can be utilized to assemble functional tissue from cells and scaffold-forming materials.
For tissue engineering, the ideal 3D printed construct would be a growth-directing structure on which cells could migrate and proliferate to form a functional tissue. While genetics can control cell fate, the research in this field has proven to be tedious and rather complex.[17,18] In addition to the difficulty of using genetic tools to direct cell fate, epigenetics has shown that covalent and noncovalent modifications (e.g., DNA methylation), both to the DNA and histone protein organization in chromatin, act as a liaison between the inherited genotype and resulting phenotype.[19,20] Without diving into the genetic and epigenetic world, the local environment of the cell, or microenvironment, is the natural grounds of influencing cell fate, as seen in developmental biology.[21] It is the extra-cellular matrix (ECM) of the cellular microenvironment that serves as a platform for mechanical and chemical cues, which can be created in vitro through 3D printing. Though widely debated, the material selection (e.g., synthetic ECM) is crucial in creating this bottom-up, assembled microenvironment. Soft tissue engineering has examined synthetic biodegradable polymers,[11,12,22,23] natural polymers,[24–32] and various combinations,[14,33,34] that can be printed using layer-by-layer solid freeform fabrication (SFF) methods, such as 3D blotting, among others.[3,26,30,33] Hard tissue engineering (e.g., bone) has explored ceramic materials, such as hydroxyapatite, that are known to be favorable for bone ingrowth[35–37] and are also typically fabricated through SFF methods, such as powder-based 3D printing.[38] In contrast, high concentrations of cells and cell spheroids (i.e. cellular aggregates) have been proposed as bioink for dispensing-based printers, relying on the biophysics of cellular self-assembly.[2,3,16,39]
While 3D printed tissue engineering constructs have been developed based on the fundamental characteristics of biodegradability, biocompatibility, and rapid-prototyping, further attention must be given to tissue integration.[40,41] Host tissue remodeling and integration has often been approached through modification of polymer-cell adhesion and scaffold biodegradability timed with cellular maturation.[7,26,37,40,42] Given the design control in 3D printing, the challenge of vascularization has been approached by printing a network of channels that are later seeded or printed with vascular cell types.[3,13,25,43–46] In this review, we discuss various scaffold-based and scaffold-free approaches in 3D printing that address these major hurdles in tissue engineering. Lastly, we conclude with design considerations and future perspectives of 3D printing research.
2. Scaffold-based Approach
2.1. Polymeric Scaffolds
Tissue-engineered constructs require a microenvironment that mimics the structural support of the ECM while being biodegradable so as to make room for the natural cellular ECM production. Synthetic polymer scaffolds have fine-tunable properties, giving them the ability to be biodegradable, structurally defined to the nano-scale, and be applied to soft and hard tissue printing.[33,47] Taking the printing design from the original ceramic models, Griffith and others used the SFF method to bind a mixed biodegradable polymer powder of 25% poly-l-lactide (PLLA) and 75% polylactide-coglycolide (PLGA) using chloroform as a binder[48] to print a branched liver construct with internal architecture.[12] This design took host implantation into consideration by creating an artery- and vein-like inlet and outlet. This “mini-liver” model was later improved with successful in vitro hepatocyte and nonparenchymal liver cell seeding under continuous flow conditions that enhanced the liver cell metabolism.[11]
The mechanical potential of synthetic polymers has lent itself to applications in bone and cartilage tissue engineering as well. Being able to adjust polymer concentrations and SFF design criteria (e.g., porogen concentration, drying time, drying method) allow for precise control over the final construct characteristics. An osteochondral composite scaffold was successfully designed by Sherwood and others using an SFF approach that contained a cartilage region made of d,l-PLGA/L-PLA and a bone portion of l-PLGA/TCP composite with a gradient of materials and porosity between both sections.[22] This novel design showed preferential attachment of chondrocytes to the cartilage region and comparable orthopedic mechanical properties. As is true for industrial 3D fabrication of inorganic materials, macro-scale and micro-scale control is offered through design resolution and porosity control. Also, starch-based polymers allow for increased degradation time and consequently expanded porosity as cellular integration increases, optimal for bone tissue engineering applications.[49] To provide greater control over hierarchal structure, Martins and others integrated 3D plotted scaffolds of starch-ε-polycaprolactone (SPCL) microfibers with layers of electrospun nanofiber mesh of PCL.[33] The combination of a starch-based polymer and a nanoscale component allowed for homogeneous distribution of cells and osteoblastic cell preferential adherence to the nanofiber mesh, favorable to bone tissue engineering strategies.[33]
In addition to directly printing polymeric scaffolds, 3D printing sacrificial molds (i.e., indirect 3D printing) has been proposed for polymer scaffold fabrication. For example, Sachlos and others showed that natural polymers (i.e., collagen) can be cast into a 3D printed, ethanol-dissolvable mold with predefined internal morphology.[25] After casting the dispersion of collagen, the construct was frozen, immersed in ethanol to dissolve the mold, and critical point dried to replace ethanol with liquid CO2. By freezing, ice crystals act as interstitial spacers and force collagen to create a network of fibrils to promote cell attachment.
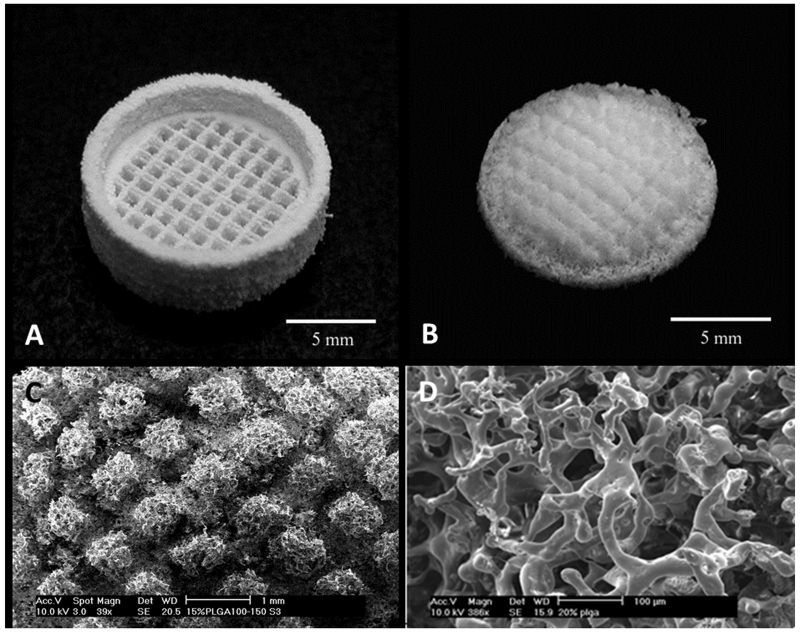
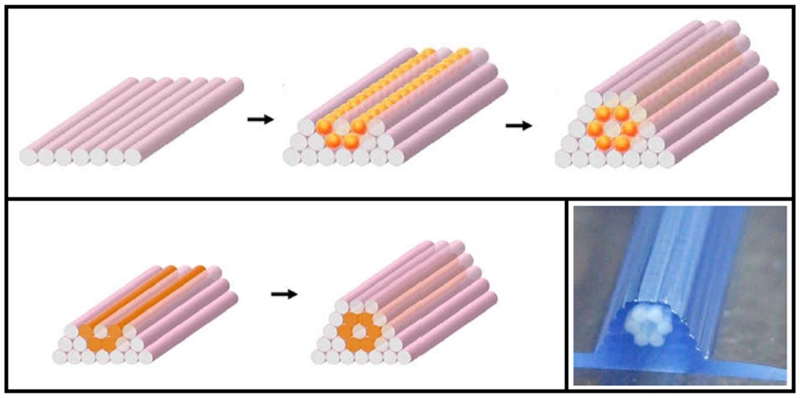
An advantage of indirect 3D printing is that it avoids utilizing organic solvents of direct 3D printing as binder for polymer scaffold construction, which can limit the printing resolution. To this end, Lee and others demonstrated the potential of indirect printing for complex constructs on the micro- and macro- scale by forming small villi constructs and a zygomatic arch defect from medical imaging data (Figure 2).[23] They used calcium sulfate hemihydrate plaster powder with water-based binder to 3D print the mold, allowing for use of high-resolution aqueous print-heads normally not compatible with organic solvent binders. A common biodegradable polymer, PLGA, mixed with sucrose particles (i.e, porogen) was casted into the mold to fabricate scaffolds. After removal of the mold and porogen in an aqueous environment, the scaffold was incubated with a fibronectin solution to promote cell attachment and proliferation for the enhanced tissue formation.[23]
Figure 2.
Indirect printing of villi constructs provides another method of attaining high resolution based on a sacrificial mold that can be made from medical imaging. Reprinted with permission from reference [21].
In the end, indirect 3D printing is itself limited by direct printing of 3D molds. Nevertheless, many direct polymer printing methods involve steps to eliminate organic solvent binders. Research that explores natural polymers (e.g., starch) with water-based binders for use in direct 3D SFF methods have shown promising results and can be combined with synthetic polymers for desired biodegradable and mechanical properties.[13,24] More notably, Miller and others recently developed a 3D fiber-drawing system to fabricate perfusable carbohydrate glass lattices coated with a thin layer of poly(d-lactide-co-glycolide), resembling patterned vascular.[13] This allows cell-laden synthetic ECM (i.e., hydrogel system) to be poured and crosslinked upon encapsulation of the lattice minutes after initial fabrication. The formed lumens were then seeded with HUVECs (human umbilical vein endothelial cells) and sustained metabolic function during blood perfusion.
2.2. Hydrogel System
The ideal 3D printed construct would be able to mimic the complexity of natural ECM. Despite the tunable control over polymeric designs, the water-based hydrogel systems provide a matrix scaffold for 3D cell growth that have similar diffusion properties for nutrient and waste exchange, thus serving as an attractive candidate for providing cell structural support and as a cell delivery agent.[50] Gelatin, the main component of hydrolyzed collagen, has been given much attention due to its natural origins in the ECM and ability to suspend cells in a gel at low temperatures. [27,28,34] However, glutaraldehyde has often been used as crosslinker to form a stable structure which can affect cell viability despite improved hydrogel structure.[27,51] Yan and others manually printed a liver tissue construct made of gelatin and chitosan mixed with hepatocytes followed with glutaraldehyde fixation.[27] Though stability of the hydrogel could be increased with longer glutaraldehyde crosslinking, it also increased cell death on the periphery of the scaffold with single cells and spheroids.[51] To take advantage of gelatin’s innate biocompatibility, Skardal and others combined it with polyethylene glycol tetracrylates and thiolated hyaluronic acid to create a tunable modular synthetic ECM that can be printed into vessel structures through a macrofilament stacking approach previously developed.[14,16] Though cell viability and structure remained after 4 weeks, further data on vascular tissue formation was not collected.
To bypass indirect toxic effects of hydrogel crosslinkers, alginate has been utilized for controlled gelation across various printing techniques. Given its modifiable chemical and physical properties,[52,53] attachment of cell-adhesive ligands (e.g., RGD peptide) can alter cell motility and differentiation[26,54] while photocrosslinking ligands provide another means of gelation.[55] Alginate gelation commonly is performed with calcium chloride (CaCl2) solutions used as a chelating agent post-printing and have shown promising biocompatibility and processing characteristics as a cell delivery agent.[15,26,30,56] Fedorovich and others showed simultaneous delivery of scaffold and cells by using high-viscous alginate to deposit fibers of cell-laden hydrogel using a 3D plotting device into a petri dish containing CaCl2 for immediate gelation.[15] To increase the variety of shapes, layer-by-layer inkjet-based printers have been successfully adapted to print cell-containing alginate into a solution of CaCl2 with added viscosity enhancers, such as polyvinyl alcohol or hyaluronan, to fix the position of the printed construct during the printing process.[56]
To improve alginate inkjet technology, Pataky and others developed the method of printing alginate mixtures onto a gelatin substrate containing CaCl2 that allows upward diffusion of calcium ions to chelate in a timely manner.[57] Because alginate is a fast-gelling hydrogel, its lack of biologically active properties can be improved by combining this technique with slow-gelling hydrogels with higher cell-directing properties, such as collagen, opening a wider physical use of alginate in 3D printing.[57] Chemical modification of alginate by adding RGD peptide can alter cell motility, but the non-adhesive properties of unmodified alginate may be conductive to host tissue integration. This was beautifully demonstrated by Gaetani and others when they demonstrated the ability of human cardiomyocyte progenitor cells embedded in an unmodified alginate matrix to migrate and fully colonize an adjacent matrigel layer.[26]
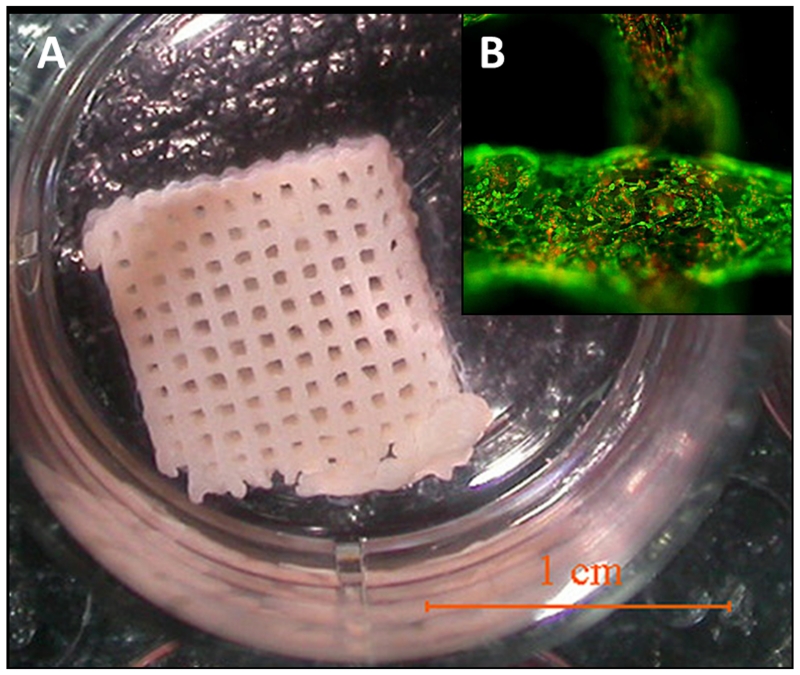
Apart from single polymer hydrogels, combinations of various polymers have been considered in efforts to take advantage of the separate qualities of each. A printed gelatin/alginate/fibrinogen matrix later seeded with adipose-derived stromal cells was proposed by Xu and others to create an adequate 3D model for studying the pathological pathways of the metabolic syndrome (Figure 3).[32] Their perspective combined ECM-like matrices of gelatin and alginate with the body’s natural scaffold material, fibrinogen. Gelation occurred through initial crosslinking of the alginate component with CaCl2 followed by polymerization (fibrinogen to fibrin) with thrombin. Culturing with VEGF (vascular endothelial growth factor) allowed seeded cells on the exterior to differentiate into endothelial cells, which provides a new technique for tissue engineering vessel networks.[32] The hydrogel system creates an aqueous, ECM-like matrix that provides a microenvironment for cellular development as well as a platform for cell and drug delivery in soft tissue engineering.
Figure 3.
3D-printed gelatin/alginate/fibrinogen hydrogel construct containing adipose-derived stromal cells with open channels. (A) The 3D structure cultured in a plate. (B) Immunostaining of the construct with cell structure in green and nuclei in red. Reprinted and adjusted with permission from reference [32].
2.3. Inorganic Scaffolds
Given the initial fabrication nature of 3D printing, some of the first applications of 3D bioprinting were to orthopedic designs and hard tissue engineering. Orthopedic implant research has used 3D printing methods, such as powder-based selective laser sintering[58] and laser engineered net shaping (LENS),[59] as a source for metal implant fabrication techniques, which have a specific and direct effect on mechanical properties. In contrast to soft tissue engineering, hard tissue scaffold designs typically use the powder-based SFF method to print ceramic, resorbable materials (e.g., hydroxyapatite) that contain varying degrees of porosity to promote cell infiltration and proliferation. Pores reduce the mechanical strength of a construct yet provide space for cellular integration and vascularization. Though typically combined with a biomolecular approach, vascularization and cellular integration can be supported through precise 3D design of porosity in trade off with mechanical strength.[42] Regular and geometrically ordered scaffolds have been shown to be more inductive of organized bone growth,[60] and thus calls for 3D printing methods.
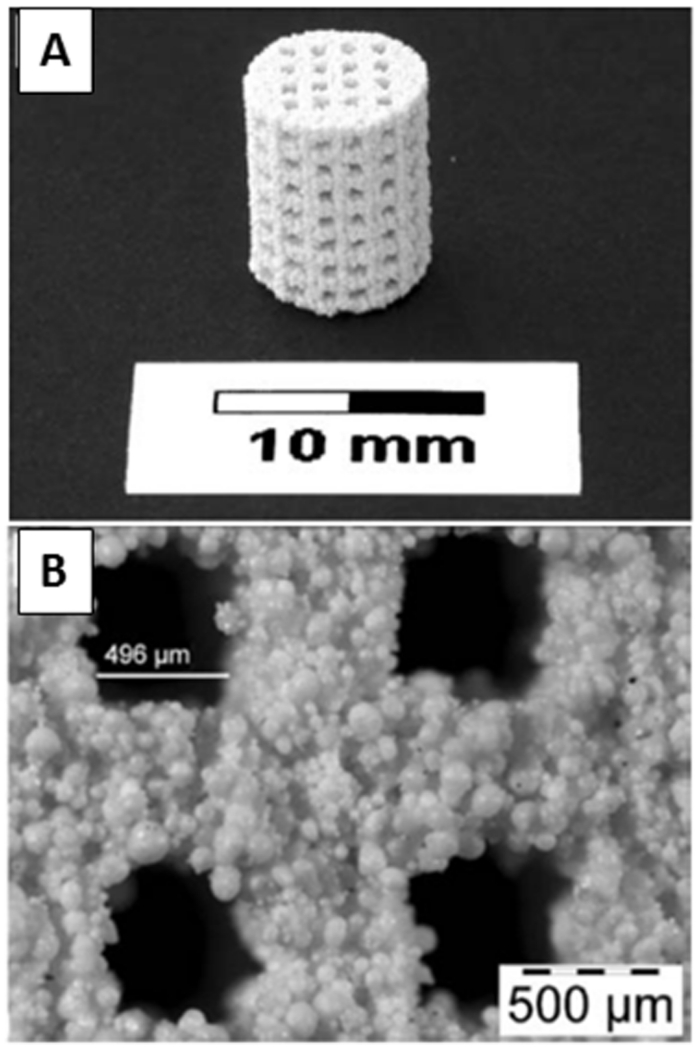
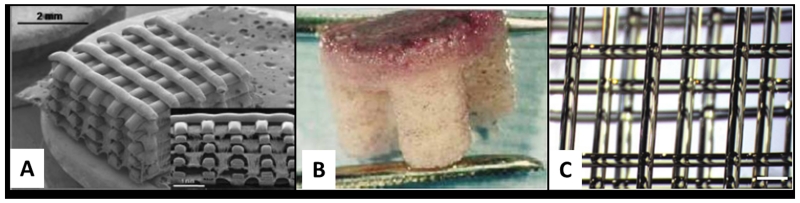
As is used in many orthopedic applications, hydroxyapatite (HA) and similarly tricalcium phosphate (TCP) are often used in ceramic scaffolds, as they are derivatives of the inorganic component of bone.[61] In 3D printing, a layer of powder is sprayed with a specific binder, according to a 2D layer breakdown of a 3D computer-aided design (CAD) file. Upon completion, the construct’s mechanical properties are improved through high-temperature heating (i.e., process of sintering), increasing the density and thus size of the construct.[42] Using HA mixed with a soluble polymer and a polymer-based binder, Leukers and others printed a porous 3D scaffold that successfully promoted cell attachment and proliferation under static and dynamic perfusion conditions (Figure 4).[36] During the sintering process, the polymer components are pyrolysed creating a microporous surface, conductive of bone integration.[35] While the CAD model controls the macropores to a limited resolution, adjusting powder composition (fine to course powder content) and sintering temperature controls micropores.[62] Teixeira and others attempted to improve osteogenic differentiation and cellular integration of human mescenchymal stem cells in HA porous scaffolds by introducing collagen but found no significant enhancement of proliferation,[63] possibly due to the hyper complexity of the microstructure.[60] Resolution and organization of micropores remains rather elusive and precise control is eventually limited by the sintering process. Rather than relying on surface heating of conventional sintering that create material-dependent heat fluxes, Tarafder and others recently used microwave sintering as a way to improve heat uniformity and short processing time.[37] This provides enhanced control over grain growth, pore size, and higher densification leading to improved mechanical properties.
Figure 4.
3D printed hydroxyapatite scaffold with interconnecting channels. (A) The macro-structure contains interconnecting channels with (B) visible porous structures resulting from polymeric additives. Reprinted with permission from reference [36].
In terms of the vascularization of scaffolds, the sintering process limits the incorporation of cells or biomolecules until post-printing. Gbureck and others fabricated brushite (i.e., hydrated calcium phosphate) and HA scaffolds using a low temperature method that allows for incorporation of biomolecules (e.g. growth factors) for enhanced cellular and vascular response.[64] With a similar approach of providing vascular cues from within the scaffold, a novel method of adding small amounts inorganic copper, alone or in combination with VEGF, has shown promising vascular induction properties. Barralet and others used a 3D printed calcium phosphate scaffold with a Y-shaped pore and loaded the edge of the macropore with VEGF and/or copper sulfate.[65] This was implanted into a mouse model and later explanted upon sacrifice to reveal wound tissue capillary formation suggesting a chemotactic effect of copper sulfate. Despite major progress in 3D printing of inorganic scaffolds and positive in vitro tests and preliminary orthotopic applications,[66–68] major challenges are still being addressed in design resolution[37,38] and vascular induction.[62,64,65] Nonetheless, given the progress made in material chemistry, material characterization, as well as the innovation in printing methods of scaffold designs, both in synthetic and natural biomaterial applications, 3D printing of scaffold-based constructs hold promise in overcoming major challenges in tissue formation and integration.
3. Scaffold-free Approach
Despite significant advancement in the areas of scaffold-based tissue engineering designs, the scaffold-free approach aims to take advantage of the natural self-assembly process common in developmental biology.[21] The process of cellular self-assembly has been thoroughly examined[39,45,69,70] and applied in non-3D printing methods, such as L’Heureux’s cell-sheet method of vessel assembly.[71–73] The laboratory of Gabor Forgac has examined the self-assembly characteristics of a 100% cellular bioink in vessel construction. Using agarose rods as building blocks for the mold template, the bioink, in the form of spheroids or cylindrical cellular rods, is dispensed using a layer-by-layer extrusion 3D printer (Figure 5).[74] Fusion of bioink occurred in two to four days post-printing. Agarose rods were then manually removed, and the vessel constructs were put into a perfusion bioreactor. To expand this bioink method beyond vessel shapes, Jakab and others manually engineered topologically defined structures using high cellular-density aggregates to achieve viable tissue, noting the importance of the “biopaper,” or dispensing surface, but also showing its potential for 3D printing applications.[2,3]
Figure 5.
Scaffold-free approach to 3D printing using either spheroids or cylinder rods of cells as bioink. The structure is supported by agarose rods that are printed layer-by-layer. Reprinted and adjusted with permission from reference [74].
4. Design Considerations
4.1. Scaffold Materials
As examined above, material selection plays a decisive role in the performance of 3D printed tissue engineering constructs, including cellular integration, maturation, and proliferation (Figure 6). Natural polymers (e.g., collagen) contain cell-interactive properties that can induce differentiation or enhance cellular motility while synthetic polymers can be precisely modified to fit a specific tissue engineering application. Inorganic scaffolds require balance of mechanical support and cellular integration. Within hydrogel systems, crosslinking agents can determine or limit the method of printing evidenced by various alginate models[30,57] and photocrosslinking gels.[55]
Figure 6.
Variety of polymeric designs addressing cellular integration. (A) The 3D plotted construct of starch-ε-polycaprolactone (SPCL) and an electrospun nanofiber mesh of allowed for homogeneous distribution of cells and osteoblastic cell preferential adherence to the nanofiber mesh. (B) An osteochondral scaffold after chondrocyte seeding comprises a cartilage region made of D,L-PLGA/L-PLA (red upper section) and a bone portion of L-PLGA/TCP composite (lower white section) with a gradient of materials and porosity between both section that showed preferential attachment of chondrocytes to the cartilage portion. Height = 4mm. (C) A multilayered lattice of carbohydrate-glass material provides a micro-structured, perfusable, dissolvable scaffold for vascular engineering. Scale bar = 1mm. Reprinted and adjusted with permission from references [31, 20, 13], respectively.
However, it is apparent through the heterogeneous make-up of the living tissues that combinations of materials and fabrication techniques may provide the optimal characteristics for tissue constructs. Xu and others combined polyurethane and gelatin using a double-nozzle low-temperature deposition system to simultaneously layer synthetic and natural polymers that create a mechanically stable structure with enhanced matrix-like space for cell implantation and proliferation.[34] Material properties, such as biodegradability, can be combined to optimize an cellular interactive mold, as seen in the design of Phipps and others where electrospinning was used to fabricate bone-mimetic fiber meshes of PCL, collagen I, and hydroxyapatite.[75] Researching a wide variety of biomaterials for 3D printing develops individual approaches that can be used to individually address specific tissues or pathologies.
4.2. Bioink
Scaffold-based designs use materials as a cell delivery agent to provide structural cues and support as the tissue develops. Scaffold-free approaches propose using cell aggregates as their own delivery vehicle without using ECM-mimetic materials. The work of Norotte, Jakab, and others has shown that cellular aggregates (i.e., spheroids or cylindrical rods) fuse and self-assemble into organized tissues.[2,3,74] In contrast, it has been shown that monodispersed cells can form complex shapes at similar efficiencies when compared to spheroids.[76] In the same study, nonetheless, tissue structural properties and cellular sorting behavior can be controlled by pre-culture time of spheroids, where shorter time results in more compact fusion, and by different combinations of mixed spheroids and cells.[76] Thus, bioink selection, whether as single cells or spheroids, overcomes cell sorting tendencies and provides an adjustable criterion for cell position in heterotopic 3D tissues in scaffold and scaffold-free designs.
4.3. Bioprinters
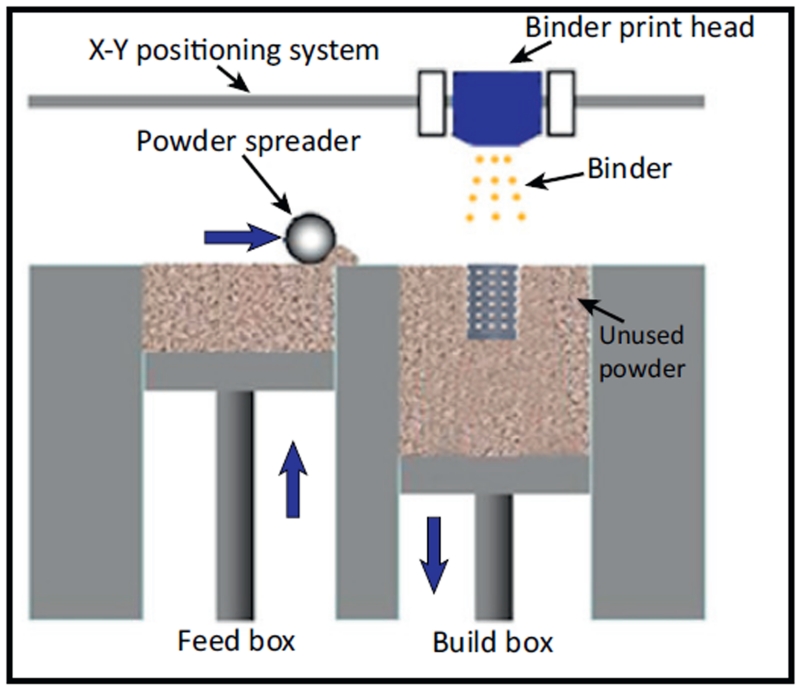
The advent of 3D printing allows researchers to fabricate specific shapes that can ideally be patient-specific through medical imaging data and CAD models[6] (Table 1). One of the major challenges to reconstructing tissues is controlling the architecture down to the micro- and nano-scale while retaining cytocompatibility. Hydrogel systems that rely on organic solvents as crosslinkers risk toxic exposure to cells or increase steps of fabrication. Printers that involve UV crosslinking risk genetic alteration. Dispensing and inkjet printers place volume-defined droplets whose spatial control is dictated by droplet dispersion. The common SFF method uses powder and binder to assemble a layer-by-layer construct and significant progress has been made in this field (Figure 7). Indirect printing proposes higher resolution scaffolds through casting into SFF printed molds. However, constructs are limited by resolution, which can be attributed to physical properties of the materials, such as droplet dispersion and powder flowability.[38] Guillotin and others propose that the highest resolution can be defined by one-by-one cell placement and have experimentally proven this capability through laser assisted bioprinting (LAB).[5,77] However, though this technique may be useful in research applications, its application to tissue genesis is impractical due to lack of timely fabrication.[40] To decrease tissue fabrication time and design micromanagement, the scaffold-free method of cellular aggregate dispensing promotes high-cell density, self-assembling bioink yet resolution is still being developed.[74] Independent of the printing method, post-printing handling (i.e., dynamic bioreactors) can also provide tissue construct maturation for orthotopic application.[78–81] Additionally, future research in cellular positioning control will improve tissue-specific bioprinter methods.[76,82–84] In addition to 3D printing, other fabrication methods may have promising potential. For instance, the research of Du and others have shown the directed self-assembly of cell-laden microgels with defined micro-architectures.[82–84] The PRINT (particle replication in nonwetting templates) technology developed by the DeSimone laboratory also holds great potential in tissue engineering applications.[85,86]
Table 1.
3D Bioprinting Approaches
| Material | Binder/Crosslinker | Resolution (μm) |
Reference (s) |
|---|---|---|---|
| Powder-based 3D Printing | |||
| Synthetic polymer powder | Chloroform binder | 38-150* | [12], [22] |
| Plaster powder | ZB7 water-based binder | 300 | [23] |
| Organic compounds | BioAct™ binder | 181 ± 8 | [25] |
| HA/soluble polymer | Polymer-based binder | 500 | [36] |
| TCP powder | Water-based binder | 400 | [37] |
| DCPD; HA | Phosphoric acid binder | N/A | [64] |
| Liquid-based 3D Dispensing | |||
| Molten polymer/starch | 300 | [33] | |
| Gelatin/chitosan | TPP and glutaraldehyde crosslinker |
10 | [27] |
| Alginate | CaCl2 solution | 320±41 | [25] |
| CaCl2/gelatin surface | 200 | [57] | |
| Gelatin/alginate/fibrinogen | CaCl2/thrombin | N/A | [32] |
| 3D fiber-drawing | |||
| Glucose/sucrose/dextran | 200 | [13] | |
| Macrofilament stacking | |||
| Gelatin/PEG tetracrylate/thiolated HA filament/agarose rods |
500 | [14] | |
| Cells/agarose rods | 900 | [2], [3], [74] |
indicates use of milling to achieve resoultion; HA= hydroxyapatite; TCP= tricalcium phosphate; DCPD= dicalcium phosphate dihydrate (brushite); N/A indicates resolution was not mentioned
Figure 7.
A representation of the common solid freeform fabrication (SFF) method of layer-by-layer 3D printing. A powder reservoir supplies the desired material (e.g., polymer, ceramic) to the build box where the print head can apply the desired binder (e.g., organic solvent, UV) in a defined pattern to form a construct layer-by-layer. Reprinted with permission from reference [40].
5. Summary and Outlook
Tissue engineering combines engineering concepts with tissue biology to construct functional tissue. As a rapid-prototyping method with innate potential for industrial scale fabrication, 3D printing holds remarkable promise to overcome major challenges in tissue engineering.[6,21,40] The previously mentioned design considerations can play an essential role in improving cellular integration, host integration, vascularization of 3D printed tissue engineering constructs. Specifically, vascularization is a limiting factor to increasing tissue construct size, and thus limits the practical size of implantable tissue constructs.[41,87,88] Significant progress has been made from various perspectives to overcome this challenge, though continued research and expanded in vitro and in vivo models must be explored.[13,25,44,89] From a fabrication standpoint, successful designs should involve reduced steps to obtain the final product. Enhanced or simultaneous delivery of structure, cells, and biomolecules without extra purification steps is embodied in several of the mentioned methods and provide a platform for further research to streamline the process.[13,37,65,75] Apart from the pursuit of implantable tissue constructs, further research into 3D printed tissues will also provide advanced drug delivery and pathological human models reducing risk in the transition from animal to human studies.[18,90] In conclusion, 3D printing can provide a powerful approach to assemble functional tissue in vitro and accelerate the translation of tissue engineering concepts in regenerative therapy and drug testing.
Acknowledgement
The work is supported by the National Science Foundation (NSF - EPS-0903795), the startup funds from Clemson University, and the National Institutes of Health (8P20 GM103444).
References
- [1].Elbert DL. Curr. Opin. Biotechnol. 2011;22(5):674–80. doi: 10.1016/j.copbio.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Jakab K, Marga F, Norotte C, Murphy K, Vunjak G. Biofabrication. 2010;2(2):1–34. doi: 10.1088/1758-5082/2/2/022001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Jakab K, et al. Tissue. Eng. Part. A. 2008;14(3):413–21. doi: 10.1089/tea.2007.0173. [DOI] [PubMed] [Google Scholar]
- [4].Wang X, Yan Y, Zhang R. Trends. Biotechnol. 2007;25(11):505–13. doi: 10.1016/j.tibtech.2007.08.010. [DOI] [PubMed] [Google Scholar]
- [5].Guillotin B, Guillemot F. Trends. Biotechnol. 2011;29(4):183–90. doi: 10.1016/j.tibtech.2010.12.008. [DOI] [PubMed] [Google Scholar]
- [6].Tsang VL, Bhatia SN. Adv. Drug Deliv. Rev. 2004;56(11):1635–47. doi: 10.1016/j.addr.2004.05.001. [DOI] [PubMed] [Google Scholar]
- [7].Rivron NC, et al. Biomaterials. 2009;30(28):4851–8. doi: 10.1016/j.biomaterials.2009.06.037. [DOI] [PubMed] [Google Scholar]
- [8].Perez-Castillejos R. Mater. Today. 2010;13(1-2):32–41. [Google Scholar]
- [9].Karande TS, Ong JL, Agrawal CM. Ann. Biomed. Eng. 2004;32(12):1728–43. doi: 10.1007/s10439-004-7825-2. [DOI] [PubMed] [Google Scholar]
- [10].Lee M, Wu BM, Dunn JCY. J. Biomed. Mater. Res. A. 2008;87(4):1010–6. doi: 10.1002/jbm.a.31816. [DOI] [PubMed] [Google Scholar]
- [11].Kim SS, et al. Ann. Surg. 1998;228(1):8–13. doi: 10.1097/00000658-199807000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Griffith LG, et al. Ann. NY Acad. Sci. 1997;831(617):382–97. doi: 10.1111/j.1749-6632.1997.tb52212.x. [DOI] [PubMed] [Google Scholar]
- [13].Miller JS, et al. Nat. Mater. 2012;11(9):768–74. doi: 10.1038/nmat3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Skardal A, Zhang J, Prestwich GD. Biomaterials. 2010;31(24):6173–81. doi: 10.1016/j.biomaterials.2010.04.045. [DOI] [PubMed] [Google Scholar]
- [15].Fedorovich NE, De Wijn JR, Verbout AJ, Alblas J, Dhert W. J. a. Tissue. Eng. Part. A. 2008;14(1):127–33. doi: 10.1089/ten.a.2007.0158. [DOI] [PubMed] [Google Scholar]
- [16].Norotte C, Marga FS, Niklason LE, Forgacs G. Biomaterials. 2009;30(30):5910–7. doi: 10.1016/j.biomaterials.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Porteus M. Mol. Ther. 2011;19(3):439–41. doi: 10.1038/mt.2011.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Barrett AJ, Melenhorst JJ. Mol. Ther. 2011;19(2):224–7. doi: 10.1038/mt.2010.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Goldberg AD, Allis CD, Bernstein E. Cell. 2007;128(4):635–8. doi: 10.1016/j.cell.2007.02.006. [DOI] [PubMed] [Google Scholar]
- [20].Bernstein BE, Meissner A, Lander ES. Cell. 2007;128(4):669–81. doi: 10.1016/j.cell.2007.01.033. [DOI] [PubMed] [Google Scholar]
- [21].Marga F, Neagu A, Kosztin I, Forgacs G. Birth. Defects. Res. C. Embryo. Today. 2007;81(4):320–8. doi: 10.1002/bdrc.20109. [DOI] [PubMed] [Google Scholar]
- [22].Sherwood JK, et al. Biomaterials. 2002;23(24):4739–51. doi: 10.1016/s0142-9612(02)00223-5. [DOI] [PubMed] [Google Scholar]
- [23].Lee M, Dunn JCY, Wu BM. Biomaterials. 2005;26(20):4281–9. doi: 10.1016/j.biomaterials.2004.10.040. [DOI] [PubMed] [Google Scholar]
- [24].Lam CX, Mo X, Teoh S, Hutmacher D. Mater. Sci. Eng, C. 2002;20(1-2):49–56. [Google Scholar]
- [25].Sachlos E, Reis N, Ainsley C, Derby B, Czernuszka JT. Biomaterials. 2003;24(8):1487–97. doi: 10.1016/s0142-9612(02)00528-8. [DOI] [PubMed] [Google Scholar]
- [26].Gaetani R, et al. Biomaterials. 2012;33(6):1782–90. doi: 10.1016/j.biomaterials.2011.11.003. [DOI] [PubMed] [Google Scholar]
- [27].Yan Y, et al. Biomaterials. 2005;26(29):5864–71. doi: 10.1016/j.biomaterials.2005.02.027. [DOI] [PubMed] [Google Scholar]
- [28].Wang X, et al. Tissue. Eng. 2006;12(1):83–90. doi: 10.1089/ten.2006.12.83. [DOI] [PubMed] [Google Scholar]
- [29].Xu W, et al. J. Bioact. Compat. Polym. 2007;22(4):363–377. [Google Scholar]
- [30].Nishiyama Y, et al. J. Biomech. Eng. 2009;131(3):035001. doi: 10.1115/1.3002759. [DOI] [PubMed] [Google Scholar]
- [31].De Colli M, et al. Biomed. Mater. 2012;7(5):055005. doi: 10.1088/1748-6041/7/5/055005. [DOI] [PubMed] [Google Scholar]
- [32].Xu M, Wang X, Yan Y, Yao R, Ge Y. Biomaterials. 2010;31(14):3868–77. doi: 10.1016/j.biomaterials.2010.01.111. [DOI] [PubMed] [Google Scholar]
- [33].Martins A, et al. J. Tissue. Eng. Regen. Med. 2009;3:37–42. doi: 10.1002/term.132. [DOI] [PubMed] [Google Scholar]
- [34].Xu W, Wang X, Yan Y, Zhang R. J. Bioact. Compat. Polym. 2008;23(5):409–422. [Google Scholar]
- [35].Seitz H, Rieder W, Irsen S, Leukers B, Tille C. J. Biomed. Mater. Res. B. Appl. Biomater. 2005;74(2):782–8. doi: 10.1002/jbm.b.30291. [DOI] [PubMed] [Google Scholar]
- [36].Leukers B, et al. J. Mater. Sci. Mater. Med. 2005;16(12):1121–4. doi: 10.1007/s10856-005-4716-5. [DOI] [PubMed] [Google Scholar]
- [37].Tarafder S, Balla VK, Davies NM, Bandyopadhyay A, Bose S. J. Tissue. Eng. Regen. Med. 2012 doi: 10.1002/term.555. Epub ahea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Butscher a, Bohner M, Hofmann S, Gauckler L, Müller R. Acta Biomater. 2011;7(3):907–20. doi: 10.1016/j.actbio.2010.09.039. [DOI] [PubMed] [Google Scholar]
- [39].Neagu A, Jakab K, Jamison R, Forgacs G. Phys. Rev. Lett. 2005;95(17):178104. doi: 10.1103/PhysRevLett.95.178104. [DOI] [PubMed] [Google Scholar]
- [40].Mironov V, Kasyanov V, Markwald RR. Curr. Opin. Biotechnol. 2011;22(5):667–673. doi: 10.1016/j.copbio.2011.02.006. [DOI] [PubMed] [Google Scholar]
- [41].Bramfeldt H, Sabra G, Centis V, Vermette P. Curr. Med. Chem. 2010;17(33):3944–67. doi: 10.2174/092986710793205327. [DOI] [PubMed] [Google Scholar]
- [42].Bose S, Roy M, Bandyopadhyay A. Trends. Biotechnol. 2012;30(10):546–54. doi: 10.1016/j.tibtech.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kelm JM, et al. Tissue. Eng. 2006;12(8):2151–60. doi: 10.1089/ten.2006.12.2151. [DOI] [PubMed] [Google Scholar]
- [44].Cui X, Boland T. Biomaterials. 2009;30(31):6221–7. doi: 10.1016/j.biomaterials.2009.07.056. [DOI] [PubMed] [Google Scholar]
- [45].Mironov V, et al. Biomaterials. 2009;30(12):2164–74. doi: 10.1016/j.biomaterials.2008.12.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Visconti RP, et al. Expert. Opin. Biol. Ther. 2010;10(3):409–420. doi: 10.1517/14712590903563352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Place ES, George JH, Williams CK, Stevens MM. Chem. Soc. Rev. 2009;38(4):1139–51. doi: 10.1039/b811392k. [DOI] [PubMed] [Google Scholar]
- [48].Wu BM, et al. J. Control. Release. 1996;40:77–87. [Google Scholar]
- [49].Gomes ME, Azevedo HS, Moreira AR, Ell V. J. Tissue. Eng. Regen. Med. 2008;2:243–252. doi: 10.1002/term.89. [DOI] [PubMed] [Google Scholar]
- [50].Tibbitt MW, Anseth KS. Biotechnol. Bioeng. 2009;103(4):655–63. doi: 10.1002/bit.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].McGuigan AP, Sefton MV. J. Tissue. Eng. Regen. Med. 2007;1:136–145. doi: 10.1002/term.14. [DOI] [PubMed] [Google Scholar]
- [52].Augst AD, Kong HJ, Mooney DJ. Macromol. Biosci. 2006;6(8):623–33. doi: 10.1002/mabi.200600069. [DOI] [PubMed] [Google Scholar]
- [53].Lee KY, Mooney DJ. Prog. Polym. Sci. 2012;37(1):106–126. doi: 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Huebsch N, et al. Nat. Mater. 2010;9(6):518–26. doi: 10.1038/nmat2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Rouillard AD, et al. Tissue. Eng. Part. C. Methods. 2011;17(2):173–179. doi: 10.1089/ten.TEC.2009.0582. [DOI] [PubMed] [Google Scholar]
- [56].Nakamura M, et al. J. Imaging. Sci. Technol. 2008;52(6):1–6. [Google Scholar]
- [57].Pataky K, et al. Adv. Mater. 2011;24:391–396. doi: 10.1002/adma.201102800. [DOI] [PubMed] [Google Scholar]
- [58].Fukuda A, et al. Acta Biomater. 2011;7(5):2327–36. doi: 10.1016/j.actbio.2011.01.037. [DOI] [PubMed] [Google Scholar]
- [59].Xue W, Krishna BV, Bandyopadhyay A, Bose S. Acta Biomater. 2007;3(6):1007–18. doi: 10.1016/j.actbio.2007.05.009. [DOI] [PubMed] [Google Scholar]
- [60].Scaglione S, et al. Sci. Rep. 2012;2:274. doi: 10.1038/srep00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Khan Y, Yaszemski MJ, Mikos AG, Laurencin CT. J. Bone. Joint. Surg. Am. 2008;90(Suppl 1):36–42. doi: 10.2106/JBJS.G.01260. Suppl 1. [DOI] [PubMed] [Google Scholar]
- [62].Will J, et al. J. Mater. Sci. Mater. Med. 2008;19(8):2781–90. doi: 10.1007/s10856-007-3346-5. [DOI] [PubMed] [Google Scholar]
- [63].Teixeira S, et al. J. Biomed. Mater. Res. A. 2010;93(2):567–575. doi: 10.1002/jbm.a.32532. [DOI] [PubMed] [Google Scholar]
- [64].Gbureck U, Hölzel T, Doillon CJ, Müller F. a., Barralet JE. Adv. Mater. 2007;19(6):795–800. [Google Scholar]
- [65].Barralet J, et al. Tissue. Eng. Part. A. 2009;15(7):1601–9. doi: 10.1089/ten.tea.2007.0370. [DOI] [PubMed] [Google Scholar]
- [66].Becker ST, et al. J. Mater. Sci. Mater. Med. 2010;21(4):1255–62. doi: 10.1007/s10856-009-3878-y. [DOI] [PubMed] [Google Scholar]
- [67].Warnke PH, et al. J. Biomed. Mater. Res. B. Appl. Biomater. 2010;93(1):212–7. doi: 10.1002/jbm.b.31577. [DOI] [PubMed] [Google Scholar]
- [68].Tamimi F, et al. Biomaterials. 2009;30(31):6318–26. doi: 10.1016/j.biomaterials.2009.07.049. [DOI] [PubMed] [Google Scholar]
- [69].Yang X, Mironov V, Wang Q. J. Theor. Biol. 2012;303:110–8. doi: 10.1016/j.jtbi.2012.03.003. [DOI] [PubMed] [Google Scholar]
- [70].Fleming P. a, et al. Dev. Dyn. 2010;239(2):398–406. doi: 10.1002/dvdy.22161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].L’Heureux N, Paquet S, Labbe R, Germain L, Auger FA. FASEB. J. 1998;12:47–56. doi: 10.1096/fasebj.12.1.47. [DOI] [PubMed] [Google Scholar]
- [72].L’Heureux N, et al. Nat. Med. 2006;12(3):361–5. doi: 10.1038/nm1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].L’Heureux N, et al. Nat. Clin. Pract. Cardiovasc. Med. 2007;4(7):389–95. doi: 10.1038/ncpcardio0930. [DOI] [PubMed] [Google Scholar]
- [74].Norotte C, Marga F, Niklason L, Forgacs G. Biomaterials. 2009;30(30):5910–5917. doi: 10.1016/j.biomaterials.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Phipps MC, Clem WC, Grunda JM, Clines G. a, Bellis SL. Biomaterials. 2012;33(2):524–34. doi: 10.1016/j.biomaterials.2011.09.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Rago AP, Dean DM, Morgan JR. Biotechnol. Bioeng. 2009;102(4):1231–41. doi: 10.1002/bit.22162. [DOI] [PubMed] [Google Scholar]
- [77].Guillemot F, Souquet A, Catros S, Guillotin B. Nanomedicine. 2010;5:507–515. doi: 10.2217/nnm.10.14. [DOI] [PubMed] [Google Scholar]
- [78].Pei M, et al. FASEB. J. 2002;16:1691–1694. doi: 10.1096/fj.02-0083fje. [DOI] [PubMed] [Google Scholar]
- [79].Martin I, Wendt D, Heberer M. Trends. Biotechnol. 2004;22(2):80–6. doi: 10.1016/j.tibtech.2003.12.001. [DOI] [PubMed] [Google Scholar]
- [80].Pörtner R, Nagel-Heyer S, Goepfert C, Adamietz P, Meenen NM. J. Biosci. Bioeng. 2005;100(3):235–45. doi: 10.1263/jbb.100.235. [DOI] [PubMed] [Google Scholar]
- [81].Bilodeau K, Mantovani D. Tissue. Eng. 2006;12(8):2367–2383. doi: 10.1089/ten.2006.12.2367. [DOI] [PubMed] [Google Scholar]
- [82].Du Y, Lo E, Vidula MK, Khabiry M, Khademhosseini A. Cell Mol. Bioeng. 2008;1(2):157–162. doi: 10.1007/s12195-008-0020-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Du Y, et al. Biotechnol. Bioeng. 2010;105(3):655–62. doi: 10.1002/bit.22552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Zamanian B, et al. Small. 2010;6(8):937–44. doi: 10.1002/smll.200902326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Rolland JP, et al. J. Am. Chem. Soc. 2005;127(19):10096–10100. doi: 10.1021/ja051977c. [DOI] [PubMed] [Google Scholar]
- [86].Xu J, et al. Angew. Chem. Int. Ed. Engl. 2013;52(26):6580–9. doi: 10.1002/anie.201209145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Rouwkema J, Rivron NC, van Blitterswijk C. a. Trends. Biotechnol. 2008;26(8):434–41. doi: 10.1016/j.tibtech.2008.04.009. [DOI] [PubMed] [Google Scholar]
- [88].Jain RK, Au P, Tam J, Duda DG, Fukumura D. Nat. Biotechnol. 2005;23(7):821–3. doi: 10.1038/nbt0705-821. [DOI] [PubMed] [Google Scholar]
- [89].Mironov V, et al. Vitural. Phys. Prototyp. 2009;4(2):63–74. [Google Scholar]
- [90].Saltzman WM, Olbricht WL. Nat. Rev. Drug Discov. 2002;1(3):177–86. doi: 10.1038/nrd744. [DOI] [PubMed] [Google Scholar]