Abstract
Fusarium and Verticillium wilts, two of the most important diseases in cotton, pose serious threats to cotton production. Here we introduced a novel antimicrobial protein Hcm1, which comprised harpin protein from Xanthomonas oryzae pv. oryzicola (Xoc), and the chimeric protein, cecropin A-melittin, into cotton. The transgenic cotton lines with stable Hcm1 expression showed a higher resistance to Verticillium and Fusarium wilts both in greenhouse and field trials compared to controls. Hcm1 enabled the transgenic cotton to produced a microscopic hypersensitive response (micro-HR), reactive oxygen species (ROS) burst, and caused the activation of pathogenesis-related (PR) genes in response to biotic stress, indicating that the transgenic cotton was in a primed state and ready to protect the host from pathogenic infection. Simultaneously, Hcm1 protein inhibited the growth of Verticillium dahliae (V. dahliae) and Fusarium oxysporum (F. oxysporum) in vitro. The spread of fungal biomass was also inhibited in vivo since the V. dahliae biomass was decreased dramatically in transgenic cotton plants after inoculation with V. dahliae. Together, these results demonstrate that Hcm1 could activate innate immunity and inhibit the growth of V. dahliae and F. oxysporum to protect cotton against Verticillium and Fusarium wilts.
Fungal disease is a major threat to both crop yields and global food security1,2,3. Fusarium wilt and Verticillium wilt, also known as vascular wilt, pose the largest threat of disease to most economically important crops, such as tomato and cotton. In particular, Verticillium wilt has been reported in most cotton-growing areas, and is the most important disease of cotton in the world4. About half of the cotton cultivating area in China was subjected to this disease in 2009 and 2010 (National Cotton Council of America-Disease Database). Traditional methods of pathogen control rely heavily on two methods: biological control measures such as cultivar choice and crop rotation and chemical control. Intensive plant breeding and chemical controls allow farmers to overcome many common plant diseases. However, effective fungicides or alternative methods for controlling Verticillium dahliae (V. dahliae) infection is still lacking5. Transgenic technology for the control of insect herbivores and weeds offers an alternative approach to enhance plant resistance to fungal pathogens6. Genetic engineering techniques are the most economic and effective means for managing Verticillium wilt7. Some genes have been reported to confer resistance to Verticillium wilt in cotton. The Verticillium resistance genes, Ve1, were cloned using a map-based cloning strategy in tomato plants8. Overexpression of Gbve1, a cotton gene homologous to the tomato Ve gene, endowed transgenic Arabidopsis and upland cotton with resistance to both highly aggressive defoliating and non-defoliating isolates of V. dahliae9. Baculovirus anti-apoptotic genes p35 and op-iap could enhance tolerance to Verticillium wilt in transgenic cotton10. Zhao et al.11 reported that overexpression of GbRLK, a putative receptor-like kinase gene, improved cotton resistance to Verticillium wilt. Some researchers report that introducing foreign genes to cotton could enhance resistance to both Verticillium and Fusarium wilts. Expression of Arabidopsis NPR1 in cotton confers significant resistance to multiple pathogens, including V. dahliae and Fusarium oxysporum (F. oxysporum)12,13. The plant defensin NaD1, which inhibits the growth of fungal pathogens in vitro, confers resistance to Fusarium wilt and Verticillium wilt in cotton6. Hpa1Xoo, which induces the hypersensitive response (HR), improved cotton resistance to Verticillium wilt and Fusarium wilt14.
The HR is one component of plant immunity. It is a rapid, local defense-related programmed cell death triggered by the effectors produced by microbial pathogens15,16. Harpin is a type of pathogen effector that is secreted from bacteria via a type-III secretion system (T3SS)17. Harpin was first identified as an HR-elicitor18. The application of Harpin induces the HR, a reactive oxygen species (ROS) burst14,19, and activates the expression of HR markers such as HIN120 and HSR203J21, and pathogenesis-related (PR) genes such as PR1a and PR1b21,22,23 in plants. Plants treated with harpins at an early growth stage show systemic acquired resistance (SAR) to pathogens and insects, and exhibit benefits in both growth and yield15,24. However, harpins currently used as plant defense-activators have no antimicrobial properties25.
Some antimicrobial proteins against pathogens have been identified in insects. Cecropin A, isolated from the hemolymph of the cecropia moth, shows broad spectrum capability of suppressing bacteria, fungi, enveloped viruses, and tumor cells26,27,28. Another protein isolated from bee venom29, melittin, has been shown to be active against bacterial and human red blood cells30,31,32. Due to the bacterial suppression activity of melittin, researchers employed genetic engineering techniques to introduce it into plant genomes in order to improve their pathogen resistance. To suppress the hemolytic activity of melittin, an artificial protein was created by joining the α-helix structures of two peptides, cecropin A and melittin. This chimeric protein showed a better antimicrobial spectrum than cecropin A alone, and less hemolytic activity than melittin alone27,30,33. The hybrid peptide can effectively inhibit the proliferation of pathogens in plants33,34,35, however, few hybrid peptides have been shown to activate plant innate immunity.
Compounds designed for use in protecting plants against pathogenic infection are likely to be most effective if they activate innate plant immunity as well as possess antimicrobial activity36. In a previous study, a novel chimeric protein, Hcm1, consisting of Hpa1Xoc joined to the active domains of cecropin A-melittin, was constructed. It not only elicited an HR in tobacco, but also effectively inhibited the growth of Gram-negative and Gram-positive bacteria in vitro. Plants sprayed with Hcm1 or Hpa1Xoc protein show high resistance to multiple pathogens; exhibiting a broad-spectrum disease resistance. Moreover, the disease resistance of plants sprayed with Hcm1 protein is better than that of plants sprayed with Hpa1Xoc protein, showing that Hpa1Xoc and cecropin A-melittin both contribute to disease resistance22. In the present study, Hcm1 driven by the CaMV35S promoter was transformed into Gossypium hirsutum (G. hirsutum) acc. W0 using the Agrobacterium-mediated method. Hcm1 was found to confer resistance to a variety of diseases in cotton, including Verticillium wilt and Fusarium wilt, in both greenhouse and field conditions. Hcm1-transformed plants demonstrated a micro-HR and an increase in the expression of PR genes in response to biotic stress. The biomass of V. dahliae in transgenic plants was also lower than that in the parent W0 plants. Our results showed that constitutive expression of Hcm1 in cotton plants increased their resistance to two devasting diseases: Verticillium wilt and Fusarium wilt.
Results
Generation of Hcm1-expressing cotton plants
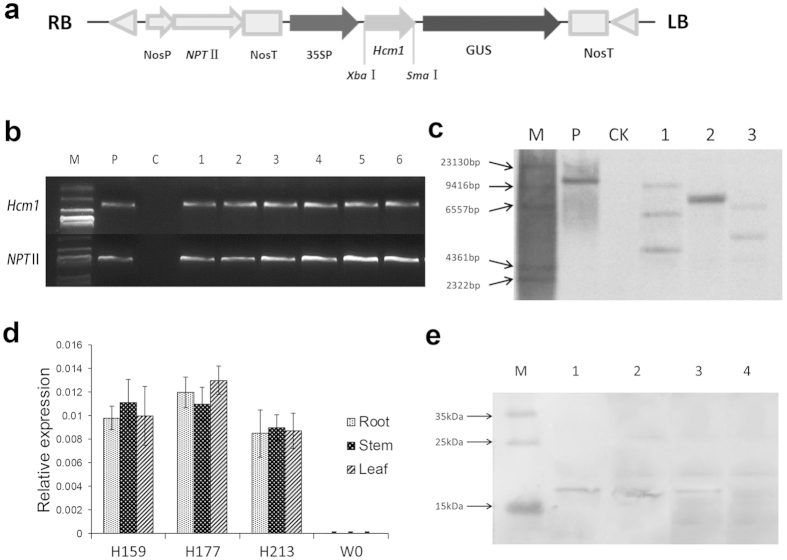
In order to improve disease resistance in cotton, a binary transformation vector carrying an Hcm1 gene cassette (CaMV35S promoter-Hcm1 ORF-Nos terminator), designated pBI121-Hcm1 (Fig. 1a), was introduced into G. hirsutum acc. W0 using the Agrobacterium-mediated transformation method. Primary transgenic plants (T0) were allowed to self-pollinate to generate seeds. From generation T1 to T6, the transgenic lines were screened for their resistance to kanamycin together with the PCR detection of the presence of NPTII and Hcm1 sequence fragments (Fig. 1b). To minimize the effects of the transgenic operation and the insertion location on the chromosome, three homogenous lines, H159, H177, and H213, without observable difference of agronomic characters with parent W0 (Table 1), were selected for further study. Southern blot analysis revealed three, one, and two copies of Hcm1 in the three homogenous lines, respectively (Fig. 1c). Real-time quantitative reverse transcript PCR (qRT-PCR) analysis found that Hcm1 was expressed in roots, stems, and leaves of three transgenic lines (Fig. 1d). To further test the expression of the artificial chimeric protein, a multi-clone antibody was generated against Hcm1 protein. Western blotting with the anti-Hcm1 antibody indicated that the Hcm1 protein at the expected molecular weight of 17 kilodalton (KD) (Fig. 1e) was expressed in the total protein extracted from the leaves of the Hcm1-transformed plants. No blotting band was observed in the parent W0 plants. All of these results indicated that Hcm1 had been successfully transformed into parent W0 plants and constitutively expressed in the transgenic plants.
Figure 1. Molecular analysis of Hcm1 in transgenic plants.
(a) Schematic representation of recombinant plasmid pBI121-35S::Hcm1-NPTII. RB and LB represent the right and left borders of T-DNA, respectively. (b) PCR analysis of the transgenic plants to detect the 35S: Hcm1 and the NPTII genes. M: Marker DL2000; P: plasmid as positive control; C: non-transformed plant W0; Lanes 1–6: positive transgenic plants. (c) Southern blot analysis of Hcm1 insertions in transgenic lines. Genomic DNA was digested with EcoRΙ and hybridized with a 0.75-kb fragment of NPTII. M: Marker, P: positive control pBI121, CK: non-transformed W0 plant, lanes 1–3: T6 generation homozygous transgenic lines H159, H177, and H213. (d) qRT-PCR analysis of expression levels of Hcm1 in roots, stems and leaves of Hcm1-transformed (lines H159, H177, and H213) and parent W0 plants. Error bars represent the standard deviation of triplicate experiments, and the EF-1α gene was amplified as a control. (e) Western blot analysis of Hcm1 in transgenic plants. M: PageRulerTM Prestained Protein Ladder, lanes 1–4: Three T6 generation homozygous transgenic lines (H159, H177, and H213) and parent W0.
Table 1. The agronomic performance of Hcm1-transformed and non-transgenic parent W0 plants at a farm known not to harbor cotton fungal pathogens in Jiangsu province.
| Lines | Height(cm) | No. fruit branch per plant | Boll number per plant | boll weight (g) | Lint percent (%) | Seed cotton yield(kg) |
| H159 | 101.9 ± 3.9 | 17.6 ± 0.5 | 19.5 ± 1.6 | 3.28 ± 0.38 | 37.1 ± 1.5 | 165.33 ± 20.9 |
| H177 | 108.2 ± 7.6 | 18.7 ± 2.2 | 20.3 ± 1.4 | 3.35 ± 0.19 | 37.5 ± 2.2 | 170.01 ± 14.9 |
| H213 | 112.3 ± 9.6 | 17.8 ± 1.2 | 22.6 ± 1.9 | 3.59 ± 0.39 | 39.3 ± 2.4 | 202.54 ± 39.2 |
| W0 | 115.9 ± 8.5 | 17.7 ± 2.9 | 20.7 ± 2.6 | 3.57 ± 0.13 | 38.1 ± 1.1 | 185.71 ± 14.2 |
The cotton plants during growth development did not show any disease. Data represent the mean ± SE (n ≥15); similar results were obtained from four independent experiments.
Resistance of Hcm1-transformed cotton plants to Fusarium wilt
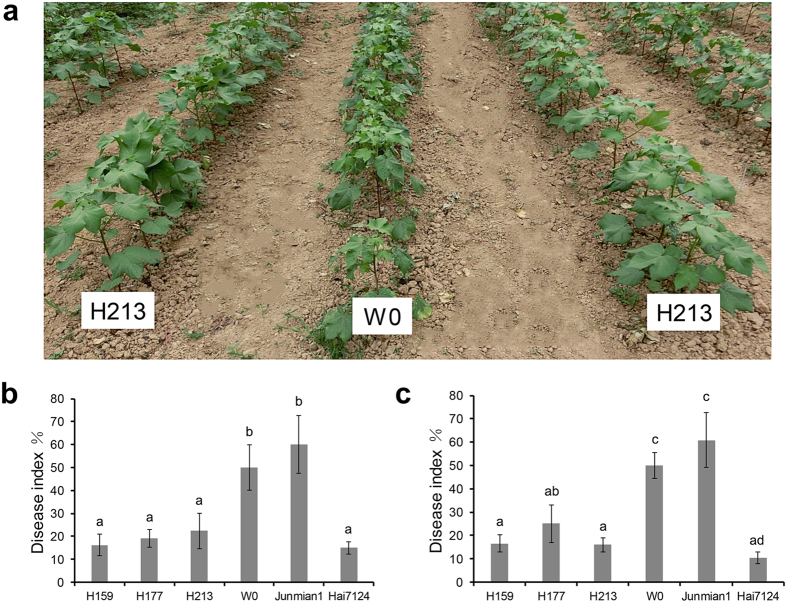
Hcm1 homozygous plants from three transgenic cotton lines, H159, H177, and H213, were first assessed in greenhouse bioassays for F. oxysporum resistance. In this bioassay, the progression of Fusarium wilt disease in the transgenic lines was compared to three control lines: the parent W0, a susceptible variety of Junmian 1, and a less susceptible variety, Hai7124. After 7 weeks, the disease progression in Hcm1-transformed lines and Hai7124 was statistically lower than in the parent W0 and the susceptible variety, Junmian 1. The susceptible variety, Junmian 1, had the highest disease index (DI) and the less susceptible variety, Hai7124, had the lowest DI. The three transgenic lines showed lower DIs compared to that of the parent W0 (Fig. 2b), implying that Hcm1 improved the cotton’s resistance to Fusarium wilt caused by F. oxysporum in greenhouse conditions. The assessment of Hcm1-transformed lines’ resistance to Fusarium wilt under field conditions took place in Shangqiu city, Henan province, China, during the 2014 cotton-growing season. Seeds from Hcm1-transformed lines, parent W0, a susceptible variety, Junmian 1, and a less susceptible variety, Hai7124, were planted in a field where Fusarium wilt occurred heavily historically. The DI was investigated on June 24, 2014 in Henan according to historical peak incidences. These results showed that the DIs of the three transgenic lines were reduced to 66.77%, 49.83%, and 67.99% compared to the parent W0 (Fig. 2a,c), indicating that expressing Hcm1 in cotton conferred resistance to Fusarium wilt in a field condition.
Figure 2. Resistance phenotypes of independent homozygous transgenic cotton lines.
(a) Resistance phenotypes of independent transgenic cotton line H213 in F. oxysporum-inoculated field in Henan province, China in 2014. (b) The DIs of the transgenic and parent W0 plants induced by F. oxysporum isolate Fnj1 in greenhouse conditions. At least 30 plants were used for each experiment. (c) Severity of Fusarium wilt in Hcm1-transformed and parent W0 plants in the nursery. H159, H177, and H213 were the transgenic lines. Junmian 1 and Hai7124 were used as the susceptible and resistant controls. At least 15 plants were used for each experiment. Average values and standard errors were calculated from four independent experiments. The letters in (a,c) indicate significant differences at P ≤ 0.01 according to a randomization one-way ANOVA test.
Resistance of Hcm1-transformed cotton plants to Verticillium wilt
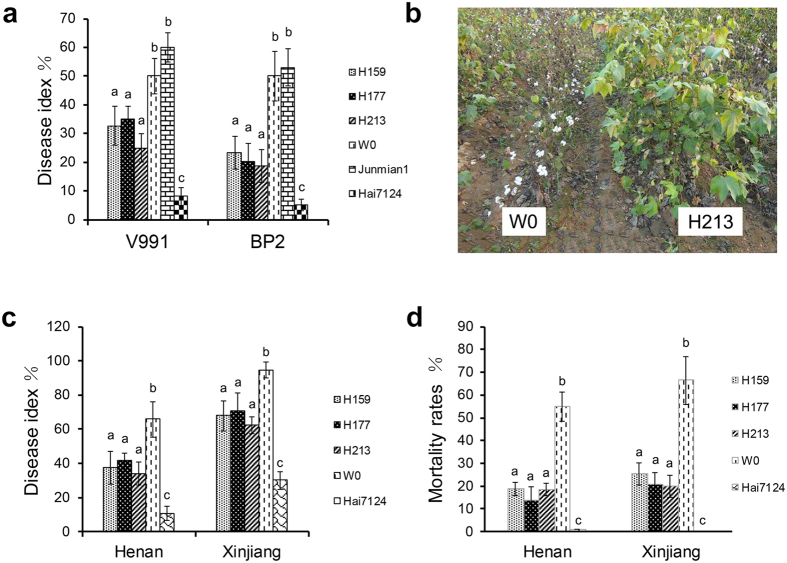
Isolates of V. dahliae can be characterized as defoliating or non-defoliating pathotypes based on symptoms expressed in cotton plants with the disease9. The defoliating V. dahliae isolate V991 and non-defoliating V. dahliae isolate BP2 were used to assess the resistance of Hcm1-transformed cotton in greenhouse conditions. Foliar damage and vascular discoloration was observed in parent W0 plants at 10 and 15 days after inoculation with V991 and BP2, respectively. With the outbreak of the disease, the Hcm1-transformed plants had only a small number of chlorotic and necrotic spots and there was almost no plant death, whereas the parent W0 and susceptible variety, Junmian 1, plants showed common large chlorotic and necrotic areas in their leaves, and some plants eventually died (Fig. S1). The results showed that the DIs of the three transgenic lines were significantly lower than that of the parent W0 after inoculation with V991 and BP2, revealing that Hcm1 improved cotton tolerance to defoliating and non-defoliating V. dahliae in greenhouse conditions (Fig. 3a).
Figure 3. Hcm1 transgenic lines improved resistance to Verticillium wilt.
(a) The DIs of the transgenic and parent W0 cotton plants induced by defoliating V. dahliae strain V991 and non-defoliating V. dahliae strain BP2 in greenhouse conditions. H159, H177, and H213 were the transgenic lines. Junmian 1 and Hai7124 were used as susceptible and resistant controls. At least 45 plants were used for each experiment. Average values and standard errors were calculated from four independent experiments. (b) Resistance phenotype of the independent transgenic cotton line H213 in Henan province, China in 2014. (c,d) The DIs and mortality rates of Hcm1-transformed and parent W0 plants in a field with a history of a high incidence of Verticillium wilt. The transgenic lines, parent W0, and the resistance variety G. barbadense cv. Hai7124 were grown at a farm in Henan and Xinjiang province, China in the 2014 cotton-growing season. At least 15 plants were used for each experiment. Average values and standard errors were calculated from four independent experiments. The letters in (a,c,d) indicate significant differences at P ≤ 0.01 according to a randomization one-way ANOVA test.
Field trials to assess the performance of the transgenic lines against Verticillium wilt were conducted in Henan and Xinjiang provinces, in China during the 2014 cotton-growing season. Disease surveys were conducted on September 5, 2014 in Henan province and August 27, 2014 in Xinjiang province, since the peak incidence of Verticillium wilt in the field generally occurs in early August to mid-September in China. The DIs of the three transgenic lines decreased from 36.95% to 58.15% and from 25.33% to 34.03% compared to parent W0 (Fig. 3b,c) in Henan and Xinjiang provinces, respectively. The mortality rates of Hcm1-transformed lines decreased from 65.70% to 74.93% and 61.72% to 70.25% in Henan and Xinjiang, respectively (Fig. 3d). However, the resistance of transgenic lines to Verticillium wilt in Xinjiang was weaker than that in Henan. The reason may be the differences in climate, geographical conditions, or physiologic races in soils between the two provinces. In addition to the DI, the agronomic performance of the transgenic lines, including the height, lint percentage, number of fruit branches, single boll weight, and lint yield, was significantly higher than that of the parent W0 (Table 2). The lower DIs and mortality rates, and the better agronomic performance of Hcm1-transformed lines demonstrated that Hcm1 conferred cotton resistance to Verticillium wilt, and improved its agronomic traits in the disease nurseries.
Table 2. The agronomic performance of Hcm1-transformed and non-transgenic parent W0 plants in a field with a history of a high incidence of V. dahliae.
| Lines | Height(cm) | No. fruit branch per plant | Boll number of per plant | Single boll weight (g) | Lint percent (%) | Seed cotton yield(kg) | |
|---|---|---|---|---|---|---|---|
| Henan | H159 | 94.9 ± 3.9a | 7.1 ± 0.7 | 11.1 ± 1.6a | 3.16 ± 0.21a | 37.6 ± 1.2a | 122.58 ± 27.4a |
| H177 | 102.2 ± 7.6b | 8.1 ± 1.5 | 12.4 ± 2.0a | 3.66 ± 0.14a | 39.3 ± 2.3a | 158.92 ± 22.7ab | |
| H213 | 101.3 ± 9.6b | 7.8 ± 0.6 | 13.6 ± 0.9a | 3.72 ± 0.79a | 38.5 ± 1.4a | 177.29 ± 16.5b | |
| W0 | 86.3 ± 7.5c | 6.5 ± 0.8 | 7.6 ± 1.1b | 2.97 ± 0.39b | 35.5 ± 2.4b | 79.01 ± 14.8c | |
| Xinjiang | H159 | 57.3 ± 2.9a | 7.8 ± 0.4 | 4.8 ± 1.6a | 3.70 ± 0.61a | 43.9 ± 1.1a | 316.3 ± 32.4a |
| H177 | 62.5 ± 2.3a | 7.8 ± 1.0 | 5.6 ± 0.6a | 3.89 ± 0.45ab | 44.0 ± 0.5a | 390.1 ± 41.6ab | |
| H213 | 60.3 ± 1.9a | 8.4 ± 1.1 | 5.8 ± 1.7a | 4.14 ± 0.57b | 44.3 ± 0.6a | 432.3 ± 48.5b | |
| W0 | 48.5 ± 2.7b | 7.6 ± 1.3 | 4.3 ± 0.8b | 3.26 ± 0.32c | 42.6 ± 0.9b | 249.5 ± 33.8c |
The transgenic lines, parent W0, and the resistance variety G. barbadense cv. Hai7124 were grown at a farm in Henan and Xjinjiang province, China in the 2014 cotton-growing season. Seeds were planted in a field with a history of a high incidence of Verticillium wilt caused by V. dahliae. Data represent the mean ± SE (n ≥ 15); similar results were obtained from four independent experiments. The letters indicate significant differences at P ≤ 0.05 according to a randomization one-way ANOVA test.
ROS burst occurred and the PR genes were activated in Hcm1-transformed plants after inoculation with V. dahliae
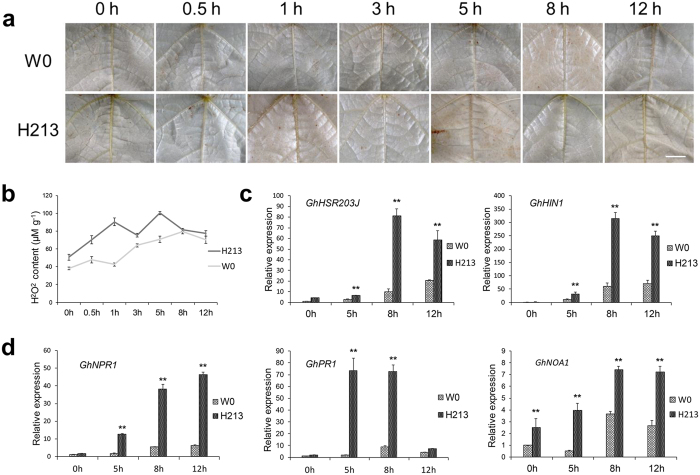
An ROS burst occurred when leaves of hpa1Xoo-transformed plants were inoculated with V. dahliae14. In the leaves of Hcm1-transformed line H213 and the parent W0 plants, a reddish-brown precipitate was observed after inoculation with V. dahliae, as detected by 3, 3′-diaminobenzidine tetrahydrochloride (DAB)37,38. However, the DAB staining in leaves of H213 plants was markedly different from that in parent W0 plants (Fig. 4a). In order to accurately observe this difference, H2O2 content was measured. The basal H2O2 content was higher in the leaves of transgenic H213 plants than in leaves of the parent W0 plants prior to inoculation. After inoculation with V. dahliae, H2O2 content gradually increased in leaves of the parent W0 plants, and peaked at 8 hours (hr), while in H213 leaves, H2O2 content was increased dramatically and appeared in two peaks at 1 hr and 5 hr (Fig. 4b). These results showed that the Hcm1 transgenic lines displayed an ROS burst in response to biotic stress.
Figure 4. ROS burst in leaves of transgenic line H213 dipped in a conidial suspension of V. dahliae.
(a) In situ observation of ROS in cotton leaves dipped in a conidial suspension of V. dahliae with DAB staining. The strong, brown precipitate was observed in leaves of parent W0 plants 8 hr after inoculation with V. dahliae and in leaves of transgenic line H213 plants 1 hr or 5 hr after inoculation with V. dahliae. At least six independent leaves were used for this experiment. A stereomicroscope (Olympus, DP72, Japan) was used for photographing the leaves under white light. Scale bars = 5mm. (b) H2O2 content (μM/g fresh weight) in leaves of transgenic line H213 and parent W0 plants dipped in a conidial suspension of V. dahliae. Error bars represent ± SE, n = 6. (c,d) The PR genes were activated in transgenic line H213 after inoculation with V. dahliae. Three biological replicates were used for each reaction with three technical replicates each. Mean values and standard errors were calculated from three biological replicates (**P ≤ 0.01, by student’s t test).
Harpins can activate the PR genes in plants14,22. GhHSR203J and GhHIN1, which are considered as marker genes for HR20,21, were up-regulated in the Hcm1-transformed line H213 and the parent W0 (Fig. 4c). NPR1, which is a key transcriptional regulator in plant defense responses involving multiple signaling pathways39, was up-regulated in transgenic line H213 after inoculation with V. dahliae. The marker genes of salicylic acid (SA) and nitric oxide (NO) signaling pathways22,40, GhPR1 and GhNOA1, were also significantly up-regulated in the transgenic line H213 compared to the parent W0 (Fig. 4d), showing that the PR genes were activated in Hcm1-transformed plants in response to biotic stress.
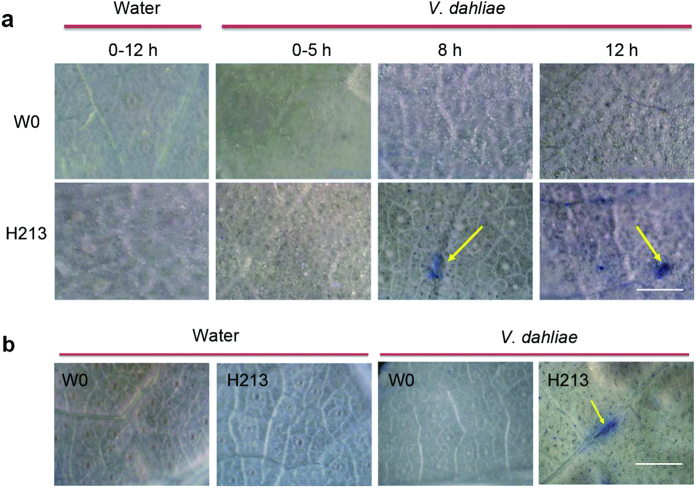
A microscopic hypersensitive response (micro-HR) was observed in transgenic line H213 after leaf and root inoculation with V. dahlia
Harpins can induce an HR in tobacco following infiltration of leaf panels2. No visible HR was observed in cotton expressing Hpa1Xoo, but a micro-HR was detected in plants after inoculation with V. dahliae14. Leaves from transgenic line H213 and parent W0 plants 0–12 hr after inoculation with V. dahliae conidia suspension were stained with trypan blue, which selectively stains dead or dying cells41. Leaves inoculated with sterile water were used as a control. No trypan blue-stained cells were observed in leaves from H213 or W0 plants inoculated with water, or in parent W0 plants inoculated with V. dahliae (Fig. 5a). However, in leaves from H213 plants, trypan blue-stained cells representing a micro-HR were observed by stereoscope at 8 hr and 12 hr after inoculation with V. dahliae (Fig. 5a). Leaves from H213 and W0 plants after root inoculation with V. dahliae conidia suspension in greenhouse conditions were also used for micro-HR detection. Leaves inoculated with sterile water were used as a control. Trypan blue-stained cells were observed in leaves from H213 plants inoculated with V. dahliae but not in leaves from parent W0 plants inoculated with sterile water or V. dahliae or in H213 plants inoculated with sterile water, revealing that micro-HR occurred in Hcm1-transformed plants in response to biotic stress (Fig. 5b). These data indicate that a micro-HR occurred when the Hcm1-transformed plants suffered biotic stress.
Figure 5. Micro-HR in leaves of Hcm1-transformed H213 plants after inoculation with V. dahliae.
(a) Trypan blue staining of leaves from Hcm1-transformed H213 and parent W0 plants after inoculation with a conidial suspension of V. dahliae. No blue-violet coloration (representative of micro-HR) was observed 0–12 hr after inoculation with sterile water or a conidial suspension of V. dahliae in leaves of parent W0 plants. The leaves of transgenic line H213 showed no blue-violet coloration 0–12 hr after inoculation with sterile water or 0–5 hr after inoculation with a conidial suspension of V. dahliae. The blue-violet coloration (5–15 lesions per leaf; indicated by the yellow arrow) was observed in all leaves (≥2 leaves per plant) collected from transgenic plants infected with V. dahliae 8 or 12 hr post inoculation with V. dahliae. (b) Trypan blue staining of leaves from Hcm1-transformed H213 and parent W0 plants 15 days after root inoculation with V. dahliae. Occurrence of micro-HR (5–10 lesions per leaf, blue-violet coloration, indicated by yellow arrow) in leaves of transgenic line H213 plants but not parent W0. Root inoculation with sterile water as a control. A stereomicroscope (Olympus, DP72, Japan) was used for photographing the leaves under white light. Scale bars = 500 μm.
Hcm1 effectively inhibits the spread of V. dahliae in cotton
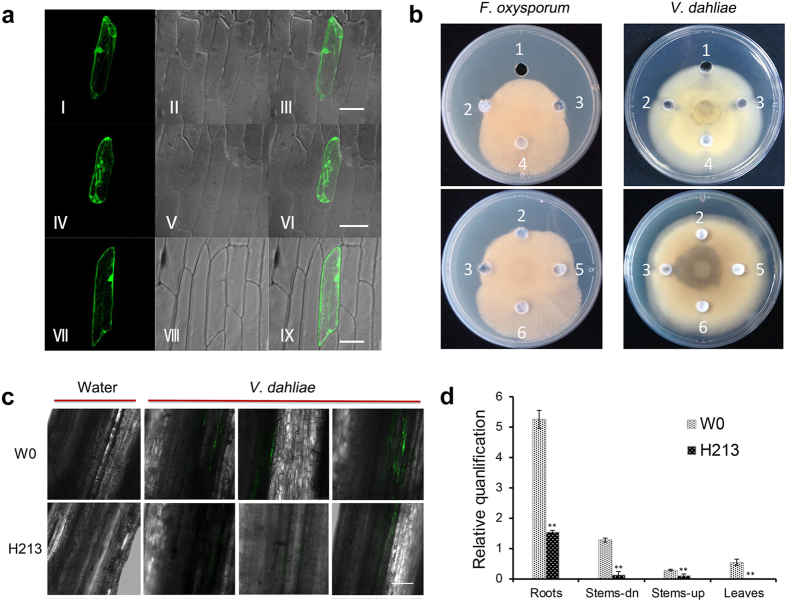
Cecropin A-melittin can normally inhibit pathogens infection when the Hcm1 protein exists in the plasma membrane. Moreover, recent studies have shown that host targets of harpins may be present in the plasma membrane20,42,43. Our unpublished data suggest that Hpa1Xocis located in the plasma membrane and nuclear membrane of plant cells. Therefore, whether the Hcm1 protein exists in the plasma membrane is very important for the function of Hpa1Xoc and cecropin A-melittin from Hcm1. We fused the Hcm1 coding region in frame with the N-terminus of GFP coding region under the control of the CaMV35S promoter to examine the subcellular localization of Hcm1. Onion epidermal cells were separately transformed with either the 35S::Hcm1::GFP fusion or the 35S::GFP plasmid control by particle bombardment. GFP-specific fluorescence was found in the cell membrane and other parts of cells transformed with the 35S::Hcm1::GFP fusion (Fig. 6a I–III). When the cell wall and cell membrane were separated by treatment with 20% sucrose for 15 min, GFP fluorescence was observed in the membrane but not in the cell wall (Fig. 6a IV–VI). GFP fluorescence was detected throughout control cells transformed with the 35S::GFP plasmid (Fig. 6a VII–IX). These results indicate that Hcm1 is present in the cell membrane when Hcm1 is expressed in plant cells.
Figure 6. Expression of Hcm1 inhibited the spread of fungal spores in cotton.
(a) Localization of the 35S::Hcm1::GFP fusion in onion epidermal cells. (I–III) Localization of 35S::Hcm1::GFP in onion epidermal cells. (IV–VI) The cell wall and membrane were separated by treatment with 20% sucrose for 15 min. (VII–IX) 35S::GFP in onion epidermal cells (positive control). (I, IV, and VII) Onion cell under excitation at 488nm. (II, V and VIII) Onion cell under bright field. (III, VI and IX) GFP in the onion cell of overlayed images. Scale bars = 50 μm. (b) Antimicrobial activities of CFEPs and transgenic Hcm1 protein against F. oxysporum Fnj1 and V. dahliae V991 on PDA plates. 1–6: 50 μg/ml Carbendazim, 1 mg/ml CFEPs of Hcm1, 1 mg/ml Hcm1-transformed protein, 1 mg/ml CFVPs, 200 μg/ml Hcm1-transformed protein, and 1 mg/ml parent W0 protein. (c) In situ observation of V. dahliae in rhizome connections of transgenic line H213 and parent W0 plants 15 days after inoculation with V. dahliae harboring the GFP gene. At least 15 plants of transgenic lines H213 and parent W0 plants were used in this study. The freehand sections were obtained and checked by laser scanning confocal microscopy. Scale bar = 200 μm. (d) Detection of the V. dahliae biomass in transgenic line H213 and parent W0 plants using qRT-PCR. DNA was extracted from roots, the lower half of stems (stem-dn), the upper half of stems (stem-up) and the first leaves of plants 15 days after inoculation with V. dahliae. The relative average fungal biomass is shown with standard errors. Asterisks indicate significant differences when compared with colonization of the parent W0 plants (**P ≤ 0.01, by student’s t test).
The Hcm1 protein shows broad antimicrobial activity in vitro22. The ability of crude cell-free elicitor preparations (CFEPs) of Hcm1 and the Hcm1-transformed proteins, which were extracted from prokaryotic expression and the leaves of transgenic line H213 plants, respectively, to inhibit the growth of V. dahliae and F. oxysporum on potato dextrose agar (PDA) and complete medium (CM) plates was tested. Carbendazim, CFEPs and Hcm1-transformed proteins caused an obvious inhibition halo on PDA and CM plates inoculated with F. oxysporum. A 5-fold dilution of Hcm1-transformed proteins also inhibited the growth of F. oxysporum compared to controls (Fig. 6b and Fig. S2). Carbendazim, CFEPs, and Hcm1-transformed proteins also inhibited the mycelial growth of V. dahliae on PDA and CM plates, while a 5-fold dilution of Hcm1-transformed protein had no effect on the growth of V. dahliae (Fig. 6b and Fig. S2).
In order to verify the antimicrobial activity of Hcm1 against V. dahliae in vivo, the strength of green fluorescence was observed in cotton plants inoculated with a V. dahliae strain V991 harboring the GFP gene , since V. dahliae harboring the GFP gene emits fluorescence in cotton tissues44. 15 days after inoculation with V. dahliae, the green fluorescent signal was observed in the leaves of parent W0 plants but not Hcm1-transformed plants (Fig. S3). Although the green fluorescent signal was observed in the rhizome connections of Hcm1-transformed and parent W0 plants, the green fluorescent signal in Hcm1-transformed plants was significantly weaker than in parent W0 plants (Fig. 6c). In addition to observing the fluorescent signal, the biomass of V. dahliae strain V991 in cotton plants was measured with qRT-PCR. Determination of the V. dahliae strain biomass showed significant differences between V. dahliae-inoculated Hcm1-transformed and parent W0 plants. The biomass of V. dahliae strain V991 in the roots, stems, and leaves of Hcm1-transformed plants was significantly lower than in parent W0 plants (Fig. 6d). These results revealed that the expression of Hcm1 reduced the biomass of V. dahliae in cotton plants. Hcm1, therefore, effectively inhibited the spread of V. dahliae in cotton.
Discussion
Hcm1 was effective at controlling Fusarium wilt and Verticillium wilt
Previous studies have shown that harpin, applied as a foliar spray or expressed in plants, confers resistance to pathogens14,24,45,46. The antimicrobial proteins, cecropin A-melittin, also effectively confer plants with a resistance to a broad spectrum of pathogens33,34,35. Resistance to tobacco mosaic virus, bacterial Ralstonia solanacearum, and fungal Magnaporthe oryzae infections can be improved by spraying Hcm1 protein on plants prior to inoculation with plant pathogens22. In the present study, Hcm1 was transformed into a susceptible cotton variety, W0. In greenhouse conditions, the Hcm1-transformed cotton lines were resistant to disease caused not only by the defoliating and non-defoliating isolates of V. dahliae, but also F. oxysporum. In the V. dahliae and F. oxysporum-inoculated field, the Hcm1-transformed plants showed lower DIs and mortality rates compared to parent W0 plants (Figs 2 and 3). The lint yields, which are regarded as the most important agronomic measurement of cultivar performance, of Hcm1-transformed and parent W0 plants were not significantly different when the plants were grown in non-infected soil in field conditions (Table 1). When planted in V. dahliae-infected soil, the lint yields of Hcm1-transformed plants were 20–77% higher in Henan province and 27–73% in Xinjiang province than parent W0 plants (Table 2). The results indicate that Hcm1 is effective at controlling Fusarium and Verticillium wilts.
Hcm1 led to an ROS primed state for plant defense activation in cotton
Higher plants are capable of inducing some stress “memory” or “stress imprinting” as a primer induced by the first exposure to a stress that leads to enhanced resistance to a later stress47,48. ROS, as signaling molecules, play a key role in such priming events49. The harpin protein, Hpa1Xoo, which is isolated from Xanthomonas oryzae, confers resistance to Verticillium wilt by activating a priming mechanism in cotton. A rapid burst of ROS was observed in Hpa1Xoo-transformed plants after inoculation with V. dahliae14. Our previous study showed that Hcm1 protein possesses the same characteristics as the harpin protein Hap1Xoc22. In addition, Hcm1, like Hpa1Xoc, is located in the plasma membrane (Fig. 6a), which may be necessary for the function of harpins20,42,43. In Hcm1-transformed plants, H2O2 content was slightly higher than in parent W0 plants and a ROS burst occurred after inoculation with V. dahliae (Fig. 4a,b). SA and ROS interplay in the transcriptional control of defense gene expression and play an important role in the disease resistance of plants50. On infection of hexanoic acid-treated plants, hexanoic acid activates the SA pathway as part of the priming mechanism51. Recent studies show that NO is another key signaling molecule involved in the induction of protection against biotic and abiotic factors through a complex network40. Harpins can activate the expression of PR genes such as NPR1, PR1-a (an SA marker)21,22,23, and HSR203J and HIN1 (HR markers)21,41,48,52. The up-regulation of GhNPR1, GhPR1, and GhNOA1 in response to pathogen infection was observed in Hcm1-transformed plants, revealing that the signaling pathways of SA and NO were activated (Fig. 4d). These results indicate that Hcm1 may lead to a primed state in cotton, and result in a faster and stronger induction of basal resistance mechanisms upon pathogenic attacks, since the priming was accompanied by an ROS burst, SA accumulation, and the induction of PR genes14,53. All harpins reported thus far, except XopA54 and truncated HrpZ155, can induce an HR in plants. Hcm1, which contains a harpin, also induces an HR in planta14,22. In Hcm1-transformed plants, the HR marker genes, HSR203J and HIN1, were activated (Fig. 4c) and a micro-HR was observed after inoculation with V. dahliae (Fig. 5). Miao et al.14 suggested that such a micro-HR may augment the response to infections caused by fungal pathogens.
Hcm1 may provide antimicrobial properties in cotton
Cecropin A-melittin of Hcm1 protein has been shown to effectively inhibit the growth of a variety of pathogens in vitro22. The Hcm1 protein extracted from prokaryotic expression or transgenic line H213 plants inhibited the growth of V. dahliae and F. oxysporum in vitro (Fig. 6b). The Hcm1 protein was located in the plasma membrane of plant cells (Fig. 6a), which may be help cecropin A-melittin to inhibit pathogens. In Hcm1-transformed plants, the biomass of V. dahliae was markedly lower than in parent W0 plants, as determined by qRT-PCR analysis and by observing the fluorescent signal strength of V. dahliae harboring the GFP gene (Fig. 6d). These results showed that the spread of V. dahliae was effectively hindered. A synthetic chimera of cecropin A and melittin CAPs with antimicrobial properties, MsrA1, effectively restricts Alternaria brassicae and Sclerotinia sclerotiorum infection in transgenic Brassica juncea and Solanum tuberosum plants34,56. The novel cecropin A-melittin hybrid peptide, CEMA, which has strong antimicrobial activity in vitro, confers resistance against Fusarium solani in transgenic tobacco57. Our previous studies have shown that the disease resistance conferred by Hcm1 protein is more effective than that of the Hpa1Xoc protein when Hcm1 or Hpa1Xoc proteins are sprayed onto plants22. These results indicate that the cecropin A-melittin of the Hcm1 protein may also contribute to resistance against Fusarium and Verticillium wilts in transgenic cotton. The improved resistance to Fusarium and Verticillium wilts in cotton plants conferred by the Hcm1 protein may be a joint action of the Hpa1Xoc protein and cecropin A-melittin.
In conclusion, these results lead us to suppose that the Hpa1Xoc protein from Hcm1 activates a ROS priming mechanism in transgenic plants in response to V. dahliae and F. oxysporum infection, and cecropin A-melittin from Hcm1, which is located in the plasma membrane, simultaneously inhibits the spread of V. dahliae and F. oxysporum to confer resistance to both Verticillium and Fusarium wilts in cotton.
Future potential for fusion protein
Verticillium wilts are among the most devastating fungal diseases worldwide and affect hundreds of different plant species including high value agricultural crops58. Economic losses of 50% or higher commonly occur in high value crops, including cotton59, lettuce60, olive61, and potato62. F. oxysporum was also described as an important fungal pathogen in a survey of plant pathologists, based on its economic and scientific importance63. Plant genetic engineering has been made possible thanks to extensive research conducted during the last three decades. Several studies have reported the control of F. oxysporum and V. dahliae infection by transgenic approaches6,7,8,9,10,11,12,13,14. Hcm1, a novel protein that induces plant defense responses and directly inhibits microbial growth, could improve cotton resistance to Verticillium and Fusarium wilts and offer a considerable yield advantage. Our previous22 and present studies showed that Hcm1 confers resistance to multiple pathogens either by spraying on plants or expressing in plants, indicating that fusion proteins like Hcm1 could be widely applied to other crops in future to improve defense against plant diseases and improve crop yields.
Materials and Methods
Plant materials and V. dahliae and F. oxysporum culture
The transgenic cotton plants, as well as their parent W0, the resistant control for Verticillium wilt and Fusarium wilt, Gossypium barbadense (G. barbadense) cv. Hai7124, and the susceptible control for Verticillium and Fusarium wilts, G. hirsutum cv. Junmian 1, were grown in the green house facility in Nanjing Agricultural University in China. The growing conditions were: a constant temperature of 28 °C, a relative humidity of 70%, and a 16 hr photoperiod. Highly aggressive defoliating V. dahliae isolate V991 was stored in our laboratory and non-defoliating V. dahliae isolate BP2 was provided by the Institute of Plant Protection, Jiangsu Academy of Agricultural Sciences. V. dahliae V991 harboring the GFP gene was provided by the Biotechnology Institute, Jiangsu Academy of Agricultural Sciences. V. dahliae was maintained on PDA at 25 °C for 5 days, inoculated into Czapek’s medium64, and then shocked at 25 °C for 5 days. F. oxysporum isolate Fnj1, which was provided by Institute of Plant Protection, Jiangsu Academy of Agricultural Sciences, was maintained on PDA at 24 °C for 3–5 days, inoculated into Czapek’s medium and shocked at 25 °C for 3 days. Before inoculation, the conidia were counted and the conidia suspension was adjusted to the required density with distilled water.
Cotton transformation and transgenic plant selection
The recombinant binary vector pBI35S-Hcm1-NPTII, which contained aneomycin phosphotransferase II (NPTII) with a nopaline synthase (Nos) promoter and terminator, a CaMV35S promoter, an Hcm1 insert, and a Nos terminator, was transformed into W0 plants. Agrobacterium-mediated cotton transformation was performed as described previously65. After induction, differentiation, and plantlet regeneration, the plantlets were grafted onto rootstocks and grown in a greenhouse. The homozygosity of transgenic plants were determined by analyzing the segregation ratio of the kanamycin selection marker and by PCR analysis. Kanamycin resistance tests, PCR analysis, Southern blots, Western blots, and resistance to Verticillium wilt were used to screen T1 to T6 progeny for Hcm1-transformed cotton lines.
Southern and Western blots analysis
The method of Southern blots analysis was conducted as described by Lv et al.66. 20 μg gDNA from the leaves of Hcm1-transformed and parent W0 plants was digested with EcoRI. Probes were prepared from purified PCR products of the NPTII coding region. The labeling of probes, prehybridization, hybridization, and detection were performed according to the protocol of the DIG High Prime DNA Labeling and Detection Starter Kit I (Roche Applied Science, Mannheim, Germany).
Total protein was extracted from the leaves of Hcm1-transformed and parent W0 plants according to the manufacturer’s instructions of the Plant Protein Extraction Kit (CWBIO, Beijing, China). Protein concentration was measured according to the manufacturer’s instructions for the BCA Protein Assay Kit (Solarbio, Beijing, China). Total proteins were separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred onto a polyvinylidene fluoride (PVDF) membrane (Roche Applied Science, Mannheim, Germany). The membrane was blotted with a polyclonal antibody developed against Hcm1 and a goat anti-rabbit IgG-HRP antibody (Sino-American Biotech, Luoyang, China). The color was developed using DAB.
qRT-PCR
Total RNA from leaves, stems and roots of Hcm1-transformed and parent W0 plants was isolated using the CTAB method67, and 2 μg of total RNA was used for reverse transcription. EF-1α (Table S1) from cotton was used as an internal control for normalization of the different cDNA samples. The PR genes in cotton are listed in Table S2. The primer sequences for PR genes are shown in Table S1. PCR was performed using the real-time PCR system (Bio-Rad) along with SYBR Green PCR Master Mix (Applied Biosystems). Each PCR was repeated three times, and the data were evaluated using the comparative cycle threshold method described by Livak and Schmittgen68.
Evaluation of resistance to Verticillium wilt and Fusarium wilt in greenhouse conditions
For the determination of Verticillium wilt resistance, after surface disinfection for 5 min with a 5% solution of sodium hypochlorite, cotton seeds were sown in a potting mixture (peat:vermiculite, 1:1, v/v). Thirty 18-day-old cotton seedlings were inoculated with defoliating V. dahliae isolate V991 and non-defoliating V. dahliae isolate BP2 by soil drenching with 20 ml conidial suspension (5 × 106 conidia/ml) for each pot (250 ml), and were grown under the following conditions: 12 hr of light at 25 °C and 70–90% relative humidity. Plants in the control group received same amount of sterile water. The DI was measured after two weeks in a greenhouse. After inoculated with non-defoliating V. dahliae isolate BP2, foliar damage was evaluated by rating the symptom on the cotyledon and leaf of inoculated plant according to the following disease grades: 0 = healthy plants, no fungal infection, 1 = 25% of the leaves showing yellowing or abnormal yellow spots, 2 = 25 to 50% of the leaves showing yellow spots, 3 = 50 to 75% of the leaves showing brown spots and curled leaf edges, and 4 = >75% of the leaves showing yellow spots or irregular yellow spots between the main vein of leaves. After inoculated with defoliating V. dahliae isolate V991, foliar damage was evaluated by rating the symptom on the cotyledon and leaf of inoculated plant according to the following disease grades: 0 = healthy plant, 1 = yellowing or necrosis of 1–2 cotyledons, 2 = yellowing or necrosis of 1 true leaf, 3 = more than 2 wilted or necrotic leaves, 4 = no leaf left or dead plant.
For the determination of F. oxysporum resistance, a spore suspension of Fnj1 was added to sterilized strain bags containing grains of wheat. The grains of wheat were removed and dried after 20 days, before being mixed with mould and sand (1:1, v/v) at a ratio of 3% and encased an aluminum skin frame (45 cm × 33 cm × 16 cm). After surface disinfection, cotton seeds were sown in the aluminum skin frame and grown with 12 hr of light, at 25 °C and 70% - 90% relative humidity. The DI was measured after seven weeks in a greenhouse. After inoculated with F. oxysporum isolate Fnj1, foliar damage was evaluated by rating the symptom on the cotyledon and leaf of inoculated plant according to the following disease grades: 0 = healthy plants, no fungal infection, 1 = 25% of the leaves showing yellowing or wilting, 2 = 25 to 50% of the leaves showing yellowing or wilting, leaf veins showing yellow, 3 = 50 to 75% of the leaves showing yellowing or wilting, cotton plants dwarf or wilting, and 4 = >75% of the leaves showing yellowing or wilting, cotton plants dead.
The disease index was calculated as according to the formula: DI = [∑disease grades × number of infected plants)/(total checked plants × 4)] × 1009,11. At least thirty individual plants per line were subjected to resistant analysis and each experiment was repeated four times.
Evaluation of resistance to Verticillium and Fusarium wilts in field conditions
For resistance assessment, the transgenic lines, the parental line W0, and the resistant variety, Hai7124 were grown in the Verticillium wilt nurseries in the Henan and Xinjiang provinces in China. To assess their agronomic performance, the transgenic lines and the parental line W0 were also planted at a farm without diseased soil in Jiangsu Province, China, in 2014. All seeds were treated with an insecticide (Pymetrozine, J&K, San Diego, USA) prior to planting to protect against thrip and aphid damage, and some seeds were also treated with dynasty fungicide to control damping off diseases. In the Verticillium wilt nurseries of Henan province and Xinjiang province, the seed densities were 5 seeds m–1 and 25 seeds m–1, respectively. Each treatment was replicated four times, and the replicates were arranged in a randomized complete block design. The DI was calculated using the above formula. At the completion of the trial, fifteen successive plants were chosen and tagged in each plot. The height of plants, number of fruit branches, and boll number per plant were investigated. Twenty-five bolls were manually harvested in each plot to investigate the weight of boll and lint percentage. Total cotton yield was assessed by hand picking all harvestable bolls.
Subcellular localization of Hcm1
The full length Hcm1 coding region was inserted between the cauliflower mosaic virus 35S promoter and terminator sequences in the pJIT166-GFP vector by PCR with linker primers (Table S1) that contained HindIII and XbaI sites. All plasmid constructs were confirmed by sequencing. The 35S::GFP control and 35S::Hcm1::GFP vectors were transiently expressed in onion epidermal cells using a biolistic particle delivery system (PDS-1000 Bio-Rad, USA). The subcellular localization of the 35S::Hcm1::GFP fusion protein was observed with a confocal laser scanning microscope (LSM 510, Zeiss, Germany).
Observation of ROS burst and quantification of H2O2 in cotton leaves
The third cotton leaves that had no visible wounds were selected when the plants were at the 4 leaf stage and the leaf surfaces were smeared with the conidial suspension of V. dahliae (1–3 × 107 ml) and incubated for 0, 0.5, 1, 3, 5, 8, or 12 hr. To visualize the accumulation of H2O2, the fresh cotton leaves were collected and incubated in 1 mg/ml of DAB (pH 3.8) for 8 hr and then decolorized in 96% ethanol. The samples were examined with a stereo microscope lens (Olympus, DP72, Germany). The accumulation of H2O2 was visible as red-brown discoloration. The production of H2O2 in leaves was also measured with a commercial H2O2 detection kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) using the method described by Jiang and Zhang69 and expressed as a percentage of fresh weight. Leaves dipped in sterile water were used as the negative control. Each experiment was repeated six times.
Microscopic investigation of micro hypersensitive response
The third cotton leaves that had no visible wounds were selected and the leaf surfaces were smeared with the conidial suspension of V. dahliae (1–3 × 107 ml). Leaves were collected at 0, 0.5, 1, 3, 5, 8, or 12 hr and stained with trypan blue using the method described by Lipka et al.41. In addition, roots of transgenic line H213 and parent W0 plants at the 2 to 3 leaf stage were inoculated with a conidial suspension of V. dahliae (5 × 106/ml) according to the method described above. Leaves were collected 15 days after inoculation with V. dahliae and then stained with trypan blue. Stained leaf samples were observed under a Leica light microscope (Leica DMRB, Leica Microsystems, Germany) and photographed with a Leica DFC camera (DM2500-3HF-FL, Leica Microsystems, Germany). Leaves without any wounds or visible symptoms of the disease from 10 independent H213 plants were examined.
Qualitative and quantitative detection of V. dahliae in cotton
Roots of transgenic line H213 and parent W0 plants at the 2 to 3 leaf stage were inoculated with a conidial suspension of V. dahliae harboring the GFP gene (5 × 106/ml) according to the method described above. 15 days post-inoculation, the fluorescence signal strength at leaves and the rhizome connections were detected using a laser scanning confocal microscope (LSM 510, Zeiss, Germany). As the quantity of V. dahliae increases, the intensity of the fluorescent signal from GFP also increases. The roots, lower half of the stems, upper half of the stems, and first true leaves were also collected to use for DNA extraction. The internal transcribed spacer region of the ribosomal DNA was targeted to generate a 200 bp amplicon to measure the biomass of V. dahliae, using the fungus-specific primer W9500F70 and the V. dahliae-specific reverse primer W9500R71. EF-1α from cotton was used as an internal control for normalization of the different DNA samples. The average fungal biomass was determined using at least six V. dahliae-inoculated plants for each genotype, and quantified in plants as described by Ellendorff Ursula et al.72.
Antimicrobial spectrum for Hcm1
CFEPs of Hcm1 were obtained using prokaryotic expression technology according to the methods described by Che et al.22. Hcm1-transformed protein was also extracted from the leaves of Hcm1-transformed plants. 10 μl spore suspension of V. dahliae isolate V991 and F. oxysporum isolate Fnj1 was placed in the center of PDA or CM plates and then 5 mm diameter holes were made around the mycelial discs using a hole puncher. One hundred microliters of Hcm1 protein was added to the holes on the PDA or CM plates. The plates were incubated at 28 °C for 3–5 days depending on the fungal growth rate. Carbendazim (50 μg ml−1) was used as the positive control for the fungi. The crude cell-free vector preparations (CFVPs) and the proteins from parent W0 plants were used as negative controls.
Additional Information
How to cite this article: Zhang, Z. et al. Constitutive expression of a novel antimicrobial protein, Hcm1, confers resistance to both Verticillium and Fusarium wilts in cotton. Sci. Rep. 6, 20773; doi: 10.1038/srep20773 (2016).
Supplementary Material
Acknowledgments
This study was financially supported in part by grants from the National Research and Development Project of Transgenic Crops of China (2011ZX08009-003), the Natural Science Foundation in Jiangsu Province (BK2010441), the National High Technology Research and Development Program of China (863 Program) (2011AA10A102), the Priority Academic Program Development of Jiangsu Higher Education Institutions, Jiangsu Collaborative Innovation Center for Modern Crop Production (JCIC-MCP) and the 111 Program (B08025).
Footnotes
Author Contributions T.Z.: Conceiving and designing the experiments, writing the paper. G.C.: Conceiving and designing the experiments. Z.Z.: Performing the experiments, writing the paper. L.D.: Transforming the cotton. J.Z.: Conceiving and analysis the data. L.Z.: Contributing materials/analysis tools. Y.L.: Contributing materials/analysis tools.
References
- Fisher M. et al. Emerging fungal threats to animal, plant and ecosystem health. Nature 484, 186–194 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennisi E. Armed and dangerous. Science 327, 804–805 (2010). [DOI] [PubMed] [Google Scholar]
- Oerke E. Crop losses to pests. J. Agr. Sci. 144, 31–43 (2006). [Google Scholar]
- Cai Y. et al. Molecular research and genetic engineering of resistance to Verticillium wilt in cotton: A review. Afr. J. Biotechnol. 8, 7363–7372 (2009). [Google Scholar]
- Klosterman S., Atallah Z., Vallad G. & Subbarao K. Diversity, pathogenicity, and management of Verticillium species. Annu. Rev. Phytopathol. 47, 39–62 (2009). [DOI] [PubMed] [Google Scholar]
- Gaspar Y. et al. Field resistance to Fusarium oxysporum and Verticillium dahliae in transgenic cotton expressing the plant defensin NaD1. J. Exp. Bot. 65, 1541–1550 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fradin E. & Thomma B. Physiology and molecular aspects of Verticillium wilt diseases caused by V. dahliae and V. albo-atrum. Mol. Plant Pathol. 7, 71–86 (2006). [DOI] [PubMed] [Google Scholar]
- Kawchuk L. M. et al. Tomato Ve disease resistance genes encode cell surface-like receptors. Proc. Natl. Acad. Sci. USA 98, 6511–6515 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B. et al. Island cotton Gbve1 gene encoding a receptor-like protein confers resistance to both defoliating and non-defoliating isolates of Verticillium dahliae. PloS one 7, e51091 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J. et al. Expression of baculovirus anti-apoptotic genes p35 and op-iap in cotton (Gossypium hirsutum L.) enhances tolerance to Verticillium wilt. PloS one 5, 3781–3793 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J. et al. Overexpression of GbRLK, a putative receptor-like kinase gene, improved cotton tolerance to Verticillium wilt. Sci. Rep. 5, 15048 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhi V., Kumar V., Campbell L., Bell A. & Rathore K. Expression of Arabidopsis NPR1 in transgenic cotton confers resistance to non-defoliating isolates of Verticillium dahliae but not the defoliating isolates. J. Phytopathol. 158, 822–825 (2010). [Google Scholar]
- Parkhi V. et al. Resistance against various fungal pathogens and reniform nematode in transgenic cotton plants expressing Arabidopsis NPR1. Transgenic Res. 19, 959–975 (2010). [DOI] [PubMed] [Google Scholar]
- Miao W. et al. Genetic transformation of cotton with a harpin-encoding gene hpaXoo confers an enhanced defense response against different pathogens through a priming mechanism. BMC Plant Biol. 10, 1471–1485(2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H., Delaney T., Bauer D. & Beer S. Harpin induces disease resistance in Arabidopsis through the systemic acquired resistance pathway mediated by salicylic acid and the NIM1 gene. Plant J. 20, 207–215 (1999). [DOI] [PubMed] [Google Scholar]
- Heath M. Hypersensitive response-related death. Plant Mol. Biol. 44, 321–334 (2000). [DOI] [PubMed] [Google Scholar]
- Alfano J. & Collmer A. Bacterial pathogens in plants: Life up against the wall. Plant Cell 8, 1683–1698 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Z. et al. Harpin, elicitor of the hypersensitive response produced by the plant pathogen Erwinia-amylovora. Science 257, 85–88 (1992). [DOI] [PubMed] [Google Scholar]
- Krause M. & Durner J. Harpin inactivates mitochondria in Arabidopsis suspension cells. Mol. Plant-Microbe Interact. 17, 131–139 (2004). [DOI] [PubMed] [Google Scholar]
- Lee J., Klessig D. & Nürnberger T. A harpin binding site in tobacco plasma membranes mediates activation of the pathogenesis-related gene HIN1 independent of extracellular calcium but dependent on mitogen-activated protein kinase activity. Plant Cell 13, 1079–1093 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y. et al. A subset of hypersensitive response marker genes, including HSR203J, is the downstream target of a spermine signal transduction pathway in tobacco. Plant J. 40, 586–595 (2004). [DOI] [PubMed] [Google Scholar]
- Che Y. et al. A novel antimicrobial protein for plant protection consisting of a Xanthomonas oryzae harpin and active domains of cecropin A and melittin. Microb. Biotechnol. 4, 777–793 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C. & Zhang S. Activation of a mitogen-activated protein kinase cascade induces WRKY family of transcription factors and defense genes in tobacco. Plant J. 38, 142–151 (2004). [DOI] [PubMed] [Google Scholar]
- Chen L. et al. A fragment of the Xanthomonas oryzae pv. oryzicola harpin HpaGXooc reduces disease and increases yield of rice in extensive grower plantings. Phytopathology 98, 792–802 (2008). [DOI] [PubMed] [Google Scholar]
- Zhao M., Wang L., Zhang B., Wang J. & Chen G. Harpin(Xooc), a proteineous elicitor of Xanthomonas oryzae pv. oryzicola, triggering hypersensitive response in tobacco and inducing disease resistance in rice. Chinese Journal of Biological Control 22, 283–289 (2006).
- Andreu D., Merrifield R., Steiner H. & Boman H. Solid-phase synthesis of Cecropin A and related peptides. Proc. Natl. Acad. Sci. USA 80, 6475–6479 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallarin L., Andreu D. & San Segundo B. Cecropin A-derived peptides are potent inhibitors of fungal plant pathogens. Mol. Plant-Microbe Interact. 11, 218–227 (1998). [DOI] [PubMed] [Google Scholar]
- Hancock R. Cationic peptides: effectors in innate immunity and novel antimicrobials. Lancet Infect. Dis. 1, 156–164 (2001). [DOI] [PubMed] [Google Scholar]
- Hristova K., Dempsey C. & White S. Structure, location, and lipid perturbations of melittin at the membrane interface. Biophys. J. 80, 801–811 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferre R. et al. Inhibition of plant-pathogenic bacteria by short synthetic Cecropin A-Melittin hybrid peptides. Appl. Environ. Microbiol. 72, 3302–3308 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glättli A., Chandrasekhar I. & Gunsteren W. A molecular dynamics study of the bee venom melittin in aqueous solution, in methanol, and inserted in a phospholipid bilayer. Eur. Biophys. J. 35, 255–267 (2006). [DOI] [PubMed] [Google Scholar]
- Raghuraman H. & Amitabha C. Orientation and dynamics of Melittin in membranes of varying composition utilizing NBD fluorescence. Biophys. J. 92, 1271–1283 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali G. & Reddy A. Inhibition of fungal and bacterial plant pathogens by synthetic peptides: in vitro growth inhibition, interaction between peptides and inhibition of disease progression. Mol. Plant-Microbe Interact. 13, 847–859 (2000). [DOI] [PubMed] [Google Scholar]
- Rustagi A. et al. Transgenic Brassica juncea plants expressing MsrA1, a synthetic cationic antimicrobial peptide, exhibit resistance to fungal phytopathogens. Mol. Biotechnol. 56, 1–11 (2014). [DOI] [PubMed] [Google Scholar]
- Yevtushenko D. et al. Pathogen-induced expression of a Cecropin A-Melittin antimicrobial peptide gene confers antifungal resistance in transgenic tobacco. J. Exp. Bot. 56, 1685–1695 (2005). [DOI] [PubMed] [Google Scholar]
- Molina A., Hunt M. & Ryals J. Impaired fungicide activity in plants blocked in disease resistance signal transduction. Plant Cell 10, 1903–1914 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres M. A., Jones J. D. & Dangl J. L. Pathogen-induced, NADPH oxidase-derived reactive oxygen intermediates suppress spread of cell death in Arabidopsis thaliana. Nat. Genet. 37, 1130–1134 (2005). [DOI] [PubMed] [Google Scholar]
- Thordal-Christensen H., Zhang Z., Wei Y. & Collinge D. Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J. 11, 1187–1194 (1997). [Google Scholar]
- Peng J., Bao Z., Ren H., Wang J. & Dong H. Expression of harpin(xoo) in transgenic tobacco induces pathogen defense in the absence of hypersensitive cell death. Phytopathology 94, 1048–1055 (2004). [DOI] [PubMed] [Google Scholar]
- Sun A. & Li Z. Regulatory role of nitric oxide in lipopolysaccharides-triggered plant innate immunity. Plant Signal. Behav. 8, 1081–1096 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipka V. et al. Pre- and postinvasion defenses both contribute to nonhost resistance in Arabidopsis. Science 310, 1180–1183 (2005). [DOI] [PubMed] [Google Scholar]
- Oh H. et al. Pseudomonas syringae lytic transglycosylases coregulated with the type III secretion system contribute to the translocation of effector proteins into plant cells. J. Bacteriol. 189, 8277–8289 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. et al. HrpZ(Psph) from the plant pathogen Pseudomonas syringae pv. phaseolicola binds to lipid bilayers and forms an ion-conducting pore in vitro. Proc. Natl. Acad. Sci. USA 98, 289–294 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M. et al. Verticillium dahliae labeled with green fluorescent protein gene. Plant Protection 39, 128–133 (2013). [Google Scholar]
- Shao M. et al. Expression of a harpin-encoding gene in rice confers durable nonspecific resistance to Magnaporthe grisea. Plant Biotech. J. 6, 73–81 (2008). [DOI] [PubMed] [Google Scholar]
- Sohn S. et al. Transgenic tobacco expressing the hrpN(EP) gene from Erwinia pyrifoliae triggers defense responses against botrytis cinerea. Mol. Cells 24, 232–239 (2007). [PubMed] [Google Scholar]
- Bruce T., Matthes M., Napier J. & Pickett J. Stressful “memories” of plants: Evidence and possible mechanisms. Plant Sci. 173, 603–608 (2007). [Google Scholar]
- Pastor V., Luna E., Mauch-Mani B., Ton J. & Flors V. Primed plants do not forget. Environ. Exp. Bot. 94, 46–56 (2013). [Google Scholar]
- Borges A., Jiménez-Arias D., Expósito-Rodríguez M., Sandalio L. & Pérez J. Priming crops against biotic and abiotic stresses: MSB as a tool for studying mechanisms. Front. Plant Sci. 5, 642 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera-Vásquez A., Salinas P. & Holuigue L. Salicylic acid and reactive oxygen species interplay in the transcriptional control of defense genes expression. Front. Plant Sci. 6, 171 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalschi L. et al. Hexanoic acid is a resistance inducer that protects tomato plants against Pseudomonas syringae by priming the jasmonic acid and salicylic acid pathways. Mol. Plant Pathol. 14, 342–355 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowling S., Clarke J., Liu Y., Klessig D. & Dong X. The cpr5 mutant of Arabidopsis expresses both NPR1-dependent and NPR1-independent resistance. Plant cell 9, 1573–1584 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee M. et al. Ascorbic acid deficiency in Arabidopsis induces constitutive priming that is dependent on hydrogen peroxide, salicylic acid, and the NPR1 gene. Mol. Plant-Microbe Interact. 23, 340–351 (2010). [DOI] [PubMed] [Google Scholar]
- Jeon E., Kim J., Hwang I., Oh J. & Moon J. Mutational analysis of Xanthomonas Harpin HpaG identifies a key functional region that elicits the hypersensitive responses in nonhost plants. J. Bacteriol. 186, 6239–6247 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunemi K. et al. Degeneration of hrpZ gene in Pseudomonas syringae pv. tabaci to evade tobacco defence: an arms race between tobacco and its bacterial pathogen. Mol. Plant Pathol. 12, 709–714 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osusky M. et al. Transgenic plants expressing cationic peptide chimeras exhibit broad-spectrum resistance to phytopathogens. Nat. Biotechnol. 18, 1162–1166 (2000). [DOI] [PubMed] [Google Scholar]
- Yevtushenko D. P. et al. Pathogen-induced expression of a cecropin A-melittin antimicrobial peptide gene confers antifungal resistance in transgenic tobacco. J. Exp. Bot. 56, 1685–1695 (2005). [DOI] [PubMed] [Google Scholar]
- Pegg G. & Brady B. Hosts in Verticillium wilts (eds Pegg G. et al.) 293–340 (CABI Publishing, 2002). [Google Scholar]
- Friebertshauser G. & Devay J. Differential effects of the defoliating and nondefoliating pathotypes of Verticillium dahliae upon the growth and development of Gossypium hirsutum. Phytopathology 72, 872–877 (1982). [Google Scholar]
- Atallah Z., Hayes R. & Subbarao K. Fifteen years of Verticillium Wilt of lettuce in America’s salad bowl: A tale of immigration, subjugation, and abatement. Plant Dis. 95, 784–792 (2011). [DOI] [PubMed] [Google Scholar]
- Jiménezdíaz R. et al. Verticillium Wilt, a major threat to olive production: current status and future prospects for its management. Plant Dis. 96, 304–329 (2012). [DOI] [PubMed] [Google Scholar]
- Davis J. & Huisman O. Comments on the feature? Potato early dying: Management challenges in a changing production environment? Plant Dis. 88, 1168–1171 (2004). [DOI] [PubMed] [Google Scholar]
- Dean R. et al. The top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 13, 414–430 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausher R., Katan J. & Ovadia S. An improved selective medium for the isolation of Verticillium dahliae. Phytoparasitica 3, 133–137 (1975). [Google Scholar]
- Wu S. et al. Enhanced Agrobacterium-mediated transformation of embryogenic calli of upland cotton via efficient selection and timely subculture of somatic embryos. Plant Mol. Biol. Rep. 26, 174–185 (2008). [DOI] [PubMed] [Google Scholar]
- Lv F. et al. GhCFE1A, a dynamic linker between the ER network and actin cytoskeleton, plays an important role in cotton fiber cell initiation and elongation. J. Exp. Bot. 66, 1877–1889 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambino G., Perrone I. & Gribaudo I. A rapid and effective method for RNA extraction from different tissues of grapevine and other woody plants. Phytochem. Anal. 19, 520–525 (2008). [DOI] [PubMed] [Google Scholar]
- Livak K. & Schmittgen T. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 25, 402–408 (2001). [DOI] [PubMed] [Google Scholar]
- Jiang M. & Zhang J. Effect of abscisic acid on active oxygen species, antioxidative defence system and oxidative damage in leaves of maize seedlings. Plant Cell Physiol. 42, 1265–1273 (2001). [DOI] [PubMed] [Google Scholar]
- Gardes M. & Bruns T. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol. Ecol. 2, 113–118 (1993). [DOI] [PubMed] [Google Scholar]
- Lievens B., Claes L., Vanachter A., Cammue B. & Thomma B. Detecting single nucleotide polymorphisms using DNA arrays for plant pathogen diagnosis. FEMS Microbiol. Lett. 255, 129–139 (2006). [DOI] [PubMed] [Google Scholar]
- Ellendorff U., Fradin E., de Jonge R. & Thomma B. RNA silencing is required for Arabidopsis defence against Verticillium wilt disease. J. Exp. Bot. 60, 591–602 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.