Summary
Next generation sequencing of spatially and temporally separated biopsies and circulating tumour DNA directs therapy in response to tumor evolution and acquired resistance in colorectal cancer.
The management of advanced colorectal cancer (CRC) uses combination systemic chemotherapy with 5-fluorouracil (5-FU), irinotecan and oxaliplatin. The EGFR targeted agents cetuximab and panitumumab are important additions to the treatment of CRC and have demonstrated survival benefits in randomized controlled trials. However patients’ cumulative exposure to drug therapy is high, with the associated toxicities and financial costs.
The concept that tumors are heterogeneous both within a single tumor lesion and between tumor lesions in the same patient (intratumour heterogeneity) has become well established over the last 30 years (1, 2). As tumors consist of diverse subclonal populations with differing genomes, epigenomes, transcriptomes and proteomes related through a common progenitor, the capacity to evolve and develop resistance in response to selective pressures such as prolonged exposure to targeted therapies presents a significant clinical challenge.
Initial studies of acquired resistance to targeted therapy have often used genomic analysis of biopsies from single sites of progression. In non-small cell lung cancer (NSCLC), analysis of biopsies following resistance to EGFR tyrosine kinase inhibitors (TKIs) shows a diverse range of resistance mechanisms (2, 3). Secondary resistance mutations (T790M) in EGFR are common and mutations in PIK3CA, MET amplification and transformation to small cell lung cancer are also observed. In CRC primary resistance to the anti-EGFR monoclonal antibodies cetuximab and panitumumab can be mediated by KRAS mutations in exon 2 & 3, as activation of MAP kinase signaling downstream of EGFR obviates any benefit from EGFR blockade (4).
The first published reports of the genetic basis of acquired resistance to anti-EGFR monoclonal antibodies in KRAS wildtype CRCs were published simultaneously by Misale et al and Diaz et al (5, 6). Misale et al demonstrated that, unlike NSCLC where secondary resistance mutations in EGFR are the predominant mechanism of acquired resistance, KRAS activating mutations were the primary abnormality found in patients progressing on EGFR blockade. Pre-existing KRAS mutations were not found in the pre-treatment biopsies despite using the most sensitive assays available at the time of the study. But even now with next generation sequencing (NGS) and digital PCR technologies the absence of a subclonal population, within a biopsy from a metastatic tumour, containing an activating KRAS mutation prior to treatment, is difficult to exclude. In the analysis of circulating tumor DNA (ctDNA) by Diaz and colleagues, mathematical modeling and correlation with clinical samples was used to predict that CRCs likely contain hundreds to thousands of KRAS mutant resistant cells prior to therapy (6). Given our current understanding of tumor heterogeneity the analysis of single biopsies in patients with multiple sites of disease may not fully reflect the complex subclonal genetic landscape and the diversity of potential resistance mechanisms that may ensue at the time of progression on therapy.
In this issue, Russo and colleagues investigated a patient with metastatic CRC and acquired resistance to cetuximab providing valuable insights into differential targeted therapy response (7). The authors leveraged the ability of next generation sequencing of multiple metastatic lesions and ctDNA to elucidate polyclonal mechanisms of resistance and guide treatment decisions. The patient relapsed with both distant liver metastasis and a local recurrence following adjuvant treatment with 5-FU and oxaliplatin. These two visible sites of relapse were excised surgically and further systemic chemotherapy was given. Both the primary tumor specimen and tumors excised at relapse were found to have a TP53 p.E171* mutation that was probably clonal, with the differing variant allele percentages between lesions likely reflective of the tumor cellularity of those samples. No mutations were seen in genes in the MAP kinase pathway downstream of EGFR, prior to treatment with cetuximab in the metastatic setting, although the authors note that these may have been present below the limit of detection by NGS.
Despite surgical resection of all visible disease the patient relapsed two months later with liver metastasis. She went onto receive irinotecan and cetuximab in the metastatic setting and had radiological disease control for 15 months. However the patient’s liver metastases eventually progressed and a biopsy of a lesion in segment 8 of the liver demonstrated the presence of an activating mutation downstream of EGFR. Interestingly this was not a mutation in KRAS but downstream at the level of MAP2K1 with an activating p.K57T mutation. Mutations in MAP2K1 have recently and infrequently been described in the setting of primary resistance to EGFR blockade but not acquired resistance. Whether the initial lack of the more common KRAS codon 2 and 3 or EGFR extracellular domain mutations indicates something unique with this patients tumor or is a reflection of the difficulty of rebiopsy and profiling of samples following acquired resistance to EGFR blockade is unclear.
Russo and colleagues have generated pre-clinical models of acquired resistance to EGFR blockade and found a mutation in MAP2K1 resulting in an amino acid substitution at the same residue (p.K57) as found in the patient. These cell lines were resistant to both cetuximab and panitumumab but when combined with the MEK inhibitor trametinib cells showed a significant reduction in viability in vitro. These models of acquired resistance are difficult to generate and require prolonged exposure to the therapy in question. Even though due to the lack of signals from a tumor microenvironment they may not generate all the possible mechanisms of acquired resistance they are a powerful resource to catalogue different mechanism and investigate the effect of therapeutic strategies before taking this into the clinic.
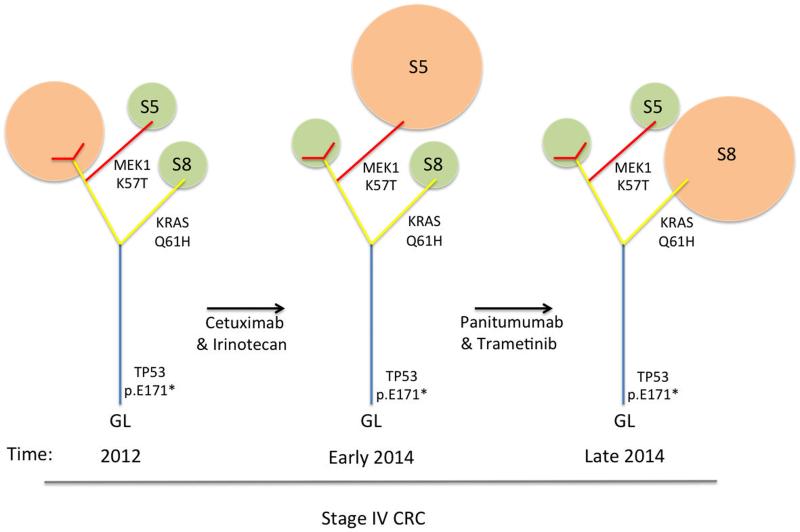
Fortunately the clinical team was able to access off license use of trametinib and the patient had a radiographic response, whilst receiving trametinib and panitumumab. The lesion in segment 8 of the liver (containing the MAP2K1 mutation) responded. In keeping with this Russo and colleagues used ctDNA to show a reduction in the level of the MAP2K1 mutation in the plasma reflecting clearance of this subclone. However the patient progressed on panitumumab and trametinib with new liver lesions and biopsy of one of these in segment 5 of the liver showed acquisition of the more classic KRAS p.Q61H substitution but complete absence of the MAP2K1 mutation. All the lesions examined contained the TP53 p.E171* mutation demonstrating that they come from a common progenitor and that subclones had acquired multiple mechanisms of resistance to therapy with potential pruning of the phylogenetic tree (Figure 1). They were also able to identify the KRAS mutation in ctDNA in the absence of the MAP2K1 mutation and rebound of the MAP2K1 mutation on withdrawal of trametinib. Unfortunately there are no effective agents to target KRAS mutations and the patient progressed on subsequent combination therapies. The lack of response to downstream MEK inhibition suggests that the KRAS mutation activity was independent of MAP kinase signaling and may have exerted its activity through other pathways which KRAS feeds into such as PI3K/Akt or Ral signaling.
Figure 1.
Phylogenetic trees of the patients tumour over the course of therapy. The detectable subclone at each time point is indicated (orange), smaller undetectable subclones are also shown (green). Clones are pruned or grow and expand in response to targeted therapy. No therapy the patient receives is effectively able to target the trunk of the tumour, consequently branches are able to evolve and those with resistance to the targeted therapies are able to expand and are detectable on biopsy or in the ctDNA. (S5 = liver segment 5 lesion, S8 = liver segment 8 lesion)
This case hints at what personalized medicine could look like in the future, with adaptive therapy in response to evolving polyclonal mechanisms of drug resistance. However for this to be a reality we need to overcome several key challenges. Firstly we need the availability of more targeted therapies to treat resistance mutations or block bypass signaling in other pathways. Even with such an armory of therapies we would need strategies in place to deal with regulatory and licensing issues in order to respond in a clinically relevant time frame. Recent technological advances in ctDNA have shown the potential to reflect the heterogeneity of a tumor and to identify polyclonal and spatially separated mechanisms of resistance to abiraterone depravation in castration resistant prostate cancer and to both ERBB2 targeted therapy and hormonal therapy in breast cancer (8, 9). If we were able to identify the spectrum of resistance mechanism to a therapy in the peripheral blood prior to treatment should we offer combination therapy with multiple targeted agents and would such combinatorial approaches be tolerated at clinically effective doses? Should we consider non-conventional dosing strategies to avoid future resistance or would sequential therapies initiated when evidence of progression and expansion of a particular subclone is evident in the ctDNA result in improved outcomes? Strategies to combat tumor evolution will require empirical evidence from clinical trials using such strategies.
Finally what should we do with residual lesions during periods of stable disease? The cancer in this case has evolved or expanded a pre-existing subclone resistant to EGFR blockade alone and in combination with MEK inhibition during periods of stable disease. Stereotactic body radiotherapy of oligoprogressive lesions in NSCLC patients on EGFR and ALK TKI’s allows patients to stay on targeted therapy for longer from retrospective studies (10). Randomized trials are planned in this area but should we be ablating or surgically excising residual lesions to remove drug persistent cells that might act as reservoirs of diversity?
In this issue Russo et al demonstrate the ability to provide lesion specific therapy in the face of tumor heterogeneity. Implementation of this adaptive response to evolving mechanisms of resistance is possible but novel approaches to forestall eventual relapse may require a paradigm shift in our use of targeted therapies and our management of stable disease.
Acknowledgments
Financial support: CH is a Rosetrees Trust fellow and is funded by the National Institute for Health Research and CS is funded by Cancer Research UK, the Rosetrees Trust, EU FP7 (projects PREDICT and RESPONSIFY, ID:259303), the Prostate Cancer Foundation, the European Research Council, the Breast Cancer Research Foundation, and is supported by the National Institute for Health Research University College London Hospital Biomedical Research Centre.
Footnotes
Conflicts of interest: Crispin Hiley is a consultant/advisory board member for AstraZeneca. Charles Swanton does not disclose any conflicts of interest.
References
- 1.Nowell PC. The clonal evolution of tumor cell populations. Science. 1976 Oct 1;194(4260):23–8. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- 2.Burrell RA, Swanton C. Tumour heterogeneity and the evolution of polyclonal drug resistance. Mol Oncol. 2014 Sep 12;8(6):1095–111. doi: 10.1016/j.molonc.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011 Mar 23;3(75):75ra26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karapetis CS, Khambata-Ford S, Jonker DJ, O’Callaghan CJ, Tu D, Tebbutt NC, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008 Oct 23;359(17):1757–65. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 5.Misale S, Yaeger R, Hobor S, Scala E, Janakiraman M, Liska D, et al. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature. 2012 Jun 28;486(7404):532–6. doi: 10.1038/nature11156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diaz LA, Jr., Williams RT, Wu J, Kinde I, Hecht JR, Berlin J, et al. The molecular evolution of acquired resistance to targeted EGFR blockade in colorectal cancers. Nature. 2012;486:537–40. doi: 10.1038/nature11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Russo, et al. Tumor heterogeneity and lesion-specific response to targeted therapy in colorectal cancer. Cancer Discovery. doi: 10.1158/2159-8290.CD-15-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carreira S, Romanel A, Goodall J, Grist E, Ferraldeschi R, Miranda S, et al. Tumor clone dynamics in lethal prostate cancer. Sci Transl Med. 2014 Sep 17;6(254):254ra125. doi: 10.1126/scitranslmed.3009448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murtaza M, Dawson SJ, Pogrebniak K, Rueda OM, Provenzano E, Grant J, et al. Multifocal clonal evolution characterized using circulating tumour DNA in a case of metastatic breast cancer. Nat Commun. 2015 Nov 4;6:8760. doi: 10.1038/ncomms9760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weickhardt AJ, Scheier B, Burke JM, Gan G, Lu X, Bunn PA, Jr, et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J Thorac Oncol. 2012 Dec;7(12):1807–14. doi: 10.1097/JTO.0b013e3182745948. [DOI] [PMC free article] [PubMed] [Google Scholar]