Abstract
Burkholderia pseudomallei, a highly pathogenic bacterium that causes melioidosis, is commonly found in soil in Southeast Asia and Northern Australia1,2. Melioidosis can be difficult to diagnose due to its diverse clinical manifestations and the inadequacy of conventional bacterial identification methods3. The bacterium is intrinsically resistant to a wide range of antimicrobials, and treatment with ineffective antimicrobials may result in case fatality rates (CFRs) exceeding 70%4,5. The importation of infected animals has, in the past, spread melioidosis to non-endemic areas6,7. The global distribution of B. pseudomallei and burden of melioidosis, however, remain poorly understood. Here, we map documented human and animal cases, and the presence of environmental B. pseudomallei, and combine this in a formal modelling framework8-10 to estimate the global burden of melioidosis. We estimate there to be 165,000 (95% credible interval 68,000-412,000) human melioidosis cases per year worldwide, of which 89,000 (36,000-227,000) die. Our estimates suggest that melioidosis is severely underreported in the 45 countries in which it is known to be endemic and that melioidosis is likely endemic in a further 34 countries which have never reported the disease. The large numbers of estimated cases and fatalities emphasise that the disease warrants renewed attention from public health officials and policy makers.
Melioidosis is a disease of public health importance in areas of Southeast Asia and Australia, and is considered a potential emerging infectious disease in many tropical developing countries11. In northeast Thailand, there are around 2,000 culture-confirmed melioidosis cases per year with a case fatality rate (CFR) of 40%12. In Singapore, 550 melioidosis cases occurred during the last ten years, of which a fifth resulted in death13. Skin inoculation is considered the main route of infection in agricultural workers in developing countries14. Recent evidence also suggests that inhalation of B. pseudomallei during extreme weather events15,16 and ingestion of B. pseudomallei contaminated water are also important routes of infection17. High-risk groups include patients with diabetes mellitus, chronic kidney disease and excessive alcohol intake12,18. Developed countries are also observing an emergence of melioidosis related to travelling and importation of cases11. No licensed vaccine for melioidosis is currently available. Strengthening of microbiological laboratories and research facilities often results in the discovery of B. pseudomallei in new areas; recent national additions include India, Southern China, Brazil and Malawi11,19. Given the diagnostic limitations3, it is likely that B. pseudomallei is present in many other tropical countries but has not yet been detected.
Importation of infected humans and animals could lead to the establishment of B. pseudomallei in previously unaffected areas because the organism can be released to and persist in the environment. Previous importation events to non-endemic regions include an outbreak of melioidosis in 1975 in Paris, resulting in the deaths of two humans and an unknown number of animals6,7. B. pseudomallei then persisted in the soil for up to six years6. A recent outbreak of melioidosis in a non-endemic area occurred at Tulane Primate Research Center, Louisiana, USA, in November 201420. The results of a CDC investigation concluded that the organism had spread from a building where mice were being infected experimentally to primates within the facility possibly through contamination of the inner garments worn by staff20. It is, however, not yet known whether B. pseudomallei could have contaminated and persisted in the environment in Louisiana.
Knowledge about the global burden of melioidosis and its potential to become established in non-endemic areas is poor. Previous maps of melioidosis simply displayed countries that had reported melioidosis cases11 and therefore provided no information on areas where melioidosis could be endemic but undiagnosed. In addition, previous maps could not estimate the global morbidity and mortality of melioidosis11, which are essential for policy makers to help determine allocation of the limited resources available for public health. Finally, previous maps could not determine the level of risk of B. pseudomallei establishment in the event that the organism was released in non-endemic areas.
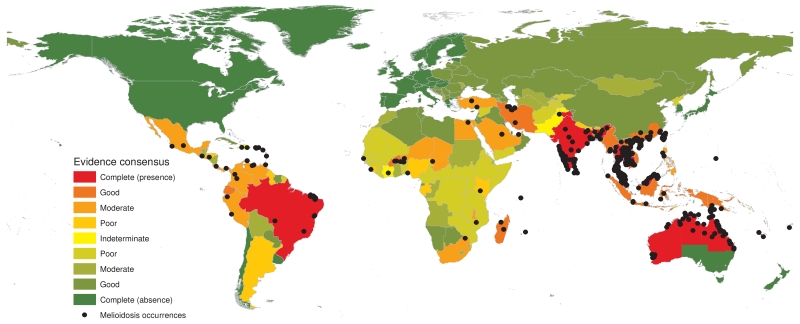
Here we present the first evidence-based predicted map of B. pseudomallei, and estimate the total incidence and mortality due to melioidosis worldwide for 2015 (see Supplementary Information Figure 1-3). A globally comprehensive database has been compiled, comprising 22,338 geo-located records of human and animal melioidosis, as well as the presence of environmental B. pseudomallei from reports published from 1910 to 2014 (Supplementary Figure 4). We assessed the strength of evidence for melioidosis endemicity at a national level, ranging from complete consensus on absence to complete consensus on presence (see Supplementary methods and Figure 1). A boosted regression tree (BRT) statistical model was used to estimate environmental suitability for B. pseudomallei globally at a resolution of 5 km × 5 km using a database of occurrence records and a set of gridded environmental covariates known, or hypothesised, to affect the presence of B. pseudomallei. We then used a multivariable negative binomial regression model to relate the environmental suitability values generated by the BRT model to geo-positioned incidence data to estimate the incidence of melioidosis cases in each 5 km × 5 km square. We similarly applied a logistic regression model to estimate the mortality in these cases. Using bootstrap resampling and Monte Carlo simulations we developed an ensemble model of 2,500 global realisations of these incidence and case fatality maps to derive spatial estimates of the numbers of cases and deaths caused by the disease in melioidosis-endemic areas, along with corresponding 95% credible intervals (CrI). We excluded countries in which there is complete consensus on B. pseudomallei absence from the estimation of global incidence and mortality of melioidosis, and lastly we evaluated environmental suitability for B. pseudomallei in those areas to identify where imported cases may lead to subsequent B. pseudomallei establishment.
Figure 1. Global evidence consensus and geographic locations of occurrence data from 1910 to 2014.
Country coloring is based on evidence-based consensus with green representing a complete consensus on absence of B. pseudomallei and red a complete consensus on presence of B. pseudomallei. Black dots represent geo-located records of melioidosis cases or presence of B. pseudomallei.
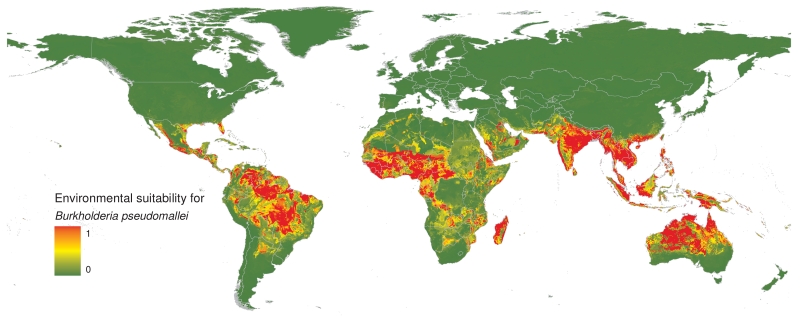
We predict that B. pseudomallei is ubiquitous throughout the tropics (Figure 2). The highest risk zones are in Southeast and South Asia, tropical Australia, Western sub-Saharan Africa and South America. Risk zones of varying sizes are also observed in Central America, Southern Africa and the Middle East. We found that high rainfall and temperature, and anthrosol and acrisol soil types were strongly associated with the presence of B. pseudomallei. Anthrosol is a soil type that has been modified profoundly by human activities particularly by irrigated agriculture, and acrisol is clay-rich soil found in tropical climates21. We also found that high salinity and high proportion of gravel were associated with the presence of B. pseudomallei (see Supplementary Information Figure 5). The association between presence of B. pseudomallei and high soil salinity is consistent with previous laboratory studies22. Although our model did not find an association between the presence of B. pseudomallei and soil pH reported by previous environmental studies23, this could be because soil pH is generally associated with other soil factors, particularly soil salinity21, reducing the capacity of our model to identify this as a geographic risk factor. Validation statistics indicated a high predictive performance of the BRT ensemble model with an area under the receiver operating characteristic curve of 0.81 (95% CrI 0.76-0.86).
Figure 2. Predicted environmental suitability for B. pseudomallei persistence at 5 km × 5 km spatial resolution.
Areas of high environmental suitability are shown in red and areas of low suitability in green.
We estimate that there will be 165,000 melioidosis cases in 2015 among the three billion people living in the areas likely to contain B. pseudomallei (incidence rate of 5.0 per 100,000 people at risk per year) (Table 1). The reported incidence rate of melioidosis was positively associated with environmental suitability for B. pseudomallei predicted by BRT, adjusted by country income level24, and prevalence of diabetes mellitus25 and indigenous ethnicity in Australia (see Supplementary Information Figure 6 and 7). Country income level was included to take account of a trend showing lower incidence rates in high-income countries (adjusted incidence rate ratio [aIRR] 0.58, 95% CrI 0.23-1.39), which could be due to comparatively lower exposure rates, better prevention in general or other residual factors14-17. The prevalence of indigenous ethnicity was significantly associated with a higher incidence rate of melioidosis (aIRR 1.23, 95% CrI 1.09-1.38, for an increase of 10% prevalence of indigenous ethnicity). Indigenous ethnicity is likely to be a proxy of other risk factors such as excessive alcohol intake and chronic kidney disease, and higher exposure rates18. The development of high-resolution geographic data for these risk factors and additional epidemiological studies outside Southeast Asia and Australia would likely improve the model further.
Table 1. Estimated burden of melioidosis in 2015, by continent.
| Population at risk | Melioidosis cases | Melioidosis deaths | |
|---|---|---|---|
|
|
|||
| Millions (credible interval) | Thousands (credible interval) | Thousands (credible interval) | |
| South Asia | 1,525 (1,402-1,595) | 73 (31-171) | 42 (18-101) |
| East Asia & Pacific | 858 (795-920) | 65 (28-161) | 31 (13-77) |
| Sub-Saharan Africa | 602 (482-695) | 24 (8-72) | 15 (6-45) |
| Latin America & Caribbean | 246 (153-334) | 2 (1-7) | 1 (< 1-3) |
| Middle East & North Africa | 49 (29-80) | < 1 | < 1 |
| Europe & Central Asia | 0 | 0 | 0 |
| North America | 0 | 0 | 0 |
| Global | 3,280 (2,862-3,624) | 165 (68-412) | 89 (36-227) |
We predict that only 40% of all melioidosis cases occur in the East Asia and Pacific region, where melioidosis is considered highly endemic. By contrast, South Asia is predicted to bear 44% of the overall burden, because large populations live in areas contaminated with B. pseudomallei. Only Australia, Brunei Darussalam and Singapore have national surveillance data for melioidosis that are comparable to our estimates. Our estimates for other countries where melioidosis is known to be endemic were higher than reported (see Supplementary Information Table 1), supporting the suggestion that the burden of melioidosis in many tropical developing countries is hidden and masked by under-development of microbiological facilities, lack of relevant clinical and laboratory expertise3, and poor reporting systems.
We estimate that 89,000 people globally will die from melioidosis in 2015. We found that the mortality of melioidosis was strongly associated with the under-5 mortality rate (odds ratio (OR), 1.88, 95%CrI 1.73-2.07, for an increase of 10 times in infant deaths per 1,000 live births)24 and used this to estimate the CFR for all predicted melioidosis cases. We predict that >99% of all deaths due to melioidosis occur in low- and middle-income countries, and <1% occur in high-income countries including Australia, Brunei Darussalam and Singapore (see Supplementary Information Figure 8).
We developed a list of priority countries where microbiological diagnostic facilities and disease reporting systems should be strengthened urgently, so that accurate diagnosis3 can be provided and the burden of melioidosis can then be defined. Appropriate prevention campaigns14 and treatment guidelines5 could then be implemented to reduce disease mortality rates. The list of priority countries includes 45 countries where melioidosis is known to be endemic but is underreported and a further 34 countries where melioidosis is probably endemic but has never been reported (Figure 3).
Figure 3. Priority countries where microbiological diagnostic facilities and disease reporting systems for melioidosis should be strengthened.
Countries where melioidosis is predicted to be endemic but is under- or never-reported are shown in red and pink, respectively.
We also predict that two (USA and Japan) of the 44 countries where B. pseudomallei is considered currently absent have areas which would be suitable for B. pseudomallei establishment. These include a geographically contiguous area covering southern parts of Florida, Louisiana and Texas in the USA, and Okinawa and Kagoshima prefectures in Japan. These areas share similar environmental values to the Caribbean islands and Taiwan where melioidosis is known to be endemic (Figure 1). Following the recent outbreak at Tulane Primate Research Center, we evaluated the B. pseudomallei suitability level in Louisiana in more detail. The B. pseudomallei suitability level is very low at the Center (suitability level 0.02) and is moderately high in New Orleans, 35 miles south of the Center (suitability level 0.55). The suitability level 0.55 is comparable to other known endemic areas such as Saravane in Laos26 (suitability level 0.54), suggesting that it would be possible for B. pseudomallei to become established in Louisiana if the bacterium were to be released widely. It is also possible that B. pseudomallei is already present in the environment in USA and Japan but has never been detected.
We have strived to be exhaustive in the assembly of contemporary data on melioidosis and have applied new modelling approaches to maximise the predictive power of these analyses. Our estimate of global mortality due to melioidosis (89,000 per year) is comparable to those due to measles (95,600 per year)27 and higher than that due to leptospirosis (50,000 per year)28 and dengue infection (9,100 – 12,500 per year)27,29 – diseases which are considered to be of high priority by many international health organizations27. The global burden of melioidosis is likely to be substantial and increasing due to population and pathogen movements increasing the likelihood of establishment in new areas, fuelled by an increase in anthrosol30 and the marked rise in the prevalence of diabetes mellitus globally27. This wide and potentially increasing geographical distribution and burden, combined with the high CFR particularly when melioidosis patients are undiagnosed and treated with ineffective antimicrobials4,5, highlight the need for public health officials and policymakers to raise the priority of this disease.
Methods
The occurrence database
The occurrence data set was comprised of geo-located records of human cases, animal cases and presence of B. pseudomallei in the environment derived from (i) peer reviewed literature and (ii) case reports (see Supplementary Information Figure 1, Panel a). For peer reviewed literature, we searched PubMed, GenBank database (http://www.ncbi.nlm.nih.gov/genbank), MLST database (http://bpseudomallei.mlst.net) and Eurosurveillance database (http://eurosurveillance.org) for studies describing human cases, animal cases or presence of B. pseudomallei in the environment between Jan 1, 1920 and Dec 31, 2014 using the MeSH terms “melioidosis” or “pseudomallei”. No language restrictions were placed on these searches, but only those citations with a full title and abstract were retrieved. We searched bibliographies from selected studies for secondary references. For case reports, we searched ProMED (http://www.promedmail.org), and Ministry of Health websites for each country. We also searched Google News archives (http://news.google.co.uk/archivesearch) using the same search terms and country name for news and reports of melioidosis at a country level.
An occurrence was defined as the reporting of a case of melioidosis infection or an identification of B. pseudomallei at an environmental sampling point. After processing, a total of 22,338 geo-located occurrences spanning a period from 1910 to 2014 were included. A summary of the data management procedure, beginning with the raw inputs and showing the proportion of data lost through the stages of quality control before reaching the final occurrence database is provided in Supplementary Information Figure 2. The final occurrence database with geo-positioned data points and the data dictionary are publicly available online (http://figshare.com/s/7f671b7610b811e59a3f06ec4b8d1f61).
Evidence for the presence of melioidosis was obtained from three types of source: health reporting organizations, peer-reviewed articles and case reports (see Supplementary Information Figure 1, Panel b). We then used a weighted scoring system to quantify evidence consensus (see Supplementary methods and Supplementary Information Figure 3).
Explanatory covariates
We assembled gridded (5 km × 5 km pixel) global data for a suite of explanatory covariates of soil characteristics, climatic conditions and other covariates. These were chosen based on factors known or hypothesised to contribute to presence of B. pseudomallei in the soil. Covariates included (i) soil characteristics from Harmonized World Soil Database (HWSD) (http://www.iiasa.ac.at)21, (ii) precipitation and land surface temperature variables from the WorldClim database (www.worldclim.org), and (iii) a vegetation/moisture index from the Advanced Very High Resolution Radiometer (AVHRR) database of the National Oceanic and Atmospheric Administration (www.noaa.gov). HWSD and WorldClim are freely available sets of global soil and climate data at a 1 km ×1 km spatial resolution, respectively. All grids were resampled to the same 5 km × 5 km grid to ensure uniformity of land/water boundaries and spatial resolution.
Predicting environmental suitability for B. pseudomallei
We used a boosted regression tree (BRT) approach31 to establish a multivariable empirical relationship between the distribution of occurrence records of B. pseudomallei and the environmental conditions at each location, as has been previously applied to mapping dengue8, avian influenza9 and Ebola10 (see Supplementary Information Figure 1, Panel c). Model accuracy was assessed by calculating the mean cross-validated area under the curve (AUC) statistic32.
Estimation of populations at risk
People living in each 5 km × 5 km pixel with a predicted environmental suitability for B. pseudomallei greater than the value of the fifth percentile of the positive occurrence records were considered at risk of acquiring melioidosis in each submodel. The population density map was derived from Global Rural-Urban Mapping Project (GRUMP) 2010 (http://sedac.ciesin.org/data/collection/grump-v1) and levelled to match national and global population projections for 2015 from UN World Population Prospects (http://esa.un.org/wpp/). World regions were categorized according to the World Bank24.
Estimation of incidence of melioidosis
Published literature reporting annual incidence rates of melioidosis from the literature search described above were used to make spatial prediction of incidence rates (see Supplementary Information Figure 1, Panel d). Inclusion criteria were restricted to the literature reporting annual incidence rate (per 100,000 population) of culture-confirmed melioidosis or equivalent in a defined area. We then used a negative binomial model to estimate the incidence of melioidosis cases based on the B. pseudomallei suitability and the prevalence of diabetes and aboriginal population. The fitted negative binomial model was then applied to the 2015 human population surface, the B. pseudomallei suitability, the prevalence of diabetes25 and the prevalence of indigenous Australians to provide a mapped estimate of incidence.
Estimation of mortality of melioidosis
Published literature reporting case fatality rates (CFRs) of melioidosis from the literature search described above were used to make spatial prediction of CFRs. Inclusion criteria were restricted to the literature reporting CFRs (%) of culture-confirmed melioidosis in a defined area. We evaluated the association of reported CFRs with national-level healthcare expenditure (HE) per capita, log10 transformed HE per capita, the national-level under-5 mortality rate (U5MR)24 and log10 transformed U5MR by constructing multivariable logistic regression models and assessing their goodness of fit to the data. Reported HE and U5MR were expected to be a proxy for the capacity of medical services in the countries and associated with the CFRs of melioidosis. The optimal logistic regression model contained only log10 transformed U5MR. This model was then used to predict the number of deaths due to melioidosis by applying it to national-level log10 transformed U5MR and multiplying this by the gridded predictions of melioidosis incidence.
Development of priority country list
Priority countries included countries where melioidosis is known to be endemic but underreported, and countries where melioidosis is probably endemic but is never reported. Countries where melioidosis is known to be endemic were defined as countries with a national level of evidence from “complete presence” to “poor presence” and having the lower limit of 95% credible interval of predicted incidence of human melioidosis as more than or equal to one case per year. Countries where melioidosis is probably endemic were defined as countries with a national level of evidence from “indeterminate” to “good absence” and having the lower limit of 95% credible interval of predicted incidence of human melioidosis as more than or equal to one case per year. Countries with a level of evidence equal to “complete absence” were considered not endemic for melioidosis, and the level of risk of B. pseudomallei establishment was evaluated in the countries within this category.
Supplementary Material
Acknowledgements
We thank Prapass Wannapinij for technical support. We thank Prof Nicholas J White and Dr Kurt Schaecher for comments on the final draft. We thank Maria Devine for proof reading. This work was funded by the Wellcome Trust (#101103). SIH is funded by a Senior Research Fellowship from the Wellcome Trust (#095066), and grants from the Bill & Melinda Gates Foundation (#OPP1119467, #OPP1106023 and #OPP1093011). SIH would also like to acknowledge funding support from the RAPIDD program of the Science & Technology Directorate, Department of Homeland Security, and the Fogarty International Center, National Institutes of Health.
Author Contributions
D.L., D.D., E.B., N.PJ.D., S.J.P. and S.I.H. conceived the research. D.L. and S.I.H. drafted the manuscript. D.L. and D.D. reviewed all the occurrence data. D.L. and N.G. carried out the modelling and analysis with advice from S.I.H., D.L., N.G., J.M. and D.P. created the maps and figures. All authors discussed the results and contributed to the revision of the final manuscript.
Footnotes
Author Information The authors declare no competing financial interests.
References
- 1.Wiersinga WJ, Currie BJ, Peacock SJ. Melioidosis. N Engl J Med. 2012;367:1035–1044. doi: 10.1056/NEJMra1204699. [DOI] [PubMed] [Google Scholar]
- 2.Aldhous P. Tropical medicine: melioidosis? Never heard of it. Nature. 2005;434:692–693. doi: 10.1038/434692a. [DOI] [PubMed] [Google Scholar]
- 3.Hoffmaster AR, et al. Melioidosis diagnostic workshop, 2013. Emerg Infect Dis. 2015;21(2) doi: 10.3201/eid2102.141045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White NJ, et al. Halving of mortality of severe melioidosis by ceftazidime. Lancet. 1989;2:697–701. doi: 10.1016/s0140-6736(89)90768-x. [DOI] [PubMed] [Google Scholar]
- 5.Lipsitz R, et al. Workshop on treatment of and postexposure prophylaxis for Burkholderia pseudomallei and B. mallei infection, 2010. Emerg Infect Dis. 2012;18:e2. doi: 10.3201/eid1812.120638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galimand M, Dodin A. Repartition de Pseudomonas pseudomallei en France et dans le monde la melioidose. Bull Soc Vet Prat de France. 1982;66:651–657. [Google Scholar]
- 7.Mollaret H. L’affaire du jardin des plantes. Medecine et Maladies Infectieuses. 1988:643–654. [Google Scholar]
- 8.Bhatt S, et al. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilbert M, et al. Predicting the risk of avian influenza A H7N9 infection in live-poultry markets across Asia. Nat Commun. 2014;5:4116. doi: 10.1038/ncomms5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pigott DM, et al. Mapping the zoonotic niche of Ebola virus disease in Africa. eLife. 2014;3:e04395. doi: 10.7554/eLife.04395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Currie BJ, Dance DA, Cheng AC. The global distribution of Burkholderia pseudomallei and melioidosis: an update. Trans R Soc Trop Med Hyg. 2008;102(Suppl 1):S1–4. doi: 10.1016/S0035-9203(08)70002-6. [DOI] [PubMed] [Google Scholar]
- 12.Limmathurotsakul D, et al. Increasing incidence of human melioidosis in Northeast Thailand. Am J Trop Med Hyg. 2010;82:1113–1117. doi: 10.4269/ajtmh.2010.10-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu X, et al. Association of melioidosis incidence with rainfall and humidity, Singapore, 2003-2012. Emerg Infect Dis. 2015;21:159–162. doi: 10.3201/eid2101.140042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Limmathurotsakul D, et al. Activities of daily living associated with acquisition of melioidosis in northeast Thailand: a matched case-control study. PLoS Negl Trop Dis. 2013;7:e2072. doi: 10.1371/journal.pntd.0002072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen PS, et al. Airborne Transmission of Melioidosis to Humans from Environmental Aerosols Contaminated with B. pseudomallei. PLoS Negl Trop Dis. 2015;9:e0003834. doi: 10.1371/journal.pntd.0003834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng AC, Jacups SP, Gal D, Mayo M, Currie BJ. Extreme weather events and environmental contamination are associated with case-clusters of melioidosis in the Northern Territory of Australia. Int J Epidemiol. 2006;35:323–329. doi: 10.1093/ije/dyi271. [DOI] [PubMed] [Google Scholar]
- 17.Limmathurotsakul D, et al. Melioidosis caused by Burkholderia pseudomallei in drinking water, Thailand, 2012. Emerg Infect Dis. 2014;20:265–268. doi: 10.3201/eid2002.121891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Currie BJ, et al. Melioidosis epidemiology and risk factors from a prospective whole-population study in northern Australia. Trop Med Int Health. 2004;9:1167–1174. doi: 10.1111/j.1365-3156.2004.01328.x. [DOI] [PubMed] [Google Scholar]
- 19.Katangwe T, et al. Human melioidosis, Malawi, 2011. Emerg Infect Dis. 2013;19:981–984. doi: 10.3201/eid1906.120717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention (CDC) Conclusion of selected agent inquiry into Burkholderia pseudomallei release at Tulane National Primate Research Center. 2015 http://www.cdc.gov/media/releases/2015/s0313-burkholderia-pseudomallei.html.
- 21.FAO. IIASA. ISRIC. ISSCAS. JR . C Harmonized world soil database (version 1.2) FAO; Rome, Italy: 2012. IIASA, Laxenburg, Austria. [Google Scholar]
- 22.Pumirat P, et al. Global transcriptional profiling of Burkholderia pseudomallei under salt stress reveals differential effects on the Bsa type III secretion system. BMC Microbiol. 2010;10:171. doi: 10.1186/1471-2180-10-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stopnisek N, et al. Genus-wide acid tolerance accounts for the biogeographical distribution of soil Burkholderia populations. Environ Microbiol. 2013;16:1503–1512. doi: 10.1111/1462-2920.12211. [DOI] [PubMed] [Google Scholar]
- 24.The World Bank World Bank open data. 2013 http://data.worldbank.org/data-catalog/world-development-indicators.
- 25. International Diabetes Federation (IDF) Diabetes Atlas. 5th edition. 2013. Country summary table: estimates for 2012 (2013); http://www.indiaenvironmentportal.org.in/files/file/diabetes%20atlas%202012.pdf
- 26.Rattanavong S, et al. Randomized soil survey of the distribution of Burkholderia pseudomallei in rice fields in Laos. Appl Environ Microbiol. 2011;77:532–536. doi: 10.1128/AEM.01822-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.GBD 2013 Mortality and Causes of Death Collaborators Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization (WHO) Report of the first meeting of the leptospirosis burden epidemiology reference group. 2010 http://whqlibdoc.who.int/publications/2010/9789241599894_eng.pdf.
- 29.World Health Organization (WHO) Dengue and severe dengue. 2015 http://www.who.int/mediacentre/factsheets/fs117/en/
- 30.Newbold T, et al. Global effects of land use on local terrestrial biodiversity. Nature. 2015;520:45–50. 14324. doi: 10.1038/nature14324. [DOI] [PubMed] [Google Scholar]
- 31.Elith J, Leathwick JR, Hastie T. A working guide to boosted regression trees. J Anim Ecol. 2008;77:802–813. doi: 10.1111/j.1365-2656.2008.01390.x. [DOI] [PubMed] [Google Scholar]
- 32.Hijmans RJ. Cross-validation of species distribution models: removing spatial sorting bias and calibration with a null model. Ecology. 2012;93:679–688. doi: 10.1890/11-0826.1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.