Abstract
Background
The pyronaridine-artesunate combination is one of the most recent oral artemisinin-based therapeutic combinations (ACTs) recommended for the treatment of uncomplicated P. falciparum malaria. The emergence of P. falciparum resistance to artemisinin has recently developed in Southeast Asia. Little data are available on the association between pyronaridine susceptibility and polymorphisms in genes involved in antimalarial drug resistance. The objective of the present study was to investigate the association between ex vivo responses to pyronaridine and the K76T mutation in the pfcrt gene in P. falciparum isolates.
Methods
The assessment of ex vivo susceptibility to pyronaridine was performed on 296 P. falciparum isolates using a standard 42-h 3H-hypoxanthine uptake inhibition method. The K76T mutation was also investigated.
Results
The pyronaridine IC50 (inhibitory concentration 50 %) ranged from 0.55 to 80.0 nM. Ex vivo responses to pyronaridine were significantly associated with the K76T mutation (p-value = 0.020). The reduced susceptibility to pyronaridine, defined as IC50 > 60 nM, was significantly associated with the K76T mutation (p-value = 0.004). Using a Bayesian mixture modelling approach, the pyronaridine IC50 were classified into three components: component A (IC50 median 15.9 nM), component B (IC50 median 34.2 nM) and component C (IC50 median 63.3 nM). The K76T mutation was represented in 46.3 % of the isolates in component A, 47.2 % of the isolates in component B and 73.3 % of the isolates in component C (p-value = 0.021).
Conclusion
These results showed the ex vivo reduced susceptibility to pyronaridine, i.e., IC50 > 60 nM, associated with the K76T mutation.
Keywords: Malaria, Plasmodium falciparum, Antimalarial, Resistance, In vitro, Molecular marker, pfcrt
In 2002, the World Health Organization (WHO) recommended the use of artemisinin-based combination therapy (ACT) for the treatment of all cases of uncomplicated malaria. The pyronaridine-artesunate combination (Pyramax®) is one of the latest oral ACTs recommended for the treatment of uncomplicated P. falciparum and P. vivax malaria [1]. The combination pyronaridine-artesunate has recently completed phase III trials in humans. The safety and efficacy of this compound were shown in four randomized clinical trials in adults and children in Africa and Asia [2–5]. Pyronaridine-artesunate showed better efficacy compared with mefloquine-artesunate for the treatment of uncomplicated falciparum malaria in Cambodia and a non-inferior efficacy compared with artemether-lumefantrine in Africa and Southeast Asia. The emergence of P. falciparum resistance to artemisinin and artemisinin derivatives has recently developed in Southeast Asia, manifesting as delayed parasite clearance following treatment with artesunate monotherapy or ACT [6, 7]. Resistance has still developed with the most recent ACT in the form of dihydroartemisinin-piperaquine, which demonstrated less than 70 % efficacy [8, 9]. In areas where the resistance of artemisinin is emerging, partner drugs are under increasing pressure for the selection of resistance, and new therapeutics are limited. Thus, it is important to use an ACT in which its partner drug shows a different mode of action or mechanism of resistance. The in vitro responses to pyronaridine and piperaquine were differently distributed in a triple normal distribution model for pyronaridine and a quadruple normal distribution model for piperaquine [10]. Significant positive in vitro cross-susceptibility was observed between pyronaridine and piperaquine (coefficient of determination of 0.20–0.23) [11, 12]. In vitro and ex vivo responses to piperaquine were not associated with the K76T mutation in the P. falciparum chloroquine resistance transporter gene (pfcrt) [13, 14]. Few data are available on the association between pyronaridine susceptibility and polymorphisms in the genes involved in antimalarial drug resistance. A study using 23 P. falciparum strains showed that there was no significant association between in vitro responses to pyronaridine and pfcrt polymorphism [15]. The objective of the present study was to investigate the association between ex vivo responses to pyronaridine and the K76T mutation in the pfcrt gene in 296 P. falciparum African isolates.
In total, 296 P. falciparum isolates were collected between April 2008 and August 2012 from patients hospitalized in France with imported malaria from African malaria-endemic countries (Angola, Benin, Burkina Faso, Cameroon, Central African Republic, Chad, Comoros, Congo, Ivory Coast, Gabon, Gambia, Ghana, Guinea, Madagascar, Mali, Mauritania, Mozambique, Niger, Senegal, Togo, and Zambia). Informed consent was not required for this study because the sampling and testing procedures were conducted according to the French national recommendations for the care and surveillance of malaria. The ex vivo responses to pyronaridine (Shin Poong Pharm Co., Seoul, Korea) and chloroquine (St. Louis, MO, USA) (control for pfcrt polymorphism) were assessed as previously described using a standard 42-h 3H-hypoxanthine uptake inhibition method [10]. Batches of plates were tested and validated using the chloroquine-susceptible 3D7 strain (West Africa) and the chloroquine-resistant W2 strain (Indochina) (MR4, Virginia, USA) in three to six independent experiments. Nucleic acid extraction and pfcrt single-nucleotide polymorphism identification were previously described [14].
The pyronaridine IC50 values (inhibitory concentration 50 %) ranged from 0.55 to 80.0 nM (Fig. 1). The geometric mean was 20.8 ± 14.6 nM (standard deviation). Ex vivo responses to pyronaridine were significantly associated with the K76T mutation (p-value = 0.020), and similar results were obtained for chloroquine IC50 (p-value < 0.001). Sixteen isolates (5.4 %) had an IC50 greater than 60 nM and were considered to display reduced susceptibility to pyronaridine in vitro [10]. The reduced susceptibility to pyronaridine, defined as IC50 > 60 nM, was significantly associated with the K76T mutation (p-value = 0.004). The odds ratio for reduced susceptibility to pyronaridine associated with the K76T mutation was 4.47 (95 % CI [1.39–18.84]). The in vitro resistance to chloroquine, defined as IC50 > 100 nM, was also significantly associated with the K76T mutation (p-value < 0.001). The odds ratio for reduced susceptibility to chloroquine associated with the K76T mutation was 96.4 (95 % CI [41.8–244.8]). Using Bayesian mixture modelling, the 296 pyronaridine IC50values were classified into three components: component A (IC50 median 15.9 nM), component B (IC50 median 34.2 nM) and component C (IC50 median 63.3 nM) (Table 1). The pyronaridine medians were significantly different in the three components (Kruskal-Wallis test, p-value < 0.001). The proportion of isolates in each group was 59.8 % for component A, 30.1 % for component B and 10.1 % for component C. The K76T mutation represented 46.3 % of the isolates in component A, 47.2 % of the isolates in component B and 73.3 % of the isolates in component C (Kruskal-Wallis test, p-value = 0.021).
Fig. 1.
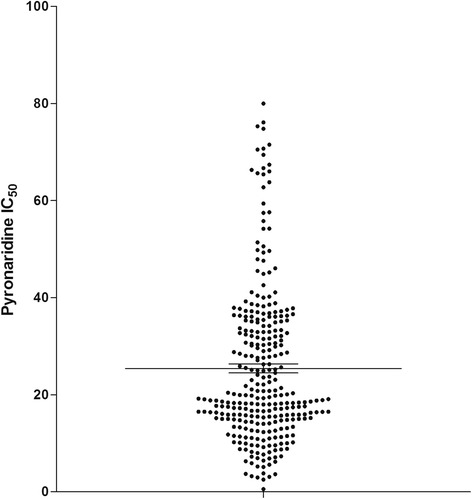
Pyronaridine median and 25 and 75 percentiles of the 50 % inhibitory concentration (IC50 in nM) of 296 African Plasmodium falciparum isolates
Table 1.
Distribution of the IC50 of pyronaridine and chloroquine and the K76T mutation according to the three components defined by the Bayesian mixture modeling approach
| Component A | Component B | Component C | P-value | |
|---|---|---|---|---|
| No of isolates | 177 | 89 | 30 | |
| Pyronaridine median IC50 | 15.9 nM | 34.2 nM | 63.3 nM | <0.001 |
| Pyronaridine 25 % percentile | 11.2 nM | 30.4 nM | 51.2 nM | |
| Pyronaridine 75 % percentile | 18.5 nM | 37.1 nM | 69.7 nM | |
| Chloroquine median IC50 | 57.0 nM | 90.6 nM | 240.5 nM | 0.003 |
| Chloroquine 25 % percentile | 18.3 nM | 19.7 nM | 71.2 nM | |
| Chloroquine 75 % percentile | 207 nM | 273 nM | 301 nM | |
| No of K76T mutation (%) | 82 (46.3) | 42 (47.2) | 22 (73.3) | 0.021 |
The results of the present study showed that ex vivo reduced susceptibility to pyronaridine, i.e., IC50 > 60 nM, was associated with the K76T mutation in the pfcrt gene. These ex vivo results are in contrast with the in vitro results in 23 P. falciparum strains in which the in vitro to pyronaridine were not associated with pfcrt polymorphisms [15]. However, none of the 23 strains showed reduced susceptibility to pyronaridine. In 59 field isolates from Kenya, pyronaridine was more active in vitro against parasites harbouring the wild-type sequence than against those harbouring the K76T mutation (IC50 of 6 versus 20 nM). However, this difference was not significant (p-value > 0.05) [16]. The odds ratio for the K76T mutation associated with reduced susceptibility to pyronaridine was 4.47, whereas the odds ratio for in vitro resistance to chloroquine was 96.4. The resistance to pyronaridine is certainly multigenic, and additional polymorphisms in other genes could also be involved in this resistance.
In contrast with piperaquine, in which its in vitro responses are not associated with pfcrt polymorphism in isolates from Africa and Asia [13, 14, 17, 18] but rather associated with repeat polymorphisms in an ABC transporter gene, pfmdr6 [19], pyronaridine mechanisms of resistance are different than those involved in piperaquine. Pyronaridine-artesunate could be used in areas where resistance to other ACTs has already emerged.
Pyronaridine-artesunate successfully treats artemisinin-resistant P. berghei parasites, while artemether-lumefantrine, artesunate-amodiaquine, artesunate-mefloquine and dihydroartemisinin-piperaquine are not effective [20]. Pyronaridine-artesunate showed better efficacy than mefloquine-artesunate for the treatment of uncomplicated falciparum malaria in Cambodia and a non-inferior efficacy compared with that of artemether-lumefantrine in Africa and Southeast Asia [2–4]. Pyronaridine-artesunate showed greater than 95 % efficacy when used as an initial falciparum malaria treatment versus the re-treatment of subsequent episodes in a multi-site trial in Mali, Burkina Faso and Guinea [21]. In addition, pyronaridine-artesunate is also affective in the treatment of acute uncomplicated P. vivax malaria [5]. Pyronaridine-artesunate is an alternative artemisinin-based combination treatment for malaria in sub-Saharan Africa.
Ethical approval
According to the French legislation, bio-banking and secondary use for scientific purposes of human clinical samples are possible as long as the corresponding patients are informed and have not indicated any objections. This requirement was fulfilled here since information is given to every patient through a hospital notice entitled “Information for Patients,” and no immediate or delayed patient opposition was reported by the hospital clinicians to the French Malaria Reference Center. Moreover, samples received at the French Malaria Reference Center were registered and declared for research purposes as a bio-bank for the French National Institute of Health Survey. No institutional review board approval is required according to French legislation (article L. 1111–7 du Code de la Santé Publique, article L. 1211–2 du Code de Santé Publique, articles 39 et suivants de la loi 78–17 du 6 janvier 1978 modifiée en 2004 relative à l’informatique, aux fichiers, et aux libertés).
Acknowledgements
We dedicate this article to our friend Julien Cren (in memoriam).
This work was financially supported by a grant from the Institut de Veille Sanitaire (grant CNR paludisme).
French National Reference Centre for Imported Malaria Study Group
V Augis, D Basset, F Benoit-Vical, A Berry, N Bourgeois, F Conquere de Monbrison, P Delaunay, J Delmont, K Ezzedine, B Faugere, C Garabedian, E Garnotel, C Lollivier, D Malvy, P Marty, D Maubon, G Menard, P Millet, P Minodier, A Mottard, P Munier, P Parola, R Piarroux, S Picot, T Pistone, C Pomares-Estran, J Puyhardy, D Raffenot, M-C Receveur, R Saidi, H Savini, F Simon, S Vedy.
Abbreviations
- ACT
artemisinin-based combination therapy
- IC50
inhibitory concentration 50 %
- pfcrt
P. falciparum chloroquine resistance transporter gene
- WHO
World Health Organization
Footnotes
Julien Cren is deceased
Competing interests
The authors declare no competing interests.
Authors’ contributions
MM, NB, HB, and JC performed the molecular genetic studies. RA and NB conducted the ex vivo evaluation of pyronaridine and chloroquine susceptibility. The French National Reference Centre for Imported Malaria Study Group supervised, performed and coordinated the collection of patient isolates. MM and BP conceived of and coordinated the study. SB and BP analysed the data. MM, SB and BP drafted the manuscript. All the authors have read and approved the final manuscript.
Contributor Information
Marylin Madamet, Email: mmadamet@gmail.com.
Sébastien Briolant, Email: sbriolant@wanadoo.fr.
Rémy Amalvict, Email: remy_alt@yahoo.fr.
Nicolas Benoit, Email: nicobenoit73@hotmail.com.
Housem Bouchiba, Email: housem.bouchiba@irba.fr.
Bruno Pradines, Email: bruno.pradines@free.fr.
References
- 1.Croft SL, Duparc S, Arbe-Barnes SJ, Craft JC, Shin CS, Fleckenstein L, et al. Review of pyronaridineanti-malarial properties and product characteristics. Malar J. 2012;11:270. doi: 10.1186/1475-2875-11-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tshefu AK, Gaye O, Kayentao K, Thompson R, Bhatt KM, Sesay SS, et al. Efficacy and safety of a fixed-dose oral combination of pyronaridine-artesunate compared with artemether-lumefantrine in children and adults with uncomplicated Plasmodium falciparum malaria: a randomised non-inferiority trial. Lancet. 2010;375:1457–67. doi: 10.1016/S0140-6736(10)60322-4. [DOI] [PubMed] [Google Scholar]
- 3.Kayentao K, Doumbo OK, Pénali LK, Offianan AT, Bhatt KM, Kimani J, et al. Pyronaridine-artesunate granules versus artemether-lumefantrine crushed tablets in children with Plasmodium falciparum malaria: a randomized controlled trial. Malar J. 2012;11:364. doi: 10.1186/1475-2875-11-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rueangweerayu R, Phyo AP, Uthaisin C, Poravuth Y, Binh TQ, Tinto H, et al. Pyronaridine–artesunate versus mefloquine plus artesunate for malaria. N Engl J Med. 2012;366:1298–309. doi: 10.1056/NEJMoa1007125. [DOI] [PubMed] [Google Scholar]
- 5.Poravuth Y, Socheat D, Rueangweerayut R, Uthaisin C, Pyae Phyo A, Valecha N, et al. Pyronaridine-artesunate versus chloroquine in patients with acute Plasmodium vivax malaria: a randomized, double-blind, non-inferiority trial. PLoS One. 2011;6:14501. doi: 10.1371/journal.pone.0014501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361:455–67. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, et al. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2015;371:411–23. doi: 10.1056/NEJMoa1314981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leang R, Taylor WRJ, MeyBouth D, Song L, Tarning J, Chuor Char M, et al. Evidence of Plasmodium falciparum malaria multidrug resistance to artemisinin and piperaquine in Western Cambodia: Dihydroartemisinin-piperaquine open-label multicentre clinical assessment. Antimicrob Agents Chemother. 2015;59:4719–26. doi: 10.1128/AAC.00835-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spring M, Lin JT, Manning JE, Vanachayangkul P, Somethy S, Bun R, et al. Dihydroartemisinin-piperaquine failure associated with a triple mutant including Kelch13 C580Y in Cambodia: an observational cohort study. Lancet Infect Dis. 2015;15:683–93. doi: 10.1016/S1473-3099(15)70049-6. [DOI] [PubMed] [Google Scholar]
- 10.Pascual A, Madamet M, Briolant S, Gaillard T, Amalvict R, Benoit N, et al. Multinormalin vitro distribution of Plasmodium falciparum susceptibility to piperaquine and pyronaridine. Malar J. 2015;14:49. doi: 10.1186/s12936-015-0586-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pascual A, Parola P, Benoit-Vical F, Simon F, Malvy D, Picot S, et al. Ex vivo activity of the ACT new components pyronaridine and piperaquine in comparison with conventional ACT drugs against isolates of Plasmodium falciparum. Malar J. 2012;11:45. doi: 10.1186/1475-2875-11-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Price RN, Marfurt J, Chalfein F, Kenagalem E, Piera KA, Tjitra E, et al. In vitro activity of pyronaridine against multidrug-resistant Plasmodium falciparumand Plasmodium vivax. Antimicrob Agents Chemother. 2010;54:5146–50. doi: 10.1128/AAC.00801-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Briolant S, Henry M, Oeuvray C, Amalvict R, Baret E, Didillon E, et al. Absence of association between piperaquine in vitro responses and polymorphisms in the pfcrt, pfmdr1, pfmrp, and pfnhe genes in Plasmodium falciparum. Antimicrob Agents Chemother. 2010;54:3537–44. doi: 10.1128/AAC.00183-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pascual A, Madamet M, Bertaux L, Amalvict R, Benoit N, Travers D, et al. In vitropiperaquine susceptibility is not associated with the Plasmodium falciparum chloroquine resistance transporter gene. Malar J. 2013;12:431. doi: 10.1186/1475-2875-12-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pradines B, Briolant S, Henry M, Oeuvray C, Baret E, Amalvict R, et al. Absence of association between pyronaridinein vitro responses and polymorphisms involved in quinoline resistance in Plasmodium falciparum. Malar J. 2010;9:339. doi: 10.1186/1475-2875-9-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okombo J, Kiara SM, Mwai L, Pole L, Ohuma E, Ochola LI, et al. Baseline In vitro activities of the antimalarialspyronaridine and methylene blue against Plasmodium falciparum isolates from Kenya. Antimicrob Agents Chemother. 2012;56:1105–7. doi: 10.1128/AAC.05454-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mwai L, Kiara SM, Abdirahman A, Pole L, Rippert A, Diriye A, et al. In vitro activities of piperaquine, lumefantrine, and dihydroartemisinin in Kenyan Plasmodium falciparum isolates and polymorphisms in pfcrt and pfmdr1. Antimicrob Agents Chemother. 2009;55:5069–73. doi: 10.1128/AAC.00638-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hao M, Jia D, Li Q, He Y, Yuan L, Xu S, et al. In vitro sensitivities of Plasmodium falciparum isolates from the China-Myanmar border to Piperaquine and association with polymorphisms in candidate genes. Antimicrob Agents Chemother. 2013;57:1723–9. doi: 10.1128/AAC.02306-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okombo J, Abdi AI, Kiara SM, Mwai L, Pole L, Sutherland CJ, et al. Repeat polymorphisms in the low-complexity regions of Plasmodium falciparum ABC transporters and associations with in vitro antimalarial responses. Antimicrob Agents Chemother. 2013;57:6196–204. doi: 10.1128/AAC.01465-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henrich PP, O'Brien C, Sáenz FE, Cremers S, Kyle DE, Fidock DA. Evidence for pyronaridine as a highly effective partner drug for treatment of artemisinin-resistant malaria in a rodent model. Antimicrob Agents Chemother. 2014;58:183–95. doi: 10.1128/AAC.01466-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sagara I, Beavogui AH, Zongo I, Soulama I, Borghini-Fuhrer I, Fofana B, et al. Safety and efficacy of re-treatments with pyronaridine-artesunate in African patients with malaria: a substudy of the WANECAM randomised trial. Lancet Infect Dis. 2015 doi: 10.1016/S1473-3099(15)00318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


