Abstract
Objective:
Femoral head avascular necrosis (FHAVN) is one of the increasingly common causes of musculoskeletal disability and poses a major diagnostic and therapeutic challenge. Although radiography, scintigraphy, computed tomography (CT), and magnetic resonance imaging (MRI) have been widely used in the diagnosis of FHAVN, positron emission tomography (PET) has recently been evaluated to assess vascularity of the femoral head. In this study, the authors compared F-18 fluoride PET/CT with MRI in the initial diagnosis of FHAVN.
Patients and Methods:
We prospectively studied 51 consecutive patients with a high clinical suspicion of FHAVN. All patients underwent MRI and F-18 fluoride PET/CT, the time interval between the two scans being 4–10 (mean 8) days. Two nuclear medicine physicians blinded to the MRI report read the PET/CT scans. Clinical assessment was also done. Final diagnoses were made by surgical pathology or clinical and radiologic follow-up.
Results:
A final diagnosis of avascular necrosis (AVN) was made in 40 patients. MRI was 96.5% sensitive, 100% specific, and 98.03% accurate while PET/CT was 100% sensitive, specific, and accurate in diagnosing FHAVN. The agreement between the two imaging modalities for the diagnosis of AVN was 96.07%.
Conclusion:
F-18 fluoride PET/CT showed good agreement with MRI in the initial diagnosis of FHAVN and can be better than MRI in detecting early disease.
Keywords: Avascular necrosis, F-18 fluoride, femoral head, magnetic resonance imaging, positron emission tomography/computed tomography
INTRODUCTION
Femoral head avascular necrosis (FHAVN) is the result of irreversible anoxia of the subchondral bone. FHAVN is one of the increasing common causes of musculoskeletal disability that poses a major diagnostic and therapeutic challenge. With approximately 20,000 new reported cases each year, it is the underlying disease in as many as 10% of 500,000 total hip replacements annually performed in the USA.[1,2,3] Affecting mainly young men in their late 30s and early 40s, it results in manpower loss and adds to morbidity. Left untreated, FHAVN will progress to secondary osteoarthritis in 80% of cases and eventually require total hip arthroplasty, hence the importance of early diagnosis is needed.[4]
The causes of FHAVN include trauma and nontraumatic causes such as long-term corticosteroid use, alcohol abuse, hematological diseases (sickle cell anemia, thalassemia, polycythemia, hemophilia, and myeloproliferative disorder), metabolic diseases (Gaucher's disease, hypercholesterolemia, pregnancy, chronic renal failure, hyperparathyroidism, and Cushing's disease), autoimmune diseases, chronic pancreatitis, Caisson's disease, radiation, congenital hip dislocation, and use of potent intravenous bisphosphonates.[4,5,6] Various pathophysiologic mechanisms have been proposed to explain the occurrence of FHAVN in these diverse conditions.[4,7] The proposed mechanisms of cell death include some form of arterial or venous insufficiency or extravascular compression in the context of a rigid bony compartment.[8]
Avascular necrosis (AVN) is characterized by areas of dead trabecular bone and marrow that extend to involve the subchondral plate. Typically, the principal weight bearing region, the anterolateral aspect of the femoral head is involved, but any region of the femoral head may be involved.
Measures to preserve the joint are associated with better prognoses when the diagnosis of FHAVN is made early in the course of the disease. The results of joint replacement therapy are poorer in younger age groups than in older patients. It is, therefore, critical to diagnose this condition as early as possible to prevent or delay progression of the disease.
Noninvasive diagnostic tests used in detecting AVN include radiography, three-phase bone scintigraphy (TPBS) with or without single photon emission computed tomography/computed tomography (SPECT/CT), conventional CT scan, and magnetic resonance imaging (MRI).
Tc-99m methylene diphosphonate (MDP) bone scintigraphy could be an ideal modality for assessing the reparative processes of osteonecrosis as the radionuclide activity reflects osteoblastic activity and blood flow. On planar scintigraphic images, the boundary of the necrotic lesion typically appears as a margin of increased tracer uptake adjacent to a photopenic area.[9] However, if the necrotic lesion is small or eccentrically located, it becomes difficult to detect the scintigraphic changes around the necrotic lesion. Thus, planar scintigraphic images suffer from important limitations such as poor spatial resolution and inability to quantify the lesion. Although SPECT, a technique involving three-dimensional scintigraphy, has been developed as a means of overcoming the limitations of planar scintigraphic images, the lack of anatomical detail makes it difficult to use often the information reliably.[10]
Uptake of F-18 fluoride, like that of Tc-99m MDP, depends on regional blood flow and osteoblastic activity. However, positron emission tomography (PET) offers superior spatial resolution, and the favorable pharmacokinetic characteristics of F-18 fluoride make this a more sensitive modality than SPECT/CT. Since PET/CT provides both structural and functional information, it has been proven useful for interpreting radionuclide uptake not only in patients with malignancies but also in nonmalignant conditions.[11] Also, PET/CT provides an accurate evaluation of the site of the lesions with the CT part of the PET providing anatomical details.[11] However, to the best of our knowledge, there are no studies to date showing the utility of F-18 fluoride PET/CT bone scan in the diagnosis of FHAVN.
The purpose of this study was to assess the utility of F-18 fluoride PET/CT bone scan in the diagnosis of FHAVN and compare it with MRI.
PATIENTS AND METHODS
Patients
The study was approved by the Institute Ethics Committee. All patients gave written informed consent before inclusion in the study. Exclusion criteria included pregnancy, severe debilitation, contraindication to MRI, and refusal to give written informed consent.
Fifty-one consecutive patients (39 male, 12 female) aged 17–48 (mean 32.5) years with high clinical suspicion of FHAVN and referred for MRI from a Tertiary Orthopedic Care Unit were prospectively evaluated. Clinical assessments done in all the patients were recorded. All patients underwent MRI and F-18 fluoride PET/CT bone scans within 4–10 (mean 8) days of each other.
Image acquisition and analysis
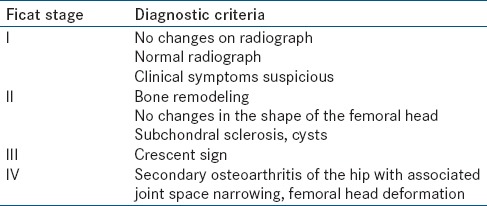
All patients underwent MRI on a 3.0 T (GE Discovery MR750w) MRI unit with a body phased array coil. The following sequences were used: T1 – Axial, sagittal, and coronal (TR/TE, 717/9.2), T2 FS-axial and coronal (TR/TE, 4528/102), PDFS-coronal (TR/TE, 275/30), 1FS-coronal (TR/TE,576/9.2). Ficat staging was used to stage FHAVN on MRI [Table 1]. One experienced radiologist (PS) and one experienced orthopedic surgeon (RKS) read and staged the MRI images.
Table 1.
Diagnostic criteria for Ficat staging
PET/CT (diagnostic CT) of the pelvis (including both hip joints) was performed 1 h after intravenous injection of 370 MBq of F-18 fluoride on a dedicated PET/CT scanner (Discovery STE 16; GE Healthcare, USA). Reconstruction of the acquired data was performed so as to obtain fused PET/CT images in transaxial, coronal, and sagittal views. Two patients with history of long-term steroid use for systemic lupus erythematosus (SLE), with pain in multiple joints underwent total body PET/CT. Two experienced nuclear medicine physicians (AB, BRM) blinded to the MRI reports read the PET/CT scans separately.
Regions of interest (ROIs) were drawn separately over the photopenic area, and the entire head of the affected femur - and the maximum standardized uptake values (SUVmax) of each of these were recorded. In patients with unilateral FHAVN, the SUVmax value of the contralateral (normal) femoral head was also recorded. All patients were followed up clinically for a mean period of 10 months (range: 7–18 months). A final diagnosis of FHAVN was made by surgical pathology or clinical and radiologic follow-up.
Statistical analysis
SPSS (Statistical Product and Service Solutions, version 16.0, IBM, USA) software was used for statistical analysis. Descriptive statistics such as mean and range were used to describe demographics and clinical profile of all patients. Sensitivity, specificity, and accuracy of MRI and PET/CT compared to composite gold standard.
RESULTS
A total of 51 patients (102 hips) were analyzed. Diagnostic criteria for FHAVN on the PET/CT included a photopenic area surrounded by areas of reactive increased tracer uptake along with the “asterisk sign” (loss of thickening of bone trabeculae in the center of the femoral head which appears similar to a star) and “crescent sign” (horizontal subchondral fracture) on CT. Findings on the CT part of the PET/CT like subchondral sclerosis, subchondral cysts, loss of contour of the femoral head, and joint space narrowing were noted whenever present.
Presence of various diagnostic features on MRI such as bone marrow edema, a circumscribed subchondral “band-like” lesion with low signal intensity on T1-weighted (T1W) sequences, double line sign (an inner bright T2 line representing granulation tissue and an outer dark line representing sclerotic bone), rim sign (a high T2 or intermediate T1 signal line sandwiched between two low signal lines), and joint effusion were noted when present.
Thirty-nine of the 51 patients evaluated were diagnosed to have FHAVN on MRI; 23 had unilateral, and 16 had bilateral femoral head involvement. Of the 55 hips diagnosed with AVN, 7 were diagnosed to be Ficat Stage I, 19 Stage II, 25 Stage III, and 4 Stage IV. Using the criteria described earlier, FHAVN was diagnosed on F-18 fluoride PET/CT in 57 hips including all 55 hips diagnosed on MRI. There was no inter-observer variation. Representative images of PET/CT and MRI in a patient with bilateral FHAVN are shown in Figure 1. One patient diagnosed with bilateral early FHAVN on PET/CT had imaging features indeterminate for AVN on MRI [Figure 2]. During 13 months of follow-up, this patient continued to have pain in both hips, and a subsequent MRI showed Ficat Stage I FHAVN on the right side and Stage II FHAVN on the left side. Of the remaining 11 patients, 3 patients had degenerative arthritis, 4 had transient osteoporosis of the hip [Figure 3], and 4 were normal on both imaging modalities.
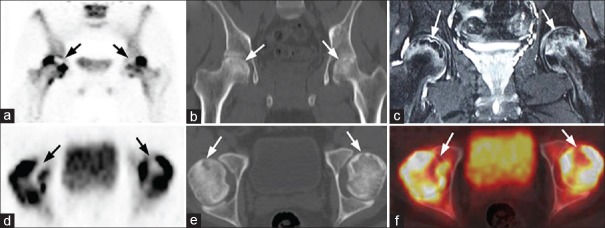
Figure 1.
A 24-year-old male patient with bilateral Ficat Stage III femoral head avascular necrosis (a) Coronal positron emission tomography, (d) Axial positron emission tomography, (f) Fused axial positron emission tomography/computed tomography images show photopenic areas (arrows) in the antero-superior regions of both femoral heads, corresponding to subchondral cysts surrounded by sclerosis on computed tomography (b and e) and decreased signal intensity on T2 weighted magnetic resonance (c). The photopenic areas are bordered by increased tracer uptake corresponding to bone marrow edema on magnetic resonance imaging. Note is also made of bilateral joint effusion, double-line sign in the right femoral head and rim sign in the left femoral head on magnetic resonance imaging (c)
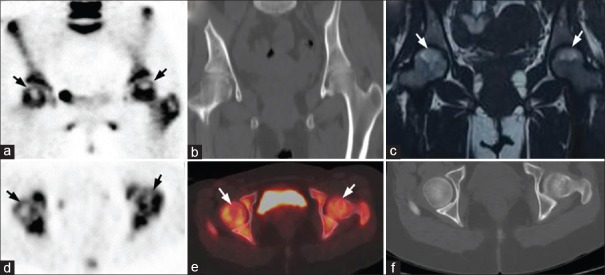
Figure 2.
A 32-year-old female patient with bilateral hip pain for 6 months: (a) Coronal positron emission tomography, (d) Axial positron emission tomography, and (e) Fused axial positron emission tomography/computed tomography images show photopenic areas (arrows) in the antero-superior regions of both femoral heads, with no definite morphological changes on computed tomography (b and f) and nonspecific marrow edema on MRI (c). A diagnosis of bilateral early femoral head avascular necrosis was made on positron emission tomography/computed tomography while magnetic resonance imaging was indeterminate for avascular necrosis. During 13 months of follow-up, the patient continued to have pain in both hips and a subsequent magnetic resonance imaging showed Ficat Stage I femoral head avascular necrosis on the right side and Stage II femoral head avascular necrosis on the left side
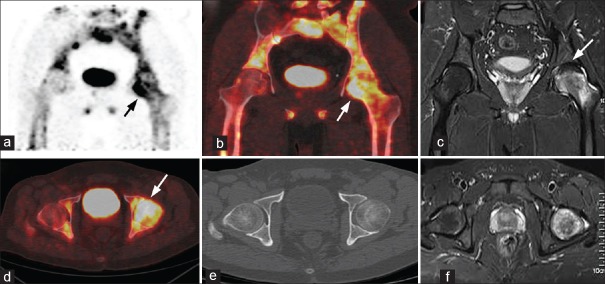
Figure 3.
A 39-year-old male patient with left sided hip pain for 1-year: (a) Coronal positron emission tomography, (b) Fused coronal positron emission tomography/computed tomography, (d) Axial fused positron emission tomography/computed tomography images show diffusely increased tracer uptake (arrow) in the head and neck of the left femur, (e) Computed tomography showed no morphological changes (c and f) coronal and axial magnetic resonance imaging images show diffuse bone marrow edema extending up to the neck and decreased signal intensity from the 11–2 O’clock position (arrow) in the left femoral head. A diagnosis of transient osteoporosis of the hip was made which was proved on clinical follow-up
By surgical pathology or clinical and radiologic follow-up, a final diagnosis of FHAVN was made in 40 patients (57 hips). Surgical pathology could be obtained in 22 patients who underwent hip preserving surgeries. Final diagnosis in the rest was made by clinical or radiological follow-up, as they refused to undergo biopsy/surgical intervention. Seventeen (43%) patients had a history of steroid use, 10 (24%) of alcohol use, 7 (17%) had trauma, 1 (2%) was pregnancy-related and in 5 (14%) no causative factor was identified. MRI was 96.5% sensitive, 100% specific, and 98.03% accurate in diagnosing FHAVN, while PET/CT was 100% sensitive, specific and accurate in diagnosing FHAVN. The agreement between the two imaging modalities for the diagnosis of FHAVN was 96.07%.
Using the ROIs drawn on the affected femoral head, the photopenic area and normal femoral head as described, mean (range) SUVmax of the photopenic area was 1.9 (0.2–2.8), affected femoral head was 21.3 (8.2–42.8) and normal femoral head was 4.4 (3.5–6.7). Mean SUVmax values for the affected femoral heads with Ficat Stage I, II, III, and IV were 10.9, 23.1, 27.8, and 35.5, respectively. As the number of hips in different stages of AVN varied, with only a few hips with Stage I and IV disease, a cut-off value to stage AVN based on SUVmax alone could not be calculated. However, it was observed that with increase in stage, SUVmax value of the femoral head was also higher, representing increased ongoing reactive changes.
Two patients with a history of long-term steroid use for SLE and pain in multiple joints underwent total body PET/CT. In addition to bilateral FHAVN, one patient showed AVN of both humeri and the other showed AVN of the right distal end of the femur.
DISCUSSION
FHAVN is a disease process for which progression is difficult to predict due to the varying etiology. However, despite the various causes, the end result is often the same. Delayed intervention in undiagnosed FHAVN leads to degenerative arthritis and eventual collapse of the femoral head, making early diagnosis crucial. Noninvasive diagnostic tests used in detecting AVN include plain radiography, CT, MRI, TPBS, and SPECT/CT.
On radiography, abnormal findings in AVN include the “crescent sign” (horizontal subchondral fracture line), cystic or sclerotic change in the femoral head, abnormal contours of the femoral head, and collapse or secondary degenerative change. However, the sensitivity of plain radiography for detecting early stages of the disease is as low as 41%. Normal radiographic findings do not necessarily mean that disease is not present.[12]
Ruland et al. demonstrated the sensitivity of Tc-99m MDP in demonstrating the absence of uptake in viable osteocytes by 72 h. They showed that initially a “cold” area is noted in the femoral head, indicating avascularity and reduced delivery of the radiopharmaceutical. There may be a “doughnut” appearance, with a photopenic center surrounded by a region of hyperemia and reactive change. Subsequently, the photopenic area diminishes as there is ingrowth of osteoblasts upward from the metaphysis into the femoral head so that ultimately, as healing progress, there will be uniform intense radioactivity throughout.[13] Using a 1.5 T magnet, Beltran et al. reported 88% sensitivity, 100% specificity, and 94% accuracy with MRI and 78% sensitivity, 75% specificity, and 76% accuracy with bone scintigraphy.[14]
A few studies have demonstrated the utility of SPECT in the diagnosis of AVN. SPECT scanning provides images of the radioactivity within the target organ in three dimensions. With this modality, overlying and underlying areas of radioactivity may be separated into sequential tomographic planes, thus providing increased image contrast and improved lesion detection and localization, as compared with planar scintigraphy. Initially, SPECT images reflect vascular integrity. Early in the disease, SPECT scans may demonstrate an avascular focus, which may be missed on MRI unless contrast is used. Collier et al. found a sensitivity of 85% for SPECT scanning.[15] With triple-head high-resolution SPECT scanning, Lee et al. reported a sensitivity of 97%.[12] Ryu et al. compared the diagnostic sensitivity of Tc-99m MDP bone SPECT and MRI in the early detection of femoral head osteonecrosis after renal transplantation and showed a sensitivity of 100% (32/32) for SPECT and only 66% for MRI.[16] Luk et al. evaluated a total of 22 patients and 24 symptomatic hips out of which seven hips (29%) were confirmed to have AVN and showed additional value of SPECT/CT versus SPECT in the diagnosis of FHAVN. They concluded that SPECT/CT conferred a benefit of improvement of area under curve in receiver operating characteristic in the diagnosis of hip AVN compared with SPECT alone.[10]
The reported sensitivity of MRI for early diagnosis of osteonecrosis ranges between 88% and 100%.[17,18,19] In the early stages of the disease, there may not be any alteration of the normal signal intensity of the femoral head. The first sign of AVN is nonspecific: Diffuse areas of decreased signal intensity are seen in the normally high-signal-intensity fatty marrow on T1W images. This is thought to be due to edema within the marrow. Hence, there are chances that early AVN may be missed in MRI.
While previous studies have demonstrated the utility of TPBS and SPECT in the diagnosis of AVN, there are not many studies using SPECT/CT for detecting AVN. Thus, the reported sensitivity and specificity of bone scintigraphy in the diagnosis of AVN in the literature is either lower or comparable to MRI.
An earlier case report has described the use of F-18 fluoride PET/CT in the diagnosis of FHAVN.[20] PET has better spatial resolution than SPECT (5 mm vs. ~1 cm respectively). The photopenic area in the affected femoral head is better appreciated on PET. F-18 fluoride has favorable kinetics such as faster blood clearance and 2-fold higher uptake in bone compared to Tc-99m MDP. It has higher capillary permeability and faster blood clearance, which results in better target to background ratio compared to MDP. Also, the high spatial resolution and contrast resolution of the CT component of PET/CT provides morphologic identification. CT scanning is appropriate in evaluating the extent of involvement, such as subchondral lucencies and sclerosis during the reparative stage, before the onset of femoral head collapse and superimposed degenerative disease. CT scanning also defines the extent of disease at Stages II and higher better than MRI and plain film radiography. With multiplanar reconstruction, CT scanning enables detection of subchondral or cancellous fractures and collapse.[12] Thus, hybrid PET/CT improves the sensitivity and specificity compared to PET alone. However, F-18 fluoride PET/CT has slightly higher radiation dosimetry than Tc-99m MDP. The estimated radiation dose in adult humans is 0.19 mGy/MBq compared to 0.03 mGy/MBq from Tc-99m MDP for the bladder wall and 0.0120 mGy/MBq compared to 0.035 mGy/MBq for the bone surfaces.[21]
The final choice of MDP SPECT/CT or 18F-NaF PET/CT would depend, to a large extent, on availability as well as costs involved. The dynamics of NaF allow for more rapid imaging (within 1 h of tracer injection) as compared to Tc-99m MDP allowing higher throughput in departments with high patient turnover. Imaging time with PET/CT is also much shorter compared to SPECT/CT. As PET/CT provides better image resolution and allows for semi-quantitative analysis, F-18 fluoride PET/CT may be the preferred investigation in the diagnosis of FHAVN. While dynamic phase PET/CT may provide important information on femoral head vascularity, the increased scan duration may be perceived as a drawback. The results of this study suggest that the 1-h static PET/CT image may be useful for a rapid evaluation of FHAVN. While the additional cost and radiation exposure of NaF PET/CT (compared to MDP SPECT/CT) may limit the use of this investigation, the accurate diagnostic information obtained would tend to justify the use of this investigation, especially in postoperative patients with orthopedic hardware. The cost will be significantly lower for institutes equipped with a medical cyclotron since no additional radiopharmaceutical preparation is required.
In the present study, PET/CT showed higher sensitivity and accuracy compared to MRI. PET/CT has the advantage of detecting abnormalities at multiple sites without increasing the cost. It can also be considered when a patient complains of pain at multiple bone and joint areas. In our study, PET/CT could diagnose additional sites of AVN in two patients. Quantitative bone scanning provides physiologic data that cannot be obtained with other modalities, including MRI and SPECT; for example, this technique allows quantification of uptake, which can be used for prognostic purpose.
MRI cannot be performed in patients with cardiac pacemakers or when intracranial clips are present and is challenging in patients with claustrophobia. It is difficult to detect FHAVN after surgery using orthopedic hardware, which creates significant image distortion. F-18 fluoride PET/CT can also be performed when results of MRI are indeterminate.
Limitations of the study
The study group included patients with high suspicion of FHAVN referred from a tertiary orthopedic care, which might have increased the sensitivity of the study. Also, histopathology, which is gold standard in the diagnosis of AVN could not be obtained in all the cases, as it is invasive.
CONCLUSION
F-18 fluoride PET/CT showed good agreement with MRI in the initial diagnosis of FHAVN. It can be especially useful in detecting early disease and also in patients in whom MRI is contraindicated.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Mankin HJ. Non-traumatic necrosis of bone (osteonecrosis) N Engl J Med. 1992;281:473–9. doi: 10.1056/NEJM199205283262206. [DOI] [PubMed] [Google Scholar]
- 2.Lavernia CJ, Sierra RJ, Grieco FR. Osteonecrosis of the femoral head. J Am Acad Orthop Surg. 1999;7:250–61. doi: 10.5435/00124635-199907000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Steinberg ME, Hayken GD, Steinberg DR. A quantitative system for staging avascular necrosis. J Bone Joint Surg Br. 1995;77:34–41. [PubMed] [Google Scholar]
- 4.Mont MA, Hungerford DS. Non-traumatic avascular necrosis of the femoral head. J Bone Joint Surg Am. 1995;77:459–74. doi: 10.2106/00004623-199503000-00018. [DOI] [PubMed] [Google Scholar]
- 5.Arlet J. Nontraumatic avascular necrosis of the femoral head. Past, present, and future. Clin Orthop Relat Res. 1992;277:12–21. [PubMed] [Google Scholar]
- 6.Coombs RR, Thomas RW. Avascular necrosis of the hip. Br J Hosp Med. 1994;51:275–80. [PubMed] [Google Scholar]
- 7.Kenzora JE, Glimcher MJ. Accumulative cell stress: The multifactorial etiology of idiopathic osteonecrosis. Orthop Clin North Am. 1985;16:669–79. [PubMed] [Google Scholar]
- 8.Hungerford DS, Lennox DW. The importance of increased intraosseous pressure in the development of osteonecrosis of the femoral head: Implications for treatment. Orthop Clin North Am. 1985;16:635–54. [PubMed] [Google Scholar]
- 9.Brenner AI, Koshy J, Morey J, Lin C, DiPoce J. The bone scan. Semin Nucl Med. 2012;42:11–26. doi: 10.1053/j.semnuclmed.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Luk WH, Au-Yeung AW, Yang MK. Diagnostic value of SPECT versus SPECT/CT in femoral avascular necrosis: Preliminary results. Nucl Med Commun. 2010;31:958–61. doi: 10.1097/MNM.0b013e32833e7732. [DOI] [PubMed] [Google Scholar]
- 11.Segall G, Delbeke D, Stabin MG, Even-Sapir E, Fair J, Sajdak R, et al. SNM practice guideline for sodium 18F-fluoride PET/CT bone scans 1.0. J Nucl Med. 2010;51:1813–20. doi: 10.2967/jnumed.110.082263. [DOI] [PubMed] [Google Scholar]
- 12.Lee MJ, Corrigan J, Stack JP, Ennis JT. A comparison of modern imaging modalities in osteonecrosis of the femoral head. Clin Radiol. 1990;42:427–32. doi: 10.1016/s0009-9260(05)80900-6. [DOI] [PubMed] [Google Scholar]
- 13.Ruland LJ, Wang GJ, Teates CD, Gay S, Rijke A. A comparison of magnetic resonance imaging to bone scintigraphy in early traumatic ischemia of the femoral head. Clin Orthop Relat Res. 1992;285:30–34. [PubMed] [Google Scholar]
- 14.Beltran J, Burk JM, Herman LJ, Clark RN, Zuelzer WA, Freedy MR, et al. Avascular necrosis of the femoral head: Early MRI detection and radiological correlation. Magn Reson Imaging. 1987;5:431–42. doi: 10.1016/0730-725x(87)90377-8. [DOI] [PubMed] [Google Scholar]
- 15.Collier BD, Carrera GF, Johnson RP, Isitman AT, Hellman RS, Knobel J, et al. Detection of femoral head avascular necrosis in adults by SPECT. J Nucl Med. 1985;26:979–87. [PubMed] [Google Scholar]
- 16.Ryu JS, Kim JS, Moon DH, Kim SM, Shin MJ, Chang JS, et al. Bone SPECT is more sensitive than MRI in the detection of early osteonecrosis of the femoral head after renal transplantation. J Nucl Med. 2002;43:1006–11. [PubMed] [Google Scholar]
- 17.Mitchell MD, Kundel HL, Steinberg ME, Kressel HY, Alavi A, Axel L. Avascular necrosis of the hip: Comparison of MR, CT, and scintigraphy. AJR Am J Roentgenol. 1986;147:67–71. doi: 10.2214/ajr.147.1.67. [DOI] [PubMed] [Google Scholar]
- 18.Coleman BG, Kressel HY, Dalinka MK, Scheibler ML, Burk DL, Cohen EK. Radiographically negative avascular necrosis: Detection with MR imaging. Radiology. 1988;168:525–8. doi: 10.1148/radiology.168.2.3393676. [DOI] [PubMed] [Google Scholar]
- 19.Fordyce MJ, Solomon L. Early detection of avascular necrosis of the femoral head by MRI. J Bone Joint Surg Br. 1993;75:365–7. doi: 10.1302/0301-620X.75B3.8496201. [DOI] [PubMed] [Google Scholar]
- 20.Gayana S, Bhattacharya A, Kashyap R, Sen RK, Mittal BR. (18) F-fluoride PET/CT in avascular necrosis of the femoral head. Clin Nucl Med. 2013;38:e265–6. doi: 10.1097/RLU.0b013e318266d036. [DOI] [PubMed] [Google Scholar]
- 21.Attachment II. Sample Formats – Labeling for Ammonia N 13 Injection, Fludeoxyglucose F18 Injection [18F] FDG, and Sodium Fluoride F18 Injection. [Last accessed on 2007 Mar 19]. Available from: http://www.fda.gov/cder/guidance/labsample.pdf .